Abstract
The ossification centers onset of the quail vertebrae, ribs, and sternum in embryos and hatchling birds was studied. Specimens were cleared, stained with Alcian Blue and Alizarin Red S and examined using stereomicroscope. The chondral rudiments of the vertebrae were observed at the 6th day of incubation (E6). The osteogenesis of the vertebrae was accomplished with both perichondral and endochondral ossifications. The cervical vertebrae began to ossify at E9-E10, whereas the thoracic ones began at E10-E11. The synsacral vertebrae began to ossify at E11-E13. In the caudal vertebrae, ossification was observed at E14 and in the pygostylous ones, at E15. The true ribs began to ossify at E7, whereas the 1st and the 2nd ribs began to ossify at E9 and E8, respectively. The uncinate processes were ossified late at E15. At E13, ossification was observed in the caudo-lateral process of the sternum. At E14, the cranio-lateral process of the sternum began to ossify, whereas late at and after hatching ossification was observed in the carina and the sternal body, respectively. The data presented here provide useful baseline information on the normal sequential pattern of ossification in the vertebral column and thoracic cage in quail.
Key Words: Ontogeny, Osteology, Quail, Skeletal development
Introduction
The chronological sequence of the centers of ossification (CO) onset during the pre-hatching period has been studied in many bird species.1 Afterwards, reference to vertebral and thoracic development is made in overall descriptions of Galliformes skeletal development both in the chicken2-4 and the turkey.5-7 However, specific attention has not been paid to the vertebral development through all embryonic stages. Additionally, Shapiro has provided an extensive account on the ossification of chicken thoracic vertebrae by means of light microscopy.8
Heideweiller has also provided information regarding the post-natal development of the neck in the chicken.9 Other studies have described the CO onset in the chicken during the post-hatching period with the aid of X-rays.10,11
Besides the chicken, the quail (Coturnix coturnix japonica) represents an avian species which is a part of the wild fauna and a domestic animal, as well. In addition, the quail is widely used in the experimental biomedical researches. According to Ainsworth et al., the Japanese quail remains one of the favoured animal models in developmental biology and is being used to investigate a variety of developmental systems. 12 Therefore, it is important that a database of the developmental characteristics of the skeleton of this species be assembled.
Comparative avian skeletal development has been examined in a phylogenetic context.6,13,14 Starck included observations on quail embryonic skeletons13 which were staged according to Starck15 classification. The embryonic development of quail skeleton in staged embryos according to Zacchei’s classification has been described by Nakane and Tsudzuki.16,17 However, the authors have recorded the ossification events in every stage through simple observation of cartilage or bone. Maxwell has observed staged embryos according to Hamburger and Hamilton classification,6,18 whereas Mitgutsch et al. have provided the ossification stages per specimen.14 Similarly, the authors recorded ossification events without detailed description and with some incomplete aspects of the ossification process.
Through the extensive study of literature, no data were found regarding the detailed description of the vertebral column and thoracic cage ossification during the pre-hatching as well as post-hatching period. The objective of this study was to provide a broad and thorough basis of anatomical information on the ossification of the vertebrae, ribs and sternum.
Materials and Methods
A total of 110 embryos were used in the present study. The fertilized quail eggs were acquired from Drosos Farm (Nea Apollonia, Thessaloniki). To evaluate the ossification during the pre-hatching period, freshly laid (< 6 to 8 hr) fertilized eggs were placed in an automatic incubator. The temperature and relative humidity of the incubator were adjusted to 37.50 ± 0.10 ˚C and 65% till the 15th day, respectively. At the 15th day, the relative humidity was augmented to 80% and the temperature was reduced to 37 ˚C. Ten eggs were collected daily from the 6th to the 16th day of incubation. The normal time of incubation is 16 days.19 The embryos were then removed from the eggs, cleared with KOH, stained with Alizarin red S and Alcian Blue20 modified by the authors and observed with the aid of stereomicroscopy.
The experiments were carried out in compliance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) for the care and use of laboratory animals and the study was approved by the Prefecture of Thessaloniki, Veterinary Directorate. All efforts were made to minimize the number of animals used and their pain or discomfort.
The terminology used for the bones and bony structures was in accordance with the Nomina Anatomica Avium.21 The E denotes the pre-hatching period.
Results
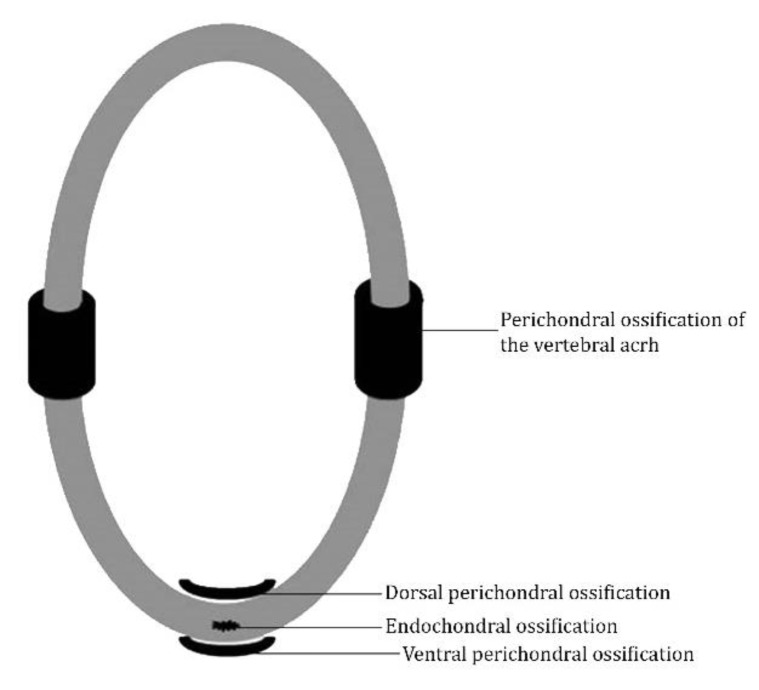
Vertebral column. At the 6th day of incubation (E6), all the rudiments of the vertebrae were observed with the form of cartilaginous rings. The vertebrae were ossified from various CO. Generally, the vertebral bodies were ossified with perichondral ossification observed on the dorsal and ventral areas and endochondral ossification within the cartilaginous body. The neural arches were ossified with perichondral ossification (Fig. 1).
Fig. 1.
Simplified schematic representation of the general pattern of vertebral ossification. Grey-shaded regions represent cartilage and black regions represent ossified tissue
The first CO of the atlas observed at the arches during the 10th day of incubation (E10). The CO had the form of bony perichondral sheaths extending gradually upwards. The unique CO of the body appeared at E14 with limited expansion.
The first CO of the axis observed in the vertebral body at E10. A bony perichondral lamina was formed at the central area of the dorsal surface of the body. At the ventral surface of the body, two perichondral bony laminae were observed which soon fused. Endochondral ossification was observed at E11 in the vertebral body. At E11, one ossification center in each arch was observed. This center of ossification had the form of a perichondral bony sheath. The two bony sheaths were extended upwards and fused at E14. At E12, in the dens, an independent endochondral ossification center was formed.
The ossification of the 3rd -15th vertebrae was firstly observed in the bodies and later in the arches. In the vertebral bodies, two perichondral CO and one endo-chondral ossification center were recorded. On the dorsal area of the bodies of the 6th, 7th, 8th and 9th vertebrae, ossification was observed at E9. The dorsal ossification center was present in all vertebrae of this group at E10.
On the ventral surface of the vertebral bodies, the first ossification center was observed in the 6th-9th vertebrae. Consequently, the ossification was expanded in the rest vertebrae of this group, both forward and rearward. However, in some of them, namely 3rd-6th and 12th two CO were observed, whereas, in the rest, one ossification center was recorded on the ventral surface of the bodies.
The main bulks of the vertebral bodies were ossified with endochondral ossification. This ossification was observed in all vertebrae of this group at E11. In the vertebral bodies of 13th-15th vertebrae, the endochondral ossification preceded of the ventral perichondral ossification. Ossification was observed at E10 in the 13th vertebra and consequently in the 14th-15th vertebrae. In these vertebrae, a dorsal perichondral lamina and two ventral perichondral laminae were recorded.
The vertebral arches were ossified after the ossification of the bodies. The first perichondral ossification was observed at E11 in the arches of 6th-9th vertebrae. Perichondral bony sheaths were formed and expanded medially and laterally. The first fusion of the bony sheaths was observed at E13 in the 7-9 vertebrae. At E14, all the neural arches of this group of vertebrae were ossified and at E15 all bony sheaths were fused.
The costal processes were ossified independently. The first ossification center was observed at E12 in the 13th vertebra. At E13, all vertebrae of this group had ossified costal processes. The ossification center had the form of a perichondral bony sheath. The ossification of the thoracic vertebrae (16-21) was observed for the first time at the vertebral bodies. In this group of vertebrae, the first ossification center at the ventral area of the bodies was observed. Two perichondral laminae were formed initially on the ventral surface of the vertebral body. Above each ventral perichondral lamina, an endochondral ossification center was observed. On the ventral surface of the body, an ossification center was formed extending laterally.
In the 18th vertebra, ossification was observed for the first time at E10. At E11, the ventral perichondral laminae of all thoracic vertebrae were formed. At E12, all CO of the vertebral bodies were present.
The ossification of the vertebral arches was achieved from two CO. The first perichondral bony sheaths were observed at E12 in the 16th vertebra. Further, these CO were observed in the rest thoracic vertebrae.
The fusion of the bony sheaths was observed in the 16th vertebra at E15. At E16, all arches of the thoracic vertebrae were fused. In the transversal processes, no independent ossification center was observed. Their ossification was the continuation of the ossification of the dorsal ossification center of the vertebral bodies.
The vertebral bodies of the synsacral vertebrae (22-28) were ossified before the vertebral arches from six CO. The first ossification center appeared at the ventral area of the bodies with the form of two bony laminae which gradually fused. Above each lamina, an endochondral ossification was observed. At the dorsal area, two bony laminae were recognized representing the perichondral CO.
At E11, the first ossification center of the 22nd vertebra was observed. Subsequently, CO was appeared at the following vertebrae. At E13, all vertebrae of this group possessed ossified vertebral bodies.
The vertebral arches of the 22nd-24th vertebrae were ossified in the same manner of the thoracic vertebrae from two perichondral bony sheaths. The ossification of the arches began at E12 in the 22nd vertebra. At E13, all bony sheaths of the neural arches were formatted.
The ossification of the vertebral arches of the 25th-28th vertebrae was accomplished from four CO. Particularly, at these arches, except for the lateral bony sheaths, two dorsal bony sheaths were observed. The first bony sheaths were observed in the 25th vertebra at E13 and subsequently in the rest vertebrae. The dorsal bony sheaths were observed at E15.
In every lateral process, a perichondral ossification center was observed in the form of bony ring transforming to the bony sheath. These sheaths were observed firstly in the 28th vertebra and later in the 27th vertebra, whereas they were absent in the 26th vertebra.
The ossification of the vertebral bodies of the synsacral vertebrae (29-33) was observed at E13 with six CO in the same manner as the previous 22nd-28th synsacral vertebrae. The first ossification center of the arches was observed in the neural arch of the 30th vertebra. The first dorsal ossification center described in the previous (25th-28th) vertebrae was observed in the 29th vertebra at E14. At E13, prior to the ossification of the neural arches, a bony sheath was observed around the lateral process.
Late at E14, CO was observed at the vertebral bodies of the caudal vertebrae (34-39). The dorsal area was ossified by two perichondral CO. Endochondral ossification was observed at the ventral position of the dorsal perichondral bony lamina. Two perichondral CO appeared at the ventral area of the bodies. In the vertebral arches, ossification was observed before the appearance of the ventral CO of the bodies. A perichondral bony sheath begun to extend from the internal surface of the arches. At hatching, the bony sheaths were incomplete dorsally.
The ossification of the transversal processes was in continuation with the ossification of the arches. The vertebrae of pygostylus (40-43) were ossified late on the 15th day of incubation. A bony perichondral lamina was observed on the dorsal surface of the vertebral bodies extending laterally towards the arches. In this way, the ossification of the arches was in continuation with the ossification of the bodies. Later, an endochondral ossification was observed in the central area of the vertebral bodies.
Ribs. In the thoracic cage, seven pairs of ribs were observed. The first two pairs possessed only the vertebral segment (costae incomplitae), whereas the next five pairs possessed the vertebral and sternal segments (costae complitae). The cartilaginous rudiments of the vertebral part of the ribs appeared at E7 across the 15th-21st vertebrae.
At the vertebral part of the 5 complete ribs, ossification was observed at the 8th day of incubation. A perichondral bony sheath appeared around the proximal half of the cartilaginous rudiments. In the 2nd rib (incomplete rib), ossification was observed at E8, whereas in the 1st one, it was observed at E9. An important periosteal activity was detected during the 11th day at the 3rd-7th ribs. The same activity was observed in the 2nd rib at the 12th day. In some embryos, a supernumerary rib was observed next to the 22nd vertebra. At E7, the cartilaginous rudiments of the sternal part of the ribs were observed across the 3rd-7th vertebral ribs. In the sternal part of 2nd, 3rd and 4th complete ribs, ossification was observed at E10, whereas in the 1st rib, it was observed at E11.
In the apical part of the 5th sternal rib, perichondral ossification was observed at E11. The cartilaginous uncinate processes of the 2nd, 3rd, 4th and 5th ribs were observed at E8. The perichondral CO appeared at E15. These CO were independent of the relevant CO of the ribs.
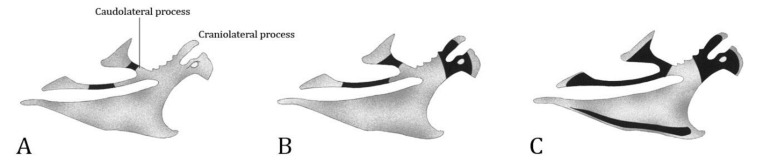
Sternum. Late, during the pre-hatching period, the first CO in the sternal processes of the cartilaginous bone were observed (Fig. 2). At E13, the first center appeared at the lateral trabecula of the caudo-lateral process. A perichondral bony sheath was observed extending both anteriorly and posteriorly. At E13-E14, an ossification center appeared at the thoracic trabecula of the caudo-lateral process. The ossification of the cranio-lateral process was observed at E14. This process remained independent from the other CO and expanded to the rostrum sterni. At the body of the sternum, an ossification center was observed immediately after hatching. At the carina, ossification was observed at the end of incubation.
Fig. 2.
Lateral view of the sternum; A) E13, B) E14, and C) E16. Grey-shaded regions represent cartilage and black regions represent ossified tissue. E: Pre-hatching period
Discussion
In the present study, the ossification events in the vertebral column as well as thorax and sternum of the quail were recorded at daily intervals during the pre-hatching period (Tables 1 and 2). There is scarce information on the ossification of Galliformes vertebral column and thoracic cage. The chicken is the model with which the quail skeleton ontogeny should be mainly compared. However, the comparison cannot be evidential. It could only be indicative due to the fact that the literature data of the quail are still incomplete.6,14,15,17 On the other hand, the chicken is a different bird species. A practical method to compare the timing of ossification is to use the percentage of the incubation period that has elapsed and the ranked ossification sequence of the bones (Tables 1 and 2).
Table 1.
The chronological order of the ossification centers onset in the vertebrae
| Order of vertebra | Anatomical order | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | IP (%) | Rank of ossification events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cervical 1 | * | x | 62.00% | 2 | |||||||||
| 2 | Cervical 2 | x | #* | 62.00% | 2 | |||||||||
| 3 | Cervical 3 | x | # | * | 62.00% | 2 | ||||||||
| 4 | Cervical 4 | x | # | * | 62.00% | 2 | ||||||||
| 5 | Cervical 5 | x | # | * | 62.00% | 2 | ||||||||
| 6 | Cervical 6 | x | #* | 56.00% | 1 | |||||||||
| 7 | Cervical 7 | x | #* | 56.00% | 1 | |||||||||
| 8 | Cervical 8 | x | #* | 56.00% | 1 | |||||||||
| 9 | Cervical 9 | x | #* | 56.00% | 1 | |||||||||
| 10 | Cervical 10 | x | # | * | 62.00% | 2 | ||||||||
| 11 | Cervical 11 | x | # | * | 62.00% | 2 | ||||||||
| 12 | Cervical 12 | x | # | * | 62.00% | 2 | ||||||||
| 13 | Cervical 13 | x | # | * | 62.00% | 2 | ||||||||
| 14 | Cervical 14 | x | # | * | 62.00% | 2 | ||||||||
| 15 | Cervical 15 | x | # | * | 62.00% | 2 | ||||||||
| 16 | Thoracic 1 | x | #* | 69.00% | 3 | |||||||||
| 17 | Thoracic 2 | x | # | * | 69.00% | 3 | ||||||||
| 18 | Thoracic 3 | x | # | # | * | 62.00% | 2 | |||||||
| 19 | Thoracic 4 | x | # | * | 69.00% | 3 | ||||||||
| 20 | Thoracic 5 | x | # | * | 69.00% | 3 | ||||||||
| 21 | Thoracic 6 | x | # | * | 69.00% | 3 | ||||||||
| 22 | Synsacral 1 | x | #* | 69.00% | 3 | |||||||||
| 23 | Synsacral 2 | x | #* | 69.00% | 3 | |||||||||
| 24 | Synsacral 3 | x | #* | 69.00% | 3 | |||||||||
| 25 | Synsacral 4 | x | # | * | 69.00% | 3 | ||||||||
| 26 | Synsacral 5 | x | # | * | 69.00% | 3 | ||||||||
| 27 | Synsacral 6 | x | # | * | 69.00% | 3 | ||||||||
| 28 | Synsacral 7 | x | # | * | 69.00% | 3 | ||||||||
| 29 | Synsacral 8 | x# | * | 81.00% | 4 | |||||||||
| 30 | Synsacral 9 | x# | * | 81.00% | 4 | |||||||||
| 31 | Synsacral 10 | x# | * | 81.00% | 4 | |||||||||
| 32 | Synsacral 11 | x# | * | 81.00% | 4 | |||||||||
| 33 | Synsacral 12 | x# | * | 81.00% | 4 | |||||||||
| 34 | Caudal 1 | x*# | 88.00% | 5 | ||||||||||
| 35 | Caudal 2 | x*# | 88.00% | 5 | ||||||||||
| 36 | Caudal 3 | x*# | 88.00% | 5 | ||||||||||
| 37 | Caudal 4 | x*# | 88.00% | 5 | ||||||||||
| 38 | Caudal 5 | x*# | 88.00% | 5 | ||||||||||
| 39 | Caudal 6 | x*# | 88.00% | 5 | ||||||||||
| 40 | Pygostylous 1 | x# | 94.00% | 6 | ||||||||||
| 41 | Pygostylous 2 | x# | 94.00% | 6 | ||||||||||
| 42 | Pygostylous 3 | x# | 94.00% | 6 | ||||||||||
| 43 | Pygostylous 4 | x# | 94.00% | 6 |
IP: Incubation period; x: Perichondral ossification of the vertebral body;*: Perichondral ossification of the vertebral arch; and #: Endochondral ossification.
Table 2.
The chronological order of the ossification centers onset in the thoracic cage
| Ossification centers | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | IP (%) | Rank of ossification events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thorax | |||||||||||||
| 1 st vertebral rib | x | 56.00% | 2 | ||||||||||
| 2 nd vertebral rib | x | 50.00% | 1 | ||||||||||
| Uncinate process | x | 50.00% | 1 | ||||||||||
| 3 rd vertebral rib | x | 50.00% | 1 | ||||||||||
| Uncinate process | x | 50.00% | 1 | ||||||||||
| 4 th vertebral rib | x | 50.00% | 1 | ||||||||||
| Uncinate process | x | 50.00% | 1 | ||||||||||
| 5 th vertebral rib | x | 50.00% | 1 | ||||||||||
| Uncinate process | x | 50.00% | 1 | ||||||||||
| 6 th vertebral rib | x | 50.00% | 1 | ||||||||||
| Uncinate process | x | 50.00% | 1 | ||||||||||
| 7 th vertebral rib | x | 50.00% | 1 | ||||||||||
| 1 st sternal rib | x | 68.00% | 4 | ||||||||||
| 2 nd sternal rib | x | 62.00% | 3 | ||||||||||
| 3 rd sternal rib | x | 62.00% | 3 | ||||||||||
| 4 th sternal rib | x | 62.00% | 3 | ||||||||||
| 5 th sternal rib | x | 68.00% | 4 | ||||||||||
| Sternum | |||||||||||||
| Craniolateral process | x | 88.00% | 6 | ||||||||||
| Caudolateral process | x | x | 81.00% | 5 | |||||||||
| Carina | x | 100% | 7 |
x: Ossification; and IP: Incubation period.
In the quail, the first ossification event of the vertebral column occurred in the C6-C9 vertebrae and subsequently continued both anteriorly and posteriorly. Similarly, Nakane and Tsudzuki17 have reported that the ossification of the vertebral column began at the medial region of the cervical vertebrae and subsequently moved anteriorly and posteriorly. Our reports coincide with the observations of Rinaldi and Caronna4 in the chicken embryo, whereas Rumpler2 has observed ossification firstly in C4-C7. In contrary, in the turkey, the ossification began from the cervical vertebrae and continued posteriorly.6,7 Even though the precise timing of the ossification events onset in other studies is different, the general pattern of anteroposterior shifting of ossification events of the vertebrae is similar.
The vertebral bodies ossified generally faster than neural arches, but in the atlas, the neural arch ossified faster. Similar observations were reported in the chicken.22 On the other hand, Rinaldi and Caronna4 have reported that the vertebral bodies ossified faster than arches in the chicken.
Regarding the vertebral ossification, in the context of the embryonic development of the quail, this occurred later in respect to the ossification of the limb long bones.23-25 During the chronological period in which the present study took place, the ossification of the bony elements occurred mainly during the embryonic development and no fusions of the vertebrae were observed in order to form the notarium and synsacrum. Regarding the pygostyle, the free caudal vertebrae began to fuse after hatching in accordance with the observations of Hogg,11 with no particular chronology.
The early appearance of ossification in the cervical vertebrae could be correlated with the fact that the neck of the quail must be structurally ready during hatching to break the eggshell. Proper integration of the structural and kinematic modifications in the developing cervical column is a prerequisite to ensure adequate pecking, drinking, preening and locomotion.9
In the present study, ossification of true ribs observed at the 8th day of incubation. In the chicken embryo, ossification was observed at the 9th-10th days of incubation.2,4,22 The presence of supernumerary ribs is in agreement with the observations of other authors in the fowl.4,10 Periosteal ossification of the ribs has also been observed in the chicken.4 The periosteal ossification observed in the ribs has also been recorded in the fore and hind limb long bones of the quail embryo.24-26 Probably, this ossification enforces the ribs which are the longest bones of the thoracic cage.
The uncinate processes have been variously claimed to ossify in the chicken embryo from the 17th day of incubation to some days after hatching.10 Starck has reported that hatchling quails do not have ossified uncinate processes. 27
According to Hogg, the ossification of the chicken sternum began from five CO, which observed till hatching.10 Our observations are in line with other authors who reported in the chicken that till hatching the carina is also ossified.2,22
The comparison of the data presented in this study together with the information provided by others,6,14,17 reveals temporal variations. This may be attributable to the fact that in each of these studies, different incubation parameters were employed and probably different strains were used.
The pattern of the ossification sequence of the studied bones during the pre-hatching period was found to be invariable in all embryos studied here. The observed segments of quail skeletons exhibited homogeneity in terms of ossification status in every daily interval. Apparently, the incubated eggs provide a constant and safe environ-ment in which the skeletal development takes place.
However, Mitgutsch et al. have claimed that in vivo observation of ossification in high numbers of specimens would be ideal to assemble the complete sequence of developmental events.14 Such an approach could reveal intra-specific variations that cannot be detected with the observation of the ossification at daily intervals.
In conclusion, the extent of ossification as revealed from the qualitative description of the bony elements of the vertebral column, thorax and sternum support the precociality of the quail. The precociality and the intra-specific consistency of the skeletal ontogeny in combination with the biological characteristics of the quail render it as an important vertebrate animal model. It is expected that the detailed description of quail skeletal development presented here will be useful in further assessment of the genetic and molecular correlation of ordered and disordered vertebral formation.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Romanoff AL. The avian embryo: Structural and functional development. New York, USA: Mac Millan ; 1960. p. 907. [Google Scholar]
- 2.Rumpler Y. Chronological appearance of the ossification points of the hen embryo skeleton [French] C R Assoc Anat. 1962;48:1175–1191. [Google Scholar]
- 3.Schumacher GH, Wolff E. comparative osteogenesis of Gallus domesticus, Larus ridibundus and Larus canus I Chronological appearance of ossifications in Gallus domesticus [German] Gegenbaurs Morphol Jahrb. 1967;110:359–373. [PubMed] [Google Scholar]
- 4.Rinaldi L, Caronna EW. The embryo ossification of the chicken (Gallusdomesticus L) [Italian] Arch Ital Anat Embriol. 1971;76:201–237. [PubMed] [Google Scholar]
- 5.Scala G, Colella G, Gentile R. Chronology of the appearance of embryonic ossification nuclei in the turkey. Boll Soc Ital Biol Sper. 1993;59(5):638–644. [PubMed] [Google Scholar]
- 6.Maxwell EE. Comparative embryonic development of the skeleton of the domestic turkey (Meleagris gallopavo) and other galliform birds. Zoology. 2008;111:242–257. doi: 10.1016/j.zool.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Atalgin SH, Kürtül I. A morphological study of skeletal development in turkey during the pre-hatching stage. Anat Histol Embryol. 2009;38(1):23–30. doi: 10.1111/j.1439-0264.2008.00887.x. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro F. Vertebral development of the chick embryo during days 3-1 9 of incubation. J Morphol. 1992;213(3):317–333. doi: 10.1002/jmor.1052130305. [DOI] [PubMed] [Google Scholar]
- 9.Heidweiller J. Post natal development of the neck system in the chicken (Gallus domesticus L) Am J Anat. 1989;186(3):258–270. doi: 10.1002/aja.1001860303. [DOI] [PubMed] [Google Scholar]
- 10.Hogg DA. A re-investigation of the centers of ossification in the avian skeleton at and after hatching. J Anat. 1980;130(Pt 4):725–743. [PMC free article] [PubMed] [Google Scholar]
- 11.Hogg DA. Fusions occurring in the postcranial skeleton of the domestic fowl. J Anat. 1982;135(Pt 3):501–512. [PMC free article] [PubMed] [Google Scholar]
- 12.Ainsworth SJ, Stanley RL Evans DJR. Developmental stages of the Japanese quail. J Anat. 2010;216(1):3–15. doi: 10.1111/j.1469-7580.2009.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starck JM. Evolution of avian ontogenies. Curr Ornithol. 1993;10:275–366. [Google Scholar]
- 14.Mitgutsch C, Wimmer C, Sanchez-Villagra MR, et al. Timing of ossification in duck, quail and zebra finch: Intraspecific variation, heterochronies, and life history evolution. Zoolog Sci. 2011;28(7):491–500. doi: 10.2108/zsj.28.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starck JM. Time pattern of ontogeneses in nest-nesting and nest-squatting birds [German] Stuttgart, Germany: Cour Forsch Inst Senckenberg. 1989;114:1–319. [Google Scholar]
- 16.Zacchei AM. The embryonic development of the Japanese quail (Coturnixcoturnix japonica T and S) [Italian] Archiv Ital Anat Embryol. 1961;66:36–62. [PubMed] [Google Scholar]
- 17.Nakane Y, Tsudzuki M. Development of the skeleton in Japanese quail embryos. Dev Growth Differ. 1999;41(5):523–534. doi: 10.1046/j.1440-169x.1999.00454.x. [DOI] [PubMed] [Google Scholar]
- 18.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 19.Padgett CS, Ivey WD. The normal embryology of the coturnix quail. Anat Rec. 1960;37(1):1–11. doi: 10.1002/ar.1091370102. [DOI] [PubMed] [Google Scholar]
- 20.Peters PWJ. Double staining of fetal skeletons for cartilage and bone. In: Neubert D, Merker HJ, Kwasogroh TE, editors. Methods in prenatal toxicology. Stuttgart, Germany: Georg Thieme ; 1977. pp. 153–154. [Google Scholar]
- 21.Baumel JJ, Witmer LM. Osteologia. In: Baumel JJ, King AS, Breazile JE, et al., editors. Handbook of avian anatomy: Nomina anatomica avium. Cambridge, USA: Publications of the Nuttall Ornithological Club ; 1993. pp. 45–132. [Google Scholar]
- 22.Fujioka T. Time and order of appearance of ossification centers in the chicken skeleton. Acta Anat Nippon. 1955;30:140–150. [Google Scholar]
- 23.Pourlis A F, Magras IN, Petridis ID. Ossification and growth rates of the limb long bones during the prehatching period in the quail (Coturnix coturnixjaponica) Anat Histol Embryol. 1998;27(1):61–63. doi: 10.1111/j.1439-0264.1998.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 24.Pourlis AF, Antonopoulos J. The ossification of the pectoral girdle and wing skeleton of the quail (Coturnix coturnix japonica) Anat Histol Embryol. 2011;40(3):219–225. doi: 10.1111/j.1439-0264.2011.01065.x. [DOI] [PubMed] [Google Scholar]
- 25.Pourlis AF, Antonopoulos J. The ossification of the pelvic girdle and leg skeleton of the quail (Coturnix coturnix japonica) Anat Histol Embryol. 2014;43(4):294–300. doi: 10.1111/ahe.12076. [DOI] [PubMed] [Google Scholar]
- 26.Pourlis AF, Antonopoulos JK, Magras IN. A light and electron microscopic study of the limb long bones perichondral ossification in the quail embryo (Coturnix coturnix japonica) Ital J Anat Embryol. 2006;111(3):159–170. [PubMed] [Google Scholar]
- 27.Starck JM. Comparative morphology and cytokinetics of skeletal growth in hatchlings of altricial and precocial birds. Zool Anz. 1996;235(1-2):53–75. [Google Scholar]