Abstract
Pathogenic Escherichia coli strains cause a wide range of extra intestinal infections including urinary tract infection in humans and colibacillosis in poultry. They are classified into uropathogenic E. coli (UPEC) and avian pathogenic E. coli (APEC) with genetic similarities and variations. Their pathogenicity is related to the virulence-encoding genes like sfa, papG II, ompT, iutA, and iss with zoonotic potentials. One hundred isolated E. coli from patients with urinary tract infection and 100 E. coli from chickens with colibacillosis were evaluated for the presence of the most common virulence-encoding genes including sfa, papG II, ompT, iutA, and iss by multiplex polymerase chain reaction. While the frequency of sfa, papG II, ompT, iutA and iss encoding genes in APEC isolates were respectively 0.00%, 67.00%, 63.00%, 89.00% and 89.00%, the frequency of these encoding genes in UPEC isolates were 18.00%, 40.00%, 40.00%, 74.00% and 48.00%, respectively. Except for sfa, the frequencies of other encoding genes in APEC were more than those in UPEC isolates. The iutA as the most common UPEC encoding gene and iss as the most common APEC encoding gene were the most prevalent virulence factors in the examined E. coli isolates. Finding out the distribution of virulence-associated genes could be helpful to identify similarities and differences between APEC and UPEC isolates in order to provide more substantial evidence of their common virulence traits and potential zoonotic threats.
Key Words: Avian colibacillosis, Escherichia coli, Urinary tract infection, Virulence
Introduction
Escherichia coli is the most significant Gram-negative facultative anaerobic bacterium with a wide range of pathogenicity and genotypic diversity.1 It is a commensal bacterium in the gut of humans and other vertebrate and is responsible for a wide range of intestinal and extra-intestinal infections.2 Accordingly, it is divided into two pathotypes of intestinal pathogenic E. coli and extra-intestinal pathogenic E. coli (ExPEC). Each pathotype is divided into sub-pathotypes based on certain common traits of host specificity, pathogenesis mechanism, and infected organ.3 For example, serogroups named O18 with large virulence plasmids and K1 capsular antigen cause meningitis.4-6 It is originated from human neonates and named as neonatal-meningitis E. coli. Human uro-pathogenic E. coli (UPEC) lacking virulence plasmids tends to be of the O2 and O6 serogroups.6 Similarly, avian pathogenic E. coli (APEC), responsible for poultry coli-septicemia, possesses large virulence plasmids and great diversity in serogroups like O1, O2, and O78.7,8
Urinary tract infections (UTIs) are among one of the highest medical costs bacterial infections affecting healthcare, with 150 million cases occurring annually worldwide.9 The virulence-associated factors involved in the establishment of UTIs enable them to survive and invade noxiously to the host tissues, induce the inflammatory response via disruption of the defense mechanisms and finally cause a variety of diseases even out of the urinary tract.10 Another virulence trait of this organism is due to the expression of a wide spectrum of antimicrobial resistance genes.11
Avian pathogenic E. coli induces multi-target infection in broilers called colibacillosis with large losses to the poultry industry. Its disseminated infection induces fibrinous lesions in internal organs from air sacs and pericardium to peritonea associated with septicemia.12,13
According to the relationship between UPEC and APEC genes for virulence and pathogenicity, the zoonotic risk of isolated avian E. coli should be considered.14-16 Despite the variable virulence gene profile engaged in the pathogenicity of APEC and human ExPEC, the similarities between these two strains may also share a common ancestor.17 A lot of studies were done to find serogroup similarity and link between genotype of virulence factors between APEC and UPEC.18 Common reported virulence-associated genes between APEC and UPEC include iron-limited urinary tract (iutA) related to iron acquisition systems, increased serum survival (iss), S-fimbrial adhesion (sfa), pyelonephritis-associated pili (papG II) and outer-membrane protein T (ompT) genes showing their common traits.10 Additionally, the prevalence of wide serological diversity was revealed in several studies with particular combinations of virulence-associated genes among APEC strains.6,8,19 Different virulence assays were mentioned in APEC with various similarities to the UPEC. The most prevalent genes in E. coli isolated from poultry colibacillosis in Iran were hly F, ompT, iss, iutA and pap G II.20 While tsh, iss, astA, iucD, vat, and papC were the most frequent virulence genes isolated from APEC, other genes including fim, aaadA1 and qnr have been reported as the most virulence genes isolated from UPEC.21,22 Likewise, the clonal relations between some APEC and human ExPEC strains have been demonstrated.23-25 Also, serotype O18:K1:H7 isolated from avian has been reported to have pathogenicity for human.26
Epidemiological studies have showed genetic similarity and variation between E. coli isolated from poultry and human.27,28 In order to get distribution of virulence-associated genes in UPEC and APEC strains, we gathered a relatively large collection of them and made a systemic comparison between the prevalence of their virulence-encoding genes. The aim of this study was to find out the distribution of virulence-associated genes to identify the similarities and differences between these groups in order to provide more substantial evidence of their common virulence traits and potential zoonotic threats.
Materials and Methods
Sampling and bacterial culture. A total of 200 E. coli isolates were studied in this survey. They were isolated from the pericardium of broiler chickens suffering or died of colibacillosis (n = 100) and from the urine samples of the patients suffering from urinary infection (n = 100). The urine isolates were collected from the patients having at least 105 colonies forming unit of a bacterium per milliliter of urine. The isolates were routinely grown in eosin methylene blue agar (Merck, Darmstadt, Germany). After confirming the bacteria like E. coli by biochemical tests, each one was incubated in trypticase soy broth (TSB; Merck) for 18 hr at 37 ˚C. Then, each culture was centrifuged at 3000 g for 15 min. After discarding the supernatant, 1.00 µL of the bacterial pellet with 1.00 µL of glycerol and 9.00 µL of TSB were cast in a microtube and stored at – 20 ˚C for the following steps.
DNA extraction. The bacterial samples were mixed with lysis buffer (Bio-Rad, Watford, UK) and incubated for 10 min in a water bath at 55 ˚C. Proteinase K (Sigma-Aldrich Co. Ltd., Dorset, UK) was added into each micro tube, vortexed carefully and incubated at 55 ˚C for 20 min. In the next step, binding buffer was added and incubation was done again at 70 ˚C for 10 min. After adding pure ethanol (Merck) and vortexing, the microtubes were then centrifuged at 8000 g for 1 min. Briefly, by using washing buffer and then centrifuging them, the ethanol was cleaned up. Finally, the elution buffer was used followed by multistage centrifuge to purify the extracted DNA.
Gene amplification. The E. coli specific gene uidA and virulence-associated genes including sfa, papG II, ompT, iutA, and iss were detected by multiplex polymerase chain reaction (PCR) assay. The oligonucleotide sequences of primers used for amplifications of the target genes are shown in Table 1. In PCR run, two virulence genes were amplified in a 30.00 µL reaction mixture. The mixture was included Taq DNA Polymerase Master Mix (12.50 µL), Ampliqon (Odense, Denmark), template DNA (8.00 µL) and each 2.00 µL of primes (Metabion international AG, Planegg, Germany) supplemented with 5.50 µL distilled water. The pure E. coli culture (ATCC 10536) harboring target genes was obtained from Tehran University, Tehran, Iran and used as a positive control and the mixture of Taq DNA Polymerase Master Mix and distilled water was used as a negative control. Each tube reaction mixture was subjected to denaturation (94 ˚C for 30 sec), annealing (58 ˚C for 30 sec) and extension (72 ˚C for 90 sec), followed by one cycle consisting of 5 min at 72 ˚C in a thermal cycler (ABI 2720; Applied Biosystems, Vilnius, Lithuania). After gene amplification, every PCR product was run on horizontal gel electrophoresis with 2.00% agarose gel, stained with SYBER safe (SinaClon, Tehran, Iran) and illuminated by ultraviolet exposure.
Table 1.
The sequence of primers used for amplifications of uidA, sfa, papG II, ompT, iutA, and iss encoding genes
| Genes | Oligonucleotide sequence | Expected size (bp) | Reference |
|---|---|---|---|
| uidA |
F: tggtaattaccgacgaaaacggc
R: acgcgtggttacagtcttgcg |
147 | |
| sfa |
F: ctccggagaactgggtgcatcttac
R: cggaggagtaattacaaacctggca |
410 | |
| papG II |
F: gggatgagcgggcctttgat
R: cgggcccccaagtaactcg |
190 | |
| ompT |
F: atctagccgaagaaggaggc
R: cccgggtcatagtgttcatc |
559 | |
| iutA |
F:
ggctggacatcatgggaactgg R: cgtcgggaacgggtagaatcg |
302 | |
| iss |
F:
cagcaacccgaaccacttgatg R: agcattgccagagcggcagaa |
323 |
Statistical analysis. In order to compare the frequency of virulence genes, Chi-square test and to investigate the relationship between the frequency of genes, Chi-square test, and Spearman correlation index were used. All analyses were performed with SPSS (version 18.0; SPSS Inc., Chicago, USA) and p value < 0.05 was the threshold for significance.
Results
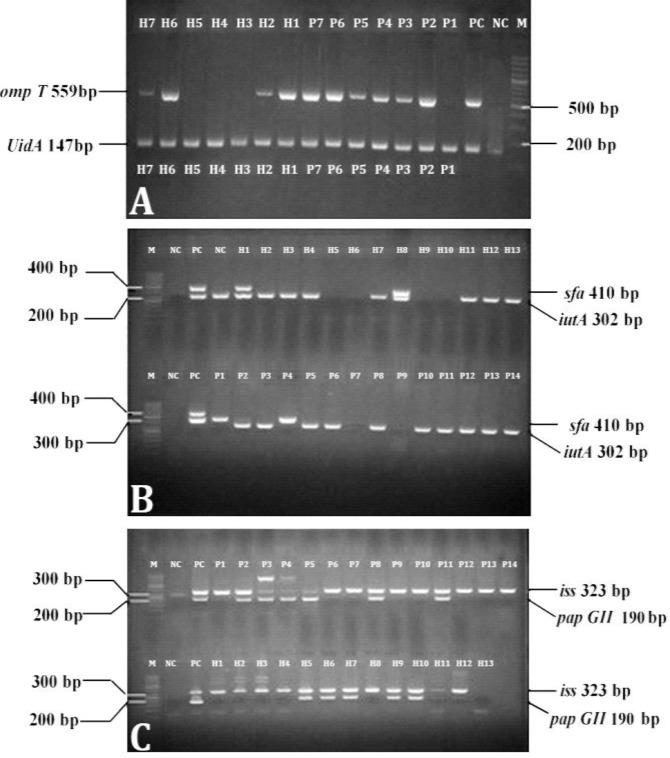
Figure 1 shows the electrophoresis of the PCR products. While the isolates from poultry colibacillosis (P1-P7) and human UTIs (H1-H7) were uidA positive, the samples of P2-P7, H1, H2, H6, and H7 were also ompT positive (Fig. 1A). The P1-P6, P8, P10-P14, H1-H4, H7, H8, and H11-H13 isolates were positive for the iutA gene (Fig. 1B). While H1 and H8 contained sfa, none of the isolated strains from poultry colibacillosis contained this virulence gene.
Fig. 1.
Gene-specific polymerase chain reaction product electrophoresis to evaluate uidA and ompT (A), sfa and iutA (B) and iss and papG II (C) genes. M: Marker; PC: Positive control; NC: Negative control; P: E. coli strains isolated from poultry colibacillosis; H: E. coli strains obtained from human urinary infection
The electrophoresis of genes products of iss and papG II from colibacillosis and human UTIs is shown in Figure 1C. The isolates P1, P2, P6-P14, H1-H10, and H12 contained iss gene. The papG II was present in P2-P5, P8, H5-H7, H9, and H10 isolates.
The frequency of the sfa, pap GII, ompT, iutA and iss genes in E. coli strains isolated from APEC and UPEC is shown in Table 2. Except for sfa gene, the frequency of other genes from APEC was significantly higher than that of UPEC (p < 0.05). According to Table 3 summarizing the virulence- encoding genes in APEC and UPEC, most isolates of APEC and UPEC had three (40.00%) and two (44.00%) virulence-encoding genes, respectively. None of them had all five virulence-encoding genes.
Table 2.
The frequency (%) of virulence encoding genes in avian pathogenic E. coli (n = 100) and human uropathogenic E. coli (n = 100)
| Genes | APEC1 isolates | UPEC2 isolates | p value |
|---|---|---|---|
| sfa | 0 | 18 | 0.000 * |
| papG II | 67 | 40 | 0.000 * |
| ompT | 63 | 40 | 0.001 * |
| iutA | 89 | 74 | 0.006 * |
| iss | 89 | 48 | 0.000 * |
indicates significant differences between APEC and UPEC isolates at p < 0.001.
Avian pathogenic Escherichia coli;
Human uropathogenic E. coli.
Table 3.
The frequency of avian pathogenic E. coli (n = 100) and human uropathogenic E. coli (n = 100) in terms of the number of virulence encoding genes
Avian pathogenic Escherichia coli;
Human uropathogenic E. coli.
Table 4 shows the simultaneous presence of two virulence-encoding genes in APEC and UPEC isolates. The correlation between the frequency of virulence- encoding genes between APEC and UPEC isolates is also listed. While in UPEC isolates, between sfa, iss, iutA, iss and papG II a significant negative and between sfa and papaG II a significant positive correlation was observed, no correlation was seen between any of these genes in APEC isolates.
Table 4.
The frequency (%) of avian pathogenic E. coli (n = 100) and human uropathogenic E. coli (n = 100) in terms of carrying two virulence encoding genes
| Genes | APEC1 | Spearman correlation index | p value | UPEC2 | Spearman correlation index | p value |
|---|---|---|---|---|---|---|
| ompT, iss | 57 | 0.03 | 0.70 | 17 | -0.09 | 0.30 |
| papG II, iss | 60 | 0.06 | 0.50 | 11 | † -0.27 | 0.007 |
| iutA, iss | 80 | 0.06 | 0.50 | 12 | † -0.31 | 0.002 |
| iss, sfa | 0 | - | - | 5 | * -0.26 | 0.01 |
| sfa, ompT | 0 | - | - | 6 | -0.26 | 0.40 |
| sfa, papG II | 0 | - | - | 12 | † 0.74 | 0.007 |
| sfa, iutA | 0 | - | - | 8 | 0.27 | 0.10 |
| ompT, papG II | 23 | 0.10 | 0.10 | 16 | 0.04 | 0.60 |
| ompT, iutA | 62 | 0.03 | 0.70 | 14 | -0.85 | 0.40 |
| papG II, iutA | 60 | 0.00 | 1.00 | 14 | -0.85 | 0.40 |
indicate significant differences between APEC and UPEC isolates at p < 0.05, p < 0.001, respectively
Avian pathogenic Escherichia coli;
Human uropathogenic E. coli.
In Table 5, the results of the simultaneous presence of three and four genes in APEC and UPEC isolates are shown. Fifty-three percent of APEC isolates had iss, iutA, and papG II and 53.00% percent of APEC isolates had also iss, ompT and iutA simultaneously. While 39.00% of APEC isolates had iutA, papG II and ompT together and 41.00% had iss, ompT, and papG II simultaneously, in UPEC isolates only one or two percent of strains had these genes together. Thirty-seven percent of UPEC isolates had four genes of papG II, iutA, ompT and iss in common.
Table 5.
The frequency (%) of avian pathogenic E. coli (n = 100) and human uropathogenic E. coli (n = 100) in terms of carrying three and four virulence encoding genes
| Virulence encoding genes | APEC1 | UPEC2 |
|---|---|---|
| sfa, iss, ompT | 0 | 0 |
| sfa, papG II, iss | 0 | 2 |
| iutA, iss, sfa | 0 | 0 |
| iss, ompT, papG II | 41 | 2 |
| iss, ompT, iutA | 53 | 1 |
| iutA, papG II, ompT | 39 | 2 |
| sfa, ompT, iutA | 0 | 1 |
| iss, iutA, papG II | 53 | 1 |
| ompT, papG II, iss | 0 | 1 |
| papG II, iutA, sfa | 0 | 1 |
| sfa, iss, ompT, papG II | 0 | 1 |
| papG II, iutA, ompT, iss | 37 | 1 |
| iutA, ompT, iss , sfa | 0 | 0 |
| iutA, ompT, papG II, sfa | 0 | 3 |
| iss, papG II, iutA, sfa | 0 | 0 |
Avian pathogenic Escherichia coli;
Human uropathogenic E. coli.
Discussion
Avian pathologic E. coli is one of the most bacterial cause of infectious diseases in poultry and the main cause of poultry colibacillosis. This infection is responsible for great losses in the poultry industry.1-3,30 Recently, the zoonotic potential of APEC strains was considered by some researchers.19,31 In several studies, the phylogenetic, genotypic and serotype relation between APEC strains and extra-intestinal E. coli strain in human, like UPEC, has been demonstrated. Similar virulence factors with the same mechanism between APEC and UPEC strains have been concluded.10,26,32
In the present study, we evaluated the frequency of sfa, papG II, ompT, iutA and iss as virulence-encoding genes of E. coli and identified four genes including papG II, ompT, iutA, and iss in both APEC and UPEC. The similar result was reported previously.10
These results evidently have concluded the relation between APEC and UPEC and confirmed the previous knowledge of the role of certain genes specified to a particular host (human versus avian and/or urinary tract versus respiratory tract).33,34 The frequency of virulence-related genes in APEC including sfa, papG II, ompT, iutA and iss in our study was respectively 0.00%, 67.00%, 63.00%, 89.00% and 89.00% versus 2.00%, 43.00%, 60.00%, 90.00% and 81.00% in the report of Zhao et al.10 In another study, iutA, iss and papG II genes had the frequencies of 50.00%, 40.00% and 15.20%, respectively.35 It was also reported that more than 97.00% of APEC had sfa and fim virulence-encoding genes.26 In our study, none of the APEC isolates had sfa, which is in agreement with Zhao et al.10 and is in contrast with Moulin-Schouleur et al. 26 Among the genes examined for this study, iutA, and iss as virulence factors encoding genes were detected in the majority of APEC isolates which was similar to the other reports.10,26,35 These results were in contrast with other study reporting papG, the virulence genes coding adhesion, had the highest frequency among the other virulence genes.27
In the present study, the presence of virulence-encoding genes from UPEC isolates was sfa (18.00%), papG II (40.00%), ompT (40.00%), iutA (74.00%) and iss (48.00%). Among these, the gene encoding iutA followed by iss were the most frequent (74.00% and 48.00%, respectively) which is almost similar to previous reports including sfa (49.00%), pap G II (38.00%), ompT (63.00%), iutA (83.00%) and iss (53.00%).10 According to these results, the genes encoding iutA and iss were more frequent in both APEC and UPEC and iutA was occurred in the majority of UPEC isolates. So, it could be considered as important virulence factors in avian colibacillosis and human UTIs caused by UPEC in Iran. For example, the high prevalence of iss in both APEC and UPEC has shown its resistance efficacy against the complement and their survival promotion in serum.36 On the other hand, because of having plasmid-linked genes,10 it can be demonstrated that most E. coli strains inducing poultry colibacillosis and human UTIs have considerable antibiotic resistance in Iran. Moreover, we showed the simultaneous presence of virulence-encoding genes iutA and iss in 80.00% of APEC isolates indicating high resistance of this strain against various antibiotics. These results were almost the same as the previous study reporting that iss, iucC, iutA, and iroN occur in almost 75.00% of APEC and UPEC isolates.6
There were variations among the frequency of APEC virulence-encoding genes in our study and the others of Zhu et al. in China and Moon et al. in Thailand and these variations were also seen in the frequency of UPEC virulence genes between our report in Iran and Zhu et al. in China.28,35 The differences between the frequencies of virulence-encoding genes in our study and previous reports could be related to the capability of E. coli strains in a high rate of genetic exchange and mutual transfer of genes related to virulence and resistance. On the other hand, the use of different antibiotics in different parts of the world would eliminate different sensitive strains in these areas. Therefore, these antibiotics could impact the frequency of E. coli virulence genes dramatically in different regions of the world.
According to the abundance of genes encoding virulence factors, papG II, ompT and iss in APEC and UPEC, it was found that the frequency of genes in APEC was significantly more than UPEC isolates which is in agreement with Zhao et al.10 who have shared the similar virulence gene profile. By contrast, UPEC contained more iutA compared to APEC in their studies which is in contrast with our results.
In the present study, most of the strains isolated from poultry had three of the five virulence-encoding genes andmore strains isolated from human UTIs had two virulence-encoding genes. Thus, none of the isolates of avian pathogenic and human uropathogenic E. coli had all five genes of virulence factors at the same time. Accordingly, they might have another gene (s) responsible for their virulence traits.
In UPEC, the negative correlation was seen between papG II and iss, between iutA and iss and between iss and sfa, unlike the positive correlation seen between sfa and papG II. The positive correlation indicates that by increasing the frequency of gene encoding virulence adhesion factors, sfa, the frequency of the gene encoding adhesion factor papG II was also increased. But, none of the virulence genes in APEC showed a significant correlation, which means that change in the frequency of none of the mentioned genes affects each other.
Generally, the results of the present study indicated the relation of genes encoding virulence factors between human and poultry and showed that both APEC and UPEC isolates encounter similar challenges in establishing infection. These results indicate the zoonotic risk of APEC. Therefore, further studies have to be done in poultry products such as chicken meat and eggs. However, due to the limitation of the present study in terms of lack of solid phylogenetic linkage between APEC and UPEC strains, the number of virulence-associated genes and investigating the ability of APEC to get passed the body's defenses to cause UTIs in humans can be considered in future studies.
Acknowledgments
The authors gratefully acknowledge the funding supports of Razi University, Kermanshah, Iran.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Croxen MA, Law RJ, Scholz R, et al. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev. 2013;26(4):822–880. doi: 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russo TA, Johnson JR. Proposal for a new inclusive designation for extra intestinal pathogenic isolates of Escherichia coli: ExPEC. J Infect Dis. 2000;181(5):1753–1754. doi: 10.1086/315418. [DOI] [PubMed] [Google Scholar]
- 3.Logue CM, Wannemuehler Y, Nicholson BA, et al. Comparative analysis of phylogenetic assignment of human and avian ExPEC and fecal commensal Escherichia coli using the (previous and revised) Clermont phylogenetic typing methods and its impact on avian pathogenic Escherichia coli (APEC) classification. Front Microbiol. 2017;8:283. doi: 10.3389/fmicb.2017.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholson BA, Wannemuehler YM, Logue CM, et al. Complete genome sequence of the neonatal meningitis-causing Escherichia coli strain NMEC O18. Genome Announc. 2016;4(6):e01239–16. doi: 10.1128/genomeA.01239-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholson BA, West AC, Mangiamele P, et al. Genetic characterization of ExPEC-Like virulence plasmids among a subset of NMEC. PLoS ONE 11(1):e0147757. doi: 10.1371/journal.pone.0147757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Siek KE, Giddings CW, Doetkott C, et al. Comparison of Escherichia coli isolates implicated in human urinary tract infection and avian colibacillosis. Microbiology. 2005;151(Pt 6):2097–2110. doi: 10.1099/mic.0.27499-0. [DOI] [PubMed] [Google Scholar]
- 7.Johnson TJ, Wannemuehler Y, Doetkott C, et al. Identification of minimal predictors of avian pathogenic Escherichia coli virulence for use as a rapid diagnostic tool. J Clin Microbiol. 2008;46(12):3987–3996. doi: 10.1128/JCM.00816-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Siek KE, Giddings CW, Doetkott C, et al. Characterizing the APEC pathotype. Vet Res. 2005;36(2):241–256. doi: 10.1051/vetres:2004057. [DOI] [PubMed] [Google Scholar]
- 9.Akram M, Shahid M, Khan AU. Etiology and antibiotic resistance patterns of community-acquired urinary tract infections in J N M C Hospital Aligarh, India. Ann Clin Microbiol Antimicrob. 2007;6(4):1–7. doi: 10.1186/1476-0711-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao L, Gao S, Huan H, et al. Comparison of virulence factors and expression of specific genes between uropathogenic Escherichia coli and avian pathogenic E coli in a murine urinary tract infection model and a chicken challenge model. Microbiology. 2009;155(Pt 5):1634–1644. doi: 10.1099/mic.0.024869-0. [DOI] [PubMed] [Google Scholar]
- 11.Jafri SA, Qasim M, Masoud MS, et al. Antibiotic resistance of E coli isolates from urine samples of urinary tract infection (UTI) patients in Pakistan. Bioinformation. 2014;10(7):419–422. doi: 10.6026/97320630010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutful Kabir SM. Avian colibacillosis and salmonellosis: A closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int J Environ Res Pub Health. 2010;7(1):89–114. doi: 10.3390/ijerph7010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dziva F, Stevens MP. Colibacillosis in poultry: Unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathol. 2008;37(4):355–366. doi: 10.1080/03079450802216652. [DOI] [PubMed] [Google Scholar]
- 14.Vincent C, Boerlin P, Daignault D, et al. Food reservoir for Escherichia coli causing urinary tract infections. Emerg Infect Dis. 2010;16(1):88–95. doi: 10.3201/eid1601.091118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mora A, Viso S, Lopez C, et al. Poultry as reservoir for extra intestinal pathogenic Escherichia coli O45:K1:H7-B2-ST95 in humans. Vet Microbiol. 2013;167(3-4):506–512. doi: 10.1016/j.vetmic.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Mokady D, Gophna U, Ron EZ. Extensive gene diversity in septicemic Escherichia coli strains. J Clin Microbiol. 2005;43(1):66–73. doi: 10.1128/JCM.43.1.66-73.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauchart P, Germon P, Bree A, et al. Pathogenomic comparison of human extra intestinal and avian pathogenic Escherichia coli-search for factors involved in host specificity or zoonotic potential. Microb Pathog. 2010;49(3):105–115. doi: 10.1016/j.micpath.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Schouler C, Schaeffer B, Bree A, et al. Diagnostic strategy for identifying avian pathogenic Escherichia coli based on four patterns of virulence genes. J Clin Microbiol. 2012;50(5):1673–1678. doi: 10.1128/JCM.05057-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moulin-Schouleur M, Reperant M, Laurent S, et al. Extra intestinal pathogenic Escherichia coli strains of avian and human origin: Link between phylogenetic relationships and common virulence patterns. J Clin Microbiol. 2007;45(10):3366–3376. doi: 10.1128/JCM.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kafshdouzan K, Zahraei-Salehi T, Nayeri B, et al. Distribution of virulence associated genes in isolated Escherichia coli from avian colibacillosis. Iran J Vet Med. 2013;7(1):1–6. [Google Scholar]
- 21.Arabi S, Jafarpour M, Mirinargesi M, et al. Molecular characterization of avian pathogenic Escherichia coli in broilers bred in northern Iran. Glob Vet. 2013;10(4):382–386. [Google Scholar]
- 22.Momtaz H, Karimian A, Madani M, et al. Uropathogenic Escherichia coli in Iran: Serogroup distributions, virulence factors and antimicrobial resistance properties. Ann Clin Microbiol Antimicrob. 2013;12:8. doi: 10.1186/1476-0711-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White DG, Wilson RA, Gabriel AS, et al. Genetic relationships among strains of avian Escherichia coli associated with swollen-head syndrome. Infect Immun. 1990;58(11):3613–3620. doi: 10.1128/iai.58.11.3613-3620.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White DG, Dho-Moulin M, Wilson RA, et al. Clonal relationships and variation in virulence among Escherichia coli strains of avian origin. Microb Pathog. 1993;14(5):399–409. doi: 10.1006/mpat.1993.1039. [DOI] [PubMed] [Google Scholar]
- 25.Achtman M, Heuzenroeder M, Kusecek B, et al. Clonal analysis of Escherichia coli O2:K1 isolated from diseased humans and animals. Infect Immun. 1986;51(1):268–276. doi: 10.1128/iai.51.1.268-276.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moulin-Schouleur M, Schouler C, Tailliez P, et al. Common virulence factors and genetic relationships between O18:K1:H7 Escherichia coli isolates of human and avian origin. J Clin Microbiol. 2006;44(10):3484–3492. doi: 10.1128/JCM.00548-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ewers C, Li G, Wilking H, et al. Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: How closely related are they? Int J Med Microbiol. 2007;297(3):163–176. doi: 10.1016/j.ijmm.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Ge X, Jiang J, Pan Z, et al. Comparative genomic analysis shows that avian pathogenic Escherichia coli isolate IMT5155 (O2:K1:H5; ST complex 95, ST140) shares close relationship with ST95 APEC O1:K1 and human ExPEC O18:K1 strains. PLoS One. 2014;9(11):e112048. doi: 10.1371/journal.pone.0112048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tantawiwat S, Tansuphasiri U, Wongwit W, et al. Development of multiplex PCR for the detection of total coliform bacteria for Escherichia coli and Clostridium perfringens in drinking water. Southeast Asian J Trop Med Pub Health. 2005;36(1):162–169. [PubMed] [Google Scholar]
- 30.Yaguchi K, Ogitani T, Osawa R, et al. Virulence factors of avian pathogenic Escherichia coli strains isolated from chickens with colisepticemia in Japan. Avian Dis. 2007;51(3):656–662. doi: 10.1637/0005-2086(2007)51[656:VFOAPE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 31.Mellata M. Human and avian extra intestinal pathogenic Escherichia coli: Infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog Dis. 2013;10(11):916–932. doi: 10.1089/fpd.2013.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li G, Kariyawasam S, Tivendale KA, et al. tkt1, located on a novel pathogenicity island, is prevalent in avian and human extra intestinal pathogenic Escherichia coli. BMC Microbiol. 2012;12:51. doi: 10.1186/1471-2180-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson JR, Kuskowski MA, Smith K, et al. Antimicrobial-resistant and extra intestinal pathogenic Escherichia coli in retail foods. J Infect Dis. 2005;191(7):1040–1049. doi: 10.1086/428451. [DOI] [PubMed] [Google Scholar]
- 34.Ramchandani M, Manges AR, DebRoy C, et al. Possible animal origin of human-associated, multidrug-resistant, uropathogenic Escherichia coli. Clin Infect Dis. 2005;40(2):251–257. doi: 10.1086/426819. [DOI] [PubMed] [Google Scholar]
- 35.Moon B, Won G, Choi Y, et al. Isolation and characteristics of avian pathogenic Escherichia coli from birds associated with colibacillosis. In proceedings: AZWMP. Bangkok, Thailand. 2006:26–29. [Google Scholar]
- 36.Nolan LK, Horne SM, Giddings CW, et al. Resistance to serum complement, iss, and virulence of avian Escherichia coli. Vet Res Commun. 2003;27(2):101–110. doi: 10.1023/a:1022854902700. [DOI] [PubMed] [Google Scholar]