Abstract
Purpose of review
Sarcoidosis is a complex disease with many faces, and the clinical manifestation and course of neurosarcoidosis are particularly variable. Although neurosarcoidosis occurs in up to 10% of sarcoidosis patients, it can lead to significant morbidity and some mortality.
Recent findings
Three criteria are usually required for a diagnosis of (neuro)sarcoidosis: clinical and radiologic manifestations, noncaseating granulomas, and no evidence of alternative disease. Recent guidelines have helped to clarify criteria for diagnosing neurosarcoidosis. No firm guidelines exist on whether, when, and how treatment should be started. Treatment depends on the presentation and distribution, extensiveness, and severity of neurosarcoidosis. As regards evidence-based treatment, only a few randomized controlled trials have been done. Hence, several aspects of (neuro)sarcoidosis management are not fully addressed by the current literature.
Summary
Significant advances have been made in the potential and accuracy of diagnostics for neurosarcoidosis. Treatment should be approached within the context of the patient's anticipated clinical course, avoidance of adverse drug effects, and, if necessary, from the perspective of the comprehensive management of a chronic disease. A multidisciplinary approach to the management of sarcoidosis is strongly recommended.
Keywords: diagnostic procedure, infliximab, multidisciplinary management, neurosarcoidosis, sarcoidosis, treatment
INTRODUCTION
Sarcoidosis is a multisystem inflammatory disorder characterized by the formation of noncaseating granulomas in various organ systems, mainly the lungs and the lymphatic system. Although the pathogenesis of sarcoidosis has not been fully elucidated, environmental and genetic factors may contribute substantially to its pathogenesis, leading to an exaggerated granulomatous reaction. Sarcoidosis occurs throughout the world, affecting all races and ages [1]. The clinical manifestation, natural history, and prognosis of sarcoidosis are highly variable. It can occur at almost any site in the body and most patients report disabling impairments, especially those with chronic disease [2]. Neurosarcoidosis occurs in 5–10% of patients with sarcoidosis, rates which are not influenced by race or sex [3–5]. The clinical manifestations of neurosarcoidosis are also heterogeneous, as granulomas can affect any part of the nervous system, such as the meninges, brain, cranial nerves, spinal cord, and peripheral nerves [6,7▪▪]. Cranial neuropathy is the most common manifestation, with facial nerves being most commonly affected, and is seen in 50–70% of cases of neurosarcoidosis [6,7▪▪].
The epidemiological assessment of sarcoidosis and its manifestations is problematic, because of the lack of consistent case definitions, lack of sensitivity and specificity of diagnostic tests, variable diagnostic intensity, and variable diagnostic methods. Here we discuss the diagnostic approach and treatment of neurosarcoidosis.
Box 1.
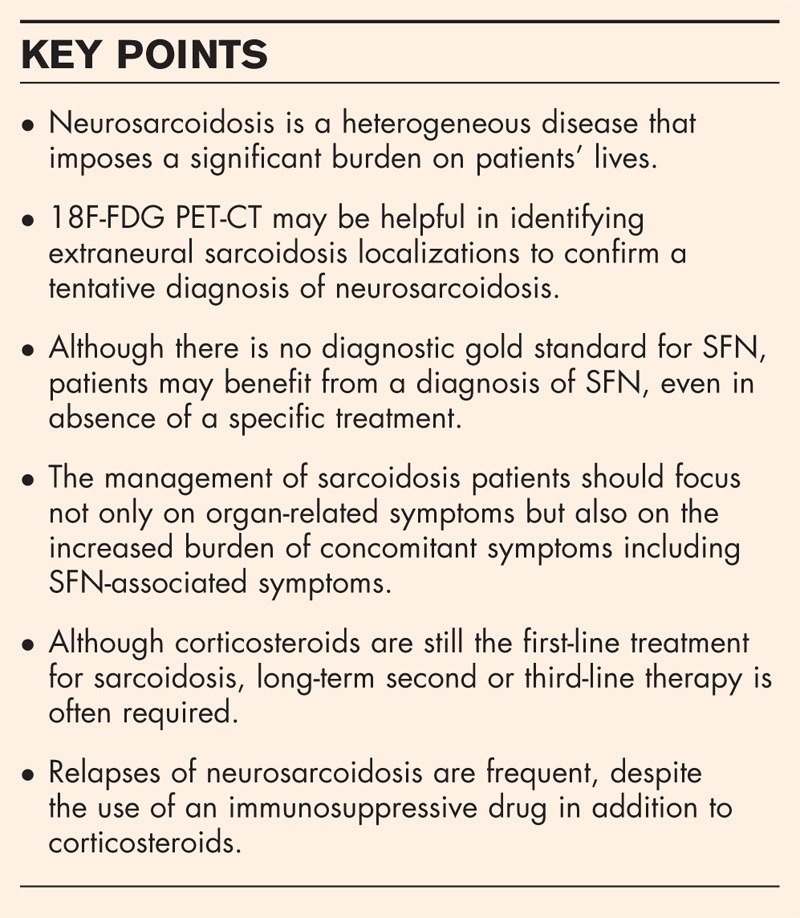
no caption available
DIAGNOSTIC APPROACH TO NEUROSARCOIDOSIS
Although the diagnosis of sarcoidosis is a complex procedure and can be difficult for clinicians, the potential and accuracy of diagnostics for sarcoidosis, especially to assess organ involvement, have been improved in recent decades. However, there is still no single diagnostic test for sarcoidosis. The finding of granuloma in a single organ is not specific for this disease, as many other conditions can cause granulomas. According to the joint statement of the American Thoracic Society, the European Respiratory Society, and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG), three criteria are usually required for a diagnosis of sarcoidosis: typical clinical and radiologic manifestations, noncaseating granulomas, and no evidence of alternative disease [8]. In case of suspected sarcoidosis, the diagnostic procedures aim to accomplish the following goals: provide histological confirmation of the disease; evaluate the extent and severity of organ involvement; assess whether the disease is stable or likely to progress; and determine if the patient will benefit from treatment.
The presence of specific clinical features, especially multiorgan involvement, can enhance the diagnostic certainty. The WASOG recently developed criteria for categorizing sarcoidosis organ involvement as ‘highly probable,’ ‘at least probable,’ or ‘possible.’ The WASOG instrument provides a structured system for identifying the clinico-radiologic findings in patients with sarcoidosis and may standardize organ involvement reporting for patients with sarcoidosis [9]. Bickett et al.[10] developed the Sarcoidosis Diagnostic Score to summarize the clinical features of patients with possible sarcoidosis. For each organ, it allocates points if the organ has a positive biopsy (5 points), if one or more features are consistent with highly probable organ involvement (3 points), or at least probable organ involvement (2 points). The resulting Sarcoidosis Diagnostic Score was found to have high sensitivity and specificity [10]. This score can be used to support the clinical diagnosis of sarcoidosis referred to in Fig. 1. Confirmation by other studies may establish the value of this scoring system.
FIGURE 1.
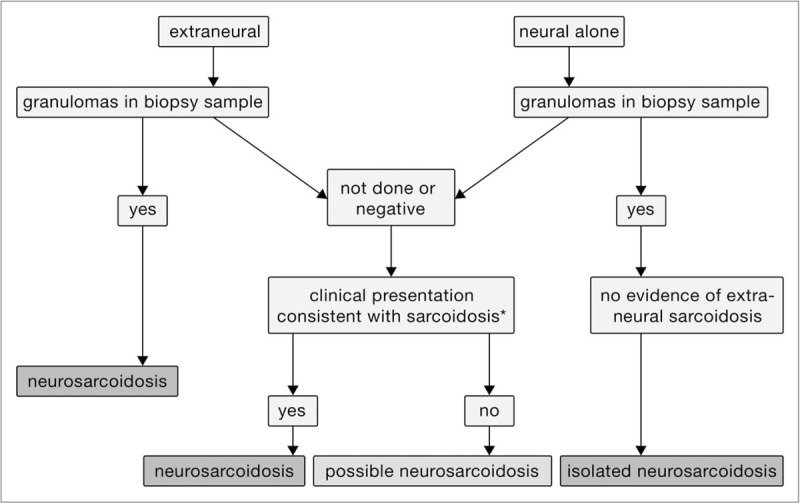
Approach to diagnosis of neurosarcoidosis.
Patients with suspected neurosarcoidosis require a careful assessment of the systemic manifestations and neurologic evaluation. As sarcoidosis is a multiorgan disease, a diagnosis of neurosarcoidosis does not rest merely on neurologic features. Table 1 lists the routine testing recommended for suspected neurosarcoidosis patients as part of their initial evaluation; chest imaging is part of this evaluation. The chest radiographic finding of bilateral hilar adenopathy with right paratracheal involvement makes sarcoidosis highly probable [11,12]. Recently, it was shown that high-resolution computed tomography (HRCT) was superior, and therefore preferable, to chest radiography for evaluating the features, pattern and distribution of the parenchymal lesions and mediastinal lymph nodes, as well as for assessing the stage and activity of the disease and aiding the detection of subtle parenchymal lesions which are liable to be missed on conventional imaging [13]. Characteristic findings are nodular infiltrates with bronchovascular and subpleural distribution, thickened interlobular septa, architectural distortion, and conglomerate masses originating from the coalescence of nodules in the perihilar, peribronchovascular, or subpleural regions [14]. Fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) can be useful in cases in which other imaging modalities have been unsuccessful in detecting sarcoidosis activity. FDG PET/CT is mainly used for the detection of extraneural localizations and the identification of extraneural biopsy sites [15–19]. The brain can be included in whole body PET/CT examinations with only marginal increases in examination time and radiation dose (because of the extended field of low-dose CT scanning) [20]. The intense physiologic uptake in the brain limits accurate evaluation of brain lesions. However, the FDG PET findings may provide the first suggestion of a diagnosis of sarcoidosis involving only neurologic symptoms.
Table 1.
Recommended tests for initial evaluation of sarcoidosis
| Type of evaluation |
| History (occupational and environmental exposure, symptoms) |
| Physical examination |
| Chest imaging: HRCT or PET-CT |
| Pulmonary function tests: spirometry and carbon monoxide diffusion capacity (respiratory symptoms) |
| Laboratory diagnostics |
| Peripheral blood counts: white blood cells, red blood cells, platelets |
| Serum chemistries: calcium, liver enzymes, creatinine, blood urea nitrogen, ACE and sIl2R |
| Electrocardiography |
| Routine ophthalmologic examination |
| Tuberculin skin test or Quantiferon Gold |
ACE, angiotensin converting enzyme; HRCT, high-resolution computer tomography; PET-CT, positron emission tomography-computer tomography; sIL2R, soluble interleukin 2 receptor.
Depending on the part of the brain involved, a sarcoidosis lesion can be hypo or hypermetabolic. In contrast, spinal cord lesions appear as hypermetabolic because the basal metabolism of the spinal cord is one-third that of the cerebral gray matter. Its capability to depict metabolic changes also enables FDG PET to be used to monitor therapy before morphologic changes are detected with conventional imaging [21]. Novel PET strategies, to overcome the limitations of FDG, have recently been introduced, including imaging of somatostatin receptors or C-X-C motif chemokine receptor (CXCR4), which are overexpressed on the cell surface of activated macrophages [22,23]. Given the specific nature of their signal, these tracers might be used in cardiac and neurosarcoidosis, as these organs have an intense physiologic uptake of FDG. It might thereby be possible to directly assess the extent of inflammatory activity, localize sites of activity, and monitor response to therapy. Further studies are needed to evaluate this new PET strategy for neurosarcoidosis.
In patients for whom extraneural tissue is needed to identify granulomas, bronchoscopy can provide multiple options for diagnosing sarcoidosis. These include bronchoalveolar lavage, endobronchial biopsy, transbronchial lung biopsy, cryobiopsy, and endobronchial ultrasound fine-needle aspiration. These tests are complementary and the combination of tests is superior to performing any single test [24,25]. Although bronchoscopy is often performed in patients with pulmonary disease, a recent study among patients presenting with manifestations of suspected cardiac sarcoidosis without a prior history of lung disease demonstrated that lung and mediastinal lymph node biopsies confirmed extracardiac sarcoidosis in 58% of patients, and bronchoalveolar lavage cellular analyses were suggestive of extracardiac sarcoidosis in 67% of patients, even those without abnormalities on preliminary HRCT [26]. Hence, the use of bronchoscopy in patients with extrapulmonary disease, even with normal CT scans, can be helpful in diagnosing sarcoidosis [26,27].
Diagnostic criteria for neurologic involvement in sarcoidosis patients have been developed by neurologists and general sarcoidosis experts [7▪▪,9]. The Neurosarcoidosis Consortium Consensus Group, an expert panel of physicians experienced in the management of patients with sarcoidosis and/or neurosarcoidosis, engaged in an iterative process to define neurosarcoidosis, and developed a practical clinical diagnostics approach for patients with suspected neurosarcoidosis. The resulting consensus clinical definition of neurosarcoidosis aimed to enhance the clinical care of patients with suspected neurosarcoidosis and to encourage standardization of research initiatives to address this disease manifestation. The authors identified criteria indicating possible, probable, or definite neurologic involvement (see Table 2).
Table 2.
Diagnostic criteria for neurosarcoidosis (central and peripheral nervous system involvement) [7▪▪]
| Possible |
| The clinical presentation and diagnostic evaluation suggest neurosarcoidosis, as defined by the clinical manifestations and MRI, CSF, and/or EMG/NCS findings typical of granulomatous inflammation of the nervous system and after rigorous exclusion of other causes |
| There is no pathological confirmation of granulomatous disease |
| Probable |
| The clinical presentation and diagnostic evaluation suggest neurosarcoidosis, as defined by the clinical manifestations and MRI, CSF, and/or EMG/NCS findings typical of granulomatous inflammation of the nervous system and after rigorous exclusion of other causes |
| There is pathological confirmation of systemic granulomatous disease consistent with sarcoidosis |
| Definite |
| The clinical presentation and diagnostic evaluation suggest neurosarcoidosis, as defined by the clinical manifestations, MRI, CSF, and/or EMG/NCS findings typical of granulomatous inflammation of the nervous system, after rigorous exclusion of other causes |
| The nervous system abnormality is consistent with neurosarcoidosis |
| Type a. Extraneural sarcoidosis is evident |
| Type b. No extraneural sarcoidosis is evident (isolated neurosarcoidosis) |
CSF, cerebrospinal fluid; EMG, electromyography; MRI, magnetic resonance imaging; NCS, nerve conduction studies.
Gadolinium-enhanced MRI is the modality of choice for the diagnosis of neurosarcoidosis. The abnormalities seen at MRI include periventricular white matter lesions, meningitis or meningoencephalitis, solid parenchymal enhancing lesions, cranial neuritis, and myelopathy [28,29]. As bone sarcoidosis often involves the spine, patients undergoing MRI scanning for back pain or radiculopathy may be found to have an infiltrative lesion [30]. Such an MRI pattern is often confused with malignancy and in some cases a bone biopsy may be required to confirm sarcoidosis [31].
Figure 1 illustrates the approach to diagnosing neurosarcoidosis. In Table 3 the prevalence of various manifestations of neurosarcoidosis including myelitis (see Fig. 2) are summarized. For a patient with neurologic signs and symptoms consistent with neurosarcoidosis, the next step is to check for extraneural disease. If there is evidence of such disease based on a biopsy showing granuloma, the patient most likely has neurosarcoidosis. If the patient has not had a biopsy or the biopsy was negative for granuloma, the extraneural features are then evaluated. If the patient's clinical presentation is consistent with sarcoidosis and other causes have been excluded, the patient is assumed to have neurosarcoidosis. If there are insufficient clinical features to support the diagnosis, the patient is said to have possible neurosarcoidosis. Isolated neurosarcoidosis is diagnosed in a patient who presents with only neural symptoms, a biopsy which demonstrates granulomas, and no clinical features of extraneural sarcoidosis. Patients who present with neurologic disease should always be evaluated for extraneural sarcoidosis.
Table 3.
Summary of the prevalence of various neurosarcoidosis manifestations or site of neurological involvement of neurosarcoidosis and associated comments [7▪▪,32,33]
| Neurosarcoidosis manifestationa | Prevalence | Comments |
| Cranial nerve palsy | 31–55% | Facial and optic nerves are the most commonly affected; uni or bilateral involvement |
| Chronic aseptic meningitis | 16–37% | Subacute or chronic lymphocytic meningitis; dural involvement including pachymeningitis, dural mass mimicking meningioma |
| Spinal cord disease/myelitis | 18–23% | Subpial intramedullary lesions, typically longitudinally extensive; myelitis predilection cervicothoracic |
| Cerebral parenchymal disease | 21% | Small cortical or periventricular white matter lesions; mimicking multiple sclerosis or micro-ischemic lesions, larger solitary aggregates of granulomas can masquerade as neoplasms |
| Neuroendocrine (hypothalamo-pituitary) involvement | 6–9% | Hormonal disturbances including hypothyroidism, hypogonadism, panhypopituitarism, SIADH |
| Hydrocephalus | 9–10% | Communicating and noncommunicating hydrocephalus; combination with leptomeningeal enhancement along the skull base |
| Cerebral infarction | 6% | Stroke can be because of in situ thrombosis, compression of a large vessel by a granulomatous mass, sinovenous thrombosis, and intracerebral hemorrhage |
| Peripheral nervous system | 17% | Large fiber involvement: most commonly axonal distal sensorimotor polyneuropathy or asymmetric polyradiculoneuropathy (nonlength dependent distribution) |
SIADH, syndrome of inappropriate antidiuretic hormone.
aone individual patient can have one or more neurosarcoidosis manifestation(s).
FIGURE 2.
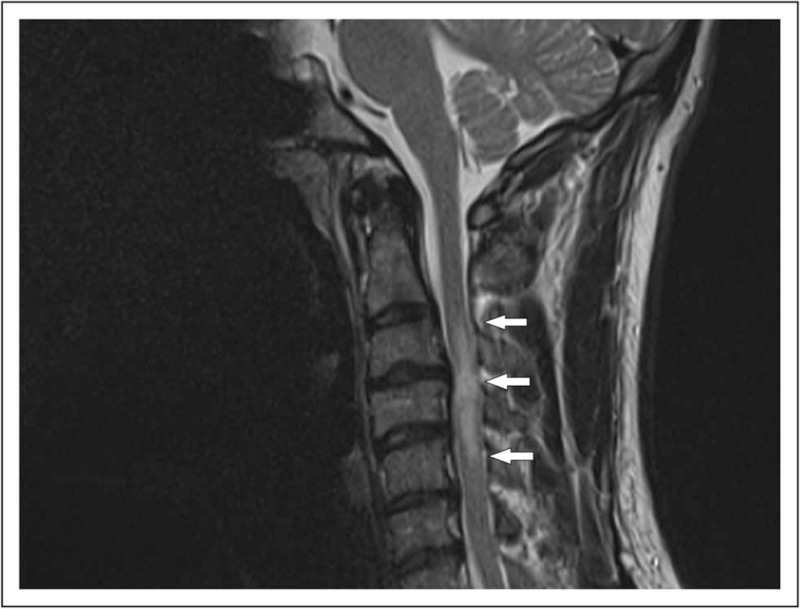
A 36-year-old man, presenting with numbness in both arms and cervical pain. MRI showed a cervical myelitis (C3–C5, arrows). Bilateral mediastinal lymph-adenopathy was seen on a chest X-ray and the diagnosis sarcoidosis was confirmed with an endobronchial ultrasound fine-needle aspiration (EBUS-FNA).
NONORGAN-RELATED SARCOIDOSIS-ASSOCIATED SYMPTOMS
There are several consequences of sarcoidosis, including fatigue, cognitive failure, and small fiber neuropathy (SFN), which are not directly related to granulomatous involvement [2,34].
Sarcoidosis-associated fatigue is reported in more than half of patients [35,36]. It is commonly associated with other nonorgan-related problems, including SFN and cognitive failure [37,38,39▪]. Questionnaires can be helpful in objectifying these complaints [40–42]. A multidisciplinary approach including psychological counseling is recommended [43,44].
SFN, which is found in many conditions [45,46▪], was originally described for sarcoidosis by Hoitsma et al.[47] in 2002. There is no diagnostic gold standard for SFN. Nerve conduction studies are performed as the first diagnostic test to exclude large-fiber disorders. Skin biopsies with quantitative counting of the nerve fibers have been proposed. The fewer the nerve fibers, the greater the likelihood of SFN [45]. However, the disease can be patchy, leading to sampling errors and false negative findings. The disease is also invasive.
An SFN screening tool has been developed for patients with sarcoidosis, called the SFN screening list [42]. This screening tool can distinguish between patients who are very likely to have SFN-associated symptoms and those without such symptoms. A substantial percentage of patients have intermediate scores (between highly likely and unlikely SFN), and these patients require additional tests to confirm the diagnosis of SFN. Despite its limitations, the SFN screening list has proved useful in assessing response to therapy [48].
An interesting new test for SFN is corneal confocal microscopy. Photographs are taken of the cornea and a quantitative assessment of nerve fibers is made. A correlation has been established between the severity of neuropathy and progressive corneal nerve degeneration [49,50]. Unfortunately, this technique is not widely available, and has not yet been validated against other measures of SFN. The changes demonstrated during treatment for SFN did not correlate with other markers of SFN [51].
TREATMENT OF NEUROSARCOIDOSIS
Treatment is almost always warranted in neurosarcoidosis. Treatment strategies are mainly based on expert opinion and small retrospective studies. So far, no randomized controlled trials have been performed [6]. The intensity of treatment depends on the severity of the neurosarcoidosis manifestations. For example, a facial nerve paralysis can most often be treated with only a few weeks of prednisone monotherapy, and rarely recurs [52], whereas in the case of spinal cord disease a second-line (methotrexate (MTX), azathioprine, mycophenolate mofetil) or third-line (tumor necrosis factor (TNF)-α inhibitors; infliximab or adalimumab) agent is initiated earlier on in the treatment [53,54]. Limited data are available on the optimal treatment options for neurosarcoidosis.
Treatment of sarcoidosis has commonly been empirical. However, an increasing number of studies have been published which provide a level of confidence regarding general therapeutic recommendations [55▪▪]. These recommendations have to be modified for neurosarcoidosis. Figure 3 shows a proposed stepwise approach to the treatment of neurosarcoidosis other than that involving the seventh cranial nerve alone.
FIGURE 3.
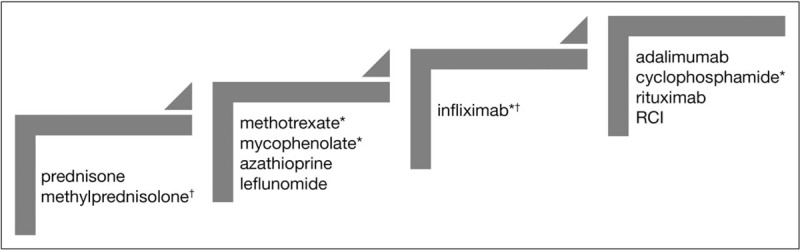
Stepwise treatment for neurosarcoidosis. RCI, repository corticotropin injection. ∗indicates drugs that have been reported specifically for neurosarcoidosis. †indicates therapies which may be used for severe or progressive disease. See text for further details.
Initial therapy consists of glucocorticoids, usually oral prednisone. For a patient with severe disease or disease progressing despite oral therapy, high-dose intravenous methylprednisolone may be useful. High-dose oral prednisone has been shown to be as effective as high-dose intravenous steroids [56]. Most of the prednisone recommendations are based on retrospective studies, and the dosage of prednisone varies. Failure of steroid monotherapy is common, because of severity of the disease or because of the toxicity of the high-dose prednisone used in neurosarcoidosis. In one study, over 80% of patients went on to second and third-line therapy [52].
The next step involves a steroid-sparing or second-line agent. MTX has been the most widely used steroid-sparing agent for sarcoidosis [57]. In one of the few case series examining the use of MTX specifically for neurosarcoidosis, the response rate was 63% [52]. More recently, neurosarcoidosis patients treated with MTX were successfully weaned down to prednisone in half of cases and maintained on that regimen for years [53]. Mycophenolate, another steroid-sparing second-line agent, has a different toxicity profile than methotrexate and may be more rapidly effective in treating neurosarcoidosis [58]. However, in one study, mycophenolate treatment was associated with a significantly higher relapse rate when prednisone was withdrawn [53].
The use of two other cytotoxic agents, azathioprine and leflunomide, is based on studies of nonneurosarcoidosis patients. In one large study, azathioprine was as effective as methotrexate but associated with some more toxicity [59]. Leflunomide has been reported to be an effective alternative to methotrexate with a different toxicity profile [60,61]. However, leflunomide can cause a peripheral neuropathy, which limits its use in neurosarcoidosis [62].
The next step is the use of TNF-α inhibitors. Although not all TNF-α inhibitors have been successful in sarcoidosis, the monoclonal anti-TNFα antibody infliximab was found to be superior to placebo in treating advanced pulmonary sarcoidosis [63,64]. A Delphi study amongst the world's leading sarcoidologists resulted in practical recommendations for the use of TNF-α inhibitors in sarcoidosis, to support clinicians in the management of patients with refractory sarcoidosis [65]. Based on expert experience and recent studies, infliximab is now considered the main third-line treatment option in sarcoidosis. Two recent studies reported on relatively large studies of infliximab for neurosarcoidosis in the United States and France [66▪,67]. In both of these series, response to treatment was seen even when other therapies had failed. Unfortunately, infections and other toxicities were encountered in a significant number of cases [66▪], although these were similar to those for second-line treatment options [59]. Treatment withdrawal was associated with a high rate of relapse of the disease [66▪,67]. Treatment with infliximab is expensive, creating a barrier limiting universal access to this effective therapeutic agent. Recently, biosimilars of infliximab have become available. In view of the working mechanism of the original biological and that of the biosimilars, it is highly likely that the therapeutic effect of both agents is comparable. Hence, inclusion of biosimilars in the treatment regime could lower the costs of TNF-α inhibitors in sarcoidosis [68]. The infliximab biosimilar inflectra proved to be effective in the treatment of refractory sarcoidosis, with a safety profile comparable to that of the reference product infliximab [69,70▪].
For patients for whom infliximab fails because of intolerance or lack of efficacy, or for whom TNF-α inhibitors are contraindicated, several other potential agents have been reported. Adalimumab is usually reserved for patients who develop reactions to infliximab, and the drug has been shown to be effective in that situation [71]. Intravenous cyclophosphamide was reported to be effective in the treatment of refractory neurosarcoidosis, although less so than infliximab [52,72–74]. It may still be an alternative for refractory neurosarcoidosis patients. For patients for whom TNF-α inhibitors are contraindicated, such as those with malignancy or advanced congestive heart failure, rituximab has also been reported to be useful [75,76].
TREATMENT OF SARCOIDOSIS-ASSOCIATED SMALL FIBER NEUROPATHY
Currently, there is no cure for SFN, and only symptomatic relief of complaints is achieved [46▪]. Unfortunately, symptomatic treatment for neuropathic pain often provides only partial relief from pain, without effects on autonomic dysfunction, and is often associated with (sometimes severe) side-effects. Some data suggest some effectiveness of immunoglobulins and TNF-α inhibitors [38,77,78▪]. Whether these expensive treatments should be initiated as a causative treatment for SFN is unclear and is currently being investigated. Cibinetide (ARA290) seems a promising new drug to relieve pain and increase corneal and skin nerve fiber density in sarcoidosis-associated SFN [48,79,80]. However, a recent placebo-controlled trial demonstrated changes in corneal nerve fibers, but not in symptoms [51]. Further studies, including a wider dose range, are needed to clarify the role of this drug.
CONCLUSION
As sarcoidosis is a multiorgan disorder that imposes a burden on patients’ lives, patients may initially present to various organ specialists, depending on the presenting symptoms. Therefore, a multidisciplinary approach is recommended for the management of sarcoidosis, with considerable patient participation focusing on somatic as well as psychosocial aspects of this erratic disorder. Neurosarcoidosis can be extreme burdensome for patients and their families. Recent reports have helped to clarify a diagnostic strategy for this disease entity. Treatment options, especially with infliximab or biosimilars, may prove an effective way to control the disease.
Acknowledgements
The authors wish to thank Marjon Elfferich of the ild care foundation (www.ildcare.nl) for critically reviewing this paper.
Financial support and sponsorship
The preparation of this review was supported by a research grant from the Netherlands Organization for Health Research and Development, ZonMw (project number 842002005) and the ild care foundation:www.ildcare.nl.
R.P.B. has grant support for studies in sarcoidosis from Actelion, Bayer, Celgene, Genentech, Gilead, Mallinckrodt, Novartis, Foundation for Sarcoidosis Research, and National Institutes of Health. He has also been a consultant and speaker for Actelion, Genentech, and Mallinckrodt.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet 2014; 383:1155–1167. [DOI] [PubMed] [Google Scholar]
- 2.Drent M, Strookappe B, Hoitsma E, De Vries J. Consequences of sarcoidosis. Clin Chest Med 2015; 36:727–737. [DOI] [PubMed] [Google Scholar]
- 3.Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001; 164:1885–1889. [DOI] [PubMed] [Google Scholar]
- 4.Judson MA, Boan AD, Lackland DT. The clinical course of sarcoidosis: presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis 2012; 29:119–127. [PubMed] [Google Scholar]
- 5.Pietinalho A, Ohmichi M, Hiraga Y, et al. The mode of presentation of sarcoidosis in Finland and Hokkaido, Japan. A comparative analysis of 571 Finnish and 686 Japanese patients. Sarcoidosis Vasc Diffuse Lung Dis 1996; 13:159–166. [DOI] [PubMed] [Google Scholar]
- 6.Fritz D, Voortman M, van de Beek D, et al. Many faces of neurosarcoidosis: from chronic meningitis to myelopathy. Curr Opin Pulm Med 2017; 23:439–446. [DOI] [PubMed] [Google Scholar]
- 7▪▪.Stern BJ, Royal W, 3rd, Gelfand JM, et al. Definition and consensus diagnostic criteria for neurosarcoidosis: from the Neurosarcoidosis Consortium Consensus Group. JAMA Neurol 2018. [DOI] [PubMed] [Google Scholar]; The Neurosarcoidosis Consortium Consensus Group, an expert panel of physicians experienced in the management of patients with sarcoidosis and/or neurosarcoidosis, engaged in an iterative process to define neurosarcoidosis and developed a practical clinical diagnostic approach to patients with suspected neurosarcoidosis.
- 8.Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis 1999; 16:149–173. [PubMed] [Google Scholar]
- 9.Judson MA, Costabel U, Drent M, et al. The WASOG sarcoidosis organ assessment instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis 2014; 31:19–27. [PubMed] [Google Scholar]
- 10.Bickett AN, Lower EE, Baughman RP. Sarcoidosis diagnostic score: a systematic evaluation to enhance the diagnosis of sarcoidosis. Chest 2018; 154:1052–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winterbauer RH, Belic N, Moores KD. Clinical interpretation of bilateral hilar adenopathy. Ann Intern Med 1973; 78:65–71. [DOI] [PubMed] [Google Scholar]
- 12.Reich JM, Brouns MC, O’Connor EA, Edwards MJ. Mediastinoscopy in patients with presumptive stage I sarcoidosis: a risk/benefit, cost/benefit analysis. Chest 1998; 113:147–153. [DOI] [PubMed] [Google Scholar]
- 13.Dhagat PK, Singh S, Jain M, et al. Thoracic Sarcoidosis: imaging with high resolution computed tomography. J Clin Diagn Res 2017; 11:TC15–TC18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drent M, De Vries J, Lenters M, et al. Sarcoidosis: assessment of disease severity using HRCT. Eur Radiol 2003; 13:2462–2471. [DOI] [PubMed] [Google Scholar]
- 15.Teirstein AS, Machac J, Almeida O, et al. Results of 188 whole-body fluorodeoxyglucose positron emission tomography scans in 137 patients with sarcoidosis. Chest 2007; 132:1949–1953. [DOI] [PubMed] [Google Scholar]
- 16.Sakushima K, Yabe I, Shiga T, et al. FDG-PET SUV can distinguish between spinal sarcoidosis and myelopathy with canal stenosis. J Neurol 2011; 258:227–230. [DOI] [PubMed] [Google Scholar]
- 17.Mostard RL, van Kroonenburgh MJ, Drent M. The role of the PET scan in the management of sarcoidosis. Curr Opin Pulm Med 2013; 19:538–544. [DOI] [PubMed] [Google Scholar]
- 18.Keijsers RG, Grutters JC, Thomeer M, et al. Imaging the inflammatory activity of sarcoidosis: sensitivity and inter observer agreement of (67)Ga imaging and (18)F-FDG PET. Q J Nucl Med Mol Imaging 2011; 55:66–71. [PubMed] [Google Scholar]
- 19.Akaike G, Itani M, Shah H, et al. PET/CT in the diagnosis and workup of sarcoidosis: focus on atypical manifestations. Radiographics 2018; 38:1536–1549. [DOI] [PubMed] [Google Scholar]
- 20.Ganeshan D, Menias CO, Lubner MG, et al. Sarcoidosis from head to toe: what the radiologist needs to know. Radiographics 2018; 38:1180–1200. [DOI] [PubMed] [Google Scholar]
- 21.Vorselaars AD, Verwoerd A, van Moorsel CH, et al. Prediction of relapse after discontinuation of infliximab therapy in severe sarcoidosis. Eur Respir J 2014; 43:602–609. [DOI] [PubMed] [Google Scholar]
- 22.Armani C, Catalani E, Balbarini A, et al. Expression, pharmacology, and functional role of somatostatin receptor subtypes 1 and 2 in human macrophages. J Leukoc Biol 2007; 81:845–855. [DOI] [PubMed] [Google Scholar]
- 23.Kircher M, Lapa C. Novel noninvasive nuclear medicine imaging techniques for cardiac inflammation. Curr Cardiovasc Imaging Rep 2017; 10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu LX, Chen RX, Huang H, et al. Endobronchial ultrasound-guided transbronchial needle aspiration versus standard bronchoscopic modalities for diagnosis of sarcoidosis: a meta-analysis. Chin Med J (Engl) 2016; 129:1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aragaki-Nakahodo AA, Baughman RP, Shipley RT, Benzaquen S. The complimentary role of transbronchial lung cryobiopsy and endobronchial ultrasound fine needle aspiration in the diagnosis of sarcoidosis. Respir Med 2017; 131:65–69. [DOI] [PubMed] [Google Scholar]
- 26.Petek BJ, Rosenthal DG, Patton KK, et al. Cardiac sarcoidosis: diagnosis confirmation by bronchoalveolar lavage and lung biopsy. Respir Med 2018; 144S:S13–S19. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi T, Azuma A, Abe S, et al. Significance of lymphocytosis in bronchoalveolar lavage in suspected ocular sarcoidosis. Eur Respir J 2001; 18:515–521. [DOI] [PubMed] [Google Scholar]
- 28.Tavee JO, Stern BJ. Neurosarcoidosis. Continuum (Minneap Minn) 2014; 20:545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bathla G, Singh AK, Policeni B, et al. Imaging of neurosarcoidosis: common, uncommon, and rare. Clin Radiol 2016; 71:96–106. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Lower EE, Li H, et al. Clinical characteristics of patients with bone sarcoidosis. Semin Arthritis Rheum 2017; 47:143–148. [DOI] [PubMed] [Google Scholar]
- 31.Moore SL, Teirstein A, Golimbu C. MRI of sarcoidosis patients with musculoskeletal symptoms. AJR Am J Roentgenol 2005; 185:154–159. [DOI] [PubMed] [Google Scholar]
- 32.Fritz D, van de Beek D, Brouwer MC. Clinical features, treatment and outcome in neurosarcoidosis: systematic review and meta-analysis. BMC Neurol 2016; 16:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leonhard SE, Fritz D, Eftimov F, et al. Neurosarcoidosis in a tertiary referral center: a cross-sectional cohort study. Medicine (Baltimore) 2016; 95:e3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoitsma E, De Vries J, van Santen-Hoeufft M, et al. Impact of pain in a Dutch sarcoidosis patient population. Sarcoidosis Vasc Diffuse Lung Dis 2003; 20:33–39. [PubMed] [Google Scholar]
- 35.Gvozdenovic BS, Mihailovic-Vucinic V, Ilic-Dudvarski A, et al. Differences in symptom severity and health status impairment between patients with pulmonary and pulmonary plus extrapulmonary sarcoidosis. Respir Med 2008; 102:1636–1642. [DOI] [PubMed] [Google Scholar]
- 36.Drent M, Lower EE, De Vries J. Sarcoidosis-associated fatigue. Eur Respir J 2012; 40:255–263. [DOI] [PubMed] [Google Scholar]
- 37.Hendriks C, Drent M, De Kleijn W, et al. Everyday cognitive failure and depressive symptoms predict fatigue in sarcoidosis: a prospective follow-up study. Respir Med 2018; 138S:S24–S30. [DOI] [PubMed] [Google Scholar]
- 38.Elfferich MD, Nelemans PJ, Ponds RW, et al. Everyday cognitive failure in sarcoidosis: the prevalence and the effect of anti-TNF-alpha treatment. Respiration 2010; 80:212–219. [DOI] [PubMed] [Google Scholar]
- 39▪.Voortman M, De Vries J, Hendriks CMR, et al. Everyday cognitive failure in patients with neurosarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2019, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated the burden of cognitive failure in neurosarcoidosis.
- 40.Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol 1982; 21:1–16. [DOI] [PubMed] [Google Scholar]
- 41.De Vries J, Michielsen H, Van Heck GL, Drent M. Measuring fatigue in sarcoidosis: the fatigue assessment scale (FAS). Br J Health Psychol 2004; 9:279–291. [DOI] [PubMed] [Google Scholar]
- 42.Hoitsma E, De Vries J, Drent M. The small fiber neuropathy screening list: construction and cross-validation in sarcoidosis. Respir Med 2011; 105:95–100. [DOI] [PubMed] [Google Scholar]
- 43.Drent M. Sarcoidosis: benefits of a multidisciplinary approach. Eur J Intern Med 2003; 14:217–220. [DOI] [PubMed] [Google Scholar]
- 44.Moor CC, van Manen MJ, van Hagen PM, et al. Needs, perceptions and education in sarcoidosis: a live interactive survey of patients and partners. Lung 2018; 196:569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tavee J, Zhou L. Small fiber neuropathy: a burning problem. Cleve Clin J Med 2009; 76:297–305. [DOI] [PubMed] [Google Scholar]
- 46▪.Voortman M, Fritz D, Vogels OJ, et al. Small fiber neuropathy: a disabling and underrecognized syndrome. Curr Opin Pulm Med 2017; 23:447–457. [DOI] [PubMed] [Google Scholar]; This review underlines that SFN is difficult to diagnose, as a gold standard is still lacking. In the majority of cases, the only option is symptomatic treatment.
- 47.Hoitsma E, Marziniak M, Faber CG, et al. Small fibre neuropathy in sarcoidosis. Lancet 2002; 359:2085–2086. [DOI] [PubMed] [Google Scholar]
- 48.Heij L, Niesters M, Swartjes M, et al. Safety and efficacy of ARA 290 in sarcoidosis patients with symptoms of small fiber neuropathy: a randomized, double-blind pilot study. Mol Med 2012; 18:1430–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stettner M, Hinrichs L, Guthoff R, et al. Corneal confocal microscopy in chronic inflammatory demyelinating polyneuropathy. Ann Clin Transl Neurol 2016; 3:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tavakoli M, Quattrini C, Abbott C, et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care 2010; 33:1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brines M, Culver DA, Ferdousi M, et al. Corneal nerve fiber size adds utility to the diagnosis and assessment of therapeutic response in patients with small fiber neuropathy. Sci Rep 2018; 8:4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lower EE, Broderick JP, Brott TG, Baughman RP. Diagnosis and management of neurological sarcoidosis. Arch Intern Med 1997; 157:1864–1868. [PubMed] [Google Scholar]
- 53.Bitoun S, Bouvry D, Borie R, et al. Treatment of neurosarcoidosis: a comparative study of methotrexate and mycophenolate mofetil. Neurology 2016; 87:2517–2521. [DOI] [PubMed] [Google Scholar]
- 54.Bradley DA, Lower EE, Baughman RP. Diagnosis and management of spinal cord sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2006; 23:58–65. [PubMed] [Google Scholar]
- 55▪▪.James WE, Baughman R. Treatment of sarcoidosis: grading the evidence. Expert Rev Clin Pharmacol 2018; 11:677–687. [DOI] [PubMed] [Google Scholar]; Excellent review discussing the current therapeutic options for sarcoidosis.
- 56.Ramo-Tello C, Grau-Lopez L, Tintore M, et al. A randomized clinical trial of oral versus intravenous methylprednisolone for relapse of MS. Mult Scler 2014; 20:717–725. [DOI] [PubMed] [Google Scholar]
- 57.Cremers JP, Drent M, Bast A, et al. Multinational evidence-based World Association of Sarcoidosis and Other Granulomatous Disorders recommendations for the use of methotrexate in sarcoidosis: integrating systematic literature research and expert opinion of sarcoidologists worldwide. Curr Opin Pulm Med 2013; 19:545–561. [DOI] [PubMed] [Google Scholar]
- 58.Androdias G, Maillet D, Marignier R, et al. Mycophenolate mofetil may be effective in CNS sarcoidosis but not in sarcoid myopathy. Neurology 2011; 76:1168–1172. [DOI] [PubMed] [Google Scholar]
- 59.Vorselaars AD, Wuyts WA, Vorselaars VM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest 2013; 144:805–812. [DOI] [PubMed] [Google Scholar]
- 60.Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2004; 21:43–48. [DOI] [PubMed] [Google Scholar]
- 61.Sahoo DH, Bandyopadhyay D, Xu M, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J 2011; 38:1145–1150. [DOI] [PubMed] [Google Scholar]
- 62.Kho LK, Kermode AG. Leflunomide-induced peripheral neuropathy. J Clin Neurosci 2007; 14:179–181. [DOI] [PubMed] [Google Scholar]
- 63.Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med 2006; 174:795–802. [DOI] [PubMed] [Google Scholar]
- 64.Baughman RP, Lower EE. Infliximab for refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2001; 18:70–74. [PubMed] [Google Scholar]
- 65.Drent M, Cremers JP, Jansen TL, Baughman RP. Practical eminence and experience-based recommendations for use of TNF-alpha inhibitors in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2014; 31:91–107. [PubMed] [Google Scholar]
- 66▪.Cohen Aubart F, Bouvry D, Galanaud D, et al. Long-term outcomes of refractory neurosarcoidosis treated with infliximab. J Neurol 2017; 264:891–897. [DOI] [PubMed] [Google Scholar]; A retrospective cohort study describing the long-term outcomes of treatment with infliximab in patients with biopsy-proven neurosarcoidosis. At 6 months after initiation of infliximab treatment, 33% had complete remission and 56% partial remission, whereas stable disease was observed in 11%. After a median follow-up of 20 months, 50% of patients had a relapse. Side-effects were observed in eight patients, seven of whom had infections.
- 67.Gelfand JM, Bradshaw MJ, Stern BJ, et al. Infliximab for the treatment of CNS sarcoidosis: a multiinstitutional series. Neurology 2017; 89:2092–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Veltkamp M, Drent M, Baughman RP. Infliximab or biosimilars in sarcoidosis; to switch or not to switch? Sarcoidosis Vasc Diffuse Lung Dis 2016; 32:280–283. [PubMed] [Google Scholar]
- 69.Merinopoulos D, Hayes F, Gallagher DA, Dasgupta B. A case report of neurosarcoidosis successfully treated with an infliximab biosimilar after a relapse while on dual therapy. Clin Exp Rheumatol 2017; 35:356–357. [PubMed] [Google Scholar]
- 70▪.Schimmelpennink MC, Vorselaars AD, van Beek FT, et al. Efficacy and safety of infliximab biosimilar Inflectra® in severe sarcoidosis. Respir Med 2018; 138S:S7–S13. [DOI] [PubMed] [Google Scholar]; First study demonstrating the efficacy and safety of a biosimilar in sarcoidosis.
- 71.Crommelin HA, van der Burg LM, Vorselaars AD, et al. Efficacy of adalimumab in sarcoidosis patients who developed intolerance to infliximab. Respir Med 2016; 115:72–77. [DOI] [PubMed] [Google Scholar]
- 72.Doty JD, Mazur JE, Judson MA. Treatment of corticosteroid-resistant neurosarcoidosis with a short-course cyclophosphamide regimen. Chest 2003; 124:2023–2026. [DOI] [PubMed] [Google Scholar]
- 73.Sodhi M, Pearson K, White ES, Culver DA. Infliximab therapy rescues cyclophosphamide failure in severe central nervous system sarcoidosis. Respir Med 2009; 103:268–273. [DOI] [PubMed] [Google Scholar]
- 74.Talar-Williams C, Hijazi YM, Walther MM, et al. Cyclophosphamide-induced cystitis and bladder cancer in patients with Wegener granulomatosis. Ann Intern Med 1996; 124:477–484. [DOI] [PubMed] [Google Scholar]
- 75.Lower EE, Baughman RP, Kaufman AH. Rituximab for refractory granulomatous eye disease. Clin Ophthalmol 2012; 6:1613–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J 2014; 43:1525–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parambil JG, Tavee JO, Zhou L, et al. Efficacy of intravenous immunoglobulin for small fiber neuropathy associated with sarcoidosis. Respir Med 2011; 105:101–105. [DOI] [PubMed] [Google Scholar]
- 78▪.Tavee JO, Karwa K, Ahmed Z, et al. Sarcoidosis-associated small fiber neuropathy in a large cohort: Clinical aspects and response to IVIG and anti-TNF alpha treatment. Respir Med 2017; 126:135–138. [DOI] [PubMed] [Google Scholar]; A very interesting article describing responses to intravenous immunogloblulin (IVIG) and anti-TNF-α treatment in sarcoidosis-associated SFN. Among 115 patients, symptomatic improvement was seen in 75% with IVIG, in 67% with anti-TNF-α, and in 71% with both treatments.
- 79.Culver DA, Dahan A, Bajorunas D, et al. Cibinetide improves corneal nerve fiber abundance in patients with sarcoidosis-associated small nerve fiber loss and neuropathic pain. Invest Ophthalmol Vis Sci 2017; 58:BIO52–BIO60. [DOI] [PubMed] [Google Scholar]
- 80.Dahan A, Dunne A, Swartjes M, et al. ARA 290 improves symptoms in patients with sarcoidosis-associated small nerve fiber loss and increases corneal nerve fiber density. Mol Med 2013; 19:334–345. [DOI] [PMC free article] [PubMed] [Google Scholar]


