Abstract
The Xenopus oocyte expression system is ideal for electrophysiological characterization of voltage-dependent and ligand-dependent ion channels due to its relatively low background of endogenous channels and the large size of the cell. Here we present a protocol to study voltage and ligand dependent activation of ion channels expressed in Xenopus oocytes using patch clamp techniques. The protocol is designed to control both the membrane voltage and the intracellular solution. In this protocol the large conductance, voltage and Ca2+-activated K+ (BK) channel is studied as an example. After injection of BK channel mRNA, oocytes are incubated for 2–7 days at 18°C. Inside-out membrane patches containing single or multiple BK channels are excised with perfusion of different solutions during recording. The protocol can be used to study structure-function relations for ion channels and neurotransmitter receptors.
MATERIALS
Reagents:
ND96 solution
Oocyte Dissociation Solution <R>
Bath solution (0Ca2+ 0Mg2+ solution)
Pipette Solution
Oocytes Stripping Solution
Xenopus oocytes (Newman et al. 2017)
Oocytes can be surgically extracted from Xenopus ovaries (Sive HL 2000; Green 2009; Sive et al. 2010b; Sive et al. 2010a) or directly bought from commercial vendors (e.g., EcoCyte Bioscience and Nasco)
Solution of mRNA that is transcribed in vitro from the gene of interest (0.001 µg/µL to 1 µg/µL)
Basal Internal (+EGTA) Solution
This solution is used to make solutions containing desired free Ca2+ concentration ([Ca2+]i) between 0 and 500μM (excluding 0 and 500 μM) by adding different amount of 1M CaCl2 solution into this solution. Please refer to user’s manual for Calcium Combination Ion Selective Electrode (ISE) - HI4104 for how to calculate the amount of CaCl2 solution to add and how to measure final [Ca2+]i in the solution.
Equipment:
Petri dishes
48 well cell culture cluster (flat bottom with lid)
Calibrated Disposable Micropipets 100µL (VWR International, Cat # 53432–921)
Sticky Wax (Kerr Australia Pty. Ltd, Cat # 00625)
Watchmaker’s forceps (Dumont #5 or #55)
Calcium Combination Ion Selective Electrode (ISE) (HI4104 from Hanna Instruments, Inc.)
Microinjector (Drummond Nanoject II Auto-Nanoliter Injector, Drummond Scientific Company)
3.5” Drummond Replacement Tubes (#3–000-203-G/X)
Dissecting microscope
Micromanipulator
Incubator (set at 16°C–20°C)
Micropipette puller (P-97 from Sutter Instrument)
Microforge (Model X1211–1 from World Precision Instruments (WPI), newer model available from WPI)
Patch Clamp Amplifier (Axopatch 200B Amplifier from Molecular Devices, LLC, This is an old model. Check with the company for the newest model.)
Acquisition Interfaces (InstruTECH ITC-18 from HEKA Elektronik)
Acquisition Software (Pulse 8.6 from HEKA Elektronik. The new version is called Patchmaster. Check with HEKA Elektronik for more information.)
Manipulator Controller (MC1000e controllers from Siskiyou Corporation)
Perfusion System (OctaFlow II™ Multi-function Multi-valve from ALA Scientific Instruments)
Headstage (Model CV 203BU from Molecular Devices, LLC)
Inverted Microscope (Model CKX31 from Olympus Corporation)
METHOD
Injection of mRNA into Oocytes (Sive et al. 2010c; Aguero et al. 2017)
-
1
Prepare oocytes for microinjection (Sive et al. 2010a). Take 5–15 mL of oocytes from Xenopus ovaries through dissection and then separate them into small clusters using forceps (about 10–20 oocytes per cluster). Use oocyte dissociation solution for enzymatic defolliculation of cells (30–90 min at room temperature, until the follicles of some oocytes are broken by visual inspection under microscope) and then wash the cells with ND96 solution at least 5 times to remove any residual enzyme. Under a dissecting microscope, select stage V and VI oocytes (>0.8 mm in diameter) (Wasserman et al. 1984) that are visibly healthy. Injection can be performed on the same day of harvesting or on the next day, with oocytes incubated in ND96 solution at 18°C for overnight.
If fewer than 100 oocytes are needed each time, it may be better to order them from a commercial source or to manually defolliculate oocytes using watchmaker’s forceps (Sive HL 2000; Sive et al. 2010b).
-
2
Prepare needles for microinjection. Use a P97 puller to pull 3.5” Drummond replacement tubes in 1 step at 300°C. The needle should have a long shank (≥ 5mm). Break the tip of the needle using forceps under a microscope to get a final tip of ≤ 20μm in outer diameter. During the process of microinjection, use caution (wearing gloves, don’t allow the injection needles or pipette tips to contact unclean surfaces, etc.) to prevent contamination of the ribonuclease.
If the needle size is too big, it may damage oocytes. If it is too small, it may be blocked easily and mRNA cannot be injected.
-
3
To prevent oocytes from moving during injection, use a piece of nylon mesh or scratch a grid using a steel needle on the bottom of a Petri dish. Then load the selected oocytes in ND96 solution.
-
4
Load 0.5–2 µL of messenger RNA (mRNA) solution into a microinjection needle that has been back filled with mineral oil (see the manufacturer’s instructions for the microinjector). Adjust the needle to the desired injection amount for each oocyte (such as 46 nL). Position the needle against one oocyte in the Petri dish and push the needle in. The needle should penetrate the oocyte through the animal pole or where the vegetal and animal poles meet. Inject mRNA solution using the foot pedal or an injection button, pause for 3 seconds to allow mRNA to flow into oocyte, and then gently withdraw the needle. Move to the next oocyte by moving the Petri dish. Watch carefully to make sure that the mRNA solution is not used up during injection (mineral oil will come out when the mRNA solution is used up). Repeat the injection on a dozen or two oocytes for each type of mRNA.
-
5
Rinse the injected oocytes twice with ND96 solution, and then place each oocyte into a well of a 48 well cell culture dish loaded 2/3 full with ND96 solution. Incubate at 18°C for 2–7 days, depending on the desired expression level of the ion channels. Monitor oocytes daily to ensure minimal solution evaporation and condensation on the lid. Change the ND96 solution after a couple of days if a prolonged incubation period is required.
Preparing the Perfusion System
-
6
Turn on the multi-channel perfusion system (Figure 1) and open the valve of a cylinder of pressurized nitrogen. Wash each solution reservoir tube 3 times with deionized (DI) water and then add the desired solution in the tube.
-
7
Connect tubing to the perfusion pencil and open the reservoir valve. Use perfusion control software to turn on the electronic valves one at a time. The solution should emerge from the pencil, leave it on for 5–10 minutes to make sure that all of the tubing is filled with solution, without any air bubble in the system.
If the solution doesn’t emerge from the perfusion pencil, apply vacuum to the tip of pencil to remove any air bubbles that may be blocking the system. If there is still no solution coming out, the system may be clogged, and both the tubing and the pencil should be cleared by sonication in DI water. If an air bubble is visible around the reservoir valve, it can be removed by tapping the tubing with fingers.
-
8
Using a fixture or modeling clay, fix the perfusion pencil to the bath (Figure 2) that is mounted on the stage of the inverted microscope. Adjust it to position the perfusion tip in the bath, and add DI water to the bath to confirm the perfusion tip is submerged. Test the perfusion of the solution to make sure it comes out of the perfusion tip correctly: turning on a valve, you can see a jet of fluid coming out of the perfusion tip under the microscope. Repeat the same test for all the perfusion solutions you are going to use.
-
9
When all the perfusion solutions flow correctly, replace the DI water in the bath with bath solution. The perfusion system is now ready for use.
Figure 1.
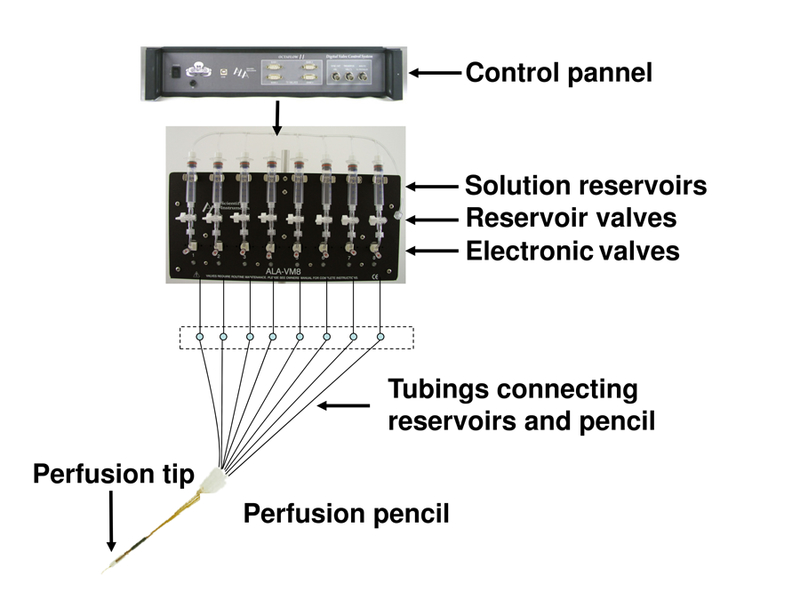
The OctaFlow II™ Multi-function Multi-valve perfusion system from ALA Scientific Instruments
Figure 2.
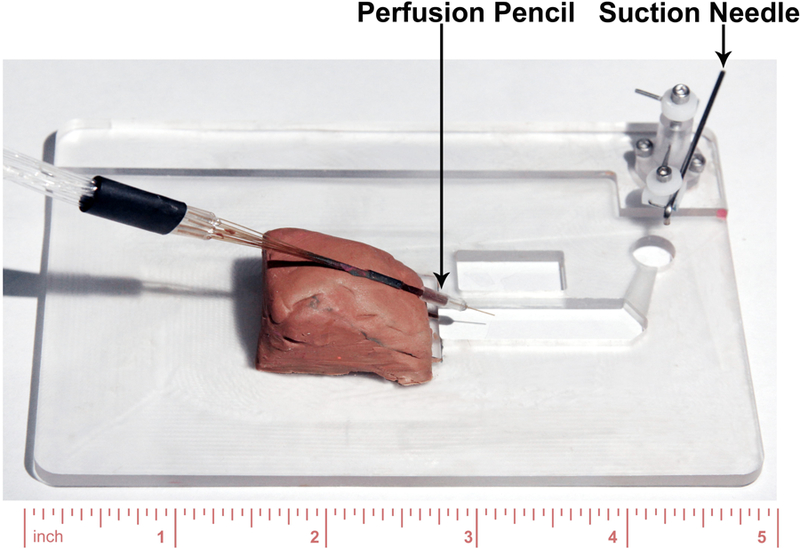
Recording bath. Perfusion pencil is mounted with modeling clay. The suction needle is used for controlling the solution level in the bath, and it is connected with vacuum.
Patch Clamping
-
10
Prepare electrodes for patch clamp recordings. First, remove the silver electrode wire from the pipette holder and immerse ¾ of the tip of this wire in a 1.5 mL Eppendorf tube containing fresh bleach for at least 30 minutes, which will deposit a layer of AgCl on the wire. Rinse the wire with DI water and blot dry. Put the Ag/AgCl electrode back in the pipette holder. At the same time, connect the ground (reference) electrode to the headstage and stabilize it in the bath with a fixture or clay and tape.
-
11
Prepare patch pipettes. Pull glass pipettes using the P97 puller. In order to get the ideal shape for patch clamp, a 5-step program has been designed. Refer to user’s manual of the puller for the design of and setting up the program. Inspect pipettes under a microscope to determine the shape and opening diameter of the tip. The outer diameter of the opening should be 2–4 micrometers, and the ideal resistance of the pipette filled with solution is around 1–1.5 MΩ. After pulling, coat the pipette tip with wax to reduce capacitive current during recordings. Melt the wax in a small beaker on a hot plate, dip the pipette tip into the wax for 2–5 mm deep. (Sylgard 184 is another good material for coating the pipette tip (Rae and Levis 1992; Levis and Rae 1998)). Then fire-polish the pipette tip using a microforge. To fill the pipette with solution, first dip the pipette tip into the pipette solution under negative pressure, and then back-fill the pipette to 2/3 full using a syringe and needle. Place the pipette in the pipette holder with the Ag/AgCl electrode inserted inside and in contact with the pipette solution.
-
12
Prepare for data acquisition. Switch on the amplifier and start the HEKA Pulse acquisition software in the computer. Load the protocol file for the experiment and adjust the configuration settings (refer to the user’s manual for Pulse acquisition software).
-
13
Prepare oocytes. Put an mRNA-injected oocyte in a Petri dish containing stripping solution for 5 – 10 min. The stripping solution detaches the vitelline membrane from the plasma membrane, which makes it easy to strip the vitelline membrane. Then gently strip the vitelline membrane from the oocyte, using two pairs of Dumont #5 forceps under a dissection microscope. Some people prefer not to use stripping solution and to directly strip the vitelline membrane using forceps. Transfer a devitellinized oocyte into a Petri dish containing bath solution that serves as the recording chamber. (Caution: the oocyte is extremely fragile after devitellinizing and therefore must be handled with extreme care.)
-
14
Excise an inside-out patch. Mount the recording chamber on the stage of the inverted microscope, and move the stage to find a clear edge of oocyte under the microscope (Figure 3). By a syringe or by mouth through a suction tube connected to the pipette holder, apply a gentle positive pressure in the pipette to prevent dirt from attaching to the pipette tip. Maintain the positive pressure in the pipette by closing the suction tube with a valve switch. Move the patch pipette close to the oocyte, using a micromanipulator (we use a MC 1000e controller). Slowly push the pipette against the oocyte until the measured resistance of the patch clamp circuit approximately doubles. Switch the positive pressure in the pipette into a negative pressure with a gentle and steady suction until a giga-ohm seal is obtained. (This is a critical step requiring practice to gain skills.) Stabilize the patch by holding it at −50 mV, which is close to the resting potential of the oocyte, for one minute. Excise the patch by quickly pushing the pipette further into the oocyte and then pulling it out to the bath solution. Now the inside-out patch is ready for patch clamp experiments.
To study ion channels that are activated by extracellular ligands, such as neurotransmitter receptors, the outside-out patch configuration is preferred. To excise an outside-out patch, apply a brief strong negative pressure to break the membrane patch after forming a giga-ohm seal, and then pull out the pipette slowly. The membrane attached to the pipette follows the retreating pipette, and a giga-ohm seal should form again when the membrane breaks and an outside-out patch is excised.
-
15
Collect data of BK channel activation in different intracellular Ca2+ concentrations. Move the pipette with the inside-out patch to about 100 µm away from the opening of the perfusion tip. Turn on both the reservoir and electrode valves to begin the perfusion with the desired solution. Run voltage protocol and record currents across the membrane patch. The experiments can be repeated by switching to different perfusion solutions and running different voltage protocols.
-
16
Clean up the system. After finishing all recordings, turn off all valves of the perfusion system and switch off the microscope and amplifier. Make sure to clean the perfusion tubing, pencil and tip by pushing deionized water through to avoid salt precipitation in the system that could clog the system.
Figure 3.
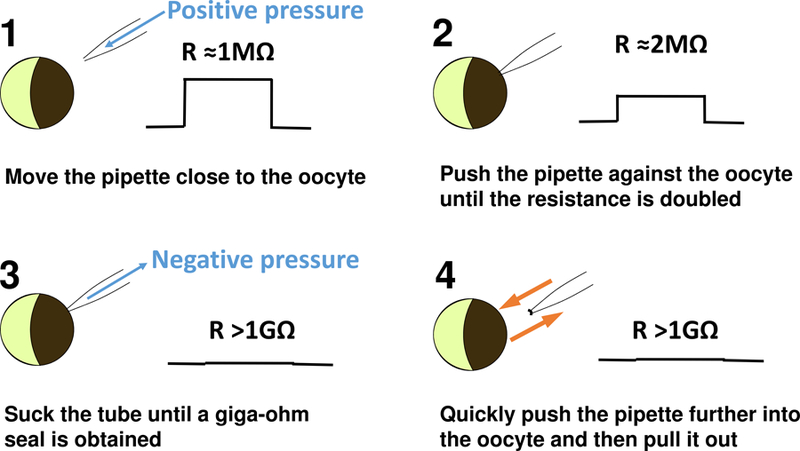
Steps of forming an inside-out patch. The right side of each panel shows the current step in response to a voltage pulse on the patch clamp monitor.
TROUBLESHOOTING:
Problem: Poor oocytes quality.
Solution: Several possible sources may contribute to poor oocytes quality. (1) The frogs’ quality may vary. If the oocytes look bad (for example, with spots of discoloration) or immature (the oocytes’ maturation stage is less than level V (Wasserman et al. 1984)) right after dissection, it is possible that the frog is not healthy. Try a new frog. (2) Unhealthy frogs may result from frog husbandry problems (water and food quality, feeding schedule, frog population density, and infections, etc.) (Sive HL 2000; Green 2009) or may be caused by microbial contamination. Improvements in husbandry and antibiotic supplementation may solve the problem (O’Connell et al. 2011). (3) Oocytes may look good before enzyme digestion, but become bad after digestion. Over-digestion (with excess enzyme or prolonged digestion time) may cause the problem. Closely monitoring the digestion process and stopping the digestion in time may avoid such a problem. (4) Oocytes may turn bad after mRNA injection and incubation. When the tip of the injection needle is too large, it may damage oocytes during injection. Too much mRNA injected into oocytes may cause overexpression. A prolonged incubation time without changing the solution may result in altered salt concentration due to solution evaporation. Microbial contamination may also damage oocytes. Caution can be used to avoid these problems.
Problem: Hard to obtain or keep patches.
Solution: Poor oocyte quality is the most probable reason. See above for the discussion of oocytes quality issues. Here we present several other possibilities and solutions.
Recording pipette. If the pipette tip is not fire-polished well or there is a leakage in pipette holder or suction tubing it is hard to obtain a patch successfully.
Pipette solution. Pipette solution should be fresh (no older than 18 months) and filtered.
Perfusion system. Too high a perfusion pressure or air bubbles in the perfusion flow can damage patches.
Problem: No current detected.
Solution: Any of the following circumstances can result in no current from a seemingly good patch with a giga-ohm seal.
The membrane in the pipette tip forms a sealed vesicle. The vesicle may break when the pipette tip is rapidly lifted out of the bath solution and then immediately lowered back into solution.
Few channels are expressed due to problems with the mRNA solution such as degradation or low concentration.
A newly made mutation of a known well-expressed ion channel may destroy channel function or shift the voltage dependence of channel activation to extreme positive voltages. The well-expressed wild type channel can be studied as a positive control.
DISCUSSION:
This protocol is based on the study of voltage and Ca2+-activated BK channels exogenously expressed in Xenopus laevis oocytes using patch-clamp techniques (Zhang et al. 2017). By excising inside-out patches, the cytosolic side of the channel can be exposed to the desired solutions, while the voltage across the membrane can be clamped at command potentials. BK channels sense various intracellular signal molecules besides Ca2+ (Yang et al. 2015), such as Mg2+ (Shi et al. 2002), ethanol (Dopico et al. 1998), and omega-3 fatty acids (Hoshi et al. 2013). This protocol provides an effective way to study the function of the channel by controlling both voltage and intracellular solutions. This protocol can be readily used for the study of other voltage- or ligand-dependent ion channels. Furthermore, the oocyte expression system provides an efficient method for the study of structure-function relations of the channel by combining it with mutagenesis and chemical modifications of the channel. Due to their large size, oocytes are also ideal for the study of ion channels using other electrophysiological techniques, such as two-electrode voltage clamp and voltage clamp fluorometry (Zaydman et al. 2014), which is an advantage when various functional aspects of an ion channel need to be examined. However, it should be noted that there may be differences in the signaling pathways for post-translational modification between oocytes and mammalian cells (Kyle and Braun 2014), and oocytes may or may not contain specific protein or chemical factors that associate with the ion channel of interest and modify channel function (Sanguinetti et al. 1996). Therefore, the results obtained from patch clamp experiments in Xenopus oocytes should be compared with those obtained from other cell types.
RECIPES:
ND96 Solution
| Reagent | Grams in 1L | Final Concentration (1X) |
|---|---|---|
| NaCl | 5.61 g | 96 mM |
| KCl | 0.1491 g | 2 mM |
| CaCl2.2H2O | 0.2646 g | 1.8 mM |
| MgCl2.6H2O | 0.2033 g | 1 mM |
| HEPES | 4.766 g | 20 mM |
| Na Pyruvate | 0.276 g | 2.5 mM |
| HEPES | 4.766 g | 20 mM |
| Penicillin-Streptomycin | 100000 U | 100U/mL |
| H2O | Add water to 1L |
Use 4 M NaOH to adjust pH to 7.5–7.6
Bath solution (0Ca2+ 0Mg2+ intracellular solution)
| Reagent | Grams in 1L | Final Concentration (1X) |
|---|---|---|
| KOH | 7.854 g | 140 mM |
| HEPES | 4.766 g | 20 mM |
| KCl | 0.1491 g | 2 mM |
| EGTA | 1.902 g | 5 mM |
| MeSO3 | 13.314 g | 140 mM |
| H2O | Add water to 1L |
Use MeSO3 to adjust pH to 7.1–7.2
Note: Xenoups oocytes express a large endogenous Ca2+-activated Cl− current that may interfere with BK channel currents when the intracellular solution contains free Ca2+. To avoid this contamination, a Cl−-free solution is desired. However, 2mM KCl is included in the solution in order to maintain steady electric connection with the Ag/AgCl ground electrode.
Pipette Solution (expires in 18 months)
| Reagent | Grams in 1L | Final Concentration (1X) |
|---|---|---|
| KOH | 7.854 g | 140 mM |
| HEPES | 4.766 g | 20 mM |
| KCl | 0.1491 g | 2 mM |
| MgCl2.6H2O | 0.4066 g | 2 mM |
| MeSO3 | 13.314 g | 140 mM |
| H2O | Add water to 1L |
Use MeSO3 to adjust pH to 7.1–7.2
Oocytes Stripping Solution
| Reagent | Grams in 1L | Final Concentration (1X) |
|---|---|---|
| N-Methyl-D-Glucamine | 39.044 g | 200 mM |
| Aspartate | 26.62 | 200 mM |
| HEPES | 4.766 g | 20 mM |
| KCl | 0.1491 g | 2 mM |
| MgCl2.6H2O | 0.2033 g | 1 mM |
| EGTA | 3.804 g | 10 mM |
| H2O | Add water to 1L |
Use 4 M NaOH to adjust pH to 7.4
Basal Internal Solution (+EGTA)
| Reagent | Grams in 1L | Final Concentration (1X) |
|---|---|---|
| KOH | 7.854 g | 140 mM |
| HEPES | 4.766 g | 20 mM |
| KCl | 0.1491 g | 2 mM |
| EGTA | 0.3804g | 1 mM |
| MeSO3 | 13.314 g | 140 mM |
| H2O | Add water to 1L |
Use MeSO3 to adjust pH to 7.1–7.2
ACKNOWLEDGMENT
This work was supported by National Institutes of Health grants HL70393, NS092570 and GM114694.
REFERENCES
- Aguero T, Newman K, King ML. 2017. Microinjection of Xenopus oocytes. Cold Spring Harbor protocols 2017: 10.1101/pdb.prot096974. [DOI] [PubMed] [Google Scholar]
- Dopico AM, Anantharam V, Treistman SN. 1998. Ethanol increases the activity of Ca(++)-dependent K+ (mslo) channels: functional interaction with cytosolic Ca++. J Pharmacol Exp Ther 284(1): 258–268. [PubMed] [Google Scholar]
- Green SL. 2009. The Laboratory Xenopus sp CRC Press Boca Raton, FL. [Google Scholar]
- Hoshi T, Tian Y, Xu R, Heinemann SH, Hou S. 2013. Mechanism of the modulation of BK potassium channel complexes with different auxiliary subunit compositions by the omega-3 fatty acid DHA. Proceedings of the National Academy of Sciences of the United States of America 110(12): 4822–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle BD, Braun AP. 2014. The regulation of BK channel activity by pre- and post-translational modifications. Frontiers in physiology 5: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis RA, Rae JL. 1998. Low-noise patch-clamp techniques. In Methods in enzymology, Vol 293, pp. 218–266. [DOI] [PubMed] [Google Scholar]
- Newman K, Aguero T, King ML. 2017. ISOLATION OF XENOPUS OOCYTES. Cold Spring Harbor protocols 2017: 10.1101/pdb.prot095851. [DOI] [PubMed] [Google Scholar]
- O’Connell D, Mruk K, Rocheleau JM, Kobertz WR. 2011. Xenopus laevis oocytes infected with multi-drug-resistant bacteria: implications for electrical recordings. The Journal of general physiology 138(2): 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae JL, Levis RA. 1992. Glass technology for patch clamp electrodes. Methods in enzymology 207: 66–92. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. 1996. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature 384(6604): 80–83. [DOI] [PubMed] [Google Scholar]
- Shi J, Krishnamoorthy G, Yang Y, Hu L, Chaturvedi N, Harilal D, Qin J, Cui J. 2002. Mechanism of magnesium activation of calcium-activated potassium channels. Nature 418(6900): 876–880. [DOI] [PubMed] [Google Scholar]
- Sive HL GR, Harland RM. 2000. Early development of Xenopus laevis : A laboratory manual Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Sive HL, Grainger RM, Harland RM. 2010a. Defolliculation of Xenopus oocytes. Cold Spring Harbor protocols 2010(12): pdb prot5535. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. 2010b. Isolation of Xenopus oocytes. Cold Spring Harbor protocols 2010(12): pdb prot5534. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. 2010c. Microinjection of RNA and preparation of secreted proteins from Xenopus oocytes. Cold Spring Harbor protocols 2010(12): pdb prot5538. [DOI] [PubMed] [Google Scholar]
- Wasserman WJ, Houle JG, Samuel D. 1984. The maturation response of stage IV, V, and VI Xenopus oocytes to progesterone stimulation in vitro. Developmental biology 105(2): 315–324. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhang G, Cui J. 2015. BK channels: multiple sensors, one activation gate. Frontiers in physiology 6: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaydman MA, Kasimova MA, McFarland K, Beller Z, Hou P, Kinser HE, Liang H, Zhang G, Shi J, Tarek M et al. 2014. Domain-domain interactions determine the gating, permeation, pharmacology, and subunit modulation of the IKs ion channel. eLife 3: e03606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Geng Y, Jin Y, Shi J, McFarland K, Magleby KL, Salkoff L, Cui J. 2017. Deletion of cytosolic gating ring decreases gate and voltage sensor coupling in BK channels. The Journal of general physiology 149(3): 373–387. [DOI] [PMC free article] [PubMed] [Google Scholar]


