Abstract
Campylobacter jejuni and C. coli are the main causes of gastrointestinal diseases in humans even in industrialized countries affecting public health. The aim of the current study was to evaluate the occurrence and antibiotic resistance of C. jejuni and C. coli in chicken meat, beef, mutton and water buffalo meat slaughtered in Ahvaz city, Iran. A total of 380 samples including chicken meat from industrial abattoirs (n = 150), chicken meat from traditional abattoirs (n = 50), fresh packed chicken meat from local markets (n = 30) and beef, mutton and water buffalo meat from industrial abattoirs (50 samples for each meat) in Ahvaz,were collected and tested for the presence of Campylobacter spp. The procedure was one-step enrichment in Preston enrichment broth followed by plating on supplemented blood agar for 24 hr under microaerophilic conditions at 42 ˚C. Suspected colonies were tested by polymerase chain reaction assay and susceptibility of the confirmed isolates to various antibiotics was investigated by the Kirby-Bauer disk diffusion method. Overall, 32 samples (8.40%) were contaminated with Campylobacter spp. Mutton was the most contaminated meat (24%), while fresh packed chicken meat were not contaminated. Among the 32 isolates, 40.60%, 34.40%, 21.90%, and 15.60% were resistant to tetracycline, ciprofloxacin, ampicillin, and streptomycin, respectively. Moreover, a high number of multi-antibiotic resistant Campylobacter spp. was determined. Since foods of animal origin are the most sources of Campylobacter infection, the presence of resistant strains to antibiotics is a potential risk to public health.
Key Words: Antibiotic resistance, Beef, Campylobacter, Chicken, Mutton, Water buffalo
Introduction
Thermophilic Campylobacters such as Campylobacter jejuni and Campylobacter coli are one of the main causes of gastrointestinal diseases in humans even in industrialized countries affecting public health.1, 2 The most predominant clinical symptoms of campylobacteriosis in humans are mild to severe bloody diarrhea, abdominal pain and sometimes fever. Most of patients recover after a week, but some cases may require treatment with antibiotics. Arthritis, meningitis, myocarditis, urinary tract infection, Guillain-Barre syndrome, and Miller Fisher syndrome are other complications in the acute phase of the disease.3,4 The low infective dose of Campylobacter cells means that even very small numbers of Campylobacter spp. in food samples can be a potential hazard to human health.5 Antibiotic therapy in animals and humans for various purposes has led to the increased bacterial resistance to many antibiotics. Antimicrobial-resistant strains could be transmitted to humans by the consumption of contaminated food and nowadays it has been a significant public health concern.6 Although poultry have been introduced as the main source of campylobacteriosis in humans, there is evidence suggesting that other animals such as cattle and sheep frequently carry C. jejuni and C. coli in their intestine.7-9 Meat may be contaminated by feces during the slaughtering process and then, consumption of the contaminated meat may pose a risk of Campylobacter transmission.10,11 Monitoring animal meats for detecting antibiotics resistant foodborne pathogens is necessary.
Isolation of Campylobacter spp. from meat samples in various provinces such as Khorasan in north,12,13 Fars in south,14 Tehran in middle-north15,16 and Kerman in central17 of Iran has been reported during the last decade. There is a little information available about Campylobacter contamination of raw meats in Ahvaz located in south-west of Iran. The aim of the current study was to investigate the presence and antimicrobial susceptibility of C. jejuni and C. coli in chicken meat, beef, mutton, and water buffalo meat slaughtered in Ahvaz, Iran.
Materials and Methods
Sampling. In this cross-sectional survey during a six month period from January to July 2016, a total of 380 samples including chicken meat from industrial abattoirs (n = 150), chicken meat from traditional abattoirs (n = 50), fresh packed chicken meat from local markets (n = 30) and beef, mutton and water buffalo meat from industrial abattoirs (50 samples for each meat) were collected. In abattoir samples were taken after washing the carcasses and before transferring to the retailers. For Sampling, 10 g meat was taken from the surface section of flank and chuck and placed in a sterile falcon tube containing 30 mL buffered peptone water. Falcon tubes were well shaken and transported to the laboratory in a cooler with an ice pack in less than 2 hr.18,19
Isolation and Identification of Campylobacter spp. In the laboratory, tubes were centrifuged at 4000 rpm for 5 min. The supernatant was discarded and the pellet was dissolved in 30 mL Preston enrichment broth base (Himedia, Mumbai, India) supplemented with 5% defibrinated horse blood and an antibiotic vial (FD042; Himedia). Tubes were incubated under microaerophilic conditions at a temperature of 42.00 ± 0.50 ˚C for 24 hr. Then, 0.10 mL of the cultures were streaked onto individual selective agar plates (blood agar base supplemented with FD 006; Himedia) and incubated for 48 hr at 42 ˚C under the same conditions. Suspected colonies (flat, non-hemolytic, gray and circular) were subjected to gram staining, catalase, oxidase, nitrate reduction, and nalidixic acid resistance tests. Positive colonies were tested by polymerase chain reaction (PCR) assay for final confirmation.20
Polymerase chain reaction procedures. Pre-sumptive colonies in two steps were subjected to the PCR assay. The first step was to confirm Campylobacter genus and the second one was to identify C. jejuni and C. coli species. Template DNA was obtained by boiling method21 from a pure culture of the suspected isolates. Briefly, the bacterial culture was centrifuged and the pellet was re-suspended in 1 mL of deionized water. The sample was boiled at 100 ˚C for 10 min and the suspension was centrifuged at 14000 rpm for 10 min. The supernatant was used as a PCR template. According to Denis et al.,22 three genes were selected for the identification of Campylobacter genus, C. jejuni and C. coli (Table 1).
Table 1.
List of target genes, the sequence of primers and product size (bp)
| Specificity | Gene | Size (bp) | Primer sequence |
|---|---|---|---|
| Campylobacter genus | 16S rRNA | 857 | Forward: ATC TAA TGG CTT AAC CAT TAA AC Reverse: GGA CGG TAA CTA GTT TAG TAT T |
| C. jejuni | mapA | 589 | Forward: CTA TTT TAT TTT TGA GTG CTT GTG Reverse: GCT TTA TTT GCC ATT TGT TTT ATT A |
| C. coli | ceuE | 462 | Forward: AAT TGA AAA TTG CTC CAA CTA TG Reverse: TGA TTT TAT TAT TTG TAG CAG CG |
Each PCR tube contained 25 μL of reaction mixture consisting of 20.00 μL master mix (CinnaGen, Tehran, Iran) and 5.00 μL of template DNA. The cycling conditions used in the thermal cycler (Bioer, Hercules, China) were as follow: the first denaturation at 95 ˚C (10 min, 1 cycle), denaturation at 95 ˚C for 30 sec, annealing at 59 ˚C for 90 sec and extension at 72 ˚C for 1 min. After 35 cycles, a final cycle comprised a 10 min extension step at 72 ˚C. The amplified PCR products were detected by agarose gel (1.20%) electrophoresis (Paya Pajoohesh Pars, Tehran, Iran), stained with 1.00% solution of ethidium bromide and visualized under ultraviolet light illumination (Kiagen, Tehran, Iran). Standard and confirmed strains of C. coli and C. jejuni (kindly provided by the University of Shiraz) were used as positive controls and deionized water was used as a negative control.
Antibiotic susceptibility testing. According to the method of Clinical and Laboratory Standards Institute,23 antibiotic susceptibility tests were carried out using the Kirby-Bauer disk diffusion method. The tested antibiotic (Padtan Teb, Tehran, Iran) were ampicillin (10 mg), erythromycin (15 mg), ciprofloxacin (15 mg), tetracycline (15 mg), gentamicin (10 mg), chloramphenicol (30 mg), streptomycin (30 mg) and enrofloxacin (10 mg). A swab was taken from each bacterial suspension (almost equal to 1 × 108 CFU mL-1 or 0.50 McFarland standards) and stroked thoroughly on Mueller-Hinton agar (Mast Diagnostics, Merseyside, UK) with 5.00% defibrinated sheep blood agar,24 and then antibiotic discs (Padtan Teb, Tehran, Iran) were placed on the agar. After incubation under a microaerophilic condition at 42 ˚C for 48 hr,25 the diameter of the inhibition zone was measured for each antibiotic. Then, the isolates were classified as resistant, intermediate (reduced susceptibility) or sensitive.
Results
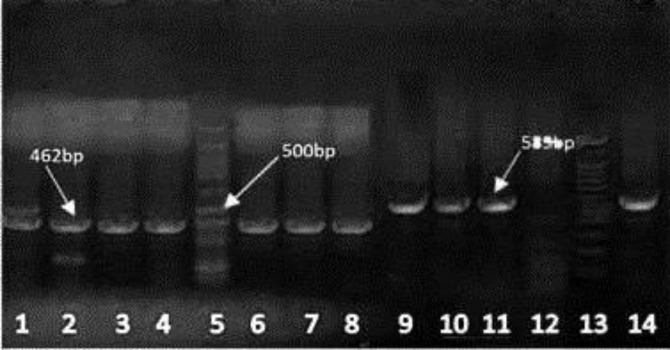
Among 380 meat samples, nine chicken meat (2.40%), seven beef (1.80%), four buffalo meat (1.10%) and 12 mutton (3.10%) samples were found to be contaminated with Campylobacter spp. The predominant species in samples were C. jejuni (81.30%) followed by C. coli (18.70%), (Fig. 1).
Fig.1.
Polymerase chain reaction results for C. jejuni and C. coli detection on gel electrophoresis. Lane 1: Positive control 462 bp (C. coli); Lanes 2-4: C. coli isolates; Lane 5: Ladder 100 bp plus; Lanes 6-8: C. coli isolates; Lanes 9-11: C. jejuni isolates; Lane 12: Negative control; Lane 13: Ladder 100 bp plus; Lane14: Positive control 589 bp (C. jejuni)
Table 2 shows the prevalence of Campylobacter spp. isolated from chicken meat, beef, mutton and buffalo meat in Ahvaz, Iran. As can be seen in Table 2, the highest prevalence (24.00%) of Campylobacter spp. was found in mutton. Chicken meat obtained from traditional abattoirs and beef had the second and third prevalence values among samples, respectively. Interestingly, the fresh packed chicken meat showed no contamination to Campylobacter spp.
Table 2.
Prevalence of Campylobacter spp. isolated from chicken meat, beef, mutton and buffalo meat in Ahvaz, Iran
| Meat sample | Number of samples |
Campylobacter
spp.
Positive (%) |
C. jejuni
(%) |
C. coli
(%) |
|---|---|---|---|---|
| Chicken meat from industrial abattoirs | 150 | 1(0.60) | 0(0) | 1(100) |
| Chicken meat from traditional abattoirs | 50 | 8(16.00) | 3(37.50) | 5(62.50) |
| Fresh packed chicken meat | 30 | 0 (0) | 0 (0) | 0 (0) |
| Beef | 50 | 7 (14.00) | 7(100) | 0 (0) |
| Mutton | 50 | 12 (24.00) | 12(100) | 0 (0) |
| Water buffalo meat | 50 | 4 (8.00) | 4(100) | 0 (0) |
| Total | 380 | 32 (8.40) | 26(81.30) | 6(18.70) |
Antibiotic susceptibility tests were carried out on all Campylobacter spp. isolated in this study. The susceptibility, intermediate and resistance profiles of the isolates to eight tested antibiotics are shown in Table 3.
Table 3.
The resistance profile of Campylobacter spp. isolated from various meats
| Antibiotic |
Chicken meat
|
Beef
|
Mutton
|
Buffalo meat
|
Total number of resistant isolates (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| profile | S | I | R | S | I | R | S | I | R | S | I | R | |
| Ampicillin | 6 | 1 | 2 | 5 | - | 2 | 10 | - | 2 | 3 | - | 1 | 7 (21.90) |
| Streptomycin | 2 | 2 | 5 | 6 | 1 | - | 12 | - | - | 4 | - | - | 5 (15.60) |
| Gentamicin | 9 | - | - | 7 | - | - | 12 | - | - | 4 | - | - | 0 (0) |
| Erythromycin | 9 | - | - | 7 | - | - | 9 | 1 | 2 | 4 | - | - | 2 (6.20) |
| Ciprofloxacin | 8 | - | 1 | 3 | - | 4 | 6 | 2 | 4 | 2 | - | 2 | 11 (34.40) |
| Tetracycline | 5 | - | 4 | 4 | - | 3 | 7 | - | 5 | 3 | - | 1 | 13 (40.60) |
| Chloramphenicol | 7 | 1 | 1 | 7 | - | - | 10 | - | 2 | 4 | - | - | 3 (9.40) |
| Enrofloxacin | 8 | 1 | - | 6 | - | 1 | 9 | 2 | 1 | 4 | - | - | 2 (6.20) |
S: Susceptible; I: Intermediate resistant; R: Resistant.
Overall, 40.60% were resistant to tetracycline, 34.40% were resistant to ciprofloxacin and 21.90% were resistant to ampicillin. Also, 15.60% of isolates were resistant to streptomycin. Surprisingly, no resistance to gentamicin was observed in the isolates.
Discussion
Campylobacter spp. is mainly transmitted to humans through contaminated foods of animal origin.26 Contamination of food to thermophilic Campylobacter spp. could occur during production, processing, distribution, and preparation in the food supply chain.26,27 Consumption of undercooked meat products contaminated with these pathogens has been identified as a risk factor for human campylobacteriosis.
In this study, 380 raw samples of chicken meat, beef, mutton and buffalo meat slaughtered in industrial and traditional abattoirs were examined. Overall, 8.40% of all the meat samples were contaminated with C. coli and C. jejuni. The results of this study showed that mutton (24.00%) and chicken carcasses slaughtered in traditional abattoirs (16.00%) are important sources for Campylobacter infections in Ahvaz, Iran. The prevalence of Campylobacter spp. in the beef, buffalo meat and chicken carcasses slaughtered in industrial abattoirs as other sources were 14.00%, 8.00%, and 0.60%, respectively.
To the best of our knowledge, there are several studies regarding isolation of Campylobacter spp. from various meat samples in Iran and in the world, but few reports could be found on the occurrence of Campylobacter spp. in the water buffalo meat. According to Andrzejewska et al. 41.60% of the poultry meats available in retail stores in the northern part of Poland were contaminated with Campylobacter spp.28 In another study in this country, thermophilic Campylobacter species were detected in poultry (49.30%), pork (10.60%) and beef (10.10%).29 In another study in northwestern Greece, Campylobacter spp. were isolated from 91 (29.40%) of the free-range chicken meat samples and from 106 (28.70%) of the conventional farming chicken meat samples,30 while only one sample (0.60%) was contaminated with C. jejuni in 175 bovine ground meat samples in Finland.31 It could be concluded that raw retail meats are potential vehicles for transmitting campylobacteriosis, however, the prevalence of these pathogens is markedly different in various meats.
In Iran, Campylobacter spp. were isolated from 110 (44.00%) of chicken meat samples and from 11 (5.50%) of beef samples,16 which are much higher and lower than our results, respectively. The contamination rate of mutton samples observed in our study (24.00%) was also much higher than the results reported by Rahimi et al. (12.00%) in central of Iran.25
Comparison of data also indicated that the contamination rate in buffalo meat (8.00%) in our work was much lower than the previous data (21.40%) in Iran.32 The difference in data suggests that time, season, place of sampling methods, and laboratory techniques used in studies may affect the results of prevalence rate. It seems that humidity and precipitation were not important predictors for Campylobacter prevalence; meanwhile a strong relationship between temperature and Campylo-bacter infections has been demonstrated.33
In our study, C. jejuni was the most prevalent Campylobacter spp. recovered from the samples which is in agreement with reports of Dabiri et al. in Iran,16 Guyard-Nicodème et al. in France34 and Han et al. in China.35
It is noticeable that slaughtering, evisceration, and skinning of large animals in Ahvaz abattoir are manual and cross-contamination during these procedures could happen. Comparison of the poultry slaughtered in industrial abattoirs and those that have been slaughtered traditionally in our study showed that slaughterhouse sanitation process could be effective in the elimination or reduction of Campylobacter in poultry meat.
The results of antimicrobial susceptibility testing in the present study showed that 41.70% (5/12) and 33.30% (4/12) of strains isolated from mutton were resistant to tetracycline and ciprofloxacin, respectively. Surprisingly, 40.60% and 34.40% of all Campylobacter isolates were resistant to these antibiotics. All isolates were sensitive to gentamicin which is in agreement with previous reports.36,37 On the contrary of results reported by Van Looveren et al. and Ge et al. a low resistance to erythromycin and enrofloxacin (6.20%) was displayed in our study.38,39
It is noticeable that we found a high number of multi-antibiotic-resistant Campylobacter spp. in different meats, mostly in mutton which has the highest interest for consumption in Iran. The existence of such strains in meat has important public health and health promotion policy implications. It is recommended that in patients with campylobacteriosis, especially in immunosuppressive cases or for those cases with severe or prolonged symptoms, an antibiotic susceptibility testing of Campylobacter can be performed and appropriate antibiotics be prescribed.
The presence of resistant strains to antibiotics in meat and other foods should be taken seriously and hygienic measures are necessary to be taken in this regard. Antibiotics prescription in livestock and poultry under the supervision of a veterinarian, considering mandatory antibiotic withdrawal times before slaughtering, application of a fully sanitized procedure during the slaughtering, permanent microbiological monitoring in abattoirs and carcasses, inhibiting the activity of traditional slaughterhouses, sanitation education of the public in abattoirs, retailers, restaurants and home environments and fully cooking of raw meat can be useful in reducing Campylobacter infection risk.
Acknowledgments
We would like to express our appreciation to the Research Council of Shahid Chamran University of Ahvaz, Ahvaz, Iran for the financial support.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Friis L, Pin C, Pearson B, et al. In vitro cell culture methods for investigating Campylobacter invasion mechanisms. J Microbiol Methds. 2005;61(2):145–160. doi: 10.1016/j.mimet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Macé S, Haddad N, Zagorec M, et al. Influence of measurement and control of microaerobic gaseous atmospheres in methods for Campylobacter growth studies. Food Microbiol. 2015;52:169–176. doi: 10.1016/j.fm.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Brooks GF, Caroll KC, Butel JS, et al. Jawetz, Melnick, & Adelberg's medical microbiology. 25th ed. Columbus, USA: McGraw-Hill Medical; 2006. p. 342. [Google Scholar]
- 4.Soltan Dallal M, Sanaei M, Taremi M, et al. Prevalence and antimicrobial resistance pattern of thermophilic Campylobacter spp (jejuni and coli) isolated from beef and raw chicken in Tehran. J Zanjan Univ Med Sci. 2009;17(68):85–92. [Google Scholar]
- 5.Liu G, Han Y, Li X, et al. Applicability of a rapid method based on immunomagnetic capture-fluorescent PCR assay for Campylobacter jejuni. Food Control. 2006;17(7):527–532. [Google Scholar]
- 6.Founou LL, Founou RC, Essack SY. Antibiotic resistance in the food chain: A developing country-perspective. Front Microbiol. 2016;7:1881. doi: 10.3389/fmicb.2016.01881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoshbakht R, Tabatabaei M, Hoseinzadeh S, et al. Prevalence and antibiotic resistance profile of thermophilic Campylobacter spp of slaughtered cattle and sheep in Shiraz, Iran. Vet Res Forum. 2016;7(3):241–246. [PMC free article] [PubMed] [Google Scholar]
- 8.Blaser MJ, LaForce FM, Wilson NA, et al. Reservoirs for human campylobacteriosis. J Infect Dis. 1980;141(5):665–669. doi: 10.1093/infdis/141.5.665. [DOI] [PubMed] [Google Scholar]
- 9.Stanley K, Jones K. Cattle and sheep farms as reservoirs of Campylobacter. J Appl Microbiol. 2003;94(s1):104–113. doi: 10.1046/j.1365-2672.94.s1.12.x. [DOI] [PubMed] [Google Scholar]
- 10.Hannu T, Mattila L, Rautelin H, et al. Three cases of cardiac complications associated with Campylobacter jejuni infection and review of the literature. Eur J Cli Microbiol Infect Dis. 2005;24(9):619–622. doi: 10.1007/s10096-005-0001-2. [DOI] [PubMed] [Google Scholar]
- 11.Man SM. The clinical importance of emerging Campylobacter species. Nature Rev Gastroenterol Hepatol. 2011;8(12):669–685. doi: 10.1038/nrgastro.2011.191. [DOI] [PubMed] [Google Scholar]
- 12.Jamshidi A, Bassami MR, Farkhondeh T. Isolation and identification of Campylobacter spp and Campylobactercoli from poultry carcasses by conventional culture method and multiplex PCR in Mashhad, Iran. Iranian J Vet Res. 2008;9(2):132–137. [Google Scholar]
- 13.Zendehbad B, Arian AA, Alipour A. Identification and antimicrobial resistance of Campylobacter species isolated from poultry meat in Khorasan province, Iran. Food Control. 2013;32(2):724–727. [Google Scholar]
- 14.Ansari-Lari M, Hosseinzadeh S, Shekarforoush S, et al. Prevalence and risk factors associated with Campylobacter infections in broiler flocks in Shiraz, southern Iran. Int J Food Microbiol. 2011;144(3):475–479. doi: 10.1016/j.ijfoodmicro.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Bakhshi B, Kalantar M, Rastegar-Lari A, et al. PFGE genotyping and molecular characterization of Campylobacter spp isolated from chicken meat. Iran J Vet Res. 2016;17(3):177–183. [PMC free article] [PubMed] [Google Scholar]
- 16.Dabiri H, Aghamohammad S, Goudarzi H, et al. Prevalence and antibiotic susceptibility of Campylobacter species isolated from chicken and beef meat. Int J Enteric Pathog. 2014;2(2):e17087. [Google Scholar]
- 17.Ashrafganjooyi SB, Saedadlei N. Isolation and determine antibiotic susceptibility of Campylobacter jejuni in poultry feces in Kerman. Iran J Medic Microbiol. 2016;9(4):95–98. [Google Scholar]
- 18.Hakkinen M, Heiska H, Hänninen ML. Prevalence of Campylobacter spp in cattle in Finland and antimicrobial susceptibilities of bovine Campylobacter jejuni strains. Appl Environ Microbiol. 2007;73(10):3232–3238. doi: 10.1128/AEM.02579-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granić K, Krčar D, Uhitil S, et al. Determination of Campylobacter spp in poultry slaughterhouses and poultry meat. Vet Arhiv. 2009;79(5):491–497. [Google Scholar]
- 20.Whyte P, McGill K, Cowley D, et al. Occurrence of Campylobacter in retail foods in Ireland. Int J Food Microbiol. 2004;95: 111–118. doi: 10.1016/j.ijfoodmicro.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Adwan K. Fast DNA isolation and PCR protocols for detection of methicillin-resistant staphylococci. Folia Microbiol. 2014;59(1):5–8. doi: 10.1007/s12223-013-0259-1. [DOI] [PubMed] [Google Scholar]
- 22.Denis M, Refrégier‐Petton J, Laisney MJ, et al. Campylobacter contamination in French chicken production from farm to consumers Use of a PCR assay for detection and identification of Campylobacter jejuni and Camp. coli. J Appl Microbiol. 2001;91(2):255–267. doi: 10.1046/j.1365-2672.2001.01380.x. [DOI] [PubMed] [Google Scholar]
- 23.Wikler MA. Wayne, USA: Clinical and Laboratory Standards Institute ; 2006. Performance standards for antimicrobial susceptibility testing: 16th informational supplement; pp. 32–38. [Google Scholar]
- 24.Ge B, Wang F, Sjölund-Karlsson M, et al. Antimicrobial resistance in Campylobacter: susceptibility testing methods and resistance trends. J Microbiol Methods. 2013;95(1):57–67. doi: 10.1016/j.mimet.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Rahimi E, Ameri M, Kazemeini HR. Prevalence and antimicrobial resistance of Campylobacter species isolated from raw camel, beef, lamb, and goat meat in Iran. Foodborne Path Dis. 2010;7(4):443–447. doi: 10.1089/fpd.2009.0421. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs-Reitsma W, Lyhs U, Wagenaar J. Campylobacter in the food supply. In: In: Nachamkin I, Szymanski CM, Blaser MJ., editors. Campylobacter. 3rd ed. Washington, USA: American Society of Microbiology ; 2008. pp. 627–644. [Google Scholar]
- 27.Kemp R, Leatherbarrow A, Williams N, et al. Prevalence and genetic diversity of Campylobacter spp in environmental water samples from a 100-square-kilometer predominantly dairy farming area. Appl Environ Microbiol. 2005;71(4):1876–1882. doi: 10.1128/AEM.71.4.1876-1882.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrzejewska M, Szczepańska B, Śpica D, et al. Trends in the occurrence and characteristics of Campylobacter jejuni and Campylobacter coli isolates from poultry meat in Northern Poland. Food Control. 2015;51:190–194. [Google Scholar]
- 29.Korsak D, Maćkiw E, Rożynek E, et al. Prevalence of Campylobacter spp in retail chicken, turkey, pork, and beef meat in Poland between 2009 and 2013. J Food Prot. 2015;78(5):1024–1028. doi: 10.4315/0362-028X.JFP-14-353. [DOI] [PubMed] [Google Scholar]
- 30.Economou V, Zisides N, Gousia P, et al. Prevalence and antimicrobial profile of Campylobacter isolates from free-range and conventional farming chicken meat during a 6-year survey. Food Control. 2015;56:161–168. [Google Scholar]
- 31.Llarena AK, Sivonen K, Hänninen ML. Campylobacter jejuni prevalence and hygienic quality of retail bovine ground meat in Finland. Let Appl Microbiol. 2014;58(5):408–413. doi: 10.1111/lam.12206. [DOI] [PubMed] [Google Scholar]
- 32.Rahimi E, Ameri M, Alimoradi M, et al. Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from raw camel, beef, and water buffalo meat in Iran. Comp Clin Pathol. 2013;22(3):467–473. [Google Scholar]
- 33.Patrick , M E, Christiansen LE, Wainø M, et al. Effects of climate on incidence of Campylobacter spp in humans and prevalence in broiler flocks in Denmark. Appl Environ Microbiol. 2004;70(12):7474–7480. doi: 10.1128/AEM.70.12.7474-7480.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guyard-Nicodème M, Rivoal K, Houard E, et al. Prevalence and characterization of Campylobacter jejuni from chicken meat sold in French retail outlets. Int J Food Microbiol. 2015;203:8–14. doi: 10.1016/j.ijfoodmicro.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Han X, Zhu D, Lai H, et al. Prevalence, antimicrobial resistance profiling and genetic diversity of Campylobacter jejuni and Campylobacter coli isolated from broilers at slaughter in China. Food Control. 2016;69:160–170. [Google Scholar]
- 36.Little CL, Richardson JF, Owen RJ, et al. Campylobacter and Salmonella in raw red meats in the United Kingdom: prevalence, characterization and antimicrobial resistance pattern, 2003–2005. Food Microbiol. 2008;25(3):538–543. doi: 10.1016/j.fm.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Taremi M, Dallal MMS, Gachkar L, et al. Prevalence and antimicrobial resistance of Campylobacter isolated from retail raw chicken and beef meat, Tehran, Iran. Int J Food Microbiol. 2006;108(3):401–403. doi: 10.1016/j.ijfoodmicro.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 38.Van Looveren M, Daube G, De Zutter L, et al. Antimicrobial susceptibilities of Campylobacter strains isolated from food animals in Belgium. J Antimicrob Chemother. 2001;48(2):235–240. doi: 10.1093/jac/48.2.235. [DOI] [PubMed] [Google Scholar]
- 39.Ge B, White DG, McDermott PF, et al. Antimicrobial-resistant Campylobacter species from retail raw meats. Appl Environ Microbiol. 2003;69(5):3005–3007. doi: 10.1128/AEM.69.5.3005-3007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]