Abstract
In order to assess possible synergistic antinociceptive interactions, the analgesic effects of intra-peritoneal tramadol and morphine administered either separately or in combination were determined using tail-flick latency test following exposure to radiant heat in rats. Groups of eight male Sprague-Dawley rats received either tramadol (3.90, 7.00, 12.50, and 22.20 mg kg-1) and morphine (1.26, 2.25, 4.00 and 7.10 mg kg-1) or a combination of tramadol and morphine (4 different combinations). The baseline latency was obtained before drug injection for each rat, then at 15, 30, 45, 60 and 75 min after injection. The effective dose (ED)50 for either tramadol or morphine individually was 11.70 mgkg-1 and 2.26 mg kg-1, respectively. Based on isobolographic analysis, the ED50 values obtained by drug combination were significantly less than the calculated additive values; which indicates that the co-administration of tramadol and morphine produces synergistic antinociception in the radiant heat tail-flick assay. Combination of morphine and tramadol administered intra-peritoneally can be used for the control of acute pain in rats.
Key Words: Effective Dose 50, Isobolography, Morphine, Tail-flick test, Tramadol
Introduction
Pain, a multidimensional sensory experience, is often defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.1 It normally functions to protect the organism, prevent/minimize tissue injury and promote healing process.
Acute pain often induces fear and anxiety resulting in behavioral, autonomic and neuroendocrine changes. Untreated pain can lead to loss of appetite, depression, aggression, tissue catabolism, immunosuppression, poor health and hyperalgesia.2,3
Different classes of drugs (opioids, alpha-2 agonists, N‐methyl D‐aspartate (NMDA) receptor antagonists and local anesthetics) have been used to alleviate acute pain associated with surgery or trauma.4 Historically, opioids are one of the most commonly used classes of drugs in humans and animals. Although opioid analgesics continue to play an important role in the treatment of moderate to severe acute pain, many other non-opioid analgesics are increasingly being used as adjuvant because of their anesthetic and analgesic-sparing effects and their ability to reduce opioid-related side effects.5,6
The concept of multimodal analgesia (so-called balanced analgesia) is to capture the effectiveness of individual agents in optimal dosages that maximize efficacy in preventing or treating acute pain and attempts to minimize side effects from one analgesic.5,7 In multimodal analgesia, a combination of drugs with different mechanisms of action, which may act at different levels of the nociceptive pathways, is used to produce enhanced (additive and supra-additive [synergism]) analgesic effects.8,9
Opioids and non-steroidal anti-inflammatory drugs (NSAIDs) are the most commonly used substances for multimodal treatment of acute pain; however, the potential adverse effects of NSAIDs such as gastro-intestinal (GI) lesions, nephropathy and impaired platelet function should be considered.10
Morphine is the prototypical opioid analgesic and acts as a full agonist at mu (μ), kappa (κ) and delta (δ) receptors. It is effective for mild to severely painful conditions in many species including laboratory animals. Morphine is commonly used as an intra- and post-operative analgesic and as a part of balanced anesthetic techniques since it induces profound analgesia and mild sedation. It is a controlled substance and requires strict storage and record keeping.11,12
Tramadol is a racemic mixture with a dual mechanism of effect resulting from opioid and non-opioid (i.e., monoamine uptake inhibition) mechanisms.12 Norepinephrine reuptake inhibition results from activity of levo-tramadol [(‐)‐enantiomer of tramadol], serotonin (5‐HT) reuptake inhibition results from activity of dextro- tramadol [(+)‐enantiomer of tramadol] and μ opioid receptor activation results from activities of the O‐desmethyl tramadol metabolite (M1) and the (+) ‐enantiomer of tramadol.13,14
It has been demonstrated that adding tramadol to morphine results in improved analgesic efficacy without increasing side effects after major abdominal surgery in human.15 Marcou et al. reported an infra-additive inter-action between tramadol and morphine in human patients with mild to moderate postoperative pain16; however, the conclusion of this study has been questioned.17
A synergistic antinociception effect between tramadol and morphine has been reported in the mouse using hot plate assay.18 One study has demonstrated that the combination of tramadol-morphine induces effective analgesia in morphine-tolerant mice.19
The aim of this study was to evaluate the analgesic effects of intraperitoneal (IP) administration of morphine (opioid) and tramadol (the atypical opioid analgesic) or their combination using tail-flick latency test. We hypothesized that a synergistic antinociceptive interaction exists between morphine and tramadol in the radiant heat tail-flick assay, a non-inflammatory model of moderate to severe pain, in the rat.
Materials and Methods
Animals. Seventy-two male (250-300 g) Sprague-Dawley rats were used in a blinded, randomized study. Rats were housed in a temperature (21 to 22 °C) and light (12 hr light: 12 hr dark) controlled environment. Standard laboratory pellet food and tap water were available ad libitum throughout the study. This experimental study was approved by the Institutional Animal Care and Use Committee (88-GR-VT-29). The animals were acclimatized to the laboratory environment for at least 2 hr before use and ethical standard guidelines were followed as previously described.19
Administration of tramadol and morphine alone. Rats were randomly assigned to 1 of 9 treatment groups (8 rats per group) for IP administration of the following drugs: saline (control group, 1 mL kg-1), morphine (Darou Pakhsh, Tehran, Iran) and tramadol (Tehran Chemie Pharmaceutical Co., Tehran, Iran). All drugs were diluted with sterile saline and a final volume of 1 mL kg-1 was administered IP into the right caudal abdominal quadrant in each rat. The dose-response relationships of IP tramadol and morphine alone were determined with sequentially increasing doses (3.90, 7.00, 12.50 and 22.20 mg kg-1 and 1.26, 2.25, 4.00 and 7.10 mg kg-1, respectively; the dose interval was approximately 0.25 log units equivalent to 1.77 of each dose) in eight groups.20 Identical coded 1 mL syringes were prepared by a person not involved in the study. Each rat received only one treatment. Anti-nociception was assessed by the tail- flick latency (TFL) test using an analgesiometer (BorjSanat; Tehran, Iran). The TFLs were measured as the time between tail exposure to radiant heat and tail withdrawal. An intensity setting of 70 on a scale of 1-100 and a cut-off time of 12 sec (to prevent tissue damage) were used throughout the study.22 The light beam was focused on the rat’s tail about 4.00 cm from the tip. The radiant intensity was adjusted to give a baseline TFL of 3 to 5 sec and animals with a baseline TFL below 3 or above 5 sec were excluded. The baseline latency was obtained before drug injection for each rat, then at 15, 30, 45, 60 and 75 min after injection. The mean of two consecutive readings with an interval of 1 min was recorded as the TFL value at the mentioned time points.
Administration of tramadol and morphine combinations. Rats were randomly assigned to one of four treatment groups (eight rats/per group) for IP co-administration of tramadol and morphine at different ED50 dose ratios (ED50, ½ ED50, ¼ ED50 or ⅛ ED50 doses).23,24 The same procedures were repeated to evaluate the TFL before injection of drug combinations (baseline) and then at 15, 30, 45, 60 and 75 min after injection.
Data and statistical analysis. Antinociceptive activity was evaluated by the means of TFLs and expressed as the percentage of maximum possible effect (% MPE). The % MPE was calculated using the following formula:25
% MPE = (post-drug TFL – baseline TFL)/( cut off time– baseline TFL) × 100
where, the cut off time is 12 sec. Dose–response curves following IP administration of morphine and tramadol were obtained using four doses for each drug. The ED50 values were calculated by using the % MPE in each rat by GraphPad Prism (version 5.0; GraphPad software Inc., San Diego, USA). Linear regression analysis of the log dose–response curves was used to calculate the doses that produced 50.00% of antinociception (ED50) when each drug was administered alone.
Similarly, a dose–response curve was also obtained and analyzed after the co-administration of morphine and tramadol in fixed ratio combinations of fractions of their respective ED50 values, i.e., combinations of each ED50, ½ ED50, ¼ ED50 and ⅛ ED50 doses, and the experimental ED50 value of drugs combination was calculated. Subsequently, ED50 values were used as the equi-effective dose for isobolographic analysis.23,24
To investigate the interaction between tramadol and morphine, the interaction index (II) was calculated as follows:
II = Experimental ED 50 / Theoretical ED 50
Additivity occurs when the II is close to 1, which means experimental and theoretical ED50 values are similar. Synergism or supra-additive refers to a significantly lower experimental ED50 than the theoretically calculated ED50 and the II is ˂1. A II ˃1 indicates an antagonistic interaction between two drugs. A total fractional dose (FD) value was calculated as follows:
FD = (ED 50 of drug 1 in combination/ ED 50 of drug 1 alone) + (ED 50 of drug 2 in combination/ ED 50 of drug 2 alone)
The interaction between tramadol and morphine was also evaluated using an isobolographic analysis.23,24,26 For isobolographic analysis, the ED50 values of each drug alone were plotted on the X and Y axes. The line joining the X and Y axes corresponds to the theoretical additive line. If the experimental ED50 falls below or above the theoretical additive line, synergy or antagonism is present, respectively.
Differences in mean of MPE among the groups were analyzed by two-way analysis of variance for repeated measures with time and drug as the main factors followed by Bonferroni multiple comparison. The GraphPad Prism program was used to perform the statistical procedures.
Results
Individual antinociceptive activity. There was no significant difference in the baseline TFL time between saline and treatment groups (3.88 ± 0.52 sec). Mean TFL had no significant differences at any time points with the baseline values in rats received IP saline. The IP administration of tramadol and morphine produced a dose-dependent antinociceptive activity measured by the tail flick test, with ED50 values of 11.70 mg kg-1 and 2.26 mg kg-1 for tramadol and morphine, respectively. The maximum antinociceptive effects following IP tramadol and morphine administration were observed at 45 min and declined afterwards (Fig. 1). Therefore, the %MPEs at 45 min were used to calculate ED50 values. Tramadol at the doses of 3.90 and 7.00 mg kg-1 and morphine at the doses of 1.26 and 2.25 mg kg-1 did not induce any significant changes from baseline in the TFL over the 75 min observational period. Four rats receiving the highest dose of morphine (7.10 mg kg-1) exhibited signs of sedation (reduced cage activity and slow movement) and were reluctant to move their tails away from the light beam at the 30 to 75 min time points.
Fig. 1.
The effects of IP administration of A) morphine (1.26, 2.25, 4.00 and 7.10 mg kg-1), B) tramadol (3.90, 7.00, 12.50 and 22.20 mg kg-1), and C) morphine and tramadol (ED50, ½ ED50, ¼ ED50 or ⅛ ED50 doses), on tail-flick latency (TFL) in rats (n = 8). The TFL (mean ± SD) was expressed as percent maximum possible effect (% MPE). * Asterisk indicates significant difference (p < 0.05) as compared with M 40 and M 7.50 values
Combination antinociceptive activity. The IP administration of combination drugs produced a dose-dependent antinociceptive activity measured by the tail flick test (Fig. 1). The isobolographic analysis of the tramadol-morphine co-administration on the basis of a fixed ratio of their ED50 values demonstrated that the experimental ED50 was significantly less than the theoretical ED50, indicating a synergistic interaction between morphine and tramadol (Fig. 2). The II for the antinociceptive activity of the IP co-administration of morphine with tramadol was 0.44. Sedation and impaired motor function were not observed in rats receiving different ED50 dose ratios (ED50, ½ ED50, ¼ ED50 or ⅛ ED50 doses) of morphine and tramadol used in this study. No adverse effects related to the treatments (anorexia, pica behavior or death) or exposure to the thermal stimulus (tail skin damage) were observed during seven days after the completion of the experiment.
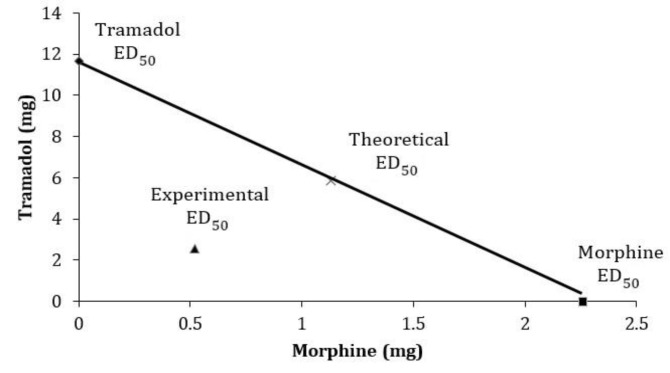
Fig. 2.
Isobologram of drugs combination of intra-peritoneal tramadol and morphine in tail-flick latency test. The oblique line between the x axis and y axis is the theoretical additive effect line of tramadol and morphine co-administration. Point ×, in the middle of the line, is the theoretical ED50 of drugs combination, which is calculated from the individual drug ED50. Point , is the experimental ED50 of drugs combination, which is actually observed after drugs co-administration. The experimental ED50 point is below theoretical ED50, suggesting a synergistic effect of morphine-tramadol combinations
Discussion
The study hypothesis was that the combinations of morphine and tramadol will provide synergistic effect when used intra-peritoneally in rats. The results of the present study demonstrated that morphine, a typical μ–receptor agonist, possesses a higher antinociceptive potency than tramadol in a model of acute pain in rats, which may be related to the higher affinity of morphine with opioid receptors. Tramadol is a centrally acting analgesic with very low affinity for μ opioid receptors.
Although tramadol weakly binds to the μ opioid receptor (6000-fold less than morphine), it also acts as serotonin and norepinephrine reuptake inhibitor.12 The O-desmethyl metabolite of tramadol (M1) has 200-300 times greater affinity for the μ-receptor than the parent compound, but still has much lower affinity than morphine. O-desmethyltramadol has 2-4 times greater analgesic potency than the parent compound and may account for part of the analgesic effect.13,14 Tramadol is commonly used in small animal practice.11,27
Tramadol is metabolized extensively in rodents and humans to M1 and it is likely to be responsible for the opioid-derived analgesic effect of tramadol.28,29 In rats administered single oral doses of tramadol, the ratio of tramadol/M1 in plasma was 0.50-1.50, indicating the high rate of metabolism of tramadol in rodents compared to that in humans.30 Since tramadol is metabolized by the liver enzyme CYP2D6 to the pharmacologically active metabolite M1 in humans, it has little analgesic effect in healthy volunteers deficient in CYP2D6 enzyme (≈8% of Caucasian population).13,14 Species-specific variation in analgesic potency may occur as a result of variations in the metabolism of tramadol. A lack of appreciable M1 metabolite concentrations of tramadol has been described in dogs.31 Lack of analgesic effect following intravenous (IV) tramadol administration in the tail-flick model in Beagle dogs has been attributed to the low concentration of the active M1 metabolite.27 A gender-related differences in pharmacokinetics of tramadol have been reported in rats, indicating that plasma concentrations of (+)-M1 are higher in females than males after a single oral dose of tramadol.32
A recent study has reported that naltrexone, an opioid antagonist at the μ, κ and δ receptors, antagonizes the antinociceptive activity of both morphine and tramadol in hot plate assay, but only partially reverses the effect of tramadol in acetic acid writhing test in mice.18 In humans, naloxone only partially inhibited the analgesic effect of tramadol,14 which suggests a greater importance of the non-opioid mechanisms in humans. Both the α2-adrenoceptor blocker yohimbine and the serotonin antagonist ritanserin significantly reduced the analgesic action of intrathecally administered tramadol in the rat tail-flick test indicating that both noradrenaline and serotonin are involved in the analgesic effect of tramadol.25
Although morphine as a full μ agonists has been shown to impair GI motility, tramadol generally has no clinically relevant effects on GI function.14 Unlike morphine, use of the recommended doses of tramadol has no clinically relevant effects on respiratory or cardiovascular parameters and does not cause histamine release when administered intravenously.13 Tramadol also has a low abuse potential and is not classified as a controlled substance in some countries. Since tramadol has minimal cardiopulmonary depression and no long term negative GI, renal or coagulation effects, it may be useful for long term analgesic treatments in patients with a risk of poor cardiopulmonary function and when NSAIDs are contra-indicated.
In the present study, the ED50 values of IP morphine and tramadol in the rat tail-flick model were 2.26 and 11.70 mg kg-1, respectively. The antinociceptive potency of morphine was approximately five times greater than tramadol. Morphine and tramadol had similar relative potency in mice using the hot plate test.19,32 Similar ED50 values for morphine (1.37 mg kg-1) and tramadol (8.97 mg kg-1) have been reported following IV administration in the rat tail-flick model.
Interestingly, ED50 value of M1 (2.94 mg kg-1) was much lower than tramadol itself.28 The ED50 of tramadol following IP administration in adult male Wistar rats was 10.30 mg kg-1using tail-flick model.33 The reported ED50 values of tramadol and morphine in the mice tail-flick model were 22.80 and 2.30 mg kg-1, respectively.25 The Ml has been shown to have analgesic activity in mice and rats as assessed by the tail flick response with two to four times greater potency than tramadol in this test.28
A multimodal (or balanced) analgesic technique uses the theory that agents with different mechanisms of analgesia may have additive or synergistic effects in preventing or treating acute pain when used in combination.5 The use of combinations of drugs from different pharmacological classes may improve analgesia and minimize the potential side effects of each drug. Tramadol is effective for the treatment of mild to moderate pain and has been used as a part of multimodal analgesic protocol for the treatment of severe pain.13,15
Combination of morphine and tramadol has been evaluated in a hot plate test in mice,18,19,32 but no specific experimental studies on the use of morphine-tramadol combination in tail flick test in rats were found in the literature. A synergistic antinociception interaction (II = 0.69) following IP administration of morphine and tramadol has been reported in hot plate tests in the mice;18 conversely, subcutaneous co-administration of morphine and tramadol has showed additive nociceptive effects in a same model of acute pain in mice.32 Interestingly, the combination of tramadol with fentanyl has been resulted only in an additive interaction (II ˃ 1) in both studies.18,32 It has been suggested that in morphine-tolerant mice, tramadol in combination with morphine could be used to induce effective analgesia.19 The administration of tramadol in combination with morphine as a part of a multimodal treatment approach for procedural or postoperative pain has been reported in human. Webb et al. have compared the morphine/tramadol combination with morphine alone after major abdominal surgery and found that tramadol decreases postoperative morphine requirements and produces superior analgesia in combination with morphine versus morphine alone without increasing side effects.15
Although opioids are generally considered to be safe, the multimodal approach to pain management may be used to provide opioid-sparing effects and minimize the potential for the undesirable adverse effects of opioids. Further studies are required to evaluate the combination of morphine and tramadol in controlling postsurgical pain and to examine opioid-sparing effects of tramadol in patients undergoing surgery.
Acknowledgments
This study was supported by Grant No. 88-GR-VT-29 from the Research Council of Shiraz University (Shiraz, Iran).
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.McKune CM, Murrell J C, Nolan AM, et al. Nociception and pain. In: In: Grimm KA, Lamont LA, Tranquilli WJ, et al., editors. Lumb and Jones’ veterinary anesthesia and analgesia. 5th ed. Ames, USA: John Wiley & Sons Inc; 2015. pp. 584–623. [Google Scholar]
- 2.Joshi GP, Ogunnaike BO. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesthesiol Clin North Am. 2005;23(1):21–36. doi: 10.1016/j.atc.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Wiese AJ, Yaksh TL. Nociception and pain mechanisms. In: In: Gaynor JS, Muir WW., editors. Handbook of veterinary pain management. 3rd ed. Saint Louis, USA: Elsevier Inc; 2015. pp. 10–41. [Google Scholar]
- 4.Dahl V, Raeder JC. Non-opioid postoperative analgesia. Acta Anaesthesiol Scand. 2000;44(10):1191–1203. doi: 10.1034/j.1399-6576.2000.441003.x. [DOI] [PubMed] [Google Scholar]
- 5.Kehlet H, Dahl JB. The value of multimodal or balanced analgesia in postoperative pain treatment. Anesth Analg. 1993;77(5):1048–1056. doi: 10.1213/00000539-199311000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Raffa RB. Pharmacology of oral combination analgesics: Rational therapy for pain. J Clin Pharm Ther. 2001;26(4):257–264. doi: 10.1046/j.1365-2710.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- 7.Williams BS, Buvanendran A. Nonopioid adjuvants in multimodal therapy for acute perioperative pain. Advances in Anesthesia. 2009;27(1):111–142. [Google Scholar]
- 8.Buvanendran A, Kroin JS. Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anaesthesiol. 2009;22(5):588–593. doi: 10.1097/ACO.0b013e328330373a. [DOI] [PubMed] [Google Scholar]
- 9.Young A, Buvanendran A. Recent advances in multi-modal analgesia. Anesthesiol Clin. 2012;30(1):91–100. doi: 10.1016/j.anclin.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Dahl JB, Kehlet H. Non-steroidal anti-inflammatory drugs: Rationale for use in severe postoperative pain. Br J Anaesth . 1991;66(6):703–712. doi: 10.1093/bja/66.6.703. [DOI] [PubMed] [Google Scholar]
- 11.Plumb DC. Plumb’s veterinary drug handbook. 7th ed. Ames, USA: Wiley-Blackwell; 2011. pp. 711–715. [Google Scholar]
- 12.KuKanich B, Wiese AJ. Opioids. In: Grimm KA, Lamont LA, Tranquilli WJ, et al., editors. Lumb and Jones’ veterinary anesthesia and analgesia. 5th ed. Ames, USA: John Wiley & Sons ; 2015. pp. 207–226. [Google Scholar]
- 13.Scott LJ, Perry CM. Tramadol - A review of its use in perioperative pain. Drugs. 2000;60(1):139–176. doi: 10.2165/00003495-200060010-00008. [DOI] [PubMed] [Google Scholar]
- 14.Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879–923. doi: 10.2165/00003088-200443130-00004. [DOI] [PubMed] [Google Scholar]
- 15.Webb AR, Leong S, Myles PS, et al. The addition of a tramadol infusion to morphine patient-controlled analgesia after abdominal surgery: A double-blinded, placebo-controlled randomized trial. Anesth Analg. 2002;95(6):1713–1718. doi: 10.1097/00000539-200212000-00045. [DOI] [PubMed] [Google Scholar]
- 16.Marcou TA, Marque S, Mazoit JX, et al. The median effective dose of tramadol and morphine for post-operative patients: a study of interactions. Anesth Analg. 2005;100(2):469–474. doi: 10.1213/01.ANE.0000142121.24052.25. [DOI] [PubMed] [Google Scholar]
- 17.Webb A, Leong S. The combination of tramadol and morphine may be recommended for postoperative analgesia. Anesth Analg. 2005;101(6):1884–1885. doi: 10.1213/01.ANE.0000180271.88987.5B. [DOI] [PubMed] [Google Scholar]
- 18.Miranda HF, Noriega V, Zanetta P, et al. Isobolographic analysis of the opioid-opioid interactions in a tonic and a phasic mouse model of induced nociceptive pain. J Biomed Sci. 2014;21(1):62–71. doi: 10.1186/s12929-014-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero A, Miranda HF, Puig MM. Antinociceptive effects of morphine, fentanyl, tramadol and their combination, in morphine-tolerant mice. Pharmacol Biochem Behav. 2010;97(2):363–369. doi: 10.1016/j.pbb.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Miranda HF, Sierralta F, Pinardi G. Previous administration of indomethacin or naloxone did not influence ketorolac antinociception in mice. Anesth Analg. 1993;77(4):750–753. doi: 10.1213/00000539-199310000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Chan SY, Ho PC. Isobolographic analysis of the analgesic interactions between ketamine and tramadol. J Pharm Pharmacol. 2002;54(5):623–631. doi: 10.1211/0022357021778934. [DOI] [PubMed] [Google Scholar]
- 22.Hernández-Delgadillo GP, Cruz SL. Dipyrone potentiates morphine-induced antinociception in dipyrone-treated and morphine-tolerant rats. Eur J Pharmacol. 2004;502(1-2):67–73. doi: 10.1016/j.ejphar.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Nishiyama T, Hanaoka K. The synergistic interaction between midazolam and clonidine in spinally-mediated analgesia in two different pain models of rats. Anesth Analg. 2001;93(4):1025–1031. doi: 10.1097/00000539-200110000-00045. [DOI] [PubMed] [Google Scholar]
- 24.Miranda HF, Puig MM, Prieto JC, et al. Synergism between paracetamol and nonsteroidal anti-inflammatory drugs in experimental acute pain. Pain. 2006;121(1-2):22–28. doi: 10.1016/j.pain.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Raffa RB, Friderichs E, Reimann W, et al. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an “atypical” opioid analgesic. J Pharmacol Exp Ther. 1992;260(1):275–285. [PubMed] [Google Scholar]
- 26.Tallarida RJ, Porreca F, Cowan A. Statistical analysis of drug-drug and site-site interactions with isobolograms. Life Sci. 1989;45(11):947–961. doi: 10.1016/0024-3205(89)90148-3. [DOI] [PubMed] [Google Scholar]
- 27.Kogel BK, Terlinden R, Schneider J. Characterisation of tramadol, morphine and tapentadol in an acute pain model in Beagle dogs. Vet Anaesth Analg. 2014;41(3):297–304. doi: 10.1111/vaa.12140. [DOI] [PubMed] [Google Scholar]
- 28.Hennies HH, Friderichs E, Schneider J. Receptor binding, analgesic and antitussive potency of tramadol and other selected opioids. Arzneimittel-Forschung/ Drug Res. 1988;38(7):877–880. [PubMed] [Google Scholar]
- 29.Gillen C, Haurand M, Kobelt DJ, et al. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human μ-opioid receptor. Naunyn-Schmiedeberg’s Arch Pharmacol. 2000;362(2):116–121. doi: 10.1007/s002100000266. [DOI] [PubMed] [Google Scholar]
- 30.Tao Q, Stone DJ, Borenstein MR, et al. Differential tramadol and O-desmethyl metabolite levels in brain vs plasma of mice and rats administered tramadol hydrochloride orally. J Clin Pharmacol Ther. 2002;27(2):99–106. doi: 10.1046/j.1365-2710.2002.00384.x. [DOI] [PubMed] [Google Scholar]
- 31.Liu HC, Jin SM, Wang YL. Gender-related differences in pharmacokinetics of enantiomers of trans-tramadol and its active metabolite, trans-O-demethyltramadol, in rats. Acta Pharmacol Sin. 2003;24(12):1265–1269. [PubMed] [Google Scholar]
- 32.Romero A, Miranda HF, Puig MM. Analysis of the opioid–opioid combinations according to the nociceptive stimulus in mice. Pharmacol Res. 2010;61(6):511–518. doi: 10.1016/j.phrs.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Guneli E, Karabay Yavasoglu NU, Apaydin S, et al. Analysis of the antinociceptive effect of systemic administration of tramadol and dexmedetomidine combination on rat models of acute and neuropathic pain. Pharmacol Biochem Behav. 2007;88(1):9–17. doi: 10.1016/j.pbb.2007.06.006. [DOI] [PubMed] [Google Scholar]