Abstract
Context
In longitudinal studies tracking recovery after concussion, researchers often have not considered the timing of return to play (RTP) as a factor in their designs, which can limit the understanding of how RTP may affect the analysis and resulting conclusions.
Objective
To evaluate the recovery of balance and gait in concussed athletes using a novel linear mixed-model design that allows an inflection point to account for changes in trend that may occur after RTP.
Design
Cohort study.
Setting
University athletics departments, applied field setting.
Patients or Other Participants
Twenty-three concussed (5 women, 18 men; age = 20.1 ± 1.3 years) and 25 healthy control (6 women, 19 men; age = 20.9 ± 1.4 years) participants were studied. Participants were referred by their team athletic trainers.
Main Outcome Measure(s)
Measures consisted of the Balance Error Scoring System (BESS) total score, sway (instrumented root mean square of mediolateral sway), single-task gait speed, gait speed while simultaneously reading a handheld article (dual-task gait speed), dual-task cost of reading on gait speed, and dual-task cost of walking on reading.
Results
We observed no significant effects or interactions for the BESS. Instrumented sway was worse in concussed participants, and a change in the recovery trend occurred after RTP. We observed group and time effects and group × time and group × RTP change interactions (P ≤ .046). No initial between-groups differences were found for single-task or dual-task gait. Both groups increased gait speed initially and then leveled off after the average RTP date. We noted time and RTP change effects and positive group × time interactions for both conditions (P ≤ .042) and a group × RTP change interaction for single-task gait speed (P = .005). No significant effects or interactions were present for the dual-task cost of reading on gait speed or the dual-task cost of walking on reading.
Conclusions
Changes in the rate of recovery were coincident with the timing of RTP. Although we cannot suggest these changes were a result of the athletes returning to play, these findings demonstrate the need for further research to evaluate the effects of RTP on concussion recovery.
Keywords: return to sport, wearable, inertial sensors, mild traumatic brain injury, postural control
Key Points
The standard and subjective Balance Error Scoring System scores did not differ between groups.
The more sensitive inertial sensor measurement indicated greater sway in the concussed group.
Sway appeared to improve initially in the concussed group and then regress without returning to normal during the 8-week study period.
Investigators should conduct more in-depth research of the effects of return to play on recovery from concussion and other injuries.
Clinicians should account for the return-to-play date when evaluating the recovery trajectory after concussion.
Research on the effects of sport-related concussion has increased over the last decade as investigators search for better ways to evaluate, diagnose, and track recovery in patients. Despite increased study, the timing of safe return to play (RTP) is still debated due to conflicting evidence associated with the recovery of clinical, physiological, and neuromotor signs and symptoms. Specifically, self-reported, subjective concussion symptoms have been observed to resolve, on average, within 6 days of injury in collegiate athletes.1 This timeline contrasts with objective findings from studies2–4 in which authors have reported subtle but persistent physiological and neuromotor deficits. Different findings across investigations may be a product of diverse experimental procedures, outcome measures, and statistical designs. Including clinically important time points, such as RTP, during analysis may affect the results and interpretations. Moreover, from an applied perspective, understanding the effect of returning to preinjury levels of play on recovery is an important step in guiding clinical protocols.
Cognitive recovery after sport-related concussion has been reported to occur within 5 to 10 days.5–7 However, some investigators have indicated a longer time course. Within-subject differences across cognitive domains (eg, reaction time) returned to baseline levels between 14 and 21 days in adolescents,8 whereas between-groups differences in reaction time relating to executive function were found in adolescents9 and adults10 up to 2 months postinjury when compared with control individuals. Researchers also have reported between-groups differences at an average of 1.6 years after concussion for simple and procedural reaction time, mathematical processing, spatial processing, and reaction time relating to executive function11 and up to 6 years postinjury across measures of memory, attention, and executive function.4
A similar pattern of evidence has been presented for postural control. In an early investigation, the authors12 observed that, when assessed clinically, balance returned to preinjury levels in adults within 3 days of injury. In contrast, signs of balance deficits in adolescents have been demonstrated within 10 days of injury and approximately 3 to 4 weeks later when conducting a concurrent cognitive task.13 Moreover, researchers have shown persistent differences between concussed collegiate athletes and healthy control participants at the time of RTP14 and ranging from 6 weeks after concussion15 to as long as 1.6 years16 and 3.7 years.3
Investigators17 have also reported differences between concussed and healthy control participants during walking under conditions of undivided (single-task) and divided (dual-task) attention. Slower gait speeds were found in concussed individuals during acute (up to 10 days)10,18–25 and more persistent (up to 90 days,21 >3 months,26,27 and up to 6 years2) time frames. Assessing gait under these conditions offers insight into subtle aspects of motor impairment (single-task gait, which is predominantly controlled through subcortical locomotor loops with little executive processing in healthy populations28) and executive control (dual-task gait, which requires executive function for simultaneous processing of cognitive and motor demands29). A dual-task paradigm can help identify prioritization between the competing cognitive and motor demands and can easily be implemented in clinical settings.
Authors of few longitudinal studies have evaluated the recovery of concussed athletes with a specific focus on how returning to play affects function in these domains. In one study of single- and dual-task walking before and after RTP, Howell et al30 identified regressed gait stability after return to activity in adolescents through some but not all measures. In addition, Howell et al31 noted correlations suggesting the length of time from injury to resumption of preinjury activity levels may be related to functional recovery from concussion. Therefore, given the limited number of investigations in which the timing of RTP has been considered, the purpose of our study was to longitudinally assess measures of balance, gait, and dual-task cost in concussed collegiate athletes over 8 weeks and compare these measures against those of healthy nonconcussed athletes. Specifically, we used a time spline and the average RTP time to track measures before and after concussed athletes returned to play. Our first hypothesis was that concussed athletes would have greater postural sway during standing, slower gait, a greater dual-task cost on gait (DTC-G), and a greater dual-task cost on reading (DTC-R) during the initial postinjury evaluation. Our second hypothesis was that these deficits would improve over time. Third, we hypothesized that any improvements over time would change trajectory after RTP.
METHODS
Participants
We recruited a total of 50 volunteers (25 concussed individuals, 25 matched healthy individuals) during the 2015 and 2016 athletic seasons from 6 sports and athletics departments of universities within the Portland, Oregon, area. Analysis of RTP dates revealed that 2 concussed participants did not RTP during the 8-week follow-up period, so we removed them from further analysis (Table 1). Athletes who had sustained concussions were referred to the study by the team athletic trainer within 24 to 48 hours postinjury. Concussed athletes were included if they were 18 years of age or older and had received a diagnosis of concussion from either their team clinician or an Oregon Health and Science University (OHSU) sports physician. Clinicians used the Sports Concussion Assessment Tool and clinical judgment to diagnose concussions in athletes. Healthy individuals serving as controls were student-athletes participating in sports in the same universities' athletics departments. They were matched for age, sex, height, mass, and, where possible, sport. Participants with a history of medical conditions that would impair cognition or mobility, including any injury or surgery within the 6 months before the study that would affect balance, were excluded. All universities gave written approval for OHSU to conduct research at their campuses. All participants provided written informed consent, and the study was approved by the OHSU Institutional Review Board.
Table 1.
Participant Demographic Information
| Characteristic |
Group |
Value |
|||
| Concussed (n = 23) |
Control (n = 25) |
t46 |
χ2 |
P |
|
| Mean ± SD |
|||||
| Age, y | 20.1 ± 1.3 | 20.9 ± 1.4 | 1.900 | .06 | |
| Height, m | 1.8 ± 0.1 | 1.8 ± 0.1 | −0.628 | .53 | |
| Mass, kg | 91.3 ± 22.0 | 83.0 ± 21.0 | −1.332 | .19 | |
| Time to return to play, d | 13.7 ± 4.4 | NA | NA | ||
| n (%) |
|||||
| Sex | 0.035 | .85 | |||
| Female | 5 (22) | 6 (24) | |||
| Male | 18 (78) | 19 (76) | |||
| Sport type | 4.680 | .03a | |||
| Contact | 18 (78) | 12 (48) | |||
| Noncontact | 5 (22) | 13 (52) | |||
| n |
|||||
| Sport | NA | ||||
| Football | 15 | 8 | |||
| Basketball | 2 | 1 | |||
| Soccer | 2 | 4 | |||
| Baseball | 1 | 3 | |||
| Track and field | 1 | 9 | |||
| Volleyball | 1 | NA | |||
| Lacrosse | 1 | NA | |||
Abbreviation: NA, not applicable.
Indicates difference (P < .05).
Procedures
Participants were assessed during 9 testing sessions over 8 weeks postinjury to evaluate the recovery of balance and gait. The timeline involved 2 testing sessions in the first week, followed by weekly testing for 7 weeks. Healthy participants were tested using the same time intervals for comparison.32 Athletes completed testing sessions at their universities of enrollment. For consistency across universities, testing was conducted in a well-lit, straight hallway with a firm surface. The testing site at each university was the same for each test day.
Each testing session included an instrumented assessment of balance and gait. To complete the assessment, we positioned 3 wireless inertial sensors (Opal; APDM Inc, Portland, OR) bilaterally on the anterior and distal aspect of each shank33 and posterior pelvis at the height of L5.34 We attached each inertial sensor to the body using an elastic belt and adjusted the belt to fit snugly enough to reduce unwanted sensor movement without being uncomfortable to the participant.
Our balance-assessment procedures have been previously described.32 Briefly, balance assessment was conducted using (1) the Balance Error Scoring System (BESS) protocol12,35 to obtain the subjective clinical error count and (2) instrumented sway metrics. The BESS test was modified to 30 seconds of stance to obtain instrumented measures,36,37 a requirement for collecting more reliable sway data.38 We only conducted the clinical error count over the initial 20 seconds of each condition. Trials were video recorded (Bloggie Touch; Sony Corporation, Tokyo, Japan), and blinded scoring was completed by a single member of our research team (C.W.S.) who was trained in scoring the BESS. Although participants completed the entire BESS protocol, we used only the double-legged–stance, firm-surface condition for the instrumented analysis because, according to King et al,37 instrumented mediolateral sway captured during this condition offers the greatest sensitivity for classifying individuals under acute concussion timelines.
We assessed gait using an instrumented 2-minute walk test under single- and dual-task conditions. Participants were instructed to walk normally back and forth down an approximately 25-m hallway. In the single-task condition, participants were instructed to walk at their normal comfortable pace. For the dual-task condition, participants were required to read words aloud from recently published sports news articles while concurrently walking at their self-selected pace. We chose this task because it is an ecological task that many people perform daily, slower gait speeds have been found when participants read text messages,39 and walking affects reading speed.40 Articles used had a Flesch reading level between 72.4 and 78.2 (ie, fairly easy to read).41 The words were printed on an A4 sheet in 12-point font and with double-spaced lines. Participants completed a timed, seated baseline reading trial and then the dual-task condition. We used an audio recorder (USB flash drive voice and audio recorder) to record the reading trials and count the number of words read. Each participant received the same articles in the same order for consistency and continued the article from where he or she finished during the previous test. The dual-task cost was calculated using the number of words read during the seated baseline and walking trials. The baseline trials (single-task gait and seated reading) allowed the calculation of DTC-G and DTC-R. The order of procedures was consistent across each testing session, and rest breaks were provided between trials.
Data Analysis
We collected data at 128 Hz using Opal sensors and used Mobility Lab Software (version 1; APDM Inc34) to export postural sway and gait metrics. The balance measures were the total BESS score and sway as measured by the root mean square of mediolateral sway (mm/s2) of the double-legged stance condition.37 Gait measures were single- and dual-task gait speed. The dual-task costs, which were the negative changes in performance associated with adding a secondary task, were assessed for gait and reading. The Equation illustrates the formula for calculating the dual-task cost:
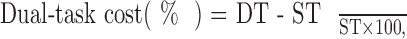 |
where DT indicates dual-task performance and ST indicates single-task performance.42
Statistical Analysis
Between-groups differences for demographic variables were assessed using independent-samples t tests. Any variables that were significantly different were accounted for in further analyses.
Our approach and statistical design have been previously reported.32 Linear mixed models were fit to each outcome measure to assess whether groups differed over the recovery period. Group was entered as a fixed factor to assess group differences. Time was included as (1) a continuous linear covariate to assess the change in each outcome measure over time and (2) a linear spline function of time, which was referred to as RTP change, to assess the change in each outcome measure after RTP. The RTP change function allowed an inflection to occur at the average RTP time. By including this inflection point, the trend before and after the average RTP day could be modeled, allowing analysis of whether a change in the trend occurred after RTP. The group × time and group × RTP change interactions were included in the model to assess whether the changes over time in each measure were different between groups. Random intercepts and random linear slopes over time were fit for each participant to account for within-subject correlations across time. The model used a full covariance matrix and Cholesky parameterization. Preliminary models included any demographic variables (eg, contact or noncontact sport type) that were significantly different between groups as covariates. If the demographic covariates had effects, they were retained in the final models. Statistical analyses were conducted using MATLAB (version R2017a Statistics and Machine Learning Toolbox; The MathWorks, Natick, MA), and the α level was set at .05.
RESULTS
The average RTP time was 13.7 days (range, 8–26 days) after concussion, with 65% (n = 15) returning between 9 and 15 days postinjury. Days tested and participants lost to follow-up are presented in Table 2. No group differences were found for age, height, or mass, but more people in the concussed group played contact sports (Table 1). Descriptive data for each measure are provided in Table 3.
Table 2.
Testing Timeline and Participants Lost to Follow-Up
| Test Day |
Concussed Group |
Control Group |
||
| Participants, n |
Time Postinjury, d, Mean ± SD |
Participants, n |
Time Postinjury, d, Mean ± SD |
|
| 1 | 23 | 2 ± 1 | 25 | 2 ± 0 |
| 2 | 23 | 5 ± 1 | 25a | 5 ± 1 |
| 3 | 20 | 10 ± 2 | 25 | 10 ± 1 |
| 4 | 21 | 16 ± 2 | 23 | 17 ± 2 |
| 5 | 20a,b | 23 ± 2 | 23 | 25 ± 1 |
| 6 | 21 | 30 ± 3 | 23 | 31 ± 1 |
| 7 | 17 | 37 ± 2 | 22 | 38 ± 2 |
| 8 | 14 | 44 ± 2 | 22 | 45 ± 1 |
| 9 | 13 | 51 ± 2 | 20 | 52 ± 1 |
Data were excluded from 1 participant due to file corruption.
One individual was absent from testing but still participating at this time.
Table 3.
Descriptive Information per Measure, Mean ± SD
| Participant Group |
Day |
||||||||
| 2 |
5 |
10 |
16 |
23 |
30 |
37 |
44 |
51 |
|
| Balance Error Scoring System, No. of total errors | |||||||||
| Control | 12.08 ± 8.06 | 12.13 ± 7.34 | 12.96 ± 8.27 | 11.26 ± 7.72 | 9.74 ± 5.55 | 9.87 ± 6.36 | 8.27 ± 4.89 | 7.82 ± 4.62 | 7.00 ± 5.88 |
| Concussed | 13.83 ± 5.51 | 12.96 ± 6.74 | 12.15 ± 7.69 | 10.19 ± 5.47 | 11.63 ± 7.28 | 9.00 ± 6.30 | 10.35 ± 6.68 | 8.07 ± 5.81 | 8.92 ± 4.54 |
| Sway,a mm/s2 | |||||||||
| Control | 53.75 ± 21.21 | 59.74 ± 20.17 | 63.32 ± 19.20 | 63.93 ± 22.83 | 59.13 ± 20.82 | 64.01 ± 22.46 | 67.75 ± 20.28 | 63.79 ± 23.75 | 60.79 ± 22.59 |
| Concussed | 72.60 ± 25.88 | 73.39 ± 30.56 | 64.67 ± 17.93 | 68.52 ± 25.51 | 74.07 ± 29.19 | 65.54 ± 16.58 | 73.60 ± 25.63 | 72.97 ± 24.05 | 68.20 ± 26.80 |
| Single-task gait speed, m/s | |||||||||
| Control | 1.34 ± 0.13 | 1.39 ± 0.13 | 1.42 ± 0.13 | 1.44 ± 0.13 | 1.43 ± 0.13 | 1.44 ± 0.11 | 1.42 ± 0.12 | 1.43 ± 0.12 | 1.45 ± 0.12 |
| Concussed | 1.32 ± 0.11 | 1.42 ± 0.11 | 1.46 ± 0.09 | 1.48 ± 0.09 | 1.47 ± 0.09 | 1.49 ± 0.08 | 1.48 ± 0.10 | 1.49 ± 0.10 | 1.51 ± 0.09 |
| Dual-task gait speed, m/s | |||||||||
| Control | 1.25 ± 0.15 | 1.29 ± 0.13 | 1.31 ± 0.13 | 1.33 ± 0.14 | 1.33 ± 0.13 | 1.32 ± 0.12 | 1.31 ± 0.13 | 1.33 ± 0.13 | 1.32 ± 0.14 |
| Concussed | 1.21 ± 0.13 | 1.29 ± 0.11 | 1.31 ± 0.12 | 1.32 ± 0.11 | 1.32 ± 0.11 | 1.32 ± 0.09 | 1.32 ± 0.12 | 1.33 ± 0.09 | 1.36 ± 0.09 |
| Dual-task cost of reading on gait speed, % | |||||||||
| Control | −6.96 ± 6.18 | −7.18 ± 3.61 | −7.31 ± 4.77 | −8.03 ± 3.56 | −7.12 ± 4.17 | −8.10 ± 4.86 | −8.25 ± 5.00 | −7.24 ± 4.50 | −8.50 ± 5.32 |
| Concussed | −7.77 ± 6.43 | −9.31 ± 5.18 | −10.24 ± 5.37 | −11.00 ± 5.20 | −9.79 ± 5.39 | −11.42 ± 5.05 | −10.41 ± 4.86 | −10.57 ± 4.04 | −9.61 ± 4.65 |
| Dual-task cost of walking on reading, % | |||||||||
| Control | −3.28 ± 6.69 | −0.54 ± 9.76 | −3.57 ± 10.03 | −1.14 ± 10.04 | −0.31 ± 11.99 | −6.94 ± 11.78 | 2.38 ± 10.44 | −6.03 ± 8.54 | −0.47 ± 9.10 |
| Concussed | −10.92 ± 9.31 | −1.97 ± 13.38 | −9.28 ± 8.92 | −2.90 ± 9.99 | −2.66 ± 10.31 | −5.98 ± 17.08 | −0.55 ± 14.88 | −2.72 ± 7.21 | −2.17 ± 9.61 |
Mediolateral root mean square sway.
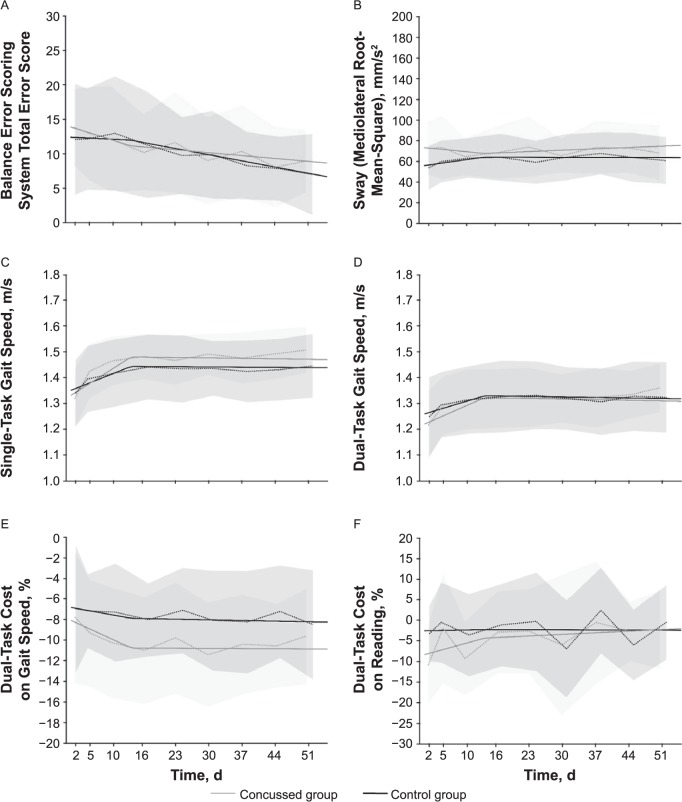
We found no significant effects for the influence of sport type (contact versus noncontact) in any analysis model (P > .05). Fixed-effects coefficients for models assessing each outcome measure are presented in Table 4 and the Figure.
Table 4.
Estimate of the Fixed Effects (β) With 95% Confidence Interval Presented for Each Outcome Measure
| Measure |
β Coefficient |
95% Confidence Interval |
P Value |
| Balance Error Scoring System total score | |||
| Group effecta | 1.69 | −2.40, 5.77 | .42 |
| Time effectb | −0.03 | −0.21, 0.14 | .72 |
| Return-to-play change effectc | −0.10 | −0.31, 0.12 | .37 |
| Group × time interactiond | −0.18 | −0.44, 0.07 | .16 |
| Group × return-to-play change interactione | 0.25 | −0.06, 0.56 | .12 |
| Sway,f mm/s2 | |||
| Group effecta | 18.61 | 5.99, 31.23 | .004g |
| Time effectb | 0.65 | 0.01, 1.30 | .046h |
| Return-to-play change effectc | −0.66 | −1.45, 0.12 | .10 |
| Group × time interactiond | −1.10 | −2.03, −0.17 | .02h |
| Group × return-to-play change interactione | 1.30 | 0.15, 2.45 | .03h |
| Single-task gait speed, m/s | |||
| Group effecta | −0.024 | −0.086, 0.038 | .45 |
| Time effectb | 0.007 | 0.006, 0.009 | <.001g |
| Return-to-play change effectc | −0.007 | −0.010, −0.005 | <.001g |
| Group × time interactiond | 0.004 | 0.002, 0.007 | <.001g |
| Group × return-to-play change interactione | −0.005 | −0.008, −0.001 | .005g |
| Dual-task gait speed, m/s | |||
| Group effecta | −0.042 | −0.114, 0.030 | .25 |
| Time effectb | 0.006 | 0.004, 0.007 | <.001g |
| Return-to-play change effectc | −0.006 | −0.008, −0.004 | <.001g |
| Group × time interactiond | 0.003 | 0.000, 0.005 | .042h |
| Group × return-to-play change interactione | −0.003 | −0.006, 0.000 | .08 |
| Dual-task cost of reading on gait speed, % | |||
| Group effecta | −1.13 | −3.99, 1.73 | .44 |
| Time effectb | −0.08 | −0.18, 0.02 | .10 |
| Return-to-play change effectc | 0.07 | −0.05, 0.19 | .23 |
| Group × time interactiond | −0.13 | −0.27, 0.02 | .09 |
| Group × return-to-play change interactione | 0.13 | −0.05, 0.31 | .14 |
| Dual-task cost of walking on reading, % | |||
| Group effecta | −6.05 | −12.26, 0.16 | .056 |
| Time effectb | 0.02 | −0.39, 0.43 | .91 |
| Return-to-play change effectc | −0.03 | −0.53, 0.48 | .92 |
| Group × time interactiond | 0.29 | −0.31, 0.89 | .34 |
| Group × return-to-play change interactione | −0.23 | −0.98, 0.51 | .54 |
Difference between the concussed and control groups at day 1.
Change across time in the control group.
Change in the time effect after return to play.
Difference in the change over time between the concussed and control groups.
Difference in the change to the time effect after return to play between the concussed and control groups.
Mediolateral root mean square sway.
P < .01.
P < .05.
Figure.
A, Balance Error Scoring System total errors. No effects or interactions were found. B, Sway (root mean square of mediolateral sway). Group and time effect and group × time and group × return-to-play (RTP) change interactions were found (P < .05). C, Single-task gait speed. Time and RTP change effects and group × time and group × RTP change interactions were present (P < .05). D, Dual-task gait speed. Time and RTP change effects and group × time interactions were found (P < .05). E, Dual-task cost on gait speed. No effects or interactions were found. F, Dual-task cost on reading. No effects or interactions were found. Dashed lines represent the group mean per session, shaded areas represent the standard deviations, and solid lines represent the group trend before and after the average RTP date.
Balance Assessment
Total BESS Errors
No significant effects or interactions were found for the BESS total error score.
Sway
We observed a group difference for sway, with the concussed group initially swaying more than the control group (positive effect of group, P = .004). We also noted a slight increase in sway in the control group over time (positive effect of time, P = .046). Sway decreased in the concussed group initially (negative group × time interaction, P = .02) but increased after RTP (positive group × RTP change interaction, P = .03).
Gait Assessment
Single-Task Gait Speed
Single-task gait speed was not different between groups; however, we found an increase in gait speed over time (positive effect of time, P < .001) that was more pronounced in the concussed group (positive group × time interaction, P < .001). At the RTP time point, gait speed stopped increasing and leveled out. This was present in both the control (negative effect of RTP change, P < .001) and concussed groups, with a greater change occurring in the concussed participants (negative group × RTP change interaction, P = .005).
Dual-Task Gait Speed
We observed no initial group difference in dual-task gait speed but demonstrated an overall increase in gait speed (positive effect of time, P < .001), which was more prominent in the concussed group (group × time interaction, P = .042). After RTP, dual-task gait speed stopped increasing in both groups (negative effect of RTP change, P < .001; no group × RTP change interaction, P = .08).
Dual-Task Cost Assessment
No effects or interactions were found for DTC-G (P > .05) or DTC-R (P > .05).
DISCUSSION
This study involved a longitudinal investigation of standing balance, gait, and dual-task cost after concussion in collegiate athletes. The analysis focused on concussed athletes both before and after RTP. Our main findings were (1) We observed no differences in BESS error count between the concussed group during recovery and the control group over the 8-week period tested. (2) Between-groups differences were detected with an instrumented measure of sway, which also indicated a change in the recovery trajectory after RTP. (3) We found no between-groups differences in single-task or dual-task gait, but a pattern of increasing gait speed that tended to level off corresponding to times after RTP was present. (4) A pattern relating to a possible shift in prioritization between gait and cognition during the recovery period for the concussed athletes was evident. Albeit not statistically different, this pattern could be clinically relevant and warrants further research. Taken together, the findings indicated that a change in objective balance and gait measures may occur in relation to RTP. Although it is unclear whether these changes are good or bad, the results suggest RTP is an important clinical feature that needs to be considered when evaluating a patient's recovery.
Balance Assessment
Total BESS Errors
The number of BESS errors appeared to decrease as the time since injury increased, suggesting that balance improved in both groups over time. However, analysis of this measure revealed null findings, which is perhaps unsurprising given that our results showed an average difference of only 1 to 2 errors between groups across the testing timeline. We find it notable that more persistent but subtle balance deficits in the concussed group were revealed by the instrumented sway measure, yet no similar findings (effect or interaction) were present for the BESS error count. This error count is known to have limited sensitivity37,43 and to be influenced by practice effects,44 which may be why our longitudinal analysis of this measure was unable to reveal any significant findings.
Sway
The concussed group exhibited more sway than the control group during quiet stance with eyes closed, a finding which is consistent with our hypothesis and previous research12,37 indicating balance deficits in individuals after concussion. The analysis suggested that sway did not improve over the 8-week study period. The lack of improvement in the concussed group would appear to be in agreement with studies14,15,36 showing more persistent balance deficits after concussion. The interactions suggested a difference in group trends before and after RTP, potentially indicating a regression in the recovery of balance control after RTP in the concussed group. Our findings prompted 2 clinical considerations. First, the current reliance on the BESS during clinical evaluation of concussion should be reevaluated given its insensitivity relative to objective measures of sway. Second, the potential benefit of instrumented procedures for assessing balance in these populations should be widely disseminated.
Gait Assessment
Single-Task Gait Speed
The lack of group effect for single-task gait speed indicated no differences in walking speed between the concussed and control groups during initial testing. This finding did not support our hypothesis that single-task gait speed would be slower in the concussed than the control group. Although this result contrasts with the results of several researchers18–23 who reported slower gait speeds in concussed college-aged students and athletes, it is consistent with the reports of other investigators10,24,45–47 who found no differences between concussed and control individuals. The effect of time and the group × time interaction for single-task gait speed suggested gait speed increased over time in the concussed group. Although not directly comparable with the previous findings, an increase in single-task gait speed over time has been documented for recovering concussed adults who initially walked more slowly than control adults.17,18,21,23 The negative effect of RTP change and group × RTP change interactions suggested a change around the period of RTP, which was more prominent in the concussed group. It is not clear whether this represents a decreased rate of improvement after RTP or perhaps the initial increase in gait speed was an outcome of familiarity with the task.
Dual-Task Gait Speed
We expected to see a slower dual-task gait speed in the concussed group during initial postinjury testing; however, no group effect was found. This result was in contrast to several previous studies, in which authors have shown slower dual-task gait speed in recently concussed college-aged students and athletes,10,19–25 albeit in agreement with other studies.23,45,46 Similar to single-task gait, dual-task gait speed increased for both groups but occurred at a greater rate in the concussed group. Recovery of dual-task gait speed is consistent with other studies.21,23,47 Our results suggested a different trend pattern before and after RTP, with dual-task gait speed remaining relatively consistent after the RTP period. However, the lack of group × RTP change interaction suggested this pattern was not different between the control and concussed groups. Therefore, without an understanding of baseline walking speeds in these athletes, it is difficult to know whether this represents true recovery in this group, and, as with single-task gait, it is possible that initial increases in gait speed were driven by task familiarity.
Dual-Task Cost Assessment
Although not significant within the statistical model, an interesting pattern emerged when we qualitatively analyzed the trends of both DTC-G and DTC-R in the concussed group. A lower cost on gait was paired initially with a greater cost on reading, which shifted after RTP. After RTP, a smaller cost on reading appeared to be concurrent with a larger cost on gait. Inconsistencies in the reporting of these measures (ie, few reports of the actual dual-task cost21,48,49), conflicting findings relating to cognitive performance while walking in concussed adolescent50,51 but not adult20,22,46,52 populations, and the lack of effects or interactions within this study for these measures make interpretation of these findings difficult. Nonetheless, given that the dynamic nature of sport participation requires continuous concurrent physical and cognitive attendance, we believe this pattern may warrant further attention and focus in future investigations.
LIMITATIONS
Testing was completed across multiple universities, making our testing environment highly representative of an applied setting. We made every effort to reduce the effect of potential environmental confounders, including keeping the testing location across universities consistent (well-lit, firm-surface hallway) and constant across the timeline. However, the following limitations should be considered when interpreting the results. First, the dual-task paradigm that we used (reading while walking) was different from the tasks used in previous studies, such as question and answer, the auditory Stroop, and mental arithmetic tasks. Our protocol involved both continuous walking and continuous dual tasking, which differs from the discrete trial methods used in many early investigations.20,22,50–52 Indeed, a DTC-G was found consistently across time for both control and concussed athletes when they were required to walk and read simultaneously; however, we observed no group effect. This lack of effect for DTC-G may have resulted from the variability of this measure, which ranged from −23% to −2% for the concussed group and from −17% to 1% for the control group on average across the recovery period. It is plausible that oculomotor function, vocabulary, reading skill, and engagement with the text, as well as other factors, could play a role in performing the reading task and, furthermore, in the level of variability in interference on the concurrent task. In addition, given that word calling (without comprehension) can occur at a more automatic level of information processing,53 it is also possible that the reading task used could not affect cognition in the same manner as tasks used in previous studies that required a more conscious level of control.
Second, longitudinal studies can be limited by participant dropout or participants missing sessions throughout the period assessed. Linear mixed modeling, as we used in this study, can more flexibly manage datasets where the number of observations per participant differ, making it a more robust method of handling longitudinal data. Nonetheless, when interpreting our findings, readers should consider that participants were lost to follow-up.
Third, having baseline balance and gait data for the concussed group would have been highly beneficial for undertaking within-subject case analyses of preinjury versus postinjury in addition to recovery. This would also have allowed the comparison between baseline and RTP values and should be considered by future investigators in this area.
CONCLUSIONS
The purpose of our study was to describe the recovery of athletes after concussion while considering changes around the RTP date. Concussed athletes had greater sway but did not walk more slowly than control athletes during single-task or dual-task gait trials. Importantly, although the standard and subjective BESS error scores were not different between groups, the more sensitive inertial sensor measurement of sway appeared to improve initially and then regress without returning to normal during the 8-week study period. We cannot suggest any change occurred due to RTP, but this novel method of analysis provides incentive for a more in-depth research agenda studying the effects of RTP on concussion recovery and any effect this may have on other injuries as well. Furthermore, these results suggested that the RTP date is an important clinical decision that should be accounted for when evaluating recovery trajectories after concussion.
ACKNOWLEDGMENTS
This study was supported by research grant R21HD080398 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (Dr King). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank the athletic departments of the participating universities (Portland State University, Lewis & Clark College, Pacific University, Linfield College, George Fox University, and Concordia University), the athletic trainers who contributed to recruiting participants, and the athletes for their participation.
REFERENCES
- 1.Williams RM, Puetz TW, Giza CC, Broglio SP. Concussion recovery time among high school and collegiate athletes: a systematic review and meta-analysis. Sports Med. 2015;45(6):893–903. doi: 10.1007/s40279-015-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martini DN, Sabin MJ, DePesa SA, et al. The chronic effects of concussion on gait. Arch Phys Med Rehabil. 2011;92(4):585–589. doi: 10.1016/j.apmr.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 3.Sosnoff JJ, Broglio SP, Shin S, Ferrara MS. Previous mild traumatic brain injury and postural-control dynamics. J Athl Train. 2011;46(1):85–91. doi: 10.4085/1062-6050-46.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konrad C, Geburek A, Rist F, et al. Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychol Med. 2011;41(6):1197–1211. doi: 10.1017/S0033291710001728. [DOI] [PubMed] [Google Scholar]
- 5.Barr WB, Prichep LS, Chabot R, Powell MR, McCrea M. Measuring brain electrical activity to track recovery from sport-related concussion. Brain Inj. 2012;26(1):58–66. doi: 10.3109/02699052.2011.608216. [DOI] [PubMed] [Google Scholar]
- 6.Bleiberg J, Warden D. Duration of cognitive impairment after sports concussion. Neurosurgery. 2005;56(5):E1166. [PubMed] [Google Scholar]
- 7.McCrea M, Guskiewicz KM, Marshall SW, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2556–2563. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- 8.Covassin T, Elbin R, Nakayama Y. Tracking neurocognitive performance following concussion in high school athletes. Phys Sportsmed. 2010;38(4):87–93. doi: 10.3810/psm.2010.12.1830. [DOI] [PubMed] [Google Scholar]
- 9.Howell D, Osternig L, Van Donkelaar P, Mayr U, Chou LS. Effects of concussion on attention and executive function in adolescents. Med Sci Sports Exerc. 2013;45(6):1030–1037. doi: 10.1249/MSS.0b013e3182814595. [DOI] [PubMed] [Google Scholar]
- 10.Yasen AL, Howell DR, Chou LS, Pazzaglia AM, Christie AD. Cortical and physical function following mild traumatic brain injury. Med Sci Sports Exerc. 2017;49(6):1066–1071. doi: 10.1249/MSS.0000000000001217. [DOI] [PubMed] [Google Scholar]
- 11.Maruta J, Spielman LA, Yarusi BB, Wang Y, Silver JM, Ghajar J. Chronic post-concussion neurocognitive deficits: II. Relationship with persistent symptoms. Front Hum Neurosci. 2016;10:45. doi: 10.3389/fnhum.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263–273. [PMC free article] [PubMed] [Google Scholar]
- 13.Dorman JC, Valentine VD, Munce TA, Tjarks BJ, Thompson PA, Bergeron MF. Tracking postural stability of young concussion patients using dual-task interference. J Sci Med Sport. 2015;18(1):2–7. doi: 10.1016/j.jsams.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Powers KC, Kalmar JM, Cinelli ME. Recovery of static stability following a concussion. Gait Posture. 2014;39(1):611–614. doi: 10.1016/j.gaitpost.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Fino PC, Nussbaum MA, Brolinson PG. Decreased high-frequency center-of-pressure complexity in recently concussed asymptomatic athletes. Gait Posture. 2016;50:69–74. doi: 10.1016/j.gaitpost.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 16.De Beaumont L, Mongeon D, Tremblay S, et al. Persistent motor system abnormalities in formerly concussed athletes. J Athl Train. 2011;46(3):234–240. doi: 10.4085/1062-6050-46.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fino PC, Parrington L, Pitt W, et al. Detecting gait abnormalities after concussion or mild traumatic brain injury: a systematic review of single-task, dual-task, and complex gait. Gait Posture. 2018;62:157–166. doi: 10.1016/j.gaitpost.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Buckley TA, Munkasy BA, Tapia-Lovler TG, Wikstrom EA. Altered gait termination strategies following a concussion. Gait Posture. 2013;38(3):549–551. doi: 10.1016/j.gaitpost.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catena RD, van Donkelaar P, Chou LS. Altered balance control following concussion is better detected with an attention test during gait. Gait Posture. 2007;25(3):406–411. doi: 10.1016/j.gaitpost.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Catena RD, van Donkelaar P, Chou LS. Cognitive task effects on gait stability following concussion. Exp Brain Res. 2007;176(1):23–31. doi: 10.1007/s00221-006-0596-2. [DOI] [PubMed] [Google Scholar]
- 21.Fino PC. A preliminary study of longitudinal differences in local dynamic stability between recently concussed and healthy athletes during single and dual-task gait. J Biomech. 2016;49(9):1983–1988. doi: 10.1016/j.jbiomech.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Chen HL, Lu TW, Chou LS. Effect of concussion on inter-joint coordination during divided-attention gait. J Med Biol Eng. 2015;35(1):28–33. [Google Scholar]
- 23.Parker TM, Osternig LR, Van Donkelaar P, Chou LS. Gait stability following concussion. Med Sci Sports Exerc. 2006;38(6):1032–1040. doi: 10.1249/01.mss.0000222828.56982.a4. [DOI] [PubMed] [Google Scholar]
- 24.Chiu SL, Osternig L, Chou LS. Concussion induces gait inter-joint coordination variability under conditions of divided attention and obstacle crossing. Gait Posture. 2013;38(4):717–722. doi: 10.1016/j.gaitpost.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Howell D, Osternig L, Chou LS. Monitoring recovery of gait balance control following concussion using an accelerometer. J Biomech. 2015;48(12):3364–3368. doi: 10.1016/j.jbiomech.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Buckley TA, Vallabhajosula S, Oldham JR, et al. Evidence of a conservative gait strategy in athletes with a history of concussions. J Sport Health Sci. 2016;5(4):417–423. doi: 10.1016/j.jshs.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pape MM, Williams K, Kodosky PN, Dretsch M. The Community Balance and Mobility Scale: a pilot study detecting impairments in military service members with comorbid mild TBI and psychological health conditions. J Head Trauma Rehabil. 2016;31(5):339–345. doi: 10.1097/HTR.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 28.Clark DJ. Automaticity of walking: functional significance, mechanisms, measurement and rehabilitation strategies. Front Hum Neurosci. 2015;9:246. doi: 10.3389/fnhum.2015.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23(3):329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howell DR, Osternig LR, Chou LS. Return to activity after concussion affects dual-task gait balance control recovery. Med Sci Sports Exerc. 2015;47(4):673–680. doi: 10.1249/MSS.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 31.Howell DR, Osternig LR, Christie AD, Chou LS. Return to physical activity timing and dual-task gait stability are associated 2 months following concussion. J Head Trauma Rehabil. 2016;31(4):262–268. doi: 10.1097/HTR.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 32.Parrington L, Fino NF, Fino PC, Murchison CF, Chesnutt JC, King LA. Inflection points in longitudinal models: tracking recovery and return to play following concussion. Scand J Med Sci Sports. 2018;28(11):2436–2442. doi: 10.1111/sms.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salarian A, Russmann H, Vingerhoets FJ, et al. Gait assessment in Parkinson's disease: toward an ambulatory system for long-term monitoring. IEEE Trans Biomed Eng. 2004;51(8):1434–1443. doi: 10.1109/TBME.2004.827933. [DOI] [PubMed] [Google Scholar]
- 34.Mancini M, King L, Salarian A, Holmstrom L, McNames J, Horak FB. Mobility lab to assess balance and gait with synchronized body-worn sensors. J Bioeng Biomed Sci. 2011;(suppl 1):007. doi: 10.4172/2155-9538.S1-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riemann BL, Guskiewicz KM, Shields EW. Relationship between clinical and forceplate measures of postural stability. J Sport Rehabil. 1999;8(2):71–82. [Google Scholar]
- 36.King LA, Horak FB, Mancini M, et al. Instrumenting the Balance Error Scoring System for use with patients reporting persistent balance problems after mild traumatic brain injury. Arch Phys Med Rehabil. 2014;95(2):353–359. doi: 10.1016/j.apmr.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.King LA, Mancini M, Fino PC, et al. Sensor-based balance measures outperform modified Balance Error Scoring System in identifying acute concussion. Ann Biomed Eng. 2017;45(9):2135–2145. doi: 10.1007/s10439-017-1856-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scoppa F, Capra R, Gallamini M, Shiffer R. Clinical stabilometry standardization: basic definitions–acquisition interval–sampling frequency. Gait Posture. 2013;37(2):290–292. doi: 10.1016/j.gaitpost.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Schabrun SM, van den Hoorn W, Moorcroft A, Greenland C, Hodges PW. Texting and walking: strategies for postural control and implications for safety. PLoS One. 2014;9(1):e84312. doi: 10.1371/journal.pone.0084312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schildbach B, Rukzio E. Proceedings of the 12th International Conference on Human Computer Interaction With Mobile Devices and Services. New York, NY: Association for Computing Machinery, Inc;; 2010. Investigating selection and reading performance on a mobile phone while walking; pp. 93–102. [Google Scholar]
- 41.Flesch R. A new readability yardstick. J Appl Psychol. 1948;32(3):221–233. doi: 10.1037/h0057532. [DOI] [PubMed] [Google Scholar]
- 42.Kelly VE, Janke AA, Shumway-Cook A. Effects of instructed focus and task difficulty on concurrent walking and cognitive task performance in healthy young adults. Exp Brain Res. 2010;207(1–2):65–73. doi: 10.1007/s00221-010-2429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCrea M, Barr WB, Guskiewicz K, et al. Standard regression-based methods for measuring recovery after sport-related concussion. J Int Neuropsychol Soc. 2005;11(1):58–69. doi: 10.1017/S1355617705050083. [DOI] [PubMed] [Google Scholar]
- 44.Mulligan IJ, Boland MA, McIlhenny CV. The Balance Error Scoring System learned response among young adults. Sports Health. 2013;5(1):22–26. doi: 10.1177/1941738112467755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parker TM, Osternig LR, Lee HJ, van Donkelaar P, Chou LS. The effect of divided attention on gait stability following concussion. Clin Biomech (Bristol, Avon) 2005;20(4):389–395. doi: 10.1016/j.clinbiomech.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Parker TM, Osternig LR, van Donkelaar P, Chou LS. Recovery of cognitive and dynamic motor function following concussion. Br J Sports Med. 2007;41(12):868–873. doi: 10.1136/bjsm.2006.033761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parker TM, Osternig LR, van Donkelaar P, Chou LS. Balance control during gait in athletes and non-athletes following concussion. Med Eng Phys. 2008;30(8):959–967. doi: 10.1016/j.medengphy.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Cossette I, Gagné MÈ, Ouellet MC, et al. Executive dysfunction following a mild traumatic brain injury revealed in early adolescence with locomotor-cognitive dual-tasks. Brain Inj. 2016;30(13–14):1648–1655. doi: 10.1080/02699052.2016.1200143. [DOI] [PubMed] [Google Scholar]
- 49.Cossette I, Ouellet MC, McFadyen BJ. A preliminary study to identify locomotor-cognitive dual tasks that reveal persistent executive dysfunction after mild traumatic brain injury. Arch Phys Med Rehabil. 2014;95(8):1594–1597. doi: 10.1016/j.apmr.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 50.Howell DR, Osternig LR, Chou LS. Dual-task effect on gait balance control in adolescents with concussion. Arch Phys Med Rehabil. 2013;94(8):1513–1520. doi: 10.1016/j.apmr.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 51.Howell DR, Osternig LR, Koester MC, Chou LS. The effect of cognitive task complexity on gait stability in adolescents following concussion. Exp Brain Res. 2014;232(6):1773–1782. doi: 10.1007/s00221-014-3869-1. [DOI] [PubMed] [Google Scholar]
- 52.Catena RD, van Donkelaar P, Chou LS. The effects of attention capacity on dynamic balance control following concussion. J Neuroeng Rehabil. 2011;8:8. doi: 10.1186/1743-0003-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LaBerge D, Samuels SJ. Toward a theory of automatic information processing in reading. Cogn Psychol. 1974;6(2):293–323. [Google Scholar]