Abstract
The aim of this study was to find a proper method for improvement of ischemic condition in the rat hind limb and also to observe the efficacy of cell engraftment with alginate/gelatin three-dimensional scaffolds. Eighteen male Wistar rats weighing 200 to 250 g were randomly divided into three groups (n = 6) including a) ischemia group; in which femoral artery was removed after ligation at the distance of 5 mm, b) scaffold group; in which hydrogel scaffold was added to the site of transected femoral artery and c) test group; in which in addition to hydrogel scaffold, mast cells (MCs) were also added (1 × 106 cells). Analysis of capillary density, artery diameter, histomorphometric parameters and immunohistochemistry in transected location were done on day 14 after femoral artery transection. The average number of blood capillary was significantly higher in the test group than other groups. Also, the average number of medium and large blood vessels was significantly higher in the test group compared to ischemia and scaffold groups. Application of MCs through the use of hydrogel scaffolds (alginate/gelatin) can be considered as a new approach in the application of stem cells for therapeutic angiogenesis under ischemic conditions which can improve the angiogenesis process in patients with peripheral artery diseases.
Key Words: Angiogenesis, Immunohistochemistry, Mast cell, Rat, Tissue engineering
Introduction
Tissue ischemia related to peripheral vascular diseases (PVD) forms more than half of cardiovascular diseases (CVDs).1 Although the life quality of CVD patients has been improved due to the advancement of medications and transplantation, there exists a serious demand for the creation and development of advanced therapeutic methods for tissue ischemia treatment. In the United States, more than eight million people suffer from atherosclerosis, which involves artery blockage by cholesterol plaques.1-3 Ischemic patients are not only exposed to high risk of amputation, but their life is threatened by CVD-induced deaths which could be attributed to heart vasculature complexity and cerebrovascular atherosclerosis.4
Movement disability, therapy-resistant ischemic ulcers, ulcer improvement disorders, and amputation are among the consequences of atherosclerosis.5 Lack of proper therapeutic methods has made patients disappointed to find relief for their pains.6
By now, no reliable method has been approved by the European Commission for treatment of motion limbs ischemia.7 Therefore, novel methods are required. Recently, clinical studies have been conducted on the basis of angiogenesis for treatment of PVD, ulcers improvements and the like.8-10 In this regard, stem cell-based methods have attracted considerable attention to enhance the angiogenesis and improvement of tissue function or blood pressure. Numerous stem cells have been applied to improve angiogenesis in CVD patients who also had ischemia; among which, adult bone marrow mononuclear cells, internal heart, and vascular membrane cells and multi-capacity stem cells can be mentioned.10-12 Shintai et al. have showed that bone marrow-derived mononuclear cells can extend new vasculatures in hind limb ischemia (HLI) of a rabbit which will stimulate lateral vasculature and increase peripheral blood.13
Jeon et al. have investigated the effect of angiogenesis treatments by mononuclear cells transplantation and also the stimulation of vascular endothelial growth in HLI model of a rat. They have reported an increase of small vasculature density and higher expression of basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) during the treatment process.14 Zhang et al. have observed a similar increase in lateral density and blood circulation in the forelimbs during application of bone marrow-derived mononuclear cells in mice with ischemic skeleton.15
Previous studies have evaluated the effect of mast cells (MCs) on angiogenesis induction.16-19 Presence of MCs in the vicinity of endothelial cells is one of the evidences of the MCs relationship with angiogenesis.20 However, for suitable and stable angiogenesis, in addition to cells and growth factors, a third coordinator factor is also needed. This third agent is the extracellular matrix (ECM). Coordination of this process is called tissue engineering and its aim is to restore, maintain and improve the function of tissues which had been injured due to different pathological factors.21
In cell therapy technique, more than 90.00% of the injected cell suspension is lost and dose not engraft.22 Most of the studies support that tissue engineering can improve this drawback of cell engraftment with the use of three-dimensional (3D) porous scaffold in tissue engineering by controlling cell attachment, mechanical support, and stimulation of new in vivo tissue growth.22-25 However, the application of synthetic and chemical materials will increase the chance of damage to cells and living tissues, therefore, the importance of natural compositions has been highlighted. One of the selection criteria for scaffolds was to prepare a 3D scaffold with proper porosity which does not interfere with angiogenesis. In this regard, the application of natural polymers such as alginate and gelatin can resolve the mentioned limitations due to their nature-based properties,22 and having no impact on angiogenesis23 in addition to their cost-effectiveness.24 The combination of alginate and gelatin has a major similarity to ECM component in animals. Thus, this characteristic makes them more appropriate material for tissue engineering.23 In the present study, angiogenesis was induced by tissue engineering along with rat bone marrow differentiated MCs in an empirical ischemic model (cut of femoral artery) by imitating physiological condition.
Materials and Methods
Experimental design and animals. Eighteen mature male Wistar rats with a weight of 200 to 250 g were randomly divided into three groups (n = 6). Before the test, rats were kept in an ambient temperature of 23.00 ± 3.00 ˚C, stable air humidity and a natural day/night cycle (14 hr light and 10 hr darkness) for one week for adapting to the environment. In ischemic group, ischemia was created in the hind limb by femoral artery transection between two ligatures with 5/0 silk (Pezeshkyaran, Tehran, Iran) in 5 mm distance. In Scaffold group, in addition to ischemia, hydrogel (alginate-gelatin) scaffold (50 µL) was placed in the location of femoral artery transection. In test group: 1 × 106 MCs were added to artery transection site along with hydrogel with the volume of 50 µL in this group.
Surgical Procedure. The procedure was carried out based on the guidelines of the Veterinary Ethics Committee of Faculty of Veterinary Medicine, Urmia University, Urmia, Iran (Reference No.: AECVU-185-2018). The Urmia University Research Council approved all of the experiments. Rats were anesthetized by intra-peritoneal administration of 90 mg kg-1 ketamine (Alfasan, Woerden, Netherlands) combined with 5 mg kg-1 xylazine (Alfasan). Approximately, a 5 mm portion of the right femoral artery was ligated and resected to create the HLI model. The proximal branches, superficial caudal epigastric and side muscular arteries and veins were also resected.6
Histological analysis. On day 14, the animals were euthanized using an overdose of ketamine-xylazine (three times of anesthetic dose, IP) and tissue samples were taken and fixed in a fixative containing 10% formaldehyde buffer.6 Afterward, the paraffin sections from fixed specimens were prepared (5-7 µm) by a rotary microtome (Microm, Walldorf, Germany). The sections were stained with hematoxylin and eosin for histomorphometric studies.
Bone marrow mast cells isolation. All rats were first anesthetized by intra-peritoneal ketamine-xylazine with the same protocol and then euthanized with above mentiond protocol.4
Bone marrow cells were immediately isolated from rat femur and tibia bones as described earlier.27,28 Then, the bones were flushed by insulin syringe using endotoxin-free culture medium and obtained materials were centrifuged for 10 min at 320 g at 4 ˚C. All chemicals were obtained from Merck, Darmstadt, Germany. The cells were then cultured at the ratio of 0.50 × 106 mL-1 in complete media (RPMI1640 containing fetal bovine serum 10%, 100 IU mL-1 penicillin, 100 µg mL-1 streptomycin, 0.10 µmol non-essential amino acids and 2.00 mmol L-glutamine (ThermoFisher Scientific, Darmstadt, Germany) and splenic mitogen pokeweed (20.00%).29 The medium has changed every five days. After 3-4 weeks, cells were washed with cold phosphate-buffered saline (PBS; 1X) 8 g NaCl, 0.20 g KCl, 1.42 g Na2HPO4, and 0.27 g KH2PO4 were added to 1 L of distilled water.
Pokeweed mitogen-stimulated spleen cell conditioned medium. Splenic cells were isolated from rat and cultured with density of two million cells mL-1 in RPMI1640 medium containing 4 µmol L-glutamine, 5 × 10-5 M 2-mercaptoethanol, 1.00 mmol sodium pyruvate (Sigma-Aldrich, Zwijndrecht, Netherlands), 100 IU mL-1 penicillin, 100 µg mL-1 streptomycin, 0.10 µmol non-essential amino acids and 8.00 µg mL-1 lectin in 75 cm2 flasks. After 5-7 days, the supernatant culture medium was centrifuged for 15 min at 3200 g and passed through 0.22 µm filter (Merck) and the obtained fluid was used as a pokeweed splenic mitogen.29-32
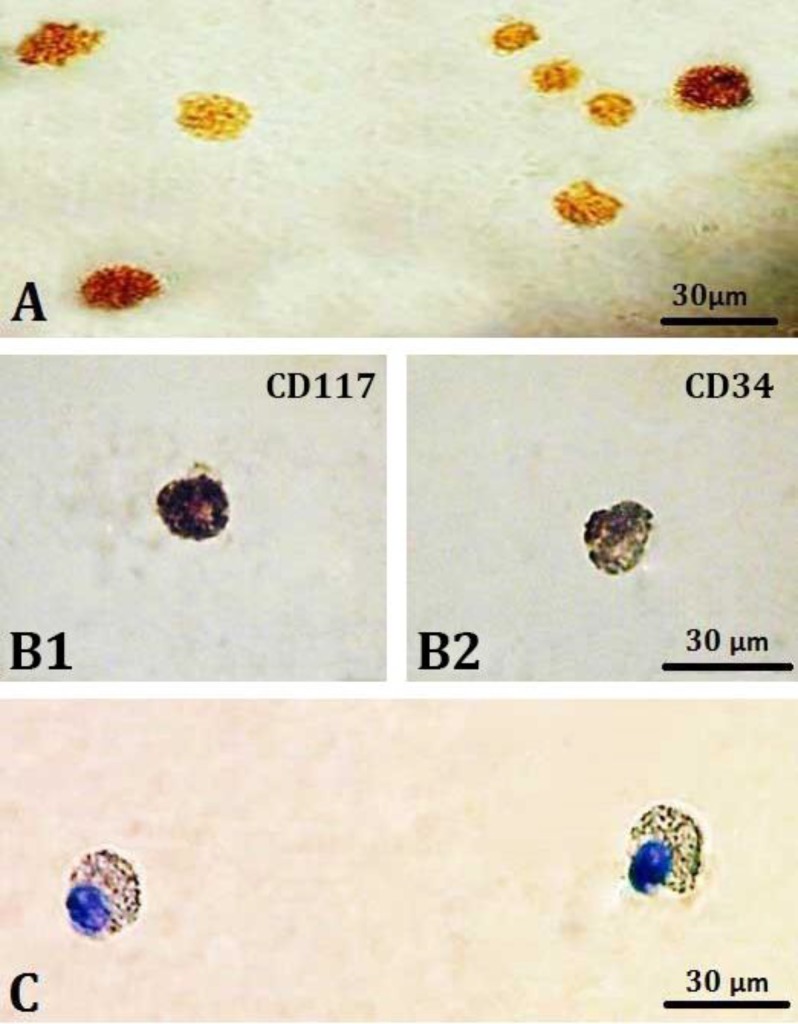
Immunocytochemistry (ICC) of tryptase: Tryptase antibody was selected to detect specific immunoreactivity against mast cells. Cells were incubated with 150 μL of rabbit anti-mouse tryptase (mouse MC protease-6; Tebu-Bio, Heerhugowaard, The Netherlands) primary antibody in predetermined optimal dilutions for 1 hr at room temperature, washed in PBS and then incubated for 30 min in 500 μL of goat anti-rabbit biotinylated secondary antibody (Dako, Glostrup, Denmark), (Fig. 1A).29,33,34
Fig. 1.
Immunohistochemistry staining for A) Tryptase reaction (brown- colored granules) in the mast cell cytoplasm; 400×, B1 and B2) The reaction for CD117 (dark-colored granules) and CD34 (light brown-colored granules); 1000×, C) Rat bone marrow mast cells stained with toluidine blue; 1000×.
Immunocytochemistry (ICC) of CD34 and CD117. The cells were placed on a slide and fixed with paraformaldehyde 4.00% and then slides were washed in PBS for 10 min and entered the specific staining stage afterward. The ICC staining steps were performed according to the protocol of the manufacturer instruction (Novocastra, Newcastle, UK). The cells were then counterstained by Gill’s II hematoxylin, fixed using crystal/mount (Biomeda) and prepared for the study (Figs. 1B1 and 1B2).35,36
Toluidine blue metachromatic staining. Toluidine blue staining used as follows: a) fixation with 4.00% neutral paraformaldehyde, and b) staining with toluidine blue solution containing 0.10 mg toluidine blue and 100.00 mL distilled water. Staining procedure has been done as follows: The cells were placed on a slide, fixed by 4.00% paraformaldehyde and then stained with toluidine blue for 1-2 min. Then, the slides were cover-slipped by finger nail polish (Fig. 1C).37
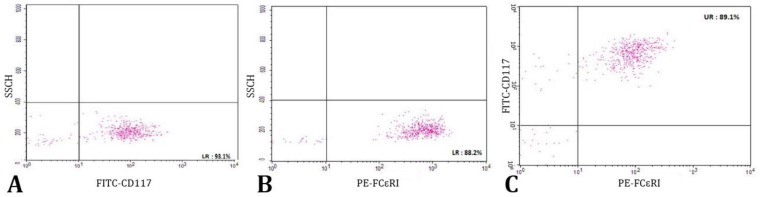
Characterization of MCs by flow cytometry. The MCs were harvested after 3 weeks of culture (about 1 × 104 cells), washed with cold PBS and the cell-surface Fc receptors were blocked with the 2.4G2 antibody (Pharmingen, San Diego, USA). The PE-conjugated anti-rat c-kit (2B8; FITC: sc-19619 F) was used to stain c-kit and Fc𝜀RI was stained with an FITC-conjugated anti-rat Fc𝜀RI antibody (BD Pharmingen, San Diego, USA) and compared with matched isotype control antibodies. The cells were incubated with antibodies in 50 µL of PBS for 1 hr at 4 ˚C, washed with PBS and identified using flow cytometer (FACS Calibour BD, New York, USA; Fig. 2).38
Fig. 2.
Flow cytometry analysis of rat bone marrow-derived mast cells. A) Positive cells for CD117 (c-kit), B) Positive cells for FCϵRI, C) Double-positive cells (89.10%).
Scaffold. Scaffolds were hydrogels (alginate-gelatin). Due to its desirable features, alginate was selected as the main component of hydrogel scaffold. After alginate modification with gelatin, phenolic groups were added for more stability and controllable gelation time.22-25 These groups were added by carbo-di-amide bonds from amine groups of tyramine to carboxylic groups of alginate and gelatin. The hydrogel was then formed in the presence of peroxidase enzyme.18-20
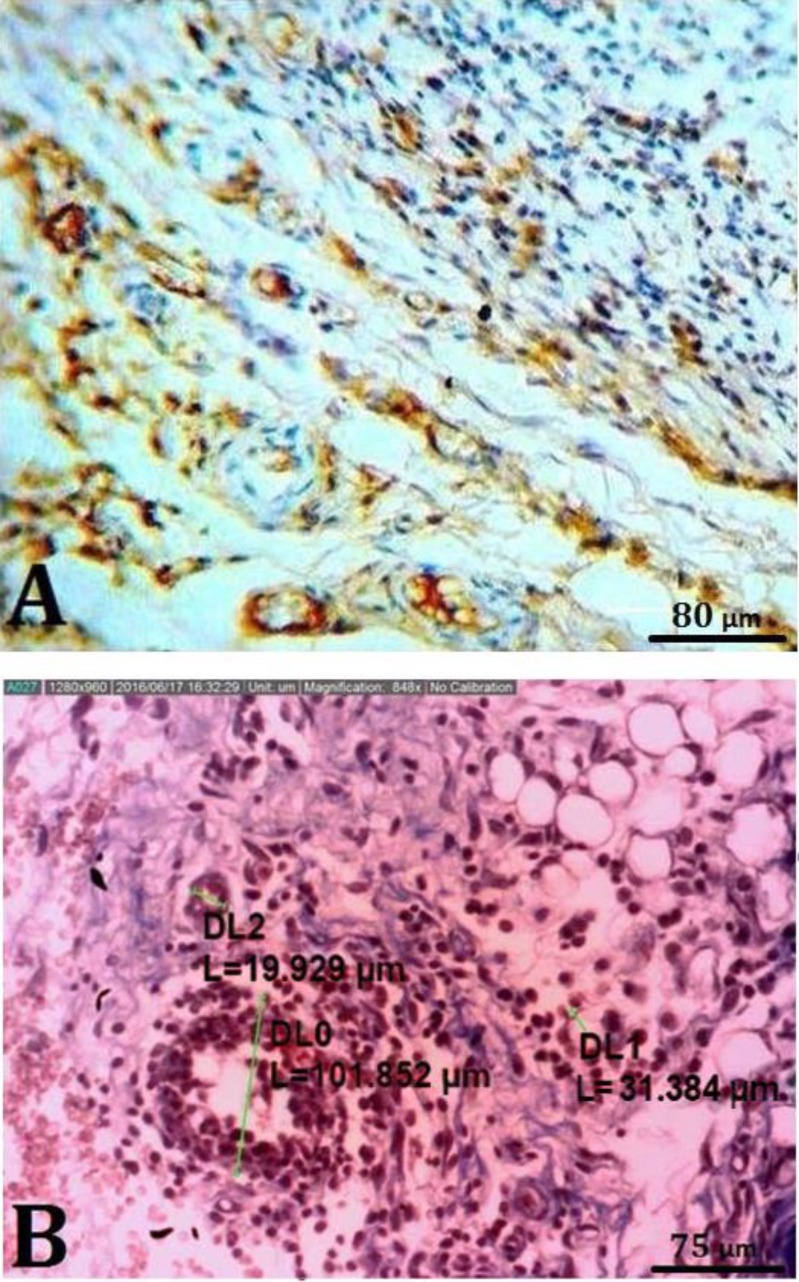
Vasculature counting. For immunohistochemical (IHC) analysis, tissue section slides were heated at 60 ˚C for approximately 25 min in a hot air oven (Venticell, Einrichtungen, Germany). The IHC staining was conducted based on the manufacturer's protocol (Biocare, Chicago, USA). In brief, the tissue sections were gently washed using a washing buffer and then incubated with CD34 (1:5,000 ab81289; Abcam, Cambridge, USA) primary antibody for 15 min. A diaminobenzidine-substrate-chromogen was added to the tissue sections and incubated for 5 min. They were then washed and counterstained using hematoxylin for 5 sec. The sections were then dipped in a weak ammonia solution (0.037 M L-1) 10 times, rinsed with distilled water and cover-slipped. Positive IHC staining was observed as brown stains under a light microscope (Fig. 3A).
Fig. 3.
A) Immunohistochemical staining for CD34. Endothelial cells are stained brownish yellow to dark brown (with chromogen); 1000×. B) Micrograph showing morphometrical analysis of blood vessels in a transected area (Hematoxylin and eosin, 352×)
The tissue sections were stained by hematoxylin and eosin.39 The capillary count was conducted by an optical microscope and digital camera (AM-7023; Dinolite, Tokyo, Japan) and related software at a magnification of 400× at the area of 0.0625 mm2. Larger vasculatures were counted at a magnification of 100× in the area of 0.88 mm2 at each tissue section. Five samples from each group were examined (Fig. 3B).
Statistical analyses. The data were analyzed by SPSS (Version 20; SPSS Inc., Chicago, USA). All values are expressed as mean ± SEM. Differences between experimental groups were analyzed using one-way ANOVA. Bonferroni test was used to specify the significant differences between the groups. The level of significance was set at p < 0.05.
Results
Specific markers for differentiated bone marrow-derived mast cells including CD117 (c-kit), CD34 and tryptase were analyzed. All three markers played an important role in confirming the specific characteristics of MCs. CD117 and CD34 markers and tryptase that evaluated by ICC method were positive 80.10%, 76.89%, and 87.90% respectively with rat splenic supernatant. The ICC results were confirmed by flow cytometry analysis of rat bone marrow-derived MCs. In this case, rat MCs, were double positive for FCԑRI and CD117 markers (89.10%).
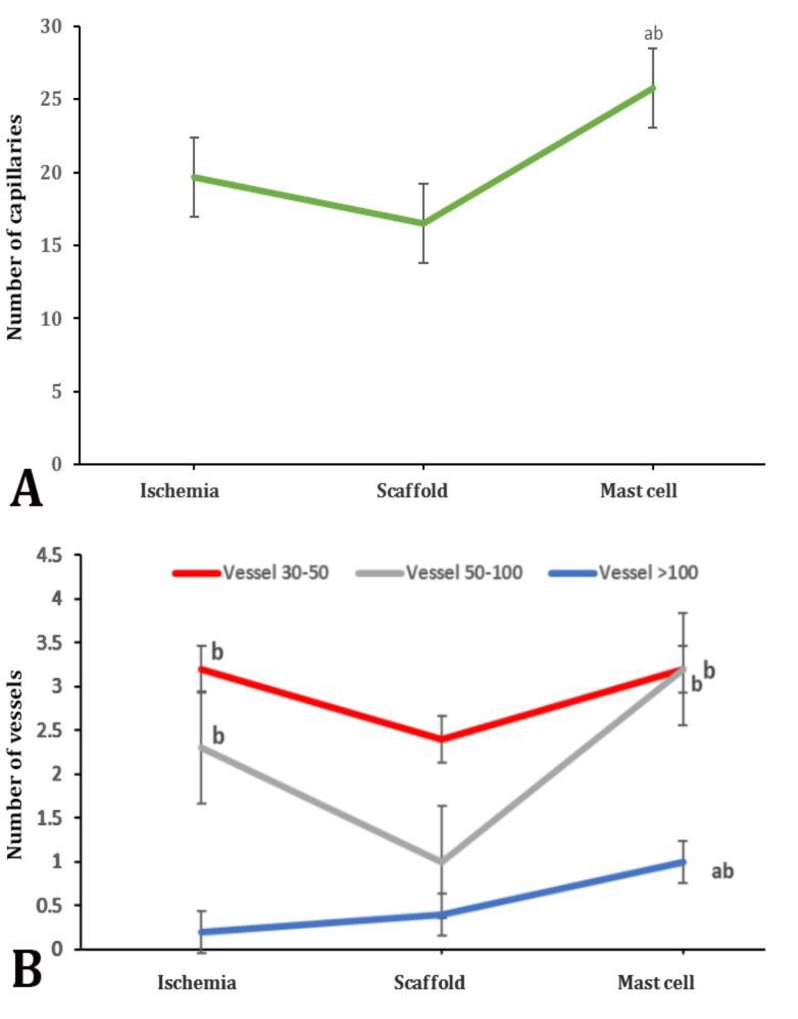
Blood capillary count results showed that the average number of blood capillary at the unit area of 0.0625 mm2 was not significantly higher in control or ischemia group in which the scaffold and cells were not applied as compared to the scaffold receiving group. Moreover, the average number of capillaries had an increase in the group receiving MCs was significant in comparison with the other groups (p < 0.05; Fig. 4A). Histomorphometric results indicated that the average number of vessels with a mean diameter of 30 to 50 μm (counted in a 0.88 mm2 area) was drastically decreased in the scaffold group as compared to other groups (p < 0.05; Fig. 4B).
Fig. 4.
A) Capillary morphometry in a transected area of all groups. B) Vessel morphometric data in a transected area of all groups. abc indicate significant differences with ischemia, scaffold and mast cells groups, respectively (p < 0.05)
The average number of vasculatures with a mean diameter of 50-100 μm (counted in a 0.88 mm2 area) was drastically decreased in scaffold group compared to ischemia and MCs groups (p < 0.05). The average number of vasculatures larger than 100 μm (at unit area of 0.88 mm2) was the lowest in ischemia group; while the numbers of these vasculatures were significantly higher in the MCs group in comparison with ischemia and scaffold groups (p < 0.05; Fig. 4B).
Discussion
The HLI is the most common pre-clinical model of PVD. In this model, the femoral arteries are blocked and reduce the blood supply of the hind limb by 20.00%. The HLI model is widely used for evaluation of the therapeutic performance of stem cells.40 Therapeutic angiogenesis using cell transplantation was experimentally and randomly used for the first time from bone marrow mononuclear cells and peripheral blood mononuclear cells.11
Investigation of angiogenesis trend by examination of capillaries morphology showed distinguished development of these vasculatures in MCs group. Numerous studies have confirmed the association between MCs and angiogenesis.16,30 The MCs presence in the vicinity of capillary formation sites can be regarded as one of the evidences.26 Paracrine factors can play a crucial role in tissue repair improvement and functional restoration after injection of stem cells. Therefore, methods have been designed and developed to enhance the cellular survival and function and delivery of paracrine factors.29 The MCs do not differentiate spontaneously, but cytokines will cause migration and differentiation of the existing MCs in the ischemic site.16 This study showed that due to the absence of MCs in the ischemic and scaffold-only groups, the number of capillaries was lower than that of MCs group. In terms of angiogenesis stimulation of capillaries, the average numbers of blood vessels in different sizes in MCs group indicated a significant increase compared to other experimental groups (p < 0.05).
In MCs group, the number of small vasculatures was relatively low and the number of medium vasculatures had a significant increase relative to the scaffold group (p < 0.05). This could be due to the effect of chemical mediators secreted from MCs which will result in more vasculatures development and their anastomosis. Angiogenesis is a multi-stage process acting in a very coordinated manner.41,42 Various factors are involved in this process. To induce angiogenesis, factors, drugs or a combination of these methods and tissue engineering have been used.
Application of bone marrow cells for angiogenesis induction in cardio-ischemic patients,43,44 and therapeutic angiogenesis by induction of human hepatocyte growth factor gene in rats are some of these methods. Improvement is directly dependent on the evaluation of the angiogenesis score by the increase of capillaries density, an increase of oxygen pressure and reduction of skin ulcers. The score of angiogenesis is a qualitative analysis of lateral vasculature by an angiogram. This score is often calculated relative to the ratio of vasculatures to a number of cross-sections in the femoral bone.44 Increase in the number of vasculatures larger than 100 μm (macro-vasculatures) in MCs receiving group showed the high potential of MCs in stimulation of angiogenesis and formation of the vascular anastomosis. It seems that by the occurrence of vascular anastomosis, a vascular shunt would be formed between general blood circulation and ischemic organ and micro-vasculatures will lose their importance. Therefore, the need for micro-vasculature will be reduced and the severity of ischemia will be ameliorated. This trend could be the reason for the variable results of micro-vasculature numbers in the MCs receiving group.11,19 Secretions of MCs can induce and increase angiogenesis via complicated paths and various MCs secretions can induce angiogenesis through sophisticated paths. These factors are bFGF, VEGF, tumor necrosis factor alpha, transforming growth factor beta, interleukin 8, proteinases, heparins, heparin-binding pro-angiogenic factors, histamines, lipid-derived mediators and so on.16,27,28 Presence of macro-vasculatures indicates the growing vascular anastomosis, therefore, if the vasculatures connect to general circulation system, the number of macro-vasculatures can also increase. This study showed that the number of vasculatures with a diameter larger than 50 μm was higher in MCs group compared to other groups.
In addition to the inherent duties of MCs in immunity, recently it has been shown that these cells can secrete immune suppressor factors too.45,46 As a result, they may prevent rejection of the transplanted cell. Also, due to the disaffiliation of MCs in a structural role in the cell trans-plantation area, there is no need to survive and prolong the activity of these cells in a cell transplantation area.
Stem cell therapy can be a proper candidate for recovery of injured vasculatures. Researchers have reached significant advances in cell injection in pre-clinical and clinical fields. However, there exist numerous problems. Application of stem cells for treatment of ischemic tissues requires a strong method for determination of cell features, storage, uniform isolation, and optimal delivery approaches. In particular, when different types of cells are substituted and revived, the integrity of long-term function is one of the main challenges of an effective clinical improvement. To overcome these limitations, vasculature networks stimulating multi-cellular structure architecture can be a key factor to enhance the function integrity and multi-cellular tissue potentials.31,32 Moreover, regarding the ischemia environment, reactivity toward immunity and host and survival of the injected cells or transplanted tissue are still challenging. Advances in imaging techniques have provided the researchers with the opportunity of better investigation of the site and survival of stem cells. Modification of delivery patterns, dosage and phenotype identification of the cells are all among the key factors in the improvement of cellular treatment effectiveness.15,32 The results of the present study showed that MCs can stimulate angiogenesis in the ischemic condition and result in vascular developing. On the other hand, it has been shown that by an increase in the number of macro-vasculatures, the micro-vasculatures will develop with lower rates.
The knowledge of stem cells has provided valuable information about CVD treatment and modification of these therapeutic methods. Although further studies are required for optimization of stem cell-based therapies for ischemic cardiovascular tissues, application of stem cells for such therapeutic purposes is very promising.
Acknowledgments
This study was supported as a Ph.D. thesis No. D-97-159 by Urmia University. We wish to thank Dr. Ali Karimi and Dr. Zahra Bakhtiari from Histology Laboratory, Mr. Asghar Aliari from Immunology Laboratory and Mr. Ali Piernejad from Central Laboratory of Faculty of Veterinary Medicine, Urmia University, Urmia, Iran for their kind technical support.
Conflict of interest
All authors declare that there exists no potential conflict of interest regarding the study described and the preparation of the article.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Executive summary: Heart disease and stroke statistics-2015 update. Circulation. 2016;131(4):434–441. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic) Circulation. 2006;113(11):e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 4.Niiyama H, Huang NF, Rollins MD, et al. Murine model of hindlimb ischemia. J Vis Exp. 2009;23:1035–1039. doi: 10.3791/1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Go AS, Mozaffarian D, Roger VL, et al. Heart and stroke statisticas- 2014 update: A report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hobo K, Shimizu T, Sekine H, et al. Therapeutic angiogenesis using tissue engineered human smooth muscle cell sheets. Arterioscler Thromb Vasc Biol. 2008;28(4):637–643. doi: 10.1161/ATVBAHA.107.151829. [DOI] [PubMed] [Google Scholar]
- 7.Lambert MA, Belch JJ. Medical management of critical limb ischemia: Where do we stand today? J Intern Med. 2013;274(4):295–307. doi: 10.1111/joim.12102. [DOI] [PubMed] [Google Scholar]
- 8.Wagoner LE, Merrill W, Jacobs J, et al. Angiogenesis protein therapy with human fibroblast growth factor (FGF-1): Results of a phase I open label, dose escalation study in subjects with CAD not eligible for PCI or CABG. Circulation. 2007;116(16):443–450. [Google Scholar]
- 9.Sifat AE, Vaidya B, Abbruscato TJ. Blood-brain barrier protection as a therapeutic strategy for acute ischemic stroke. AAPS J. 2017;19(4):957–972. doi: 10.1208/s12248-017-0091-7. [DOI] [PubMed] [Google Scholar]
- 10.Suda M, Shimizu I, Yoshida Y, et al. Therapeutic Angiogenesis. Singapore, Singapore: Springer ; 2017. Peripheral blood mononuclear cells for limb ischemia; pp. 25–43. [Google Scholar]
- 11.Hou L, Kim JJ, Woo YJ, et al. Stem cell-based therapies to promote angiogenesis in ischemic cardiovascular disease. Am J Physiol Heart Circ Physiol. 2016;310(4):455–465. doi: 10.1152/ajpheart.00726.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazarika S, Annex BH. Gene and cell therapy for critical limb ischemia. In: Dieter R, Dieter Jr R, Dieter , III R, et al., editors. Critical Limb Ischemia. 1st ed. Cham, Switzerland: Springer; 2017. pp. 491–501. [Google Scholar]
- 13.Shintani S, Murohara T, Ikeda H, et al. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation. 2001;103(6):897–903. doi: 10.1161/01.cir.103.6.897. [DOI] [PubMed] [Google Scholar]
- 14.Jeon O, Song SJ, Bhang SH, et al. Additive effect of endothelial progenitor cell mobilization and bone marrow mononuclear cell transplantation on angiogenesis in mouse ischemic limbs. J Biomed Sci. 2007;14(3):323–330. doi: 10.1007/s11373-007-9145-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Zhang N, Li M, et al. Therapeutic angiogenesis of bone marrow mononuclear cells (MNCs) and peripheral blood MNCs: Transplantation for ischemic hindlimb. Ann Vasc Surg. 2008;22(2):238–247. doi: 10.1016/j.avsg.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 16.Ribatti D. Biochemical basis and therapeutic implications of angiogenesis. Cham, Switzerland: Springer; 2017. Mast cells in angiogenesis: The role of angiogenic cytokines; pp. 157–167. [Google Scholar]
- 17.Wroblewski M, Velthaus J-L, Bauer R, et al. Effect of MCs on efficacy of anti-angiogenic therapy by secreting matrix-degrading granzyme b. J Clin Oncol. 2017;35(15):11522–11522. doi: 10.1038/s41467-017-00327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribatti D, Ranieri G. Tryptase, a novel angiogenic factor stored in MC granules. Exp Cell Res. 2015;332(2):157–162. doi: 10.1016/j.yexcr.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Norrby K. Mast cells and angiogenesis. APMIS. 2002;110(5):355–371. doi: 10.1034/j.1600-0463.2002.100501.x. [DOI] [PubMed] [Google Scholar]
- 20.De Souza Junior DA, Mazucato VM, Santana AC, et al. MCs interact with endothelial cells to accelerate in vitro angiogenesis. Int J Mol Sci. 2017;18(12):2674. doi: 10.3390/ijms18122674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanza R, Langer R, Vacanti JP. Principles of tissue engineering. 4th ed. Boston, USA: Academic Press. 2013:56–112. [Google Scholar]
- 22.Rosellini E, Cristallini C, Barbani N, et al. Preparation and characterization of alginate/gelatin blend films for cardiac tissue engineering. J Biomed Mater Res A. 2009;91(2):447–453. doi: 10.1002/jbm.a.32216. [DOI] [PubMed] [Google Scholar]
- 23.Dinescu S, Galateanu B, Radu E, et al. A 3D porous gelatin-alginate-based-IPN acts as an efficient promoter of chondrogenesis from human adipose-derived stem cells. Stem Cells Int. 2015;2015(1):1–17. doi: 10.1155/2015/252909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan T, Song W, Cao X, et al. 3D bioplotting of gelatin/alginate scaffolds for tissue engineering: Influence of crosslinking degree and pore architecture on physicochemical properties. J Mater Sci Technol. 2016;32(9):889–900. [Google Scholar]
- 25.Luo Y, Lode A, Akkineni AR, et al. Concentrated gelatin/alginate composites for fabrication of predesigned scaffolds with a favorable cell response by 3D plotting. RSC Adv. 2015;5(54):43480–43488. [Google Scholar]
- 26.Sun G, Zhang X, Shen YI, et al. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc Natl Acad Sci USA. 2011;108(52):20976–20981. doi: 10.1073/pnas.1115973108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Da Silva EZM, Jamur MC, Oliver C. Mast cell function: A new vision of an old cell. J Histochem Cytochem. 2014;62(10):608–738. doi: 10.1369/0022155414545334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dahlin JS, Hallgren J. MC progenitors: Origin, development and migration to tissues. Mol Immunol. 2015;63(1):9–17. doi: 10.1016/j.molimm.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Meurer SK, Neß M, Weiskirchen , et al. Isolation of mature (peritoneum-derived) mast cells and immature (bone marrow-derived) mast cells precursors from mice. PLoS One. 2016;11(6):e0158104. doi: 10.1371/journal.pone.0158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mortaz E, Redegeld FA, Nijkamp FP, et al. Dual effects of acetylsalicylic acid on MC degranulation, expression of cyclooxygenase-2 and release of pro-inflammatory cytokines. Biochem Pharmacol. 2005;69(7):1049–1057. doi: 10.1016/j.bcp.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Bajpai VK, Andreadis ST. Stem cell sources for vascular tissue engineering and regeneration. Tissue Eng Part B Rev. 2012;18(5):405–425. doi: 10.1089/ten.teb.2011.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fakoya AO. New delivery systems of stem cells for vascular regeneration in ischemia. Front Cardiovasc Med. 2017;4:7. doi: 10.3389/fcvm.2017.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortaz E, Givi ME, Da Silva CA, et al. A relation between TGF-β and MC tryptase in experimental emphysema models. Biochim Biophys Acta. 2012;1822(7):1154–1160. doi: 10.1016/j.bbadis.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Vukman KV, Adams PN, Metz M, et al. Fasciola hepatica tegumental coat impairs MCs’ ability to drive Th1 immune responses. J Immunol. 2013;190(6):2873–2879. doi: 10.4049/jimmunol.1203011. [DOI] [PubMed] [Google Scholar]
- 35.Drew E, Merkens H, Chelliah S, et al. CD34 is a specific marker of mature murine mast cells. Exp Hematol. 2002;30(10):1211–1218. doi: 10.1016/s0301-472x(02)00890-1. [DOI] [PubMed] [Google Scholar]
- 36.Hosseini E, Pedram B, Bahrami AM, et al. Diagnostic procedures for improving of the KIT (CD117) expressed allele burden for the liver metastases from uterus MC tumors: Prognostic value of them etastatic pattern and tumorbiology. Tumour Biol. 2015;36(2):929–937. doi: 10.1007/s13277-014-2666-6. [DOI] [PubMed] [Google Scholar]
- 37.Givi M, Blokhuis B, Da Silva C, et al. Cigarette smoke suppresses the surface expression of c-kit and FcεRI on mast cells. Mediators Inflamm. 2013;2013(1):1–7. doi: 10.1155/2013/813091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng Cx, Li Yn, Ohno H, et al. Mast cells appearing in long-term skeletal muscle cell cultures of rat. Anat Rec. 2007;290(11):1424–1430. doi: 10.1002/ar.20595. [DOI] [PubMed] [Google Scholar]
- 39.Humason GL. Animal tissue techniques. 4th ed. San Francisco, USA: WH Freeman and Company; 1979. pp. 111–131. [Google Scholar]
- 40.Dormandy JA. Management of peripheral arterial disease (PAD) TASC working group Trans Atlantic Inter-Society Consensus (TASC) J Vasc Surg. 2000;31(1 Pt 2):S1–S296. [PubMed] [Google Scholar]
- 41.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 42.Karamysheva A. Mechanisms of angiogenesis. Biochemistry (Mosc) 2008;73(7):751–762. doi: 10.1134/s0006297908070031. [DOI] [PubMed] [Google Scholar]
- 43.Hamano K, Nishida M, Hirata K, et al. Local implantation of autologous bone marrow cells for therapeutic angiogenesis in patients with ischemic heart disease. Jap Circ J. 2001;65(9):845–847. doi: 10.1253/jcj.65.845. [DOI] [PubMed] [Google Scholar]
- 44.Taniyama Y, Morishita R, Aoki M, et al. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat and rabbit hindl imb ischemia models: Preclinical study for treatment of peripheral arterial disease. Gene Ther. 2001;8(3):181–189. doi: 10.1038/sj.gt.3301379. [DOI] [PubMed] [Google Scholar]
- 45.de Vries VC, Pino-Lagos K, Nowak EC, et al. Mast cells condition dendritic cells to mediate allograft tolerance. Immunity. 2011;35(4):550–561. doi: 10.1016/j.immuni.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gan PY, Summers SA, Ooi JD, et al. Mast cells contribute to peripheral tolerance and attenuate autoimmune vasculitis. J Am Soc Nephrol. 2012;23(12):1955–1966. doi: 10.1681/ASN.2012060572. [DOI] [PMC free article] [PubMed] [Google Scholar]