Abstract
Objective
To describe the effects of proprioceptive training on pain, stiffness, function, and functional test outcomes among patients with knee osteoarthritis (OA).
Data Sources
All studies completed from 1946 to 2017 were obtained from 4 databases (PubMed, MEDLINE, CINAHL, and SPORTDiscus).
Study Selection
Three reviewers independently identified appropriate studies and extracted data.
Data Extraction
Methodologic quality and level of evidence were assessed using the Physiotherapy Evidence Database scale and Oxford Centre for Evidence-Based Medicine guidelines. The standardized mean differences (SMDs) and 95% confidence intervals (CIs) were calculated for pain, stiffness, function, and functional test outcomes.
Data Synthesis
Seven randomized controlled trials involving 558 patients with knee OA met the inclusion criteria. The selected studies had Physiotherapy Evidence Database scores of 6 to 8. All randomized controlled trials had an Oxford Centre for Evidence-Based Medicine level of evidence of 2. Meta-analysis of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale (SMD = −0.56; 95% CI = −1.06, −0.07; P = .026), function subscale (SMD = −0.40; 95% CI = −0.59, −0.21; P < .001), and non-WOMAC walking speed test (SMD = −1.07; 95% CI = −2.12, −0.01; P = .048) revealed that proprioceptive training had significant treatment effects. Proprioceptive training was not associated with reductions in WOMAC stiffness subscale scores and did not improve non-WOMAC get-up-and-go scores.
Conclusions
Proprioceptive training effectively promoted pain relief and completion of functional daily activity among patients with knee OA and should be included in rehabilitation programs. Stiffness and other mobility measures were unchanged after proprioceptive training. Modified proprioceptive training programs are needed to target stiffness and improve additional physical function domains.
Keywords: knee, pain, exercise therapy, physical function, rehabilitation
Key Points
Proprioceptive training enhanced pain relief and physical function during activities of daily living in people with knee osteoarthritis.
Evidence-based proprioceptive training should include neuromuscular control elements, with coordinated trunk and lower extremity strengthening, at a frequency of 3 to 4 times per week, for 30 to 40 minutes per session.
Approximately 250 million people worldwide (3.6% of the population) have knee osteoarthritis (OA),1 which is characterized by degradation of the joint cartilage and underlying bone, leading to joint pain, stiffness, and physical disability.2 Knee OA is the most common form of arthritis associated with functional impairment in middle-aged and elderly people.2,3 Recommended interventions for patients with knee OA include combined nonmedical and medical therapies, with surgical intervention when needed.4 Nonmedical approaches, such as therapeutic exercise, changes in lifestyle, activity pacing, and weight loss, are intended to unburden the damaged joint.5
Recent studies6–12 suggested a potential association between impaired knee proprioception and pathologic changes during the early stages of knee OA. Proprioception is provided by proprioceptors in skeletal muscles, tendons, and the fibrous capsules in joints.13 As the knee muscles, tendons, ligaments, and joint capsules in the patients with knee OA become weakened and damaged, proprioceptive sensation can also decrease.13,14 Furthermore, proprioceptive impairments may predispose patients with knee OA to pain or disability.15,16 According to Smith et al14 and Knoop et al,17 articular mechanoreceptor impairment, muscle weakness with muscle-spindle sensitivity, inflammation, and a history of knee injuries, such as anterior cruciate ligament or meniscal injury, are factors that cause impaired proprioception in patients with knee OA.18,19 However, these authors14,17 acknowledged the limitations of the available evidence and lack of consensus regarding these factors.
Several researchers14,17 have summarized the evidence on the efficacy of proprioceptive or proprioceptive-type training for knee OA. Many studies14,20,21 lacked consistent exercise protocols or longitudinal outcome data. Well-constructed and well-powered randomized controlled trials (RCTs) on proprioceptive training for knee OA are lacking.14,17 In similar review studies,14,17 the effects of general proprioceptive training for knee OA were investigated. However, no specific proprioceptive training recommendations were made. To examine the effects of the intervention training in more detail, we applied the Oxford Centre for Evidence-Based Medicine (OCEBM) guidelines for the methodologic assessment of the recent RCTs and minimum clinically important difference (MCID) to evaluate clinical effects, followed by the meta-analysis of effectiveness outcomes of proprioceptive training. Therefore, the purpose of our meta-analysis of existing studies was to determine the effects of proprioceptive training on pain, stiffness, function, and functional test results in patients with knee OA.
METHODS
Data Sources and Search Strategy
We retrieved relevant studies from the electronic databases PubMed (1951 to the present), MEDLINE (via EBSCOhost; 1946 to the present), CINAHL (via EBSCOhost; 1981 to the present), and SPORTDiscus (via EBSCOhost; 1958 to the present). All databases were searched from their implementation to the present. The following search keywords were used: (pain OR KOOS OR Tegner OR WOMAC OR IKDC OR function) AND (OA OR osteoarthritis) AND knee AND (neuromuscular OR balance OR proprioceptive) AND (therapy OR exercise OR training) NOT surgery.
Inclusion Criteria
We examined full texts of relevant articles to determine their eligibility. Inclusion criteria were as follows: (1) RCT of the effects of proprioceptive training on knee OA; (2) English language; (3) adult patients with knee OA and reported outcome measures, including pain, stiffness, function, and mobility; and (4) ≥50 years of age, grade 3 of OA or lower on the Kellgren and Lawrence plain radiograph classification, and a history of chronic knee OA. The following patient outcome measures were identified for further analysis: (1) pain (visual analog scale, numeric rating scale, Western Ontario and McMaster Universities Osteoarthritis Index [WOMAC] scale, Knee Injury and Osteoarthritis Outcome Score [KOOS], and International Knee Documentation Committee [IKDC] Knee Forms), (2) stiffness (WOMAC, KOOS, and IKDC), (3) physical function questionnaire outcome (WOMAC, KOOS, IKDC, Tegner activity scale, and Short Form-36 Health Survey), and (4) physical function test (Berg Balance Scale, walking-speed timed test [WST], get-up-and-go [GUAG] test, chair stand test, and 6-minute walk test).
Exclusion Criteria
The exclusion criteria were as follows: (1) nonhuman participants, (2) a language other than English, (3) no full text available, or (4) lack of statistical data description. We excluded all duplicate papers. Reports involving participants who had undergone knee surgery or who had a history of rheumatoid arthritis were also excluded. Studies of whole-body vibration or water training were excluded. Because the number of studies was limited, we did not restrict them based on design (ie, RCTs, cohort, and case-control studies were included). Based on the criteria for OA of the knee by Altman et al,22 these studies were selected in the category of clinical examination and laboratory tests.
Study Selection
Three reviewers independently examined article titles and abstracts and excluded irrelevant studies. We assessed the remaining full-text articles to determine whether they fulfilled the inclusion criteria. A summary of the selected studies is presented in Table 1. Any disagreements regarding study selection and critical appraisal were resolved by further discussion, and only studies that achieved a clear consensus for inclusion were analyzed and reported.
Table 1.
Characteristics of Included Studies Continued on Next Page
| Trial |
Study Design, PEDro Scale, OCEBM Level |
Participant Characteristics |
Intervention, Control, and Follow-Up |
Outcome |
Main Conclusion |
| Rogers et al36 (2012) | Single-blind, block randomized, placebo-controlled clinical trial Patient blinded? Yes Therapist blinded? No Assessor blinded? No PEDro: 6 OCEBM: Level 2 | No. (M/F): 33 (13/20) I (PrT) = 8 C (ST) = 8 C (PrT + ST) = 9 C = 8 Dropout: 11 Age, y: I (KBA) = 70.7 ± 10.7 C (RT) = 70.8 ± 6.5 C (KBA+ RT) = 68.8 ± 10.1 C = 71.2 ± 10.9 | Intervention: 4 and 8 wk (3 times/wk, 30–40 min) PrT; wedding march,a backward wedding march,a high-knees march, side stepping, semitandem walk, tandem walk, crossover walk,a modified grapevine,a toe walking, heel walking, static balance,a dynamic balancea Control: 8 wk (3 times/wk, 30–40 min) 1. ST; Ankle extensiona and flexion, knee extensiona and flexion, hip abduction and adduction, internal and external rotation, leg pressa 2. Also used in PrT + STa 3. Apply a lotion (avoid self-massage) | WOMAC (pain), WOMAC (stiffness), WOMAC (physical function), WOMAC (total), HAP: MAS, HAP: AAS, SEE: POEE, SEE: NOEE, Knee instability (KOOS-ADLS) | PrT, ST, PrT + ST program were effective in improving symptoms and quality of life among individuals with knee OA; no differences between groups in WOMAC survey. |
| Duman et al37 (2012) | Randomized controlled trial Patient blinded? No Therapist blinded? Yes Assessor blinded? No PEDro: 6 OCEBM: Level 2 | No. (M/F): 54 (5/49) I = 30 C = 24 Dropout: 0 Age, y: 64 ± 3.7 | Intervention: 3 wk (5 times/wk), NSAID meloxicam 15 mg/d + physical therapy (infrared and short wave therapy) + PrT; quadriceps, ankle extension, hip abductor, bicycling, walking by making a 45° corner at every 2 steps (zigzag), walking forward and backward by heel to toe, walking sidelong to the right and left Control: 3 wk (5 times/wk) NSAID (meloxicam 15 mg/d) + physical therapy (infrared and short wave therapy) | Proprioception (left), Proprioception (right), static balance, dynamic balance, WOMAC (pain), WOMAC (stiffness), WOMAC (physical function), WOMAC (total) | PrT had beneficial effects on static balance and to some extent on proprioceptive accuracy. |
| Fitzgerald et al33 (2011) | Single-blind, randomized controlled trial Patient blinded? Yes Therapist blinded? No Assessor blinded? No PEDro: 8 OCEBM: Level 2 | No. (M/F): 183 (61/122) I = 91 (31/60) C = 92 (30/62) Dropout: 38 Age, y: I = 63.3 ± 8.9 C = 64.6 ± 8.4 | Intervention: 8, 26, and 52 wk (3 times/wk, at least 30 min), PrT + ST PrT consisted of side stepping, braiding activity, front and back crossover steps during forward ambulation, shuttle walking, multiple change in direction during walking on therapist's command, double-legged foam balance activity, tiltboard balance training, rollerboard and platform perturbations ST consisted of calf and hamstrings and prone quadriceps stretching, long-sitting knee flexion and extension, quadriceps setting, supine straight-leg raises, prone hip extensions, seated knee-extension isometrics, single-limb seated leg press, standing hamstrings curls with cuff weights, standing calf raises Control: 8, 26, and 52 wk (3 times/wk, at least 30 min) ST consisted of calf and hamstrings and prone quadriceps stretching, long-sitting knee flexion and extension, quadriceps setting, supine straight-leg raises, prone hip extensions, seated knee-extension isometrics, single-limb seated leg press, standing hamstrings curls with cuff weights, standing calf raises | WOMAC (total), WOMAC (PF), NRS, get-up-and-go test, Knee instability (KOOS-ADLS) | No differences were present between groups with respect to these outcomes; no reduction in knee pain or improvement in performance-based function in either group. |
| Lin et al34 (2009) | Randomized clinical trial Patient blinded? No Therapist blinded? No Assessor blinded? Yes PEDro: 8 OCEBM: Level 2 | No. (M/F): 108 (33/75) I (PrT) = 36 (11/25) C (ST) = 36 (12/24) C = 36 (10/26) Dropout: 5 Age, y: I (PrT) = 63.7 ± 8.2 C (ST) = 61.6 ± 7.2 C = 62.2 ± 6.7 | Intervention: 8 wk (3 times/wk) PrT using a designed computer game foot-stepping exercise (sitting position), multiple directions (up, down, left, and right), × each 20 min × 10-min break Control: 8 wk (3 times/wk) 1. ST; full knee extension using dynamometer cable × 6 reps (per set) × 4 sets 2. No specific intervention | WOMAC (pain), WOMAC (physical function), Walking time (ground level), Walking time (stair), Walking time (spongy surface), Reposition error | The PrT and ST improved outcomes. The PrT led to greater improvements in proprioceptive function, whereas ST resulted in a greater increase in knee-extensor muscle strength. |
| Chaipinyo and Karoonsupcharoen35 (2009) | Randomized trial Patient blinded? No Therapist blinded? No Assessor blinded? Yes PEDro: 7 OCEBM: Level 2 | No. (M/F): 48 (11/31) I = 24 (9/15), C = 24 (2/22) Dropout: 6 Age, y: I = 62 ± 6 C = 70 ± 6 | Intervention: 4 wk (5 times/wk) PrT; forward, backward, and sideward step × 30 reps, bilateral mini squat × 10 reps Control: 4 wk (5 times/wk) ST Isometric knee-extension exercise × 10 reps (per set) × 5-s hold × 3 sets | KOOS (pain), KOOS (symptoms), KOOS (function in daily living), KOOS (function in sport/ recreation), KOOS (knee-related QOL), Strength (knee extensor, involved), Strength (knee extensor, uninvolved), Strength (knee flexor, involved), Strength (knee flexor, uninvolved), Mobility (walk 15 m), Mobility (get-up-and-go), Mobility (walk upstairs), Mobility (walk downstairs), | Pain, symptoms, strength, walks, and stair climbing did not differ between PrT and ST in patients with knee OA. |
| Diracoglu et al38 (2008) | Blinded, randomized controlled trial and long-term follow up clinical investigation Patient blinded? No Therapist blinded? No Assessor blinded? Yes PEDro: 6 OCEBM: Level 2 | No.: 66 (all women) I = 32 C = 28 Dropout: 6 Age, y: I = 50.3 ± 6.5 C = 50.8 ± 7.9 | Intervention: 8 and 52 wk (3 times/wk), 1-y follow-up PrT; Modified Romberg exercise (hard and soft ground), retrowalking, walking on heel and toes, walking (eyes closed), standing on 1 leg (eyes open, closed), rocker-bottom balance, sitting and standing chair, plyometric exercise (cross jumping), wide and narrow circle walking, BAPS board balance, mini trampoline (jumping and jogging), Carioca crossover maneuver Control: 8 and 52 wk (3 times/wk), 1-y follow-up ST Bike, range of motion and active stretching, isometric exercise (quads and hamstring, 6-s hold × 8 reps, 2-s rest), knee short-arc terminal extension, isometric exercise (hip joint abductors and adductors), isotonic exercise (hamstrings, 10 reps ×1 sets) | WOMAC (pain), WOMAC (stiffness), WOMAC (physical function) WOMAC (total) SF-36 Vitality | Performed for 1 y, PrT seemed to be superior to strengthening exercises only with respect to WOMAC categories in women with mild to moderate knee OA. |
| Diracoglu et al39 (2005) | Randomized controlled trial Patient blinded? No Therapist blinded? No Assessor blinded? No PEDro: 6 OCEBM: Level 2 | No.: 66 (all women) I = 32 C = 28 Dropout: 6 Age, y: I = 50.3 ± 6.5 C = 50.8 ± 7.9 | Intervention: 8 wk (3 times/wk) PrT; Modified Romberg exercise (hard and soft ground), retrowalking, walking on heel and toes, walking (eyes closed), standing on 1 leg (eyes open, closed), rocker-bottom balance, sitting and standing chair, plyometric exercise (cross jumping), wide and narrow circle walking, BAPS board balance, mini trampoline (jumping and jogging), carioca-crossover maneuver Control: 8 wk (3 d/wk) ST Bike, range of motion and active stretching, isometric exercise (quadriceps and hamstrings, 6-s hold × 8 reps, 2-s rest), knee short-arc terminal extension, isometric exercise (hip joint abductors and adductors), isotonic exercise (hamstrings, 10 reps × 1 set) | WOMAC (physical function), SF-36 (physical function), SF-36 (role-limitation, physical), SF-36 (vitality, energy, or fatigue), 10-m walking, Isokinetic muscle test | The PrT could improve functional status (WOMAC–physical function value, SF-36 Form [physical function, role limitations–physical and vitality–energy or fatigue variables]). |
Abbreviations: AAS, adjusted activity score; BAPS, Biomechanical Ankle Platform System; C, control; F, female; HAP, Human Activity Profile; I, intervention; JPS, joint position sense; KBA, kinesthesia balance agility; KOOS, Knee Injury and Osteoarthritis Outcome score; M, male; MAS, maximum activity score; NOEE, negative outcome expectancy for exercise; NRS, numeric rating scale; NSAID, nonsteroidal anti-inflammatory drug; OCEBM, Oxford Centre for Evidence-Based Medicine; PEDro, Physiotherapy Evidence Database; POEE, positive outcome expectancy for exercise; PrT, proprioceptive training; rep, repetition; RT, resistance training; SEE, self-efficacy for exercise; SF-36, Short-Form Health Survey; ST, strength training; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Indicates exercise only in PrT and ST.
Methodologic Quality Assessment
We used the Physiotherapy Evidence Database (PEDro) Scale and OCEBM guidelines. These tools have high reliability and validity and were, therefore, used to assess the methodologic quality of the individual RCTs.23,24 The OCEBM levels of evidence are arranged in a ranking system to describe the strength of the results for use in evidence-based practice.25
Data Extraction and Analysis
MedCalc software (version 17.2; MedCalc, Mariakerke, Belgium) was used for the meta-analysis. We calculated the standardized mean differences (SMDs) and 95% confidence intervals (CIs) from the postintervention means and standard deviations reported for each study.26 The SMD, a measure of the effect size, is the mean divided by the standard deviation of the difference between the values of the 2 groups. The Cohen interpretation of the SMD statistic is that a value of 0.2 indicates a small effect; 0.5, medium effect; and ≥0.8, a large effect.27 When the authors did not report standard deviations, we converted 95% CIs and standard errors to standard deviations.28 The Cochran Q and I2 test were performed to examine the heterogeneity (homogeneity) of the selected studies. When Q is larger than its expected value E[Q] under the null hypothesis of no heterogeneity, the difference Q – E[Q] can be used to obtain the best estimate of heterogeneity.29 A test to quantify heterogeneity, I2 ranges from 0% to 100%; the higher the percentage, the greater the heterogeneity. It is interpreted as follows; 20% to 50%, low; 50% to 75%, moderate; and >75%, high heterogeneity. The random-effects model was adopted to combine the studies when the I2 was significant (P < .05). Otherwise, the fixed-effects model was adopted.30 We set an a priori α level of .05 for between-groups differences, regardless of variable follow-up times. The MCID was calculated using distribution-based approaches.31
RESULTS
Literature Search
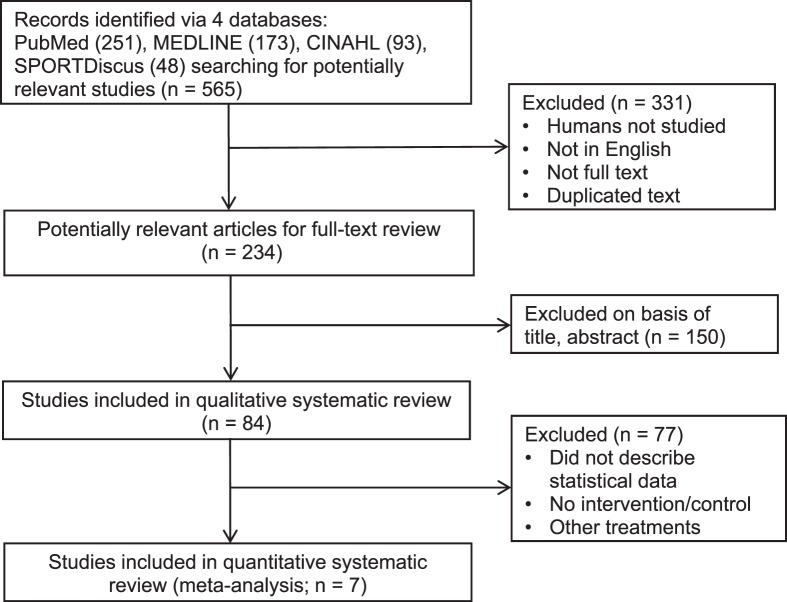
The flowchart presented in Figure 1 follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).32 The first search identified a total of 565 relevant studies. After exclusions, 7 studies (558 patients) were included in this meta-analysis.
Figure 1.
Search strategy and flowchart of this meta-analysis.
Methodologic Quality Assessment
All 7 studies received good-quality PEDro scores (≥6; Table 1). Three studies33–35 had scores of 8, which indicates high methodologic quality, and 4 studies36–39 had scores of 6. All studies displayed OCEBM level of evidence of 2, and acceptable random allocation and baseline homogeneity were reported. Participants were blinded in 2 of the selected studies, and assessors were blinded in 5 of the selected studies (Table 1). The methodologic limitations of each study are described in Table 2. All investigators reported between-groups comparisons and point estimates. Five studies33–35,38,39 used intention-to-treat analyses.
Table 2.
Allocation Concealment and Blinding
| Concealment and Blinding |
Rogers et al36 (2012) |
Duman et al37 (2012) |
Fitzgerald et al33 (2011) |
Lin et al34 (2009) |
Chaipinyo and Karoonsupcharoen35 (2009) |
Diracoglu et al38 (2008) |
Diracoglu et al39 (2005) |
| Random allocation of the subjects? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Allocation was concealed? | Yes | No | Yes | Yes | Yes | No | No |
| Blinding of all participants? | Yes | Yes | No | No | No | No | No |
| Blinding of all therapists? | No | No | No | No | No | No | No |
| Blinding of all assessors? | No | Yes | Yes | Yes | Yes | Yes | No |
Pain
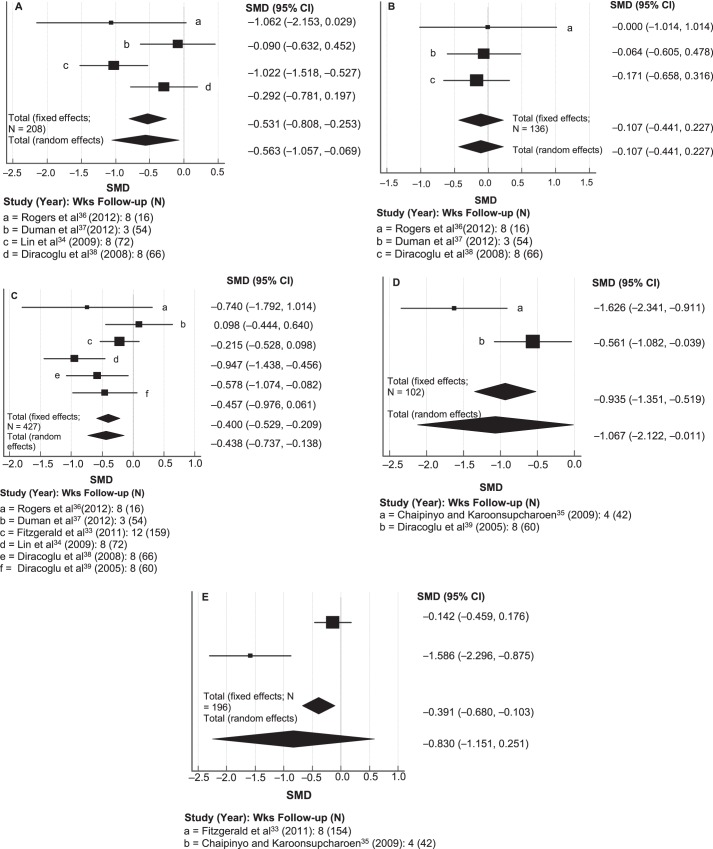
Investigators in 4 studies34,36–38 used the WOMAC pain subscale to measure the effects of proprioceptive training on self-reported pain. The meta-analysis for the effect of the intervention on pain reduction is presented via a forest plot in Figure 2A. Because Q testing showed P < .05 for pain, we used a random-effects model (N = 208, Q = 8.63, p for heterogeneity = 0.035, I2 = 65%; P = .026). A moderately strong negative effect size was found for pain (SMD = −0.56), indicating postintervention pain alleviation.27 Because the CI of the effect size did not cross zero, proprioceptive training appeared to be effective in decreasing pain among patients with knee OA. The MCID ranged from 0.3 to 2.1 for pain in each study.
Figure 2.
Forest plots of the meta-analysis for the standardized mean difference (SMD) in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) for A, pain, B, WOMAC stiffness, C, WOMAC physical function, D, walking speed time, and E, get-up and go for proprioceptive training. Abbreviation: CI, confidence interval.
Stiffness
The authors of 3 studies36–38 used the WOMAC stiffness subscale as an outcome measure to assess the effects of proprioceptive training on self-reported stiffness. The meta-analysis for the effect of the intervention on stiffness reduction is presented via a forest plot in Figure 2B. Because Q testing resulted in P > .05 for stiffness, a fixed-effects model was used (N = 136, Q = 0.15, P for heterogeneity = .93, I2 = 0.0%; P = .528). The effect size for stiffness was small (SMD = −0.11), indicating less stiffness postintervention.27 However, this value did not achieve statistical significance. The MCID ranged from 0.2 to 1.2 for stiffness in each study.
Physical Function
Researchers in 6 studies33,34,36–39 used the WOMAC physical function subscale as an outcome measure to assess the effects of proprioceptive training on self-reported physical function. The meta-analysis for the effect of the intervention on physical function is presented via a forest plot in Figure 2C. Because Q testing demonstrated P > .05 for physical function, we used a fixed-effects model (N = 427, Q = 10.74, P for heterogeneity = .057, I2 = 53%; P < .001). A moderately strong negative effect size was observed for physical function (SMD = −0.40), indicating improved postintervention physical function among patients with knee OA.27 The MCID ranged from 0.2 to 1.4 for physical function in each study.
Physical Function Tests
Investigators in 2 studies35,39 used the 10-m or 15-m WST to assess the effects of proprioceptive training on physical function and mobility. The meta-analysis for the effect of the intervention on physical function (walking speed) is presented via a forest plot in Figure 2D. Because Q testing showed P < .05 for physical function, a random-effects model was used (N = 102, Q = 5.89, P for heterogeneity = .015, I2 = 83%; P = .048). The effect size for physical function was large (SMD = −1.07), and the CI of the effect size did not cross zero, indicating improved physical functioning postintervention.27 The MCID ranged from 0.3 to 1.1 for WST in each study.
Researchers in 2 studies33,35 used the GUAG test to assess the effects of proprioceptive training on mobility. The meta-analysis for the effect of the intervention in improving physical function (GUAG test) is presented via a forest plot in Figure 2E. Because Q testing resulted in P < .05 for mobility, we used a random-effects model (N = 196, Q = 13.96, P for heterogeneity = .0002, I2 = 93%; P = .251). The effect size for mobility was large (SMD = −0.83), indicating improved mobility postintervention.27 However, because the CI of the effect size crossed zero, proprioceptive training did not significantly improve mobility. The MCID ranged from 0.3 to 0.3 for the GUAG category in each study.
DISCUSSION
The major findings of this meta-analysis were that proprioceptive training seemed to alleviate pain and improve the walking speed of patients with knee OA. All selected studies and patient outcomes demonstrated an OCEBM level of evidence of 2, and methodologic qualities were moderate. Proprioceptive training protocols included in this meta-analysis involved neuromuscular control and functional elements with both weight-bearing and non–weight-bearing tasks.33–39 Our WOMAC-based findings were as follows: proprioceptive training may be effective in reducing pain (P < .001) and improving the physical function (P = .002) of patients with knee OA. However, it may not reduce stiffness. Proprioceptive training may improve WST scores (P = .048) but may not improve mobility as measured by the GUAG test.
The RCTs included in this review involved patients older than 50 years. Data from patients who had undergone knee surgery or had rheumatoid arthritis were excluded. In other words, only patients with degenerative knee OA were investigated, not patients who developed OA as a result of joint trauma. Therefore, it is difficult to determine whether the effects of the proprioceptive training recommended in this review can be applied to patients with OA that developed after joint trauma or knee surgery. The effects of proprioceptive training for patients with OA due to joint trauma or surgery remain unclear because of pathologic differences.
Results on the WOMAC pain subscale may improve after proprioceptive training (Figure 2A). Pain is a major symptom in patients with knee OA and is commonly described as a sharp ache or burning sensation in the muscles and tendons. Pain typically worsens with prolonged activity and exercise.22,40,41 This pain may result from muscle weakness, OA-related inflammation and effusion, impaired articular mechanoreceptors, and previous ligamentous or meniscal injury.17,19 Proprioceptive training seems to be helpful in relieving pain among patients with knee OA.42 Foot-stepping exercises involving upward, downward, leftward, and rightward movements in a weight-bearing or non–weight-bearing (ie, seated) position, 3 times per week, for 30 to 40 minutes per session, is suggested for relieving pain in patients with knee OA.34,36,38 Therefore, for pain relief, proprioceptive training may be more helpful than strength training and not exercising for patients with knee OA.43 The intensity, frequency, and time of training probably affect lower extremity strength and activate neuromuscular metabolism to relieve pain.44 Irrespective of the other exercises performed, proprioceptive training including moderate-intensity foot-stepping alleviated pain. However, future authors should determine which combinations are most effective. The moderate effect size indicates that pain may not be dramatically reduced through this proprioceptive intervention.
Also, the WOMAC stiffness subscale scores may not be affected by proprioceptive training (P = .528, Figure 2B). Stiffness, particularly in the morning or after sitting for long periods, is a major symptom in patients with knee OA and typically lasts >30 minutes after beginning daily activities and returns after periods of inactivity.22 Bone swelling and friction resulting from knee-joint narrowing make the knee stiff and less flexible.45 Increased stiffness reduces the range of motion of the knee joint among patients with knee OA; those with moderate to advanced knee OA may find it difficult to straighten the knee.45 Knee OA limits performance during activities of daily living, including walking and running. Therefore, proprioceptive training of moderate intensity and frequency may help alleviate stiffness. In our analysis, this effect did not achieve statistical significance (Table 1). Large-cohort longitudinal studies of proprioceptive training will increase our understanding of the effects of this intervention. They should address stiffness in patients with knee OA or use another intervention if the treatment goal is to reduce stiffness due to a small effect size. Further research should be conducted to investigate the effectiveness of the intervention on joint stiffness.
The review revealed that the physical function of patients with knee OA might be significantly improved (P = .002, Figure 2C). The MCID for proprioceptive training was in the small range for the physical function of patients with OA. Knee OA limits physical activity because of pain and stiffness, and proprioceptive training may improve physical function by alleviating these symptoms.14,34 Proprioceptive training improved neuromuscular coordination for sensorimotor learning.34,38,46 Moreover, repeated proprioceptive training with functional elements increased cumulative neural inputs to the central nervous system via mechanoreceptors and proprioceptors in the joint capsules, ligaments, muscles, tendons, and skin.44,47,48 Konradsen et al49 reported that afferent inputs from muscles and tendons were more important than those from ligamentous mechanoreceptors, possibly because of mechanical stabilization; accordingly, in a number of studies, proprioceptive training was implemented to address neuromuscular control and coordination elements. Proprioceptive training programs should include personalized neuromuscular-control and balance exercises with a walking motion to improve proprioception in patients with knee OA.34,38
The WST and GUAG tests assess functional mobility. Proprioceptive training may have improved WST scores (P = .048, Figure 2D). This training focused on dynamic movements, such as forward, backward, and side steps, rather than isometric exercises.35,39 To test functional mobility, the GUAG requires both leg strength and gait speed. Proprioceptive training had notable but nonsignificant effects on functional mobility (P = .494, Figure 2E). Considerable statistical heterogeneity existed between the 2 studies that used the GUAG test. The MCID of proprioceptive training was in the small range for the WST and GUAG scores of patients with OA. In both studies,35,39 the sample sizes were too small to generalize the findings. Larger-cohort RCTs are recommended for continued exploration of these effects. Proprioceptive training improved the speed and motion of upright walking in patients with knee OA, even though it did not have a strength-training component. Although more work is needed to explain these findings, proprioceptive training with both dynamic exercise and coordinated trunk and lower extremity strength exercises, such as half-squats or half-lunges, may be more beneficial than simple dynamic exercises, such as footsteps.33–35,39
Our review had several limitations. First, the most common methodologic drawback of the included studies was the lack of patient and therapist blinding. Several investigations involved small sample sizes. Few patient outcomes could be analyzed in the context of a meta-analysis. Even though we considered the outcomes of the WST and GUAG tests from the Chaipinyo and Karoonsupcharoen35 and Diracoglu et al39 studies as reflecting proprioceptive interventions in our meta-analysis, fewer than 33 patients were in each group, so it may be difficult to generalize the finding of the functional mobility outcomes. In the future, researchers should include a large number of patients in the intervention group. More homogeneity (of patients and outcome measurements) should provide further evidence of the effectiveness of proprioceptive training. In addition, authors of the included studies did not provide insights about the long-term effects of proprioceptive training, but some noted that patients who performed proprioceptive training displayed better walking on soft surfaces than those who underwent strength training. This area needs further research.
Despite these limitations, our review has implications for clinical practice. Proprioceptive training consisting of a special exercise program intended to improve kinesthetic sensation and movement in the lower extremities enhanced functional movement. Improving proprioception in patients with knee OA may have positive effects on pain relief and daily activities.
CONCLUSIONS
Among people with knee OA, proprioceptive training may relieve pain and improve physical function during activities of daily living. This training should include neuromuscular-control elements, with coordinated trunk and lower extremity strengthening, at an average frequency of 3 to 4 times per week for 30 to 40 minutes per session. Under these conditions, proprioceptive training may improve the physical function of patients with knee OA. Although proprioceptive training was in the low range of MCID, it may be a useful exercise program for preventing adverse clinical symptoms associated with knee OA. Randomized controlled trials with methodologic controls should build on existing evidence. Long-term follow-up studies with standardized outcomes in a homogeneous cohort of patients with knee OA at the first clinic visit will advance our understanding of proprioceptive training.
Recommended Proprioceptive Training for Knee OA
An average of 3 to 4 times per week, 30 to 40 minutes per session
Foot stepping using the leg-press machine (sitting position, multiple-direction steps)
Modified Romberg exercise (hard and soft ground with eyes closed)
Standing on 1 leg (eyes open and closed)
Walking on heel and toes (forward, backward, left, right, carioca crossover with eyes open and closed)
Half squat on soft ground (standard and side-step position)
Knee-flexion and -extension exercise (sitting position with chair and TheraBand [Akron, OH])
Biomechanical Ankle Platform System (AliMed Inc, Dedham, MA) board balance and mini trampoline (jumping and jogging)
ACKNOWLEDGMENTS
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2015S1A5B8036349) and partly supported by the Yonsei Institute of Sports Science and Exercise Medicine (YISSEM 2015-51-0455), which is an International Olympic Committee International Research Centre for Prevention of Injury and Protection of Athlete Health.
REFERENCES
- 1.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990−2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinstein SL, Jacobs JJ, Goldberg MJ. Osteoarthritis of the knee. N Engl J Med. 2006;354(23):2508–2509. doi: 10.1056/NEJMc060815. [DOI] [PubMed] [Google Scholar]
- 3.Duncan R, Peat G, Thomas E, Hay EM, Croft P. Incidence, progression and sequence of development of radiographic knee osteoarthritis in a symptomatic population. Ann Rheum Dis. 2011;70(11):1944–1948. doi: 10.1136/ard.2011.151050. [DOI] [PubMed] [Google Scholar]
- 4.Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64(4):465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Felson DT, Gross KD, Nevitt MC, et al. The effects of impaired joint position sense on the development and progression of pain and structural damage in knee osteoarthritis. Arthritis Rheum. 2009;61(8):1070–1076. doi: 10.1002/art.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lund H, Juul-Kristensen B, Hansen K, et al. Movement detection impaired in patients with knee osteoarthritis compared to healthy controls: a cross-sectional case-control study. J Musculoskelet Neuron Interact. 2008;8(4):391–400. [PubMed] [Google Scholar]
- 8.Bayramoglu M, Toprak R, Sozay S. Effects of osteoarthritis and fatigue on proprioception of the knee joint. Arch Phys Med Rehabil. 2007;88(3):346–350. doi: 10.1016/j.apmr.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Bennell KL, Hinman RS, Metcalf BR. Association of sensorimotor function with knee joint kinematics during locomotion in knee osteoarthritis. Am J Phys Med Rehabil. 2004;83(6):455–463. doi: 10.1097/00002060-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Sharma L. Proprioceptive impairment in knee osteoarthritis. Rheum Dis Clin North Am. 1999;25(2):299–314. doi: 10.1016/s0889-857x(05)70069-7. vi. [DOI] [PubMed] [Google Scholar]
- 11.Hurley MV, Scott DL. Improvements in quadriceps sensorimotor function and disability of patients with knee osteoarthritis following a clinically practicable exercise regime. Br J Rheumatol. 1998;37(11):1181–1187. doi: 10.1093/rheumatology/37.11.1181. [DOI] [PubMed] [Google Scholar]
- 12.Barrett DS, Cobb AG, Bentley G. Joint proprioception in normal, osteoarthritic and replaced knees. J Bone Joint Surg Br. 1991;73(1):53–56. doi: 10.1302/0301-620X.73B1.1991775. [DOI] [PubMed] [Google Scholar]
- 13.Olsson L, Lund H, Henriksen M, Rogind H, Bliddal H, Danneskiold-Samsøe B. Test–retest reliability of a knee joint position sense measurement method in sitting and prone position. Adv Physiother. 2004;6(1):37–47. [Google Scholar]
- 14.Smith TO, King JJ, Hing CB. The effectiveness of proprioceptive-based exercise for osteoarthritis of the knee: a systematic review and meta-analysis. Rheumatol Int. 2012;32(11):3339–3351. doi: 10.1007/s00296-012-2480-7. [DOI] [PubMed] [Google Scholar]
- 15.van Dijk GM, Dekker J, Veenhof C, van den Ende CH, Carpa Study Group Course of functional status and pain in osteoarthritis of the hip or knee: a systematic review of the literature. Arthritis Rheum. 2006;55(5):779–785. doi: 10.1002/art.22244. [DOI] [PubMed] [Google Scholar]
- 16.Bennell KL, Hinman RS, Metcalf BR, et al. Relationship of knee joint proprioception to pain and disability in individuals with knee osteoarthritis. J Orthop Res. 2003;21(5):792–797. doi: 10.1016/S0736-0266(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 17.Knoop J, Steultjens MP, van der Leeden M, et al. Proprioception in knee osteoarthritis: a narrative review. Osteoarthritis Cartilage. 2011;19(4):381–388. doi: 10.1016/j.joca.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Pietrangelo T, Mancinelli R, Toniolo L, et al. Effects of local vibrations on skeletal muscle trophism in elderly people: mechanical, cellular, and molecular events. Int J Mol Med. 2009;24(4):503–512. doi: 10.3892/ijmm_00000259. [DOI] [PubMed] [Google Scholar]
- 19.Hurley MV. The effects of joint damage on muscle function, proprioception and rehabilitation. Man Ther. 1997;2(1):11–17. doi: 10.1054/math.1997.0281. [DOI] [PubMed] [Google Scholar]
- 20.Fransen M, McConnell S. Land-based exercise for osteoarthritis of the knee: a metaanalysis of randomized controlled trials. J Rheumatol. 2009;36(6):1109–1117. doi: 10.3899/jrheum.090058. [DOI] [PubMed] [Google Scholar]
- 21.Farrar EK, Mitchell H. Osteoarthritis and exercise: a review of the literature. J S C Med Assoc. 2009;105(1):8–11. [PubMed] [Google Scholar]
- 22.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 23.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–721. [PubMed] [Google Scholar]
- 24.de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129–133. doi: 10.1016/s0004-9514(09)70043-1. [DOI] [PubMed] [Google Scholar]
- 25.The Oxford levels of evidence 2. Oxford Centre for Evidence-Based Medicine Web site. 2015 http://www.cebm.net/index.aspx?o55653 Accessed November 23.
- 26.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillside, NJ: Lawrence Earlbaum Associates;; 1988. [Google Scholar]
- 28.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions: General Methods for Cochrane Reviews Version 5.1.0. Oxford, UK: Cochrane;; 2011. [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Copay AG, Subach BR, Glassman SD, Polly DW, Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541–546. doi: 10.1016/j.spinee.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald GK, Piva SR, Gil AB, Wisniewski SR, Oddis CV, Irrgang JJ. Agility and perturbation training techniques in exercise therapy for reducing pain and improving function in people with knee osteoarthritis: a randomized clinical trial. Phys Ther. 2011;91(4):452–469. doi: 10.2522/ptj.20100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin DH, Lin CH, Lin YF, Jan MH. Efficacy of 2 non-weight-bearing interventions, proprioception training versus strength training, for patients with knee osteoarthritis: a randomized clinical trial. J Orthop Sports Phys Ther. 2009;39(6):450–457. doi: 10.2519/jospt.2009.2923. [DOI] [PubMed] [Google Scholar]
- 35.Chaipinyo K, Karoonsupcharoen O. No difference between home-based strength training and home-based balance training on pain in patients with knee osteoarthritis: a randomised trial. Aust J Physiother. 2009;55(1):25–30. doi: 10.1016/s0004-9514(09)70057-1. [DOI] [PubMed] [Google Scholar]
- 36.Rogers MW, Tamulevicius N, Semple SJ, Krkeljas Z. Efficacy of home-based kinesthesia, balance & agility exercise training among persons with symptomatic knee osteoarthritis. J Sports Sci Med. 2012;11(4):751–758. [PMC free article] [PubMed] [Google Scholar]
- 37.Duman I, Taskaynatan MA, Mohur H, Tan AK. Assessment of the impact of proprioceptive exercises on balance and proprioception in patients with advanced knee osteoarthritis. Rheumatol Int. 2012;32(12):3793–3798. doi: 10.1007/s00296-011-2272-5. [DOI] [PubMed] [Google Scholar]
- 38.Diracoglu D, Baskent A, Celik A, Issever H, Aydin R. Long-term effects of kinesthesia/balance and strengthening exercises on patients with knee osteoarthritis: a one-year follow-up study. J Back Musculoskelet Rehabil. 2008;21(4):253–262. [Google Scholar]
- 39.Diracoglu D, Aydin R, Baskent A, Celik A. Effects of kinesthesia and balance exercises in knee osteoarthritis. J Clin Rheumatol. 2005;11(6):303–310. doi: 10.1097/01.rhu.0000191213.37853.3d. [DOI] [PubMed] [Google Scholar]
- 40.de Figueiredo EC, Figueiredo GC, Dantas RT. Influence of meteorological elements on osteoarthritis pain: a review of the literature. Rev Bras Reumatol. 2011;51(6):622–628. [PubMed] [Google Scholar]
- 41.Arden N, Blanco F, Cooper C, et al. Atlas of Osteoarthritis. London, UK: Springer; 2014. [Google Scholar]
- 42.Skou ST, Roos EM. Good Life with osteoArthritis in Denmark (GLA: D™): evidence-based education and supervised neuromuscular exercise delivered by certified physiotherapists nationwide. BMC Musculoskelel Disord. 2017;18(1):72. doi: 10.1186/s12891-017-1439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;1:CD004376. doi: 10.1002/14651858.CD004376.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashton-Miller JA, Wojtys EM, Huston LJ, Fry-Welch D. Can proprioception really be improved by exercises? Knee Surg Sports Traumatol Arthrosc. 2001;9(3):128–136. doi: 10.1007/s001670100208. [DOI] [PubMed] [Google Scholar]
- 45.Threlkeld AJ, Currier DP. Osteoarthritis: effects on synovial joint tissues. Phys Ther. 1988;68(3):364–370. doi: 10.1093/ptj/68.3.364. [DOI] [PubMed] [Google Scholar]
- 46.Tsauo JY, Cheng PF, Yang RS. The effects of sensorimotor training on knee proprioception and function for patients with knee osteoarthritis: a preliminary report. Clin Rehabil. 2008;22(5):448–457. doi: 10.1177/0269215507084597. [DOI] [PubMed] [Google Scholar]
- 47.Lin DH, Lin YF, Chai HM, Han YC, Jan MH. Comparison of proprioceptive functions between computerized proprioception facilitation exercise and closed kinetic chain exercise in patients with knee osteoarthritis. Clin Rheumatol. 2007;26(4):520–528. doi: 10.1007/s10067-006-0324-0. [DOI] [PubMed] [Google Scholar]
- 48.Hoogenboom BJ, Voight ML, Prentice WE. Musculoskeletal Interventions: Techniques for Therapeutic Exercise. New York, NY: McGraw Hill Professional;; 2014. [Google Scholar]
- 49.Konradsen L, Ravn JB, Sorensen AI. Proprioception at the ankle: the effect of anaesthetic blockade of ligament receptors. J Bone Joint Surg Br. 1993;75(3):433–436. doi: 10.1302/0301-620X.75B3.8496215. [DOI] [PubMed] [Google Scholar]