Abstract
Neospora caninum is an obligate intracellular parasite causing abortion and reproductive failure in ruminants. Here, the seroprevalence of Neospora DNA and anti-Neospora antibodies and the correlation between the DNA and the antibody using polymerase chain reaction (PCR) and a new developed whole cell-based enzyme-linked immunosorbent assay (ELISA) in water buffalo (Bubalus bubalis) were investigated. To determine the level of anti-Neospora antibody, 83 serum samples were collected from buffaloes in the northwest of Iran. Plates were coated with 2 × 106 whole Neospora tachyzoites and the anti-Neospora antibody level was determined by calculating the ratio of sample/positive control (S/P) optical densities (ODs) in the ELISA. All samples with the ration of 0.50 or above were accounted as positive. To confirm the presence of Neospora DNA, the serum samples were directly subjected to PCR and nested PCR for detection of Neospora NC5 gene without the DNA isolation process. A total number of 83 buffalo serum samples were examined for the presence of anti-N. caninum immunoglobulin G and Neospora DNA. All samples with the S/P ratio of 0.50 or above (16 samples, 19.27%) were also positive for Neospora DNA. All samples with OD less than 0.50 (34 samples, 40.96%) were negative for Neospora DNA. However, 33 samples with the S/P ratio of bellow 0.50 (39.75%) showed a significant level of antibody. A 100% correlation was observed between high levels of the anti-Neospora antibody and Neospora DNA in the serum of water buffalo, and the whole N. caninum tachyzoites have the potency to be used as antigens for detection of the parasite in ELISA.
Key Words: Antibody, ELISA, Neospora caninum, Polymerase chain reaction, Water buffalo
Introduction
Neospora caninum is now accounted as a major cause of abortion and congenital diseases in the dairy animal industry worldwide.1 The infection can pass via placenta causing serious economic losses among dairy and beef cattle due to a decrease in milk and meat production.2 In the Neospora life cycle, dogs play roles as definitive hosts and cattle and a wide range of domestic and wild animals including water buffalo (Bubalus bubalis) act as intermediate hosts.3 Water buffalo is naturally susceptible to Neospora caninum and can be experimentally infected with the parasite.4 Due to the economical importance of water buffaloes in the livestock industry, different surveys have been performed to study the seroprevalence of N. caninum in these merit animals around the world.5,6 The N. caninum infection is regularly diagnosed by serological tests performed on the blood samples like indirect fluorescent antibody test, agglutination test and enzyme-linked immunosorbent assay (ELISA). Due to the difficulties associated with a clinical diagnosis of neosporosis, serological tests are necessary for Neospora detection. These tests propose information about the seroprevalence of the parasite in herds, regions and countries.7 On the other hand, the interaction of the parasite with the immune system, the stages of parasitemia and the correlation between parasitemia and antibody production in water buffalo or even other intermediate hosts are still unclear. The northwest region of Iran is one of the most important poles of buffalo breeding in Iran and buffalo breeding plays an important role in the regional economy.
The main objective of this study was to determine the prevalence rate of Neospora infection among buffaloes in a survey study and illustrate the concurrency between the presences of anti-N. caninum antibodies and Neospora DNA in the serum of these animals using a new developed whole tachyzoite-based ELISA and polymerase chain reaction (PCR) techniques.
Materials and Methods
Samples . Blood samples were collected from 83 water buffaloes (32 males and 51 females) in Urmia abattoir in the northwest of Iran. The samples were centrifuged at 1600 g for 10 min and sera were collected and stored at – 20 ˚C until used.
Parasites. The N. caninum tachyzoites were obtained from Razi Vaccine and Serum Research Institute, Shiraz, Iran. The parasites were washed twice in phosphate-buffered saline (PBS) and reproduced in Vero cell line (Razi Vaccine and Serum Research Institute, Shiraz, Iran) cultured in Dulbecco's modified Eagle medium (Bio-Idea, Tehran, Iran) containing 10.00% fetal bovine serum (BIO-IDEA, Tehran, Iran) and 100 mg mL-1 gentamicin (Sigma-Aldrich, Stelnhelm, Germany).
ELISA. Tachyzoites were washed twice with PBS and counted by Neubauer chamber, then ELISA plates (Biofil, Indore, India) were coated with 2 × 106 tachyzoites suspended in 1 mL PBS per well and incubated at 25 ˚C for three days. The plates were then washed three times with a washing buffer (PBS and 0.05% Tween 20 (Merck, Darmstadt, Germany) and then blocked with a blocking buffer (PBS and marvel milk 5.00%) at 37 ˚C for 1 hr. A number of serum samples collected from Neospora positive cows was used as positive controls and the blocking buffer was used as a negative control. The serum samples (positive controls or buffaloes samples) were first diluted in the blocking buffer (1:100) and put onto the plates in duplicate followed by incubation at 37 ˚C for 1 hr. After three times washing, horseradish peroxidase-conjugated sheep anti-bovine immunoglobulin G (IgG)–heavy chain antibody (Bethyl Laboratories, Montgomery, USA) was diluted in PBS (1:1000) containing 5.00% marvel milk and added to the plates incubating at 37 ˚C for 1 hr. After washing three times with the blocking buffer and two times with distilled water, a substrate including 3,3´,5,5´-tetramethylbenzidine (Sigma-Aldrich), 10 µg mL-1 dimethyl sulfoxide (Merck), 0.10% sodium acetate (Merck) and 10% hydrogen peroxide (Merck) was added and the plates were incubated at room temperature for 40 min. To stop the reaction, 2M H2SO4 (Merck) was used and the plate was then read at 450 nm on a micro-plate ELISA reader (ELX808, Bio Tec, Winooski, USA). The ratio of sample/positive control (S/P) optical densities (ODs) was calculated according to the following equation:8,9
S/P = (Sample – NC) / (PC – NC)
where, NC was negative control and PC was positive control.
PCR. Samples with the S/P ratio of 0.50 or above were accounted as positive for N. caninum infection. Buffalo serum samples were analyzed for N. caninum NC5 gene using Np6 forward (5′-CTCGCCAGTCAACCTACGTCTT CCT>-3′) and Np21 reverse (5′-CCCAGTGCGTCCAATCCTG TAACC>-3′) primers.10 The PCR was programmed with ASTEC pc708 thermal cycler (ASTEC, Chattanooga, USA) as 10 min at 95 ˚C for primary denaturation and 35 cycles of 95 ˚C for 1 min, 65 ˚C for 1 min and 72 ˚C for 2 min for denaturation, annealing and extension respectively and a final extension at 72 ˚C for 10 min. To confirm the presence of N. caninum NC5 gene, 1.00 µL of the PCR products were subjected to nested PCR using 5'-GTGTTGCTCTGCTGACGTGT-3' forward and 5'-TACCAACT CCCTCGGTTCAC-3' reverse primers. After 10 min primary denaturation at 95 ˚C, 35 cycles of PCR were run with 1 min denaturation at 95 ˚C, 45 sec annealing at 54 ˚C and 1 min extension at 72 ˚C. Finally, the reaction was completed with 10 min final extension at 72 ˚C. The ELISA results were statistically analyzed using Excel (version 15.0; Microsoft Corporation, Redmond, USA) and SPSS (version 22.0; IBM, Chicago, USA) and the significance of the results was determined using student’s t-test with 95.00% confidence. Data are presented as mean ± SD.
Results
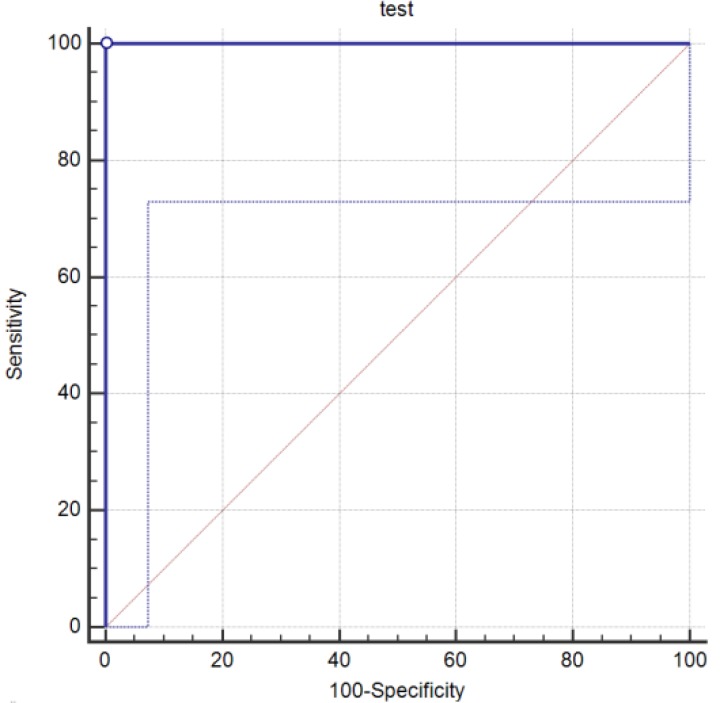
A method of ELISA based by using the whole Neospora cell as an antigen was set up to detect the level of anti-N. caninum IgG in the serum. The ratio of S/P was calculated and the cut-off value of 0.50 was determined to detect the Neospora positive samples using a receiver operator characteristic curve analysis (Fig. 1). This cutoff point distinguished nearly 100% of Neospora positive cases. The positive and negative controls were already validated with a commercial kit. The S/P ratio of 16 samples (19.27%) including three males (3.61%) and 13 females (15.66%) and also all positive controls were ≥ 0.50. The OD of samples in this group ranged from 0.52 to 2.13, 0.86 ± 0.43.
Fig. 1.
A receiver operator characteristic (ROC) curve analysis of whole cell-based ELISA against PCR confirmation of DNA. Area under the ROC curve (p < 0.0001). The pointed line shows the area with 95.00% confidence
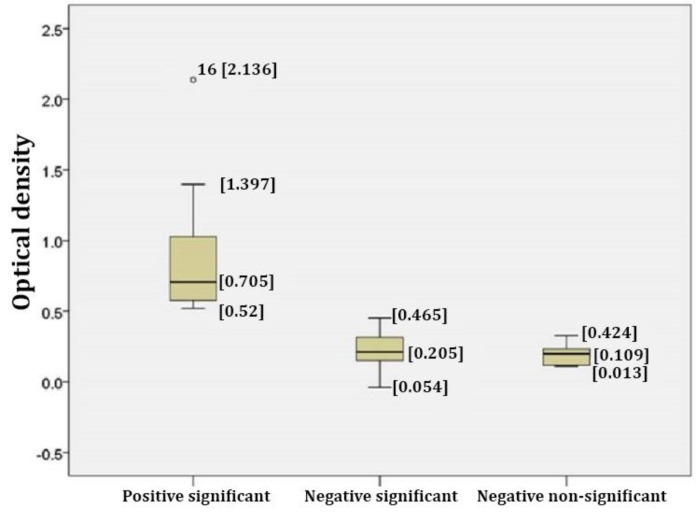
The statistical analysis showed that the level of anti-body in all samples with S/P ratio ≥ 0.50 and also in the positive controls were significantly higher than those of the negative controls (p < 0.05). Amongst the negative samples, the levels of anti-Neospora antibody in 33 samples (39.75%) were significantly higher than those of the negative controls (S/P < 0.50). The range of OD in this group was between 0.054 to 0.465, 0.16 to 0.20, while in non-significant negative samples (34 samples, 40.96%) the range of OD was between 0.013 to 0.424 and 0.11 to 0.16.
All statistical analyses were calculated with 95.00% confidence interval (Fig. 2). The prevalence of the parasitemia was evaluated by detection of the parasite DNA in the serum samples using PCR (Fig. 3) and nested PCR (Fig. 4).
Fig. 2.
Box plot illustrating S/P ratios in Neospora positive and negative samples. A number of 83 serum samples were examined for the presence of anti-N. caninum IgG by a new ELISA-based method using the whole parasite. The results were analyzed with student t-test with 95.00% confidence interval. Samples with the S/P ratio ≥ 0.50 were postulated as Neospora positive. The result of t-test for all samples with the S/P ratio ≥ 0.50 was significant. Three groups of significant positive, significant negative and non-significant negative are shown in the plot
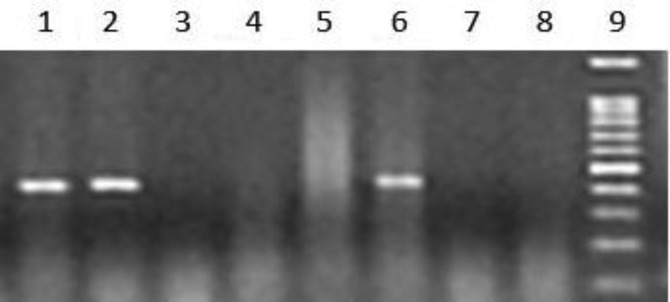
Fig. 3.
Detection of Neospora DNA in water buffalo serum. A number of 83 serum samples were examined for the presence of Neospora DNA. Lanes 1, 2 and 6: Neospora positive serum samples; Lanes 3, 4, 5 and 7: Neospora negative serum samples; Lane 8: Negative control; Lane 9: Standard DNA. The PCR product produced a 330 bp band
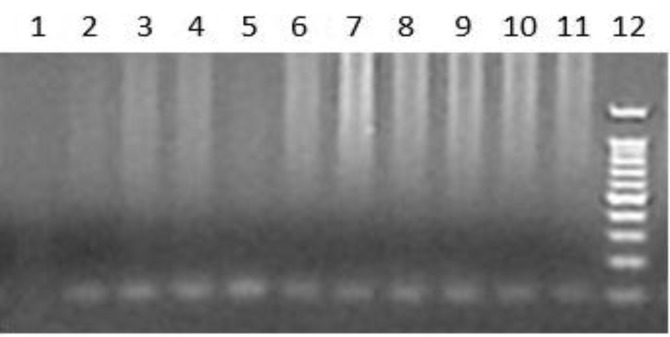
Fig.4.
Confirmation of Neospora DNA by nested PCR. The Neospora DNA PCR products were validated with nested PCR using specific primers designed for amplification of 100bp of the products. Lane 1: Negative control; Lanes 2-11: Neospora DNA PCR product; Lane 12: Standard DNA
The parasite DNA was detected in all samples with the S/P ratio ≥ 0.50 and all samples with the S/P ratio bellow 0.50 were negative for the parasite DNA.
Discussion
Because of the economical importance of water buffalo in the livestock industry and the lack of comprehensive information about Neospora infection in buffalo,8 the rate of infection as well as the relationship between parasitemia and production of anti-Neospora antibody in the serum of water buffalo in northwest of Iran was investigated. For detection of anti-Neospora antibody, an ELISA method was developed to detect the level of antibody in the serum using the whole Neospora cells. In this method of ELISA, instead of using the parasite proteins, the plates were coated with Neospora cells for 72 hr. The ELISA is shown to be a rapid and reliable method to analyze a large number of samples in a short time11 and the new whole cell-based ELISA showed to be capable of detecting anti-Neospora antibody in the water buffalo serum; since all positive samples tested by other commercial kits were positive and also negative samples were negative. The results showed that the rate of infection of water buffalo in the northwest of Iran was 19.27%. In another study, the infection rate of Neospora in buffalo was reported as 37.10% in the southwest of Iran.8 The different rate of Neospora infection between various regions is also reported in Thailand.12 Indeed, the difference in the rate of infection between the regions could be due to the epidemiology of the parasite in the regions including the rate of infection in dogs and other definitive hosts. In previous studies, the prevalence rate of N. caninum in water buffalo has been reported around the world; up to 1.50% in southern Vietnam3, 64.00% in Argentina,5 52.30% in India6, 64.00% in southern Brazil13, 70.90% in the northern region of Brazil,14 34.60% in Italy,15 28.00% in Turkey16 and 68.00% in Egypt.17 The difference in the infection rate is probably due to the environmental and management conditions or differences in the methods used for detection of the infection. The rate of infection in other intermediate hosts has also been evaluated in Iran and other countries. Many studies have shown that the rate of Neospora infection in water buffalo is higher than that of other intermediate hosts. The seroprevalence of antibody to N. caninum in cattle was 10.50% in Tabriz (northwest of Iran).18 In Mashhad (northeast of Iran), the infection rate of N. caninum in dairy cattle and camels was 15.18%19 and 5.83%,20 respectively. In northern Australia, N. caninum infection in water buffalo was highly endemic and the infection rate was higher than that for cattle.21 It is though that the higher rate of the infection in buffalo comes from this fact that this animal is probably more sensitive and its life style is slightly different than other intermediate hosts.
The PCR is a technique which has recently been widely used for detection of Neospora infections.22,23 In most of Neospora studies, PCR is performed using tissues of aborted fetus24,25 or blood cells.22,26 In the present study, to determine the presence of the parasite in the blood, serum samples were directly examined by PCR and nested PCR for amplification of Neospora Nc-5 gene which is specific for detection of Neospra.27,28 The results showed that the Neospra DNA was detectable in all samples with the S/P ratio ≥ 0.50. None of the samples with the S/P ratio less than 0.50 contained Neospora DNA, however, some of the DNA negative samples with the cutoff value of less than 0.50 showed significant levels of antibody (p-value in t-test below 0.05). The Neospora DNA has yet been isolated from sheep15 and cattle29 serum, but this is the first report on the isolation of Neospora from the serum of water buffalo and concurrent detection of anti-Neospora antibody and Neospora DNA in the serum. Stages of parasitemia in Neospora infections during the parasite life cycle and the potency of the immune system in immunity production and parasite removal from the blood have still remained unclear. Detection of the parasite DNA in the serum of positive samples clearly proved the entrance of the parasite into the blood and a period of parasitemia in water buffalo which is in line with some of previous studies confirming the entry of the parasite into the blood by detection of N. caninum DNA in the serum of infected animals.23,30 These findings showed a 100% concurrency between high levels of the antibody and the parasite DNA in the sera. In the lower levels of the antibody (below the cut-off point, but statistically significant compared to the negative controls), the Neospora DNA was disappeared indicating the potency of the immune system in antibody production to remove the parasite from the bloodstream or converting the acute phase of the disease into the chronic phase which may fall down the level of anti-Neospora antibody. The presence of DNA in the blood after parasitemia and high levels of antibody in acute phases of the infection were also addressed in other studies.26 Anyway, for detection of Neospora infection, ELISA is preferable to PCR as it can detect acute and chronic phases of the disease. However, it still needs more investigation. Other researchers have shown no association between the presence of Neospora DNA and anti-Neospora antibody in the serum in Neospora infection in cattle.30 By the way, they have detected the DNA only from white blood cells and not from the serum.22,26 This was in contrast with our findings in buffalo, where we used the serum as a DNA template in PCR reaction without any purification process. This controversy indicates that after an increase in the level of antibody in the serum, the parasites are removed from the blood, but they still grow inside the blood cells. However, the relation between the parasitemia and level of anti-Neospora antibody in the serum is more complicated and still needs to be more clear. The concurrent presence of the parasite and antibody in the serum is an important phenomenon in buffalo and needs to be investigated in cattle as the main intermediate hosts for the parasite. Our results showed a rate of 19.27% for N.caninum infection in water buffalo in the northwest of Iran. A 100% concurrency was observed between the high levels of anti-Neospora antibody and parasitemia during Neospora life stages in buffalo.
Acknowledgments
The authors would like to thank the Department of Clinical Sciences, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran for helping in the collection of samples, Razi Vaccine and Serum Research Institute, Shiraz, Iran for the generous donation of the N. caninum isolate and Miss Sakineh Azami and Mr. Hossein Khoshrouzi for their technical assistance.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Dubey JP. Review of Neospora caninum and neosporosis in animals. Korean J Parasitol. 2003;41(1):1–16. doi: 10.3347/kjp.2003.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaapan RM. The common zoonotic protozoal diseases causing abortion. J Parasit Dis. 2016;40(4):1116–1129. doi: 10.1007/s12639-015-0661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huong L, Ljungström BL, Uggla A, et al. Prevalence of antibodies to Neospora caninum and Toxoplasma gondii in cattle and water buffaloes in southern Vietnam. Vet Parasitol. 1998;75(1):53–57. doi: 10.1016/s0304-4017(97)00178-7. [DOI] [PubMed] [Google Scholar]
- 4.Chryssafidis AL, Cantón G, Chianini F, et al. Abortion and foetal lesions induced by Neospora caninum in experimentally infected water buffalos (Bubalus bubalis) Parasitol Res. 2015;114(1):193–199. doi: 10.1007/s00436-014-4178-0. [DOI] [PubMed] [Google Scholar]
- 5.Campero CM, Pérez A, Moore D, et al. Occurrence of antibodies against Neospora caninum in water buffaloes (Bubalus bubalis) on four ranches in Corrientes province, Argentina. Vet Parasitol. 2007;150(1-2):155–158. doi: 10.1016/j.vetpar.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Meenakshi , Sandhu K, Ball M, Kumar H, et al. Seroprevalence of Neospora caninum antibodies in cattle and water buffaloes in india. J Parasitol. 2007;93(6):1374–1377. doi: 10.1645/GE-1317.1. [DOI] [PubMed] [Google Scholar]
- 7.Björkman C, Uggla A. Serological diagnosis of Neospora caninum infection. Int J Parasitol. 1999;29(10):1497–1507. doi: 10.1016/s0020-7519(99)00115-0. [DOI] [PubMed] [Google Scholar]
- 8.Hajikolaei MRH, Goraninejad S, Hamidinejat H, et al. Occurrence of Neospora caninum antibodies in water buffaloes (Bubalus bubalis) from the south-western region of Iran. Bull Vet Inst Pulawy. 2007;51(2):233–235. [Google Scholar]
- 9.Yu J, Xia Z, Liu Q, et al. Seroepidemiology of Neospora caninum and Toxoplasma gondii in cattle and water buffaloes (Bubalus bubalis) in the people's Republic of China. Vet Parasitol. 2007;143(1):79–85. doi: 10.1016/j.vetpar.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 10.Müller N, Zimmermann V, Hentrich B, et al. Diagnosis of Neospora caninum and Toxoplasma gondii infection by PCR and DNA hybridization immunoassay. J Clin Microbiol. 1996;34(11):2850–2852. doi: 10.1128/jcm.34.11.2850-2852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trees AJ, Williams DJ. Endogenous and exogenous transplacental infection in Neospora caninum and Toxoplasma gondii. Trends Parasitol. 2005;21(12):558–561. doi: 10.1016/j.pt.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Kengradomkij C, Inpankaew T, Kamyingkird K, et al. Seroprevalence and risk factors associated with exposure of water buffalo (Bubalus bubalis) to Neospora caninum in northeast Thailand. Vet Parasitol. 2015;207(1-2):156–160. doi: 10.1016/j.vetpar.2014.10.034. [DOI] [PubMed] [Google Scholar]
- 13.Fujii TU, Kasai N, Nishi SM, et al. Seroprevalence of Neospora caninum in female water buffaloes (Bubalus bubalis) from the southeastern region of Brazil. Vet Parasitol. 2001;99(4):331–334. doi: 10.1016/s0304-4017(01)00474-5. [DOI] [PubMed] [Google Scholar]
- 14.Gennari SM, Rodrigues AA, Viana RB, et al. Occurrence of anti-Neospora caninum antibodies in water buffaloes (Bubalus bubalis) from the Northern region of Brazil. Vet Parasitol. 2005;134(1-2):169–171. doi: 10.1016/j.vetpar.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 15.Guarino A, Fusco G, Savini G, et al. Neosporosis in water buffalo (Bubalus bubalis) in southern Italy. Vet Parasitol. 2000;91(1-2):15–21. doi: 10.1016/s0304-4017(00)00239-9. [DOI] [PubMed] [Google Scholar]
- 16.Albayrak H, Emre Ö, Beyhan Ye, et al. A Serological investigation of some aetiological agents associated with abortion in domestic water buffalo (Bubalus bubalis Linneaus, 1758) in Samsun province of northern Turkey. Atatürk Üniversitesi Vet Bil Derg. 2012;7(3):155–160. [Google Scholar]
- 17.Dubey JP, Romand S, Hilali M, et al. Seroprevalence of antibodies to Neospora caniuum and Toxoplasma gondii in water buffaloes (Bubalus bubalis) from Egypt. Int J Parasitol. 1998;28(3):527–529. doi: 10.1016/s0020-7519(97)00190-2. [DOI] [PubMed] [Google Scholar]
- 18.Nematollahi A, Jaafari R, Moghaddam G. Sero-prevalence of Neospora caninum infection in dairy cattle in Tabriz, northwest Iran. Iran J Parasitol. 2011;6(4):95–98. [PMC free article] [PubMed] [Google Scholar]
- 19.Sadrebazzaz A, Haddadzadeh H, Esmailnia K, et al. Serological prevalence of Neospora caninum in healthy and aborted dairy cattle in Mashhad, Iran. Vet Parasitol. 2004;124(3-4):201–204. doi: 10.1016/j.vetpar.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 20.Sadrebazzaz A, Haddadzadeh H, Shayan P. Sero-prevalence of Neospora caninum and Toxoplasma gondii in camels (Camelus dromedarius) in Mashhad, Iran. Parasitol Res. 2006;98(6):600–601. doi: 10.1007/s00436-005-0118-3. [DOI] [PubMed] [Google Scholar]
- 21.Neverauskas CE, Nasir A, Reichel MP. Prevalence and distribution of Neospora caninum in water buffalo (Bubalus bubalis) and cattle in the Northern Territory of Australia. Parasitol Int. 2015;64(5):392–396. doi: 10.1016/j.parint.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Okeoma C, Williamson N, Pomroy W, et al. The use of PCR to detect Neospora caninum DNA in the blood of naturally infected cows. Vet parasitol. 2004;122(4):307–315. doi: 10.1016/j.vetpar.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Castañeda-Hernández A, Cruz-Vázquez C, Medina- Esparza L. Neospora caninum: Seroprevalence and DNA detection in blood of sheep from Aguascalientes, Mexico. Small Rumin Res. 2014;119(1):182–186. [Google Scholar]
- 24.Asmare K, Skjerve E, Bekele J, et al. Molecular identification of Neospora caninum from calf/foetal brain tissue and among oocysts recovered from faeces of naturally infected dogs in southern Ethiopia. Acta Trop. 2014;130:88–93. doi: 10.1016/j.actatropica.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Nematollahi A, Moghaddam G, Jaafari R, et al. Study on outbreak of Neospora caninum-associated abortion in dairy cows in Tabriz (Northwest Iran) by serological, molecular and histopathologic methods. Asian Pac J Trop Med. 2013;6(12):942–946. doi: 10.1016/S1995-7645(13)60168-6. [DOI] [PubMed] [Google Scholar]
- 26.Ferre I, Aduriz G, del-Pozo I, et al. Detection of Neospora caninum in the semen and blood of naturally infected bulls. Theriogenology. 2005;63(5):1504–1518. doi: 10.1016/j.theriogenology.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Baszler TV, Gay LJC, Long MT, et al. Detection by PCR of Neospora caninum in fetal tissues from spontaneous bovine abortions. J Clin Microbiol. 1999;37(12):4059–4064. doi: 10.1128/jcm.37.12.4059-4064.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collantes-Fernández E, Zaballos Á, Álvarez-García G, et al. Quantitative detection of Neospora caninum in bovine aborted fetuses and experimentally infected mice by real-time PCR. J Clin Microbiol. 2002;40(4):1194–1198. doi: 10.1128/JCM.40.4.1194-1198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okeoma CM, Stowell KM, Williamson NB, et al. Neospora caninum: Quantification of DNA in the blood of naturally infected aborted and pregnant cows using real-time PCR. Exp Parasitol. 2005;110(1):48–55. doi: 10.1016/j.exppara.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 30.McInnes LM, Ryan UM, O’Handley R, et al. Diagnostic significance of Neospora caninum DNA detected by PCR in cattle serum. Vet Parasitol. 2006;142(3-4):207–213. doi: 10.1016/j.vetpar.2006.07.013. [DOI] [PubMed] [Google Scholar]