Abstract
Plant in vitro vegetative propagation using classical semi-solid culture medium is limited due to the low degree of automation, suboptimal nutrient availability and induced physiological stress which often reduce its efficiency. Temporary Immersion System (TIS) emerged as an innovative approach to optimize and eliminate the drawbacks associated with the conventional system of micropropagation. In this study, both Dioscorea and Musa spp. were subjected to conventional semi-solid culture media, complete immersion in shaking liquid culture media and TIS using RITA bioreactor. In vitro grown plantlets were screened for possible vegetative changes using agro-morphological descriptors while genetic and methylation differences were assessed using amplified fragment length polymorphism (AFLP) and methylation-sensitive amplification polymorphism (MSAP). In vitro results showed that the number of shoots produced in Musa spp. varied significantly (P≤0.001) with the type of culture system. The highest mean shoot produced was observed with TIS (28.40) and the least using semi-solid culture medium (1.13). For Dioscorea spp., there was no significant interaction between the hormone combination and the culture system. However, the lowest mean shoot value (1.55) was observed in the semi-solid culture medium. Genetic analysis via AFLP using 15 primer pair combinations revealed that the 3 culture systems maintained genetic variation for Musa and Dioscorea spp. under in vitro and field conditions. Results showed 99% and 91% of the total bands were polymorphic under in vitro and field conditions respectively for Musa and 100% polymorphism for Dioscorea under in vitro and field conditions. Methylation investigation via MSAP using 12 primer pair combinations showed 25% and 46% polymorphic methylated-sensitive loci, 100% and 78% of non-methylated loci of the total bands generated under in vitro and field conditions respectively. Unmethylated (HPA+/MSP+) levels were highest in TIS (0.0842) as compared to CI (0.0227) and SS (0.0161) while full methylation or absence of target (HPA-/MSP-) was lowest in TIS (0.5890) and highest in SS (0.7138). For Dioscorea, 52% and 53% methylated sensitive loci and 100% non-methylated loci were polymorphic under in vitro and field conditions respectively. Although in vitro plant tissue culture techniques led to methylation at some loci of both species, there were no observable changes in the phenotype of both crops under field conditions. This also confirmed that not all methylation events lead to phenotypic changes.
Introduction
In vitro plant tissue culture is recognized as one of the most valuable biotechnology tools for rapid multiplication of disease-free and true-to-type genotypes. The technology is used extensively in clonally propagated horticultural, food and tree crops. There are, however some challenges related to formation of aberrant plantlets and low survival during acclimatization stages in the field [1–3]. In both Dioscorea and Musa spp., in vitro clonal propagation can be used either for large scale propagation or conservation. However, somaclonal variation in plant material under in vitro plant tissue culture is significantly influenced by DNA methylation changes, although the occurrence of such events is unclear [4–6].
Culture media is a crucial aspect of in vitro plant propagation. It determines its effectiveness and can be targeted for improvement. The response of plant tissue to in vitro culture medium depends on several factors including the genotype itself, the nutrient content of the culture medium, the source and physiological state of the explant and the physical culture conditions such as temperature, pH, photoperiod and aeration [7–9] of the culture systems. Semi-solid and liquid culture are some of the common culture media systems used for in vitro plant propagation [10]. Although these methods have certain advantages but there are limitations too. The major disadvantages are asphyxiation, hyperhydricity, induced stress on agitated cultures, explant blackening (oxidation), poor diffusion rate and sub-optimal nutrient uptake which may lead to severe physiological disorder [10].
The temporary immersion system (TIS) that involves automated system provides an optimal environment for plant tissue and organ in vitro cultures. The method emerged as an approach to scale up the conventional method of propagation [11]. Over the years, several TIS have been developed and successfully used in various plant in vitro systems [12–14]. In 1993, Center de Cooperation Internationale en Recherche Agronomique pour le Development (CIRAD) developed a new temporary immersion system named RITA (recipient á immersion temporaire automatique) which eliminated the limitations of previously developed bioreactors, thus promoting massive in vitro plant production.
The objective of this study was therefore to explore the efficiency of TIS (RITA bioreactor) on Musa and Dioscorea spp. with respect to its comparative advantage over other conventional culture systems and to assess probable genetic and methylation modifications in the regenerated plants using amplified fragment length polymorphism (AFLP) and methylation-sensitive amplified polymorphism (MSAP) markers.
Materials and methods
The accessions of Musa and Dioscorea spp. (Table 1) were collected from the Genetic Resources Center (GRC) of the International Institute of Tropical Agriculture (IITA) Ibadan, Nigeria.
Table 1. List of accessions of Yam and Musa with genome information and cultivar names.
| Accessions | Cultivar | Genome |
|---|---|---|
| TMb 19 | IJAU LAGADA | AA |
| TMb 26 | MALACCENSIS HOLOTYPE | AA |
| TMb 28 | MONJET | AA |
| TMb 42 | PISANG BERLIN | AA |
| TMp 59 | AGBAGBA | AAB |
| TMp 100 | ESSANG | AAB |
| TMp 82 | KLUE ROI WEE | AAB |
| TDr 1228 | Dioscorea rotundata | N/A |
In vitro culture
Shoot tips of seven accessions of Musa spp. (Table 1) were cultured on Murashige and Skoog (MS) mineral-based culture medium [15] supplemented with 4.0 mg/l 6-Benylamionopurine (BAP) and 0.18mg/l Indole Acetic Acid (IAA) [16] (S1 Table). While one accession of Dioscorea rotundata (TDr 1228) (yam) was selected and screened using four hormone combinations to check its multiplication rate (S1 Table).
Initiation of multiple shoots for Musa spp. was enhanced by wounding the apical meristem during subculture, a technique described by Jarret et al. [17] and Gupta [18] which involves vertical cuts through the meristematic dome while keeping the base of the explant intact.
The culture systems used for both species were: semisolid culture medium in test tube (SS), complete immersion in liquid culture medium in baby food jar (CI) with shaking on a rotary shaker at 100 rpm, and temporary immersion system (TIS) in RITA bioreactor with immersion time of 15min every 2 hours for both crops.
Five replicates of each of the seven accessions of Musa and one accession of Dioscorea were cultured under in vitro conditions in the three culture systems (SS, CI, TIS). The cultures were kept in growth chamber at 26°C ± 2.0, 38 μmol/m2/s and 12-hour photoperiod for three and six weeks for Musa spp. and Dioscorea rotundata respectively. The number of shoots were determined by counting the number of shoots (for Musa) and number of nodes (for yam) per single plant for each of the three culture systems. For better rooting system development, MS mineral-based culture medium was supplemented with either 0.18 mg/l IAA or no auxin for 3 weeks.
Agro-morphological characterization
The in vitro grown planting materials were acclimatized (using sterilized top soil and chicken manure in ratio 2:1 for Musa and Jiffy peat pellet for Dioscorea) in the screen-house. After 12 weeks, the established plants were transplanted to the field at IITA (Latitude 7.50338° Longitude 3.90427°, Altitude 248.00m) following usual agronomic management practices such as regular watering, weeding and mulching. Data of agro-morphological parameters were captured every month following the available descriptor list for both Musa and Dioscorea [19–20] using 33 and 83 traits respectively until the crop senesced completely. However, data on majority of the traits were recorded at harvest for Musa spp. while for Dioscorea rotundata was carried out through the entire growth period. Mini tubers obtained from yam, were planted the second year and characterization data were collected on selected traits (S2 and S3 Tables).
Molecular characterization
a) Sample collection and DNA isolation
About 100 mg of young leaf samples of Musa (cigar-like leaf) and Dioscorea (first fully expanded) genotypes were collected both from field and in vitro grown plants and labeled accordingly in sterile eppendorf tubes containing steel beads and immersed in liquid nitrogen. The samples were immediately homogenized to fine powder using a Geno Grinder (Retch MM 200) for 2 mins at a frequency of about 25 Hz. Genomic DNA was extracted from the ground samples using DNeasy plant Mini Kit (QIAGEN, 69106) and modified Dellaporta protocol [21] with the addition of DIECA and ascorbic acid to inhibit phenoloxidase and other impurities. Ground yam samples were first washed with 1000 μl HEPES buffer (10ml of 0.1 M HEPES + 90mg L-ascorbic acid + 102 mg PVP + 200μl β-mercaptoethanol) to remove secondary metabolites prior to extraction procedure. The quality of the DNA was determined by 1% agarose gel electrophoresis while the quantity and purity were measured through absorbance ratio (240/280) using NanoDrop spectrophotometer (Thermo scientific Nanodrop 2000 spectrophotometer).
b) AFLP and MSAP analysis
The AFLP and MSAP analysis followed a modified version of Vos et al., Vroh-Bi et al. and Reyna-Lopez et al. [22–24]. The isoschizomer restriction enzyme pair HpaII and MspI was used for MSAP, which recognizes CCGG site with differential sensitivity to methylation at cytosine, while MseI was used for AFLP. When the internal cytosine is fully (methylation on both DNA strand) or hemi (methylation on one DNA strand) methylated, MspI recognizes and cleaves the motif but it cannot cleave an outer cytosine. However, HpaII has the capacity to recognize and cleave outer cytosine. Profiles generated from MspI and HpaII isochizomeric pair not only provide events associated with inner and outer methylation but give a comprehensive picture of genetic and epigenetic variations linked to methylation. Accordingly, we generated MSAP profiles of in vitro and field grown plants of both Musa and Dioscorea spp. to understand the methylation pattern associated with different culture system used in the study.
A restriction digest of 250ng genomic DNA (5 μl) with 20U/μl EcoRI (0.25μl), 10U/μl MseI/MspI/HpaII (0.5μl), 10 X buffer 4 (5.0μl), 100X BSA (0.5μl) and ultra-pure molecular water was carried out in a thermo cycler for 3 hours at 37°C and the enzymes were inactivated (EcoRI/MseI, EcoRI/MspI and EcoRI/HpaII) at 70°C for 15 minutes. This was followed by addition of 10μl of freshly prepared ligated mixtures [5 pmol EcoRI adapter (1.0 μl) + 50 pmol MspI/MseI adapter (1.0 μl) + 10X T4 Ligase buffer (1.0μl) + 100X BSA (0.5 μl) + T4 Ligase (0.5 μl) + ultra-pure molecular water (6.0 μl] to the digested sample to make a total of 50 μl reaction. The incubation was continued with a ligation process at 22°C for 5 hours, 70°C for 15 minutes and kept until further use. The ligated DNA fragment (2 μl) was used as template for pre-amplification in a thermocycler using the following composition and program: 10mM dNTP mix (0.5 μl), 25mM MgCl2 (0.6 μl), 10X standard Taq buffer (1.0 μl), 100 mg/μl BSA (1.0 μl), 5U/μl Taq polymerase (0.2 μl), 10nmol AFLP preselected primer pair (EcoRI/MseI, EcoRI/MspI) (1.0 μl), ligated DNA fragment (2.0 μl), ultra-pure water; 72°C for 2 minutes, 20 cycles at 94°C for 20 seconds, 56°C for 30 seconds and 72°C for 2 minutes, 4°C for ∞. The pre-amplification PCR product was then diluted with ultra-pure water in ratio of 1:10. The diluted pre-amplification product was further used as a template for the selective amplification PCR with a reaction volume of 10 μl comprising of 2.0 μl diluted pre-amplified DNA, 10 nmol/μl MseI/MspI/HpaII primer (0.6 μl), 10nmol/ μl EcoRI primer (0.5 μl), 10mM dNTP mix (0.2 μl), 25mM MgCl2 (0.6 μl), 10X Taq buffer (1.0 μl), 5U/μl Taq polymerase (0.125 μl) and ultra-pure molecular grade water. The PCR program used for amplification is as follows: 95°C for 3 minutes, 36 cycles of 95°C for 30 seconds, 56°C for 1 minute, and 72°C for 1 minute and final extension at 72°C for 2 minutes. The PCR product (3 μl) and 7 μl of internal standard mix (HIDI and Liz) were vortexed, centrifuged and denatured for 5 minutes at 95°C prior to size fractioning in a capillary electrophoresis on ABI 3730. GeneScan 500 LIZ (applied Biosystems) was used as a size standard and POP 5 polymer (Applied Biosystems) was used for fluorescent labeling.
c) Scoring
The fragment peaks and intensity from the AFLP and MSAP analysis were evaluated using GeneScan software (Applied Biosystems) after scanning the signals from all samples for each crop separately. Following fragment analysis on ABI3730, AFLP and MSAP profiles were visualized using GeneMapper software v4 (Applied Biosystems, Foster City, CA, USA). Raw data generated were scored following a band-based strategy described by Bonin et al. [25] using the GeneMapper v4.0 software (applied Biosystems). Allelic profile was transformed into binary matrixes which were scored as ‘1’ for presence of allele and ‘0’ for absence of allele. In order to reduce eventual impact of size homoplasy [26], binning of allelic sizes was followed with a size range between 100–500 bp with peak height ≥100. All reactions were repeated twice and only distinct, polymorphic and informative bands across all samples were considered for analysis. Fragments that could not be visually distinguished with low intensity were regarded as ambiguous and were not scored.
Statistical analysis
Phenotypic data generated from field grown plants were subjected to generalized linear model (PROC GLM and PROC GENMOD) in Statistical Analysis System (SAS-V9.2 and V9.3) [27] to obtain the variance components. The least significant mean (LSMEANS) was used to compare the means of different traits across both species. Principal coordinate analysis (PCoA) was carried out on standardized morphological data, eigen values and eigen vectors were calculated to generate two-dimensional plots under different growth conditions (in vitro and field). Similarly, genotypic (presence/absence) data was analyzed with msap software using R script (msap_score.r) [28–29] and GenAlEx version 6.5 [30]. Different statistical parameters such as PCoA, population differentiation test using analysis of molecular variance were also estimated using the Shannon diversity index.
Results
In vitro performance of genotypes in different culture systems
The efficiency of the three culture systems used in the study were examined on two tropical crop species (Musa spp. and Dioscorea rotundata) after three and six weeks respectively. The number of shoots per explant were counted for each plant cultured on these culture systems. For Musa spp. the multiplication rate varied significantly both with the type of culture system and the genotype tested. However, TIS (RITA bioreactor) gave significantly higher shoot mean across the genotypes, followed by complete immersion in liquid media system with shaking while the least was observed with semi-solid medium (Fig 1). There was a significant interaction between the performance of the accessions and culture system (Table 2). On average, a higher multiplication rate was observed with genotypes having AA genome as compared to those with AAB genome. In terms of number of shoots produced under TIS (RITA bioreactor), the highest mean shoot of 28.40 was observed in TMb 28, while least mean shoot value of 3.90 was observed for TMp 100 in RITA bioreactor.
Fig 1. Effect of culture systems on Musa spp. under in vitro conditions.
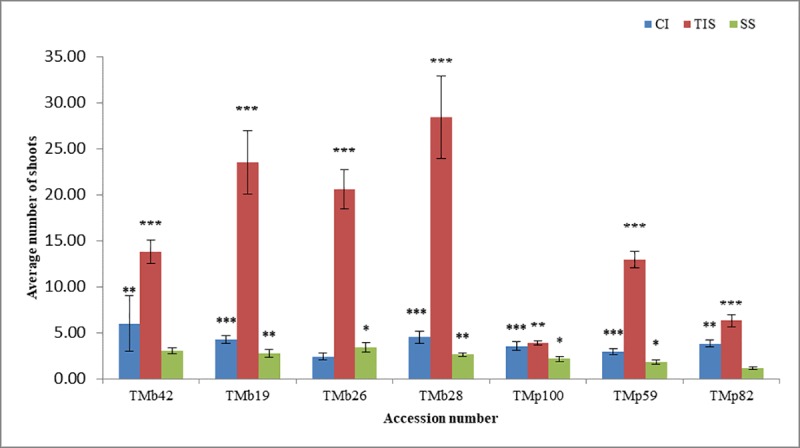
CI: Complete Immersion. TIS: Temporary Immersion System. SS: Semi-Solid.
Table 2. ANOVA summary for in vitro Musa spp. and Dioscorea rotundata.
| Musa | ||||||
| Source of variation | df | Mean square | Treatment | NBS LSMEAN | ||
| CI | 3.92*** | |||||
| Accession | 6 | 324.96*** | TIS | 15.63*** | ||
| Treatment | 2 | 2799.27*** | SS | 2.40*** | ||
| Accession*Treatment | 12 | 221.53*** | ||||
| Mean | 6.04 | |||||
| Error | 15.90 | |||||
| CV | 66.01 | |||||
| Dioscorea rotundata | ||||||
| Source of Variation | df | Mean Square | LSMean | |||
| NBS | NBC | NBS | NBC | |||
| System | 2 | 4.19** | 19.55*** | TIS | 2.45*** | 2.88*** |
| Treatment | 3 | 0.89ns | 0.69ns | CI | 2.20*** | 3.62*** |
| Treatment*System | 6 | 0.42ns | 0.54ns | SS | 1.55*** | 1.55*** |
| Mean | 2.05 | 2.59 | ||||
| Error | 0.82 | 0.97 | ||||
| CV | 44.07 | 38.13 | ||||
NBS, Number of shoot; NBC, Number of nodal cutting; TIS, Temporary Immersion System; CI, Complete Immersion; SS, Semi-solid.
*, **, *** p values significance at 0.05, 0.01 and 0.001 respectively; ns, not significant
For yam (Dioscorea rotundata), the result indicated that there was no significant difference in performance between TIS and CI within 6 weeks of plant culture while they differed significantly to SS across all the treatments used in the study (Table 2). Similarly, TIS and CI favored better multiplication rate than SS culture system (Fig 2). Culture media supplemented with low sucrose without hormone or with low level of Kinetin (T1, T2 & T4) promoted better shoot growth in TIS and CI. However, a previous study indicated that an increase in the culture duration in T1 and T4 favored shoot vigour in TIS (Jekayinoluwa et al. Unpublished, S4 Table).
Fig 2. Treatment and culture system effect on in vitro Dioscorea rotundata.
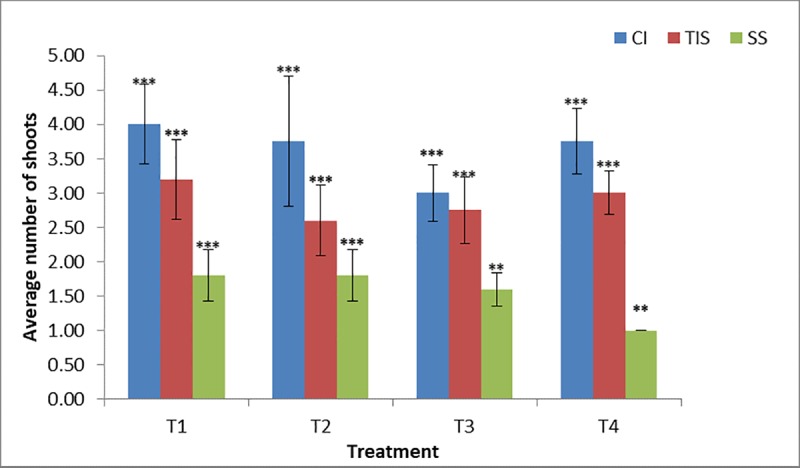
CI, Complete Immersion; TIS, Temporary Immersion System; SS, Semi-Solid.
Agro morphological characterization
a) Screen house acclimatization
The in vitro plantlets were transferred to the screen-house for acclimatization and no significant difference was observed for the parameters (NL: Number of leaves, PH: plant height, LW: leaf width, LL: leaf length) measured (S5 Table) between the different culture systems for Musa spp.
There was significant difference in growth parameters of Dioscorea spp. when transferred to screen-house across the plants generated from different culture systems. (S5 Table). TIS showed better acclimatization success rate which differed significantly in terms of leaf width and plant height in comparison to other culture systems under treatment 4 (T4). However, T4 in CI produced higher number of leaves compared to plants cultured in other systems (S5 Table).
b) Field morphological characterization
The acclimatized plantlets of both Musa and Dioscorea accessions were transplanted under field conditions for morphological characterization. The Musa plants were screened based on thirty-three agro-morphological parameters. Only 1 (Weight of Bunch (WB)) variable out of 33 agro-morphological descriptors showed significant differences between the plants derived using the three culture systems. TIS recorded the highest value for WB in comparison to SS and CI (S6A & S6B Table) for TMb 19, TMp 59 and TMp 100. While for qualitative variables, the p-value of the Chi-square distribution showed no significant difference across the three culture systems.
For Dioscorea, about 8% of the total (85) agro-morphological variables showed a varying level of differences across the culture systems used. However, six qualitative traits such as leaf density, plant vigour, spine length, number of inflorescence, tuber shape and place of root on tuber were observed on plants cultured on TIS (S7A–S7E Table).
c) Molecular characterization
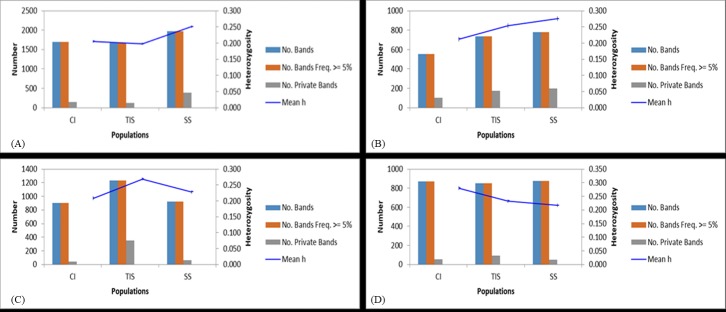
Amplified fragment length polymorphism (AFLP) profiles were generated for in vitro and field-grown plants of Musa and Dioscorea spp. with respect to different culture systems and treatments (Table 3). The profiles recorded 99–100% polymorphism regardless of the type of culture systems in both crops indicating the polymorphic nature of the markers used in the study. Principal Coordinate Analysis (PCoA) revealed the relatedness of the culture systems across the genotype used (Fig 3). The banding pattern for Musa spp. under field conditions showed clearly that TIS and CI have similar number of private bands (unique alleles) while SS recorded the highest with 387 private bands. Under in vitro conditions, SS recorded the highest number of private bands (200) while CI had the least number of private bands (103). Similarly, in Dioscorea spp., TIS derived field-grown plants recorded highest number (350) of private bands (Fig 4). The variance among the three culture systems (SS, CI & TIS) for Musa spp. under field condition was up to 3% (S8 Table) while there was no variation under in vitro conditions (S8 Table) indicating similar growth patterns among plantlets with negligible variances across different culture systems under study. Conversely, Dioscorea plants cultured under in vitro conditions recorded 17% variation among the different culture systems (S8 Table) while no variation was observed under field conditions (S8 Table).
Table 3. AFLP profile summary for Musa and Yam under in vitro and field conditions.
| S/N | Sample Group | No. primer combination | No. Loci | polymorphic AFLP |
% polymorphic AFLP |
phi_ST (AFLP) | p_phi_ST (AFLP) |
|---|---|---|---|---|---|---|---|
| 1 | Musa In vitro | 15 | 1115 | 1107 | 99 | -0.02688 | 0.7383 |
| 2 | Musa field | 15 | 2566 | 2327 | 91 | 0.0255 | 0.0620 |
| 3 | Yam In vitro | 15 | 1050 | 1046 | 100 | 0.1670 | 0.0019 |
| 4 | Yam field | 15 | 1375 | 1375 | 100 | 0.001968 | 0.4227 |
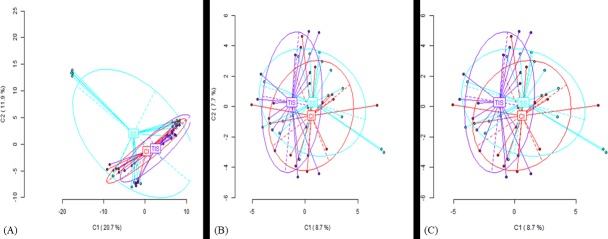
Fig 3. PCoA for Musa plants.
(A) PCoA MseI for Musa field plants. CI, Complete Immersion; TIS, Temporary Immersion System; SS, Semi-Solid, (B) PCoA on MSL for Musa field plants CI: Complete Immersion, TIS: Temporary Immersion System, SS: Semi-Solid, (C) PCoA on NML for Musa field plants CI: Complete Immersion, TIS: Temporary Immersion System, SS: Semi-Solid.
Fig 4. Band Patterns for Musa and Yam.
(A) Band patterns across populations for Musa under field conditions (MseI). CI, Complete Immersion; TIS, Temporary Immersion System; SS, Semi-Solid, (B) Band patterns across populations for Musa under in vitro conditions (MseI). CI, Complete Immersion; TIS, Temporary Immersion System; SS, Semi-Solid, (C) Band patterns across populations for Yam under field conditions (MseI). CI, Complete Immersion; TIS, Temporary Immersion System; SS, Semi-Solid, (D) Band patterns across populations for Yam under in vitro conditions (MseI). CI, Complete Immersion; TIS, Temporary Immersion System; SS, Semi-Solid.
Methylation event was revealed by the PCoA analysis developed from binary matrix of combined MSAP profiles using EcoRI/MspI and EcoRI/HpaII. For Musa spp., the profile (Table 4) revealed similarity in both methylation susceptible loci (MSL) and non-methylated loci (NML) for plants grown using all the three culture systems, and in the field (Fig 3B and 3C). There were 564 (46% of total MSL) polymorphic methylated susceptible loci (MSL) and 797 (78% of total NML) non-methylated loci (NML) for field grown Musa plants. On the other hand, in vitro plants recorded 137 (25% of total MSL) polymorphic methylated susceptible loci and 15 polymorphic NML. The profiles of isoschizomer pair HpaII, (m CCGG) for field grown plants showed a variation of 1% among the culture systems (S8 Table) and CI culture system recorded the highest number of private bands (143) while SS had the least (100 bands). For MspI (CmCGG), there was no observable variation among the three culture systems. However, samples collected from SS culture system recorded the highest private bands (141) while those from CI showed the least with 100 private bands. On the other hand, SS-derived plants recorded the least private bands in both profiles of the HpaII and MspI isoschizomer for in vitro grown Musa plants. The PCoA of HpaII (m CCGG) isoschizomer showed clustering of SS and CI grown plantlets together regardless of the genotype whereas, TIS grown plantlets are separated (Fig 5).
Table 4. MSAP profile summary for Musa and Yam.
| Sample Group | No. Loci | NML | MSL | PNML (%) | PMSL (%) | p. wilcoxon | p_PhiST_MSL | p_PhiST_NML | SIMSL | SDMSL | SINML | SDNML |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Musa In vitro | 573 | 15 | 558 | 25 | 100 | <0.0001 | 0.9897 | 1 | 0.5799 | 1.7859 | 0.3488 | 1.4174 |
| Musa field | 2246 | 1019 | 1227 | 78 | 46 | <0.0001 | 0.3518 | 0.1567 | 0.4675 | 1.5960 | 0.1744 | 1.1905 |
| Yam In vitro_CS | 1563 | 367 | 1196 | 100 | 52 | <0.0001 | 0.4597 | 3.00E-04 | 0.5910 | 1.8058 | 0.3518 | 1.4216 |
| Yam field_CS | 1993 | 563 | 1430 | 100 | 53 | <0.0001 | 0.7763 | 0.4461 | 0.5231 | 1.6872 | 0.2044 | 1.2268 |
NML, non-methylated loci; MSL, methylated sensitive loci; PNML, polymorphic non-methylated loci; PMSL, polymorphic methylated sensitive loci; SIMSL, Shannon Index_MSL; SDMSL, Shannon diversity_MSL; SINML, Shannon Index_NML; SDNML, Shannon diversity_NML
Fig 5. PCoA for Musa in vitro with HpaII.
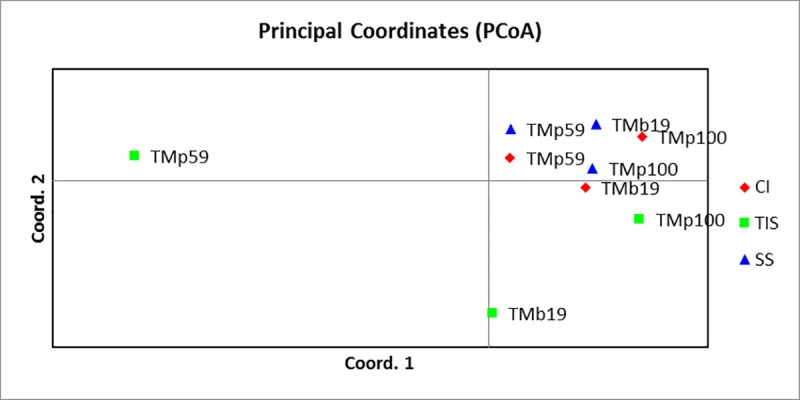
CI: Complete Immersion, TIS: Temporary Immersion System, SS: Semi-Solid.
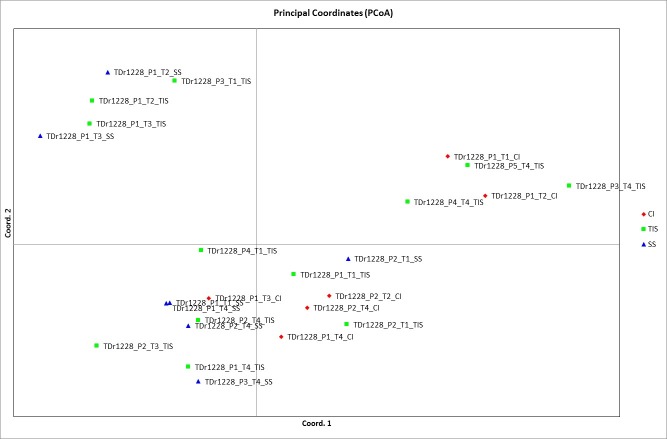
Twenty-five field-grown yam plants of TDr 1228 accession generated 757 polymorphic MSL bands and 563 polymorphic NML bands. The PCoA generated from HpaII profile, showed clustering based on different treatments used in culture system (Fig 6). T1 and T4 were closely related while T2 and T3 clustered together. There were 627 polymorphic MSL and 367 polymorphic NML for in vitro grown plants. Further clustering based on culture system in relation to the treatment used revealed TIS with minimum MSL across all treatments. Of all the treatments, T1 and T4 recorded 334 and 338 MSL while 311 and 270 NML, respectively (Table 5). The measure of genetic diversity was estimated by the Shannon diversity index and comparison with Wilcoxon test revealed a significant difference (P<0.0001) between MSL and NML for both crops.
Fig 6. PCoA for Yam in field with HpaII.
CI: Complete Immersion, TIS: Temporary Immersion System, SS: Semi-Solid.
Table 5. Summary of band pattern of in vitro Dioscorea rotundata.
| S/N | Accession | Treatment | Culture system | MSL | NML |
|---|---|---|---|---|---|
| 1 | TDr 1228 | T1 | CI | 518 | 231 |
| 2 | TDr 1228 | T1 | TIS | 334 | 311 |
| 3 | TDr 1228 | T1 | SS | 542 | 271 |
| 4 | TDr 1228 | T2 | CI | 468 | 129 |
| 5 | TDr 1228 | T2 | TIS | 366 | 130 |
| 6 | TDr 1228 | T2 | SS | 573 | 258 |
| 7 | TDr 1228 | T3 | CI | 397 | 176 |
| 8 | TDr 1228 | T3 | TIS | 380 | 242 |
| 9 | TDr 1228 | T3 | SS | 577 | 243 |
| 10 | TDr 1228 | T4 | CI | 375 | 197 |
| 11 | TDr 1228 | T4 | TIS | 338 | 270 |
| 12 | TDr 1228 | T4 | SS | 572 | 260 |
MSL: Methylation sensitive loci, NML: Non-methylation loci, CI: Complete Immersion, TIS: Temporary Immersion System, SS: Semi-Solid
Discussion
In this study, three in vitro culture systems were used, including semisolid medium in test tube, complete immersion in liquid culture medium with shaking in baby food jar and temporary immersion system in RITA bioreactor for Musa and Dioscorea spp. It was noted that the type of culture system had a significant effect on the number of polyshoot produced in vitro. In general, the temporary immersion system was more efficient for rapid propagation of Musa spp., owing to the higher number of shoots produced within a relatively short period of time compared to other conventional (semi-solid) methods. An optimal relatively short period of 21 days generated a multiplication rate of 28.40 and 12.93 for Musa AA and AAB respectively in TIS (RITA bioreactor). This can enable a faster rate of availability of plantlets for use and conservation. Similar results were observed by Roels et al. [31] at 28 days with multiplication rate of 13 and Hui et al. [32] at 5 weeks with an average rate of 4.9. The studies of Businge et al. [33], Georgieva et al., [34] and Steinmacher et al., [35] also confirmed the positive effect of TIS over conventional in vitro propagation in other crops. This may be because TIS has the advantage of allowing better contact between the explant and the culture medium, thus easy diffusion and uptake of nutrient is achieved. The TIS nullifies the drawbacks of liquid culture medium by making the explant immersed only for a while and aerating them inducing relatively less stress on the tissues [36]. Conversely, there was no significant effect of culture system on the multiplication rate of Dioscorea spp. TDr 1228 within 6 weeks of culture. However, the lowest multiplication rate was observed in semi-solid culture medium followed by TIS and CI. This suggested that an increased nutrient-to-plant contact period was needed for increased multiplication rate of TDr 1228. Also, culture media composition influenced the multiplication rate of TDr 1228. The multiplication rate was highest in MS medium supplemented with 0.5mg/l Kinetin (T1) in CI, TIS and SS. While the absence of hormone supplement (T4) also supported growth in CI and TIS but minimal in SS. Polizin et al. [37], observed a similar trend, wherein there was no significant difference between TIS and SS at 8 weeks and suggested increasing immersion frequency as a possible way of optimizing the potential of TIS for Dioscorea spp. However, Yan et al. [38], observed significant difference in the growth rate of Dioscorea fordii and Dioscorea alata in TIS indicating that the multiplication rate in Dioscorea spp. may be genotype/cultivar dependent.
TIS is also known to improve plant quality and increase shoot vigor as well as quantity of morphologically normal somatic embryos [39]. Hyperhydricity that seriously affects cultures in liquid medium is eliminated in TIS [10] as the explants are not permanently immersed. Hvoself-Eide et al., [40] confirmed that in TIS, there is increased multiplication rate when shoots are appropriately exposed to culture media at correct intervals. TIS provides an excellent way of using liquid medium at the same time controlling the gaseous environment thereby increasing the growth and multiplication rate of cultures. Also due to lack of agitation, the mechanical stress on plant tissues are low compared to other micropropagation methods.
For in vitro plant tissue culture, type of growth regulator plays an important role for the physiological response of explants. A higher level of BAP supplement in MS medium promoted the production of polyshoot for Musa spp. As reported by several authors [41–44], polyshoot production in Musa spp. was improved by wounding the apical meristem to break the apical dominance thereby stimulating the axillary buds to produce multiple shoots in Musa. For Dioscorea rotundata, low sucrose concentration has been identified to reduce exudation of phenolic compounds, which may hamper regeneration and growth [45]. This in combination with no or low levels of plant hormone have been identified to promote its multiplication rate [46–48].
Molecular characterization of plants obtained from the three culture systems was essential to compare their genetic differences in relation to phenotypic characteristics. Amplified Fragment Length Polymorphism (AFLP) and Methylation Sensitive Amplification Polymorphism (MSAP) are useful molecular markers that help to understand effect of methylation on genetic diversity within and among a population. This is possible because AFLP marker is highly polymorphic and evenly distributed in the genome, giving a broad understanding of genomic variation. MSAP is a modification of AFLP marker that reveals methylation pattern in a population.
AFLP profiles for Dioscorea and Musa spp. under in vitro and subsequent field conditions revealed a level of conserved genetic variability across the genotypes. A 3% variation among the different culture systems was explained on the basis of presence of private alleles peculiar to each type of culture system. In addition, the pairwise genetic distances were calculated to investigate the allelic differences among the 3 culture systems. A low genetic distance was observed between SS and TIS for both Dioscorea and Musa spp. either under in vitro or field conditions, indicating the relatedness between the two culture systems.
In this study, MSAP using EcoRI/HpaII and EcoRI/MSpI as restriction enzymes to identify and cleave methylation regions, thus generating methylation profiles which can help in understanding genetic diversity between and among a population. In plant genome, DNA methylation is a common phenomenon that does not alter the main genetic code but may show somatic or phenotypic variations. Schulz et al [49] and Herrera and Bazaga [50] described different forms of cytosine methylation that explained the basic principle of methylation scoring and profiling. Cleavage of methylated cytosine by MspI and HpaII could either result in full methylation (when the internal cytosine of the double stranded DNA is methylated) or hemi-methylation (methylation of internal cytosine on one DNA strand) with the exception that HpaII cleaves external cytosine. CG methylation is said to be an important factor for promoter function [51]. This is evident in the work of Hafiz et al [52] who made it clear that DNA methylation plays a significant role in the transition from vegetative to reproductive growth and ploidy level of plants. Polymorphism in DNA methylation is an important form of genetic variation which plays a significant role in cell division, higher growth rate of plants, rooting ability and a potential capacity of silencing plant viruses [53–54].
Methylation profiles generated from Musa and Dioscorea spp. under field and in vitro conditions revealed a significant level of full and partial methylation pattern. The value of Shannon diversity accounts for the richness and evenness of MSL and NML for Musa and Dioscorea spp. under field and in vitro conditions. The level of diversity of MSL under in vitro (1.78) condition-was higher than field (1.59) condition for Musa spp. A similar trend was observed for Dioscorea spp. The relative lower diversity value under field condition may be a reflection of environmental influence on the crops. It has been reported that factors such as plant growth hormone, increased level of salt, biotic or abiotic stress may contribute to methylation/genetic variation in crops. This was confirmed with the number of MSL for Dioscorea spp. across four hormone treatments and the 3 culture systems used under in vitro conditions. Plants grown in TIS recorded lowest number of MSL across all hormone treatment used while SS system had the highest number of MSL. A closer look at the hormone combination revealed that T1 and T4 had a lower number of MSL compared to other hormone combinations. LoSchiavo et al [55] and Arnholdt-Schmitt [56] reported hypo-methylation with increasing level of cytokinin (kinetin) in carrot root while higher concentration of auxin (2, 4-D) increased methylation level from 15 to 70%. This explained how plants react or adjust to stressful conditions when developing different cell types [57–58]. Rico et al [59] also confirmed increase in hemi-methylation level and decrease in full methylation of drought effect on forest trees. The DNA methylation highlights the capacity of plants to acclimatize and adapt to changing environmental conditions. An exponential of the Shannon diversity index provided information on the effective number of species, which is the actual measure of diversity as it shows the richness and evenness of a population [60–61].
Conclusion
The present study confirmed the advantage of temporary immersion system (TIS, RITA bioreactor) in improving the multiplication rate of polyshoot production in both Musa and Dioscorea spp. The adoption of TIS over other propagation system is to assist in overcoming the challenges of mass production of good quality planting materials within a relatively short period of time and at a lower cost. The suitability of tissue culture-based system depends on their effect on genetic uniformity. Methylation-sensitive amplification polymorphism (MSAP) is a valuable tool for detecting methylation, which could be a potential indicative signal of possible somaclonal variation in clonal crops with respect to the culture duration and/or systems. The culture systems used in this study did not show significant alteration on the genetic integrity of Musa and Dioscorea spp. The high level of genetic polymorphism showed the ability of the culture system to conserve crop genetic variability, which can make the crop adaptable and promote their use and conservation in genebanks, breeding and biotechnology programs. However, factors such as plant growth hormone, culture system type, mode of propagation and induced stress revealed the cause of variation in plants. It was also observed that certain type of plant growth hormone could either trigger increase or decrease in methylation, which could lead to activation or deactivation of certain genes in the plant genome. The variation observed is marked by increase or decrease in methylation events and could be further explored to understand and assess epigenetic changes in these two plants.
Supporting information
MSCM, multiple shoot culture medium; RCM, rooting culture medium; T1, Treatment 1; T2, Treatment 2; T3, Treatment 3; T4, Treatment 4.
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
*, **, ***, p values significance at 0.05, 0.01 and 0.001 respectively; WB, weight of bunch; NHWB, Number of hands on whole bunch; NFTH, Number of fruit on third hand; FL, Fruit length; NFD, Number of days to flowering; TIS, Temporary Immersion System; SS, Semi-Solid; CI, Complete Immersion.
(DOC)
DOH, Date of harvest; NTY1, Number of tuber year 1; NTY2, Number of Tuber year 2; WTY1, Weight of tuber year 1; WTY2, Weight of tuber year 2; LOT, Length of tuber; WOT, width of tuber; IL, internode length, DOFAE, days to flowering after emergence; MFL, male flower length; NOSPP, Number of stem per plant; NOI, Number of internode, *, **, ***, p values significance at 0.05, 0.01, 0.001 respectively; ns, not significant; TIS, temporary immersion system; SS, Semi-Solid, CI, complete immersion.
(DOC)
(A) AMOVA summary for Musa under field condition using MSeI restriction enzyme, (B) AMOVA summary for Musa under in vitro conditions using MSeI restriction enzyme, (C) AMOVA summary for Dioscorea under in vitro condition using MSeI restriction enzyme (D) AMOVA summary for Dioscorea under field condition using MSeI restriction enzyme (E) AMOVA summary for Musa under field condition using HpaII restriction enzyme. Df, degree of freedom; SS, sum of squares; MS, mean squares; Est. var, estimated variance.
(DOC)
Acknowledgments
The authors will like to thank Dr. Mercy Kitavi, Mr. Sam Ofodile, Dr. Tessema Gezahegn, Mrs. Owoeye Temitope and Mr. Tchamba Marimagne for their technical assistance.
Data Availability
All relevant data are within the manuscript and its supporting information files.
Funding Statement
The research was funded by the IITA Genetic Resources Center.
References
- 1.Bairu MW, Kane ME. Physiological and developmental problems encountered by in vitro cultured plants. Plant Growth Regul 2011. 63: 101–103 10.1007/s10725-011-9565-2 [DOI] [Google Scholar]
- 2.Gasppar T, Kevers C, Penel C, Greppin H, Reid DM, Thorpe TA. Plant hormones and plant growth regulators in plant tissue culture. In: In vitro Cellular & Developmental Biology-Plant. 1996. October, Volume 32, Issue 4, pp 272–289 [Google Scholar]
- 3.Sahay NS, Varma A. A biological approach towards increasing the rates of survival of micropropagated plants. In: Current Science Vol. 78, No. 2 (25 January 2000), pp. 126–129 [Google Scholar]
- 4.Baránek M, Cechová J, Raddová J, Holleinová V, Ondrusikova E, Pidra M. Dynamics and Reversibility of the DNA methylation Landscape of Grapevine Plants (Vitis Vinifera) Stressed by In vitro Cultivation and Thermotherapy. PLoS ONE 2015. 10(5): e0126638 10.1371/journal.pone.0126638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dann AL, Wilson CR. Comparative assessment of genetic and epigenetic variation among regenerants of potato (Solanum tuberosum) derived from long-term nodal tissue-culture and cell selection. Plant Cell Rep 2011. 30: 631 10.1007/s00299-010-0983-9 [DOI] [PubMed] [Google Scholar]
- 6.Rathore MS, Mastan SG, Agarwal PK. Evaluation of DNA methylation using methylation sensitive amplification polymorphism in plant tissues grown in vivo and in vitro Plant Growth Regul 2015. 75: 11 10.1007/s10725-014-9926-8 [DOI] [Google Scholar]
- 7.Chen CC, Bates R, Carlson J. Effect of environmental and cultural conditions in medium pH and plant growth performance of Douglas-fir (Pseudotsuga menziesii) shoot culture F1000Research 2014, 3:298 10.12688/11000research.5919.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai CC, Lin HM, Nalawade SM, Fang W, Tsay HS. Hyperhydricity in shoot cultures of Scrophularia yoshimurae can be effectively reduced by ventilation of culture vessels. Journal of Plant Pathology 2005. 162:355–361. 10.1016/j.jplph.2004.07.015 [DOI] [PubMed] [Google Scholar]
- 9.Li W, Masilamany P, Kasha KJ, Pauls KP. Developmental Tissue culture, and Genotypic Factors Affecting Plant Regeneration From Shoot Apical Meristems of Germinated Zea mays L. Seedlings. In vitro Cell. Dev. Biol.-Plant 2007. 38:285–292 1054-5476/02 [Google Scholar]
- 10.Berthouly M, Etienne H. Temporary immersion system: A new concept for use liquid medium in mass propagation. In: Hvoself-Eide AKand Preil W. Liquid Culture Systems for in vitro Plant Propagation. Netherlands: Department of Plant and Environmental Science; 2005. p.165–195. [Google Scholar]
- 11.Teisson C, Alvard D. A new concept of plant in vitro cultivation liquid medium: temporary immersion. In: Terzi M et al. (eds) current issues in plant molecular and cellular biology: 1995. pp.105–110. [Google Scholar]
- 12.Aitken-Christie J, Davies HE. Development of semi-automated micropropagation system. ISHS Acta Horticulturae 230: symposium on High Technology in Protected Cultivation. 10.17660/ActaHortic 1988.230.7 [DOI] [Google Scholar]
- 13.Simonton A, Robacker C, Krueger S. A programmable micropropagation apparatus using cycled liquid medium. Plant Cell Tiss Organ Cult 1991. 27:211 10.1007/BF00041292 [DOI] [Google Scholar]
- 14.Tisserat B, Vandercook CE. Development of an automated plant culture system. Plant Cell Tiss Organ Cult 1985. 5:107 10.1007/BF00040307 [DOI] [Google Scholar]
- 15.Murashige T, Skoog F. 1962. A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiologia plantarum [Google Scholar]
- 16.Vuylsteke DR. Shoot-tip culture for the propagation, conservation and distribution of musa germplasm International Institute of Tropical Agriculture, Ibadan, Nigeria: 1998. pp. 82 [Google Scholar]
- 17.Jarret RL, Rodriguez W, Fernandez R. Evaluation, tissue culture propagation, and dissemination of ‘Saba’ and ‘Pelipita’ plantains in Costa Rica. Scientia Horticulturae 1985. 25: 137–147. 10.1016/0304-4238(85)90085-8 [DOI] [Google Scholar]
- 18.Gupta PP. Eradication of mosaic disease and rapid clonal multiplication of bananas and plantains through meristem-tip culture. Plant Cell Tiss Organ Cult. 1986; 6: 33 10.1007/BF00037756 [DOI] [Google Scholar]
- 19.IPGRI/IITA. Descriptors for Yam (Dioscorea spp.). International Institute of Tropical Agriculture, Ibadan, Nigeria/International Plant Genetic Resources Institute, Rome, Italy. 1997. ISBN 92-9043-353-1
- 20.Channeliere S, Van den houwe I, Arnaud E, Horry JP, Ruas M, Roux N. Standardised Procedure or Musa Germplasm Characterization. ISHS Ata Horticulturae 897: 11 10.17660/ActaHortic.2011.897.11 [DOI] [Google Scholar]
- 21.Dellaporta SL, Wood J, Hicks JB. A Plant DNA minipreparation: Version II. Plant Molecular Biology Reporter, 1983. Volume 1, issue 4, pp 19–21. 10.1007/BF02712670 [DOI] [Google Scholar]
- 22.Vos P, Hogers R, Bleeker M, reijans M, van de Lee T, Hornes M, et al. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 1995. November 11;23 (21):4407–14 10.1093/nar/23.21.4407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vroh-Bi I, Anagbogu C, Nandi S, Tenkouano A. Genomic characterization of natural and somaclonal variations in bananas (Musa spp.). Plant Mol Biol Rep 2011. 29: 440 10.1007/s11105-010-250-9 [DOI] [Google Scholar]
- 24.Reyna-Lopez GE, Simpson J, Ruiz-Herrera J. Differences in DNA methylation patterns are detectable during the dimorphic transition of fungi by amplification of restriction polymorphisms. Mol Gen Genet 1997. 253:703–710 [DOI] [PubMed] [Google Scholar]
- 25.Bonin A, Ehrich D, Manel S. Statistical analysis of amplified fragment length polymorphism data: a toolbox for molecular ecologists and evolutionists. Molecular ecology 2007. 16, 3737–3758. 10.1111/j.1365-294X.2007.03435.x [DOI] [PubMed] [Google Scholar]
- 26.Caballero A, Quesada H, Rolan-Alvarex E. Impact of amplified fragment length polymorphism size homoplasy on the estimation of population genetic diversity and the detection of selective loci. Genetics 179, 539–554. 10.1534/genetics.107.083246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SAS Institute Inc., 2007. SAS OnlineDoc 9.2. SAS Institute Inc., Cary, NC
- 28.Perez-Figueroa A. msap (v. 1.1.8): 2014—User’s Guide http://cran.r-project.org
- 29.Perez-Figueroa A. msap: a tool for the statistical analysis of methylation-sensitive amplified polymorphism data. Molecular ecology resources 2013. 10.1111/1755-0998.12064 [DOI] [PubMed] [Google Scholar]
- 30.Peakall R, Smouse PE. 2012. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research–an update. Bioinformatics 28, 2537–2539 10.1093/bioinformatics/bts460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roels S, Escalona M, Cejas I, Noceda C, Rodriguez R, Canal MJ et al. Optimization of plantain (Musa AAB) micropropagation by temporary immersion system. Plant Cell Tissue Organ Culture (2005) 82: 57 10.1007/s11240-004-6746-y [DOI] [Google Scholar]
- 32.Hui AV, Arvind B, Sreeramanan S, Keng CL. Establishment of a shoot proliferation protocol for banana (abb group) cv. ‘pisang awak’ via temporary immersion system, Journal of Plant Nutrition, (2013) 36:4, 529–538, 10.1080/01904167.2012.748068 [DOI] [Google Scholar]
- 33.Businge E, Trifonova A, Schneider C, Rodel P, Egertsdotter U. Evaluation of a New Temporary Immersion Bioreactor System for Micropropagation of Cultivars of Eucalyptus, Birch and Fir. Forests 2017, 8, 196; 10.3390/f8060196 [DOI] [Google Scholar]
- 34.Georgieva L, Tsvetkov I, Georgieva M, Kondakova V. New protocol for in vitro propagation of berry plants by TIS bioreactor. Bulg. J. Agric. Sci. 2016; 22: 745–751 [Google Scholar]
- 35.Steinmacher DA, Guerra MP, Saare-Surminski K, Lieberei R. A temporary immersion system improves in vitro regeneration of peach palm through secondary somatic embryogenesis. Annals of Botany, Volume 108, Issue 8, 1 December 2011, Pages 1463–1475. 10.1093/aob/mcr033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kana EBG, Oloke JK, Lateef A, Kenfack RHA, Adeyemi A. Implementation Details of Computerized Temporary Immersion Bioreactor (TIB): A Fermentation Case of Pleurotos Pulmonarius. Biotechnology & Biotehnological Equipment, 24:4, 2149–2153, 10.2478/V10133-010-0093-4 [DOI] [Google Scholar]
- 37.Polzin F, Sylvestre I, Déchamp E, Ilbert P, Etienne H, Engelmann F. Effect of activated charcoal on multiplication of African yam (Dioscorea cayenensis-rotundata) nodal segments using a temporary immersion bioreactor (RITA) In Vitro Cell. Dev.Biol.(2014)—Plant 10.1007/s11627-013-9552-6 50:210–216 [DOI] [Google Scholar]
- 38.Yan H, Yang L, Li Y. Improved growth and quality of Dioscorea fordii Prain et Burk and Dioscorea alata plantlets using a temporary immersion system. African Journal of Biotechnology Vol. 10(83), pp. 19444–19448, 21 December, 2011. 10.5897/AJB11.2684 ISSN 1684–5315 2011 Academic Journals [DOI] [Google Scholar]
- 39.Jova MC, Kosky RG, Cuellar EE. 2011. Effect of liquid media culture systems on yam plant growth (Dioscorea alata L. ‘Pacala Duclos’). Biotechnol. Agron. Soc. Environ. 2011. 15(4), 515–521 [Google Scholar]
- 40.Hvoself-Eide AK, Olsen OAS, Lyngved R, Munster C, Heyerdahl PH. Bioreactor design for propagation of somatic embryos In: Hvoself-Eide AK and Preil W. Liquid Culture Systems for in vitro Plant Propagation. Nertherlands: Department of Plant and Environmental Science; 2005. pp. 41–59 [Google Scholar]
- 41.Arinaitwe G, Rubaihayo PR, Magambo MJS. Proliferation rate effect of cytokinins on banana (Musa spp.) cultivars. Scientia Horticulturae Volume 86, Issue 1, 8 September 2000, Pages 13–21. 10.1016/S0304-4238(00)00124-2 [DOI] [Google Scholar]
- 42.Buah JN, Danso E, Taah KJ, Abole EA, Bediako EA, Asiedu J et al. The effect of different concentrations of cytokinins on the in vitro multiplication of plantain (Musa sp.). Biotechnology, 9: 343–347. 10.3923/bioteh.2010.343.347 [DOI] [Google Scholar]
- 43.Muhammad A, Rashid H, Hussain I, Naqvi SMS. Proliferation-rate Effects of BAP and Kinetin on Banana (Musa spp. AAA Group) ‘Basrai’. HORTSCIENCE 42(5): 1255 2007 [Google Scholar]
- 44.Ngomuo M, Mneney E, Ndakidemi P. The effect of Auxins and Cytokinin on Growth and Development of (Musa sp.) Var. ‘Yangambi’ Explants in Tissue Culture. American Journal of Plant Sciences, 2013, 4, 2174–2180. 10.4236/ajps.2013.411269 [DOI] [Google Scholar]
- 45.Anike FN, Konan K, Oliver K, Dodo H. Efficient shoot organogenesis in petioles of yam (Diocorea spp). Plant cell Tissue and organ Culture 11(3) December 2011. 10.1007/s11240-012-0195-9 [DOI] [Google Scholar]
- 46.Bernabe-Antonio A, Santacruz-Ruvalcaba F, Cruz-Sosa F. Plant Growth Regul 2012. 68: 293 10.1007/s10725-012-9717-z [DOI] [Google Scholar]
- 47.Ezeibekew IO, Ezenwaka CL, Mbagwu FN, Unamba CIN. Effects of Combination of Different Levels of Auxin (NAA) and Cytokinin (BAP) on In vitro Propagation of Dioscorea Rotundata L. (White Yam). Journal of Molecular Genetics, 1: 18–22 http://medwelljournals.com/abstract/?doi=jmolgene.2009.18.22 [Google Scholar]
- 48.Das S, Choudhury MD, Mazumdar PB. Micropropagation of Dioscorea alata L. through nodal segments. 10.5897/AJB2013.12191 [DOI] [Google Scholar]
- 49.Schulz B, Eckstein LR, Durka W. Scoring and analysis of methylation-sensitive amplification polymorphism for epigenetic population studies. Molecular Ecology Resources 2013. 13, 642–653 10.1111/1755-0998.12100 [DOI] [PubMed] [Google Scholar]
- 50.Herrera CM, Bazaga P. Epigenetic differentiation and relationship to adaptive genetic divergence in discrete populations of the violet viola carzorlensis. New Phytol: 2010. August; 187(3):867–76. Epub 2010 May 20 10.1111/j.1469-8137.2010.03298.x [DOI] [PubMed] [Google Scholar]
- 51.Vinson C, Chatterjee R. CG methylation. Epigenomics. 2012. December; 4(6): 655–663. 10.2217/epi.12.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hafiz IA, Anjum MA, Grewal AG, Chaudhary GA. DNA methylation- an essential mechanism in plant molecular biology. Acta Physiol Plant 2001. 23: 491 10.1007/s11738-001-0060-7 [DOI] [Google Scholar]
- 53.Messeguer R, Ganal MW, Steffens JC, Tanksley SD. Characterization of the level, target sites and inheritance of cytosine methylation in tomato nuclear DNA. Plant Mol Biol 1991. 16: 753 10.1007/BF00015069 [DOI] [PubMed] [Google Scholar]
- 54.Watson JC, Kaufman LS, Thompson WF. Developmental regulation of cytosine methylation in the nuclear ribosomal RNA genes of Pisum sativum 1987. Jmb 193: 15–26 10.1016/0022-2836(87)90622-X [DOI] [PubMed] [Google Scholar]
- 55.LoSchiavo F, Pitto L, Giuliano G, Torti G, Nuti-Ronchi V, Marazziti D et al. DNA methylation of embryogenic carrot cell cultures and its variations as caused by mutation, differentiation, hormones and hypomethylating drugs. Theoret Appl. Genetics 1989. 77: 325 10.1007/BF00305823 [DOI] [PubMed] [Google Scholar]
- 56.Arnholdt-Schmitt B. Rapid changes in amplification and methylation pattern of genomic DNA in cultured carrot root explants (Daucs carota L.). Theor Appl Genet 1993. 85:793–800 10.1007/BF00225021 [DOI] [PubMed] [Google Scholar]
- 57.Kitimu SR, Taylor J, March TJ, Tairo F, Wilkinson MJ, Lopez CMR. Meristem micropropagation of cassava (Manihot esculenta) evokes genome-wide changes in DNA methylation. Frontiers in Plant Science. 10.3389/fpls.2015.00590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature genetics supplement, volume 33, March 2003. 10.1038/ng1089 [DOI] [PubMed] [Google Scholar]
- 59.Rico L, Ogaya R. Barbeta A, Penuelas J. Changes in DNA methylation fingerprint of Quercus ilex trees in respponse to experimental field drought simulation projected climate change. Plant biology: 2013 10.1111/plb.12049 [DOI] [PubMed] [Google Scholar]
- 60.Jost L. The relationship between Evenness and Diversity. Diversity 2010, 2, 207–232; 10.3390/d2020207 [DOI] [Google Scholar]
- 61.Colwell RK. Biodiversity: concepts, Patterns, and Measurement. The Princeton Guide to Ecology, 2009. Princeton, NJ, USA: Princeton University Press; p. 257–63 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MSCM, multiple shoot culture medium; RCM, rooting culture medium; T1, Treatment 1; T2, Treatment 2; T3, Treatment 3; T4, Treatment 4.
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
*, **, ***, p values significance at 0.05, 0.01 and 0.001 respectively; WB, weight of bunch; NHWB, Number of hands on whole bunch; NFTH, Number of fruit on third hand; FL, Fruit length; NFD, Number of days to flowering; TIS, Temporary Immersion System; SS, Semi-Solid; CI, Complete Immersion.
(DOC)
DOH, Date of harvest; NTY1, Number of tuber year 1; NTY2, Number of Tuber year 2; WTY1, Weight of tuber year 1; WTY2, Weight of tuber year 2; LOT, Length of tuber; WOT, width of tuber; IL, internode length, DOFAE, days to flowering after emergence; MFL, male flower length; NOSPP, Number of stem per plant; NOI, Number of internode, *, **, ***, p values significance at 0.05, 0.01, 0.001 respectively; ns, not significant; TIS, temporary immersion system; SS, Semi-Solid, CI, complete immersion.
(DOC)
(A) AMOVA summary for Musa under field condition using MSeI restriction enzyme, (B) AMOVA summary for Musa under in vitro conditions using MSeI restriction enzyme, (C) AMOVA summary for Dioscorea under in vitro condition using MSeI restriction enzyme (D) AMOVA summary for Dioscorea under field condition using MSeI restriction enzyme (E) AMOVA summary for Musa under field condition using HpaII restriction enzyme. Df, degree of freedom; SS, sum of squares; MS, mean squares; Est. var, estimated variance.
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its supporting information files.