Abstract
Background:
The prevalence of growth disorders among school-aged children in Indonesia is high (30.7%). Pesticides have been massively used in Indonesian agricultural areas.
Objective:
Objective: To determine if exposure to pesticides is associated with stunting among children in agricultural areas.
Methods:
This case-control study included 160 children (48 cases and 112 controls) aged 8–12 years. Exposure to pesticides was measured based on the history of the exposure since perinatal period, infancy, and childhood of the participants. Stunting was determined as a height for age z-score (HAZ) < -2 SD. Other variables measured were levels of thyroid stimulating hormone (TSH), insulin-like growth factor-1 (IGF-1), hemoglobin, zinc, albumin, nutrient adequacy level (energy and protein), and history of infection, low-birth weight (LBW), and mother's height.
Results:
There were no significant difference between the cases and controls in terms of in the baseline characteristics, except for the median IGF-1 level; it was significantly (p<0.001) lower in the cases (66.73 ng/mL) than the controls (112.57 ng/mL). High level of pesticide exposure (p=0.029) and low IGF-1 levels (p<0.001) were significantly associated with stunting. After adjusting for confounding variables, these variables were found to be independent risk factors for stunting in children (aOR 3.90, 95% CI 1.15 to 13.26; and aOR 8.35, 95% CI 3.65 to 19.14, respectively).
Conclusion:
Pesticide exposure could be a risk factor for the occurrence of growth disorders in children living in agricultural areas. Necessary actions should be taken to protect children living in agricultural areas from exposure to pesticides.
Keywords: Pesticides, Growth disorders, Child, Agriculture
TAKE-HOME MESSAGE
The prevalence of stunting is high among school-aged children in Indonesia. Among districts in Central Java, Indonesia, the prevalence is the highest in Brebes district.
Pesticides are commonly used in Indonesian agricultural areas. Pesticides are among toxic substances in the environment that might interfere in the synthesis of insulin-like growth factor-1.
Exposure to pesticides was associated with growth disorders in children living in agricultural areas.
Introduction
Stunting, or poor linear growth (low length- or height-for-age) in young children is the result of poor nutritional intake, in terms of both quality and quantity, high morbidity, or a combination of both. These conditions are often found in low- and middle-income countries.1 Low consumption of macronutrients and micronutrients, especially during the growth period, will disrupt the process, and result in stunting.2 In addition to food consumption factors, stunting is also influenced by genetic factors,3,4 recurrent (chronic) infections, such as acute respiratory infections (ARI) and diarrhea.5 Normal growth is the result of a complex interaction of genetic factors, nutritional factors, and hormonal factors. These three factors work together at the cellular level resulting in growth. Growth hormone (GH) plays an important role in this process; other hormones including thyroid, sex steroid, and glucocorticoid hormones, and psychosocial factors also play a role in growth, partly through interaction with hypothalamopituitary-GH-IGF (insulin-like growth factor) axis.6
History of the growth of children is a sensitive indicator of the health and well-being, and thus, it is an important component of healthy child care. Endocrine disorders include important causes to think about in differential diagnosis of growth disorders in children.3 The problem of stunting in children should be a concern because it is a reflection of the quality of human resources in the future. Some studies showed an association between stunting and impaired cognitive function7,8 and the learning achievement of children of school age.9 Impaired cognitive function in children with stunting, in the long term, will affect their economic potential. Stunting in childhood generally continues into adulthood and will affect their work capacity and productivity.10
The prevalence of stunting in 5–12-year-old children in Indonesia is 30.7%.11 The prevalence of stunting in children of the same age in Central Java is 28.6%; in Brebes district, it is 40.7%, the highest among all districts in Central Java.11 Brebes district is one of the districts in Central Java province that relies on agricultural products as the source of local revenue, especially onion, which requires pesticide spraying 2–3 times per week even everyday in the rainy season.12
Exposure to toxic substances in the environment might also interfere with the synthesis of insulin-like growth factor 1 (IGF-1).13 Research in Spain showed that serum IGF-1 levels in 6–15-year-old boys exposed to organochlorine pesticides were significantly lower than that in the unexposed boys.14 Low IGF-1 levels in serum were found to be associated with growth disorders. The results of the study in preschool children in Senegal showed that there is a relationship between IGF-1 levels and stunting.15
A study in Brebes district showed that the exposure to organophosphate pesticides is a risk factor for hypothyroidism in women at child-bearing age in agricultural areas.12 Exposure to organophosphate pesticides may also cause neurodevelopmental disorders.16,17
Pesticides exposure in children may occur directly, because of their direct involvement in agricultural activities, or indirectly through contact with the environment—water, soil, or foods contaminated with pesticide residues. A study conducted in one of the primary schools in Brebes onion farming areas indicated that 81.3% of the students were involved in agricultural activities, such as finding pests, helping with the harvest, and removing onions from stalks.18 Our previous study concluded that a history of pesticide exposure is a risk factor for thyroid dysfunction in children living in agricultural areas.12 Meanwhile, another study also proved that children whose fathers work as farmers are at higher risk of suffering from goiter compared with those who are not farmers.19 Pesticide is one of the chemicals classified as endocrine disrupting chemicals (EDCs);20 human exposure to these chemicals can interfere in the function of hormones, such as thyroid hormones, insulin, and IGF-1, which are very important in the growth process.15,21
We conducted this study to determine if exposure to pesticides is associated with growth disorders in children living in agricultural areas.
Materials and Methods
A case-control study was conducted in four primary schools in Bulakamba sub-district, where the prevalence of stunting in school children is high and the onion farming is quite common. The minimum sample size was calculated based on the case-control formula proposed by Lemeshow, et al.22 We assumed an acceptable type I error of 0.05, study power of 80%, an pesticide exposure proportion in the non-stunting children of 22.9%, and an OR of 3.26.5 The minimum sample size obtained for each case and control groups was 49.
Children aged 8–12 years included in the study. Those with spinal abnormalities (such as scoliosis), and girls who had had menarche, those who refused to give blood samples, and participants with incomplete interview data were excluded from the study.
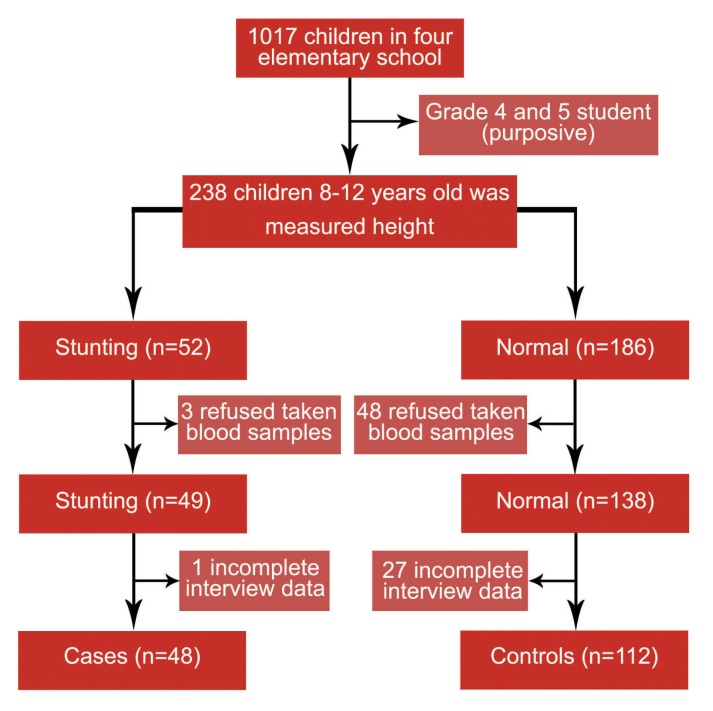
Screening of the studied children's height for stunting was conducted on 238 (119 male and 119 female) elementary school students. Based on the WHO growth standard, 52 (21.8%, 95% CI 16.6% to 27.1%) students had stunting.23 Of these 52 students, three refused to give blood samples and one had incomplete data on interviews, leaving 48 students in the case group for further analyses. Meanwhile, of 186 non-stunting students, 48 refused to give blood samples; 27 had incomplete data on interviews, leaving 112 students in the control group (Figure 1), leading to a case and the control group sample size ratio of approximately 1:2 in accordance with the theory of sample size comparison for cases and controls in the case-control study.24
Figure 1.
Selection of study participants
Data Collection and Measurements
The studied students and their mothers/caregivers were interviewed. Structured questionnaires were used to collect the baseline characteristics (ie, sex, age, parents' educational, and occupational status), pesticide exposure, history of acute respiratory infections and diarrhea in the last month, and history of low-birth weight (LBW, <2500 g). History of pesticide exposure was a composite variable of three sub-variables: (1) mother's involvement in agricultural activity during pregnancy; (2) if subjects were brought to farm by the mother when they were infant or toddler; and (3) subjects' involvement in agricultural activities at their school age. Exposure level was considered “high,” “moderate,” “low,” or “none” if the total number of answers to the above three questions was ‘4,’ ‘3,’ ‘2,’ ‘1,’ or ‘0,’ respectively.
Age of the children was derived from the school register. Height was measured by two postgraduate nutrition students who had been trained on the standard procedure of measuring height. The height was measured using a Seca® 213 stadiometer to the nearest 0.1 cm. The measurements were made on each child without wearing shoes, standing in erect position, looking horizontally, with the feet together on a horizontal surface. Stunting was defined as a height for age z-score (HAZ) < -2 SD. Normal HAZ was defined as a HAZ between -2 SD and +2 SD of the WHO Child Growth standards.23 Mother's height was measured with a stadiometer in the same way as it was measured for the children.
Non-fasting peripheral venous blood samples were taken from 160 students between 9:00 and 11.00 am. The TSH level was measured with a mini VIDAS® (bioMérieux S.A.), a compact automated immunoassay system based on enzyme-linked fluorescent assay (ELFA). A TSH level >4.5 mIU/mL was considered “hypothyroidism.”21
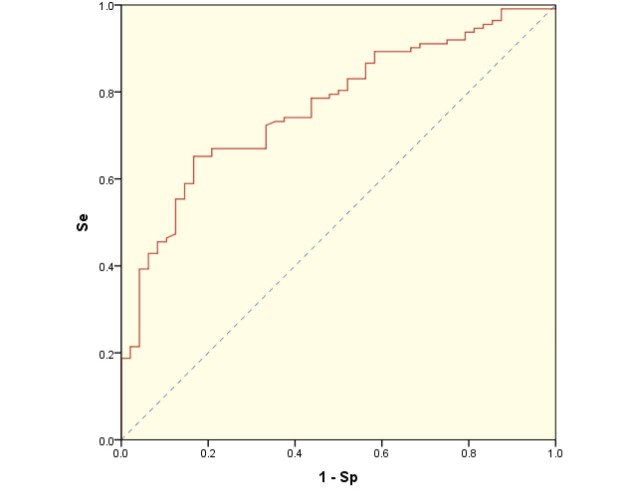
Serum concentration of IGF-1 was measured with a Quantikine® Human IGF-I ELISA kit (R&D Systems). The cut-off value for IGF-1 level for the diagnosis of students with stunting was determined using ROC curve analysis with maximizing the Youden's index,25,26 that revealed a cut-off value of 97.325 ng/mL, with a sensitivity and specificity of 0.652 and 0.833, respectively (Figure 2). IGF-1 levels was therefore considered “low” if it was <97.325 ng/mL.
Figure 2.
ROC curve for various cut-off values of IGF-1. Se: Sensitivity, Sp: Specificity
Hemoglobin was measured using a KX-21 automated hematology analyzer (Sysmex). Anemia was defined if the measured hemoglobin level was <12 g/dL.27 Serum zinc concentration was measured using a Shimadzu® Atomic Absorption Flame Emission Spectrophotometers (AAS, Model: AA-6401F); levels <70 μg/dL was considered “low.”28 Serum albumin level was measured using the bromocresol green (BCG) method. Serum albumin concentrations <3.5 g/dL were considered “low.”29
The amount of daily energy intake of the studied participants in kcal and the daily protein intake in g were assessed using a semi-quantitative food frequency questionnaire (FFQ). The level of energy and protein adequacy was considered “low” if the level was <90% recommended dietary allowance (RDA); otherwise it was considered “sufficient.” Iron adequacy level was considered “low” if it was <77% RDA; otherwise it was considered “sufficient.”30
Ethics
Ethical approval and clearance were obtained from the Diponegoro University Medical Faculty Ethical Committee. The teachers, studied students, and parents were well-informed of the purpose and benefits of the study. The parents signed a written informed consent form.
Statistical Analysis
Data were analyzed with SPSS® for Windows® ver 18. Means of continuous variables were compared using Mann-Whitney U test or Student's t test for independent samples. Categorical variables were compared with χ2 test. Binary logistic regression was used to identify the independent predictors with stunting as dependent variable and pesticide exposure level as the independent variable. The model was adjusted for confounders. A p value <0.05 was considered statistically significant.
Results
The participants were mostly 10 years old. There were four mothers in the case group and 10 in the control group who had no records and forgot the birth weight of their children. The mean mother's height of cases and controls was almost the same (Table 1). We did not measure the height of a few mothers at the time of the study because they were working outside the study area. The majority of parents had a low level of education. Many of the studied parents were farmers (Table 1).
| Table 1: Characteristics of the participants. Values are either mean (SD) or n (%). | |||
| Variables | Cases (n=48) | Controls (n=112) | |
| Age (yrs) | 9.9 (1.0) | 10.0 (1.0) | |
| Height (cm) | 123.8 (4.8) | 134.9 (6.2) | |
| HAZ (SD) | -2.50 (0.48) | -0.80 (0.75) | |
| Birth weight* (g) | 3008 (540) | 3174 (533) | |
| Mother's height† (cm) | 150.2 (6.0) | 150.2 (5.7) | |
| Sex | |||
| Male | 25 (52%) | 55 (49.1%) | |
| Female | 23 (48%) | 57 (50.9%) | |
| Mother's Education level | |||
| Illiterate | 1 (2%) | 4 (3.6%) | |
| Middle school or below | 45 (94%) | 104 (92.8%) | |
| High school | 2 (4%) | 4 (3.6%) | |
| Father's Education level | |||
| Illiterate | 0 (0%) | 1 (0.9%) | |
| Middle school or below | 46 (96%) | 101 (90.2%) | |
| High school | 2 (4%) | 10 (8.9%) | |
| Mother's Occupations | |||
| Unemployed | 7 (15%) | 30 (26.8%) | |
| Farmers | 23 (48%) | 39 (34.8%) | |
| Merchants | 13 (27%) | 34 (30.4%) | |
| Others | 5 (10%) | 9 (8.0%) | |
| Father's Occupations | |||
| Farmers | 21 (44%) | 46 (41.1%) | |
| Merchants | 20 (42%) | 52 (46.4%) | |
| Government employees | 0 (0%) | 1 (0.9%) | |
| Others | 7 (15%) | 13 (11.6%) | |
| *Cases=44, Controls=102; †Cases=37, Controls=75 | |||
The median TSH level in the case group (3.31 mIU/mL) was not significantly different from that in the control group (3.24 mIU/mL). The median IGF-1 level in the case group (66.73 ng/mL) was however lower than that in the control group (112.57 ng/mL). The prevalence of low zinc level and anemia was not significantly different between the two groups (Table 2). All studied participants had normal albumin levels. The majority of subjects in both groups did not have a history of chronic infection and low birth weight (LBW) (Table 2).
| Table 2: Comparison of confounding variables between cases and controls. Values are mean (SD), median (IQR), or n (%) | |||
| Variable | Cases (n=48) | Controls (n=112) | p value |
| TSH (mIU/mL) | 3.31 (6.76 to 2.64) | 3.24 (4.56 to 2.14) | 0.299 |
| IGF-1 (ng/mL) | 66.74 (95.27 to 50.70) | 112.57 (174.27 to 74.87) | <0.001 |
| Zn (mg/dL)* | 98.4 (24.3) | 107.7 (26.9) | 0.078 |
| Hb (g/dL) | 12.8 (0.9) | 13.1 (1.0) | 0.024 |
| Albumin (g/dL) | 4.5 (0.3) | 4.6 (0.3) | 0.409 |
| Energy intake (kcal) | 1408 (464) | 1327 (480) | 0.174 |
| Protein intake (g) | 52.3 (21.7) | 50.0 (21.9) | 0.472 |
| TSH level | |||
| Hypothyroid | 16 (33%) | 28 (25.0%) | 0.374 |
| Non-hypothyroid | 32 (67%) | 84 (75.0%) | |
| IGF-1 level | |||
| Low | 38 (79%) | 37 (33.0%) | <0.001 |
| Normal | 10 (21%) | 75 (67.0%) | |
| Zinc level* | |||
| Low | 3 (10%) | 9 (9%) | 1.000 |
| Normal | 28 (90%) | 87 (91%) | |
| Hemoglobin level | |||
| Anemia | 8 (17%) | 10 (8.9%) | 0.252 |
| Normal | 40 (83%) | 102 (91.1%) | |
| Albumin level | |||
| Low | 0 (0%) | 0 (0.0%) | NA |
| Normal | 48 (100%) | 112 (100.0%) | |
| Iron adequacy level | |||
| Low | 40 (83%) | 104 (92.9%) | 0.121 |
| Sufficient | 8 (17%) | 8 (7.1%) | |
| Energy adequacy level | |||
| Low | 26 (54%) | 91 (81.3%) | 0.001 |
| Sufficient | 22 (46%) | 21 (18.7%) | |
| Protein adequacy level | |||
| Low | 14 (29%) | 56 (50.0%) | 0.024 |
| Sufficient | 34 (71%) | 56 (50.0%) | |
| History of infections | |||
| Yes | 3 (6%) | 15 (13.4%) | 0.300 |
| No | 45 (94%) | 97 (86.6%) | |
| History of LBW† | |||
| Yes | 4 (9%) | 6 (5.9%) | 0.728 |
| No | 40 (91%) | 96 (94.1%) | |
| *Cases=31, Controls=96; †Cases=44, Controls=102; NA=Not analyzed | |||
Involvement of children in agricultural activities at school age and having a high level of exposure to pesticides were found to be risk factors of stunting in children in univariate analysis (Table 3). After adjustment for the level of IGF-1, it was found that children with high pesticide exposure carried a risk of more than three times for stunting compared with the unexposed children (OR 3.90, 95% CI 1.15 to 13.26) (Table 4).
| Table 3: Association between history of pesticide exposure and stunting. Values are n (%). | ||||
| Variable |
Cases (n=48) |
Controls (n=112) |
Crude OR (95% CI) |
|
| Mothers involvement in agricultural activity during pregnancy | ||||
| Yes | 26 (54) | 50 (44.6) | 1.47 (0.74 to 2.89) | |
| No | 22 (46) | 62 (55.4) | 1 | |
| Subjects at the time of infant/toddler brought the mother to farm/stall | ||||
| Yes | 19 (40) | 29 (25.9) | 1.88 (0.92 to 3.84) | |
| No | 29 (60) | 83 (74.1) | 1 | |
| Involvement of subjects in agricultural activities at school age | ||||
| Yes | 35 (73) | 64 (57.1) | 2.02 (1.97 to 4.23) | |
| No | 13 (27) | 48 (42.9) | 1 | |
| History of pesticide exposure (composite variable) | ||||
| High | 13 (27) | 14 (12.5) | 3.25 (1.09 to 9.66) | |
| Moderate | 14 (29) | 31 (27.7) | 1.58 (0.58 to 4.33) | |
| Low | 13 (27) | 39 (34.8) | 1.17 (0.43 to 3.19) | |
| Unexposed | 8 (17) | 28 (25.0) | 1 | |
| Table 4: Results of logistic regression analysis | ||
| Variable | Adj OR (95% CI) | |
| History of pesticide exposure | ||
| High | 3.90 (1.15 to 13.26) | |
| Moderate | 1.96 (0.64 to 5.98) | |
| Low | 1.18 (0.39 to 3.51) | |
| Low IGF-1 | 8.35 (3.65 to 19.14) | |
Discussion
Stunting is a condition of linear growth disorder31 and has more often been associated with poor nutritional intake.32 Less intake of macronutrients, especially energy and protein, was the cause of growth disorders in children.33 Amino acids present in the body as well as those derived from food, are essential ingredients in the formation of proteins as well as collagen. Food sources of energy (amino acids, carbohydrates, fats) support the growth process directly or indirectly through the provision of fuel for neutrophils, macrophages, lymphocytes, and other cells that function in cell or tissue regeneration.35 The role of nutrition in linear growth occurs through various mechanisms. Experimental studies showed that the restriction of energy and protein in the diet lowered the plasma concentration of IGF-1, a hormone needed in the process of linear growth35; it returns to normal level after energy and protein refeeding. The effect of intake restriction on IGF-1 levels, was more apparent after protein restriction rather than energy restriction. IGF-1 level also decreases in patients with kwashiorkor (acute protein deficiency) and in children with protein-energy malnutrition (PEM).34
In addition to macronutrients, several micronutrients, namely zinc,37 iron and vitamin A, are essential for growth.33 Zinc deficiency in rats decreases IGF-1 and growth hormone (GH) plasma concentrations.37 Zinc is associated with bone metabolism.38 it plays a role in DNA and RNA synthesis and also interacts with important hormones involved in bone growth such as somatomedin-c, osteocalcin, testosterone, thyroid hormones, and insulin.39 Other micronutrient deficiencies, such as iron and magnesium (Mg), can cause anorexia and result in impaired growth indirectly due to reduced dietary intake including energy and protein. Zinc, iron, and selenium are also affect the immune function, which can interfere with growth.40
The mean energy and protein intake in cases was higher than the controls. This observation was probably attributed to the recall bias. To anticipate this, serum albumin levels were examined to assess the protein intake more objectively.41 The results showed no difference in the mean albumin levels between cases and controls (Table 2).
Some theories can be used to explain why the energy and protein adequacy levels in the case group were higher than the control group. This might be due to the occurrence of nutrient absorption disorders caused by pathogenic bacterial infections and mycotoxins42 or exposure to toxic substances in the environment,43 causing a condition the so-called “environmental enteric dysfunction” (EED), which is a sub-clinical disorder characterized by morphological and physiological abnormalities in the small intestine in the form of increased permeability, impaired nutritional absorption and growth faltering.44,45 Pesticides are among toxic substances in the environment that can cause EED. Exposure to pesticides through breast milk, food, and drinking water is thought to be a major cause of EED in children.46 High intake of nutrients, energy and protein, will not provide enough benefits for growth and development, if EED occurs. Several studies have linked the high prevalence of stunting with EED.46,47 Research in Zimbabwe proves that the presence of gastrointestinal inflammatory biomarkers is associated with stunting in infants.48 Nonetheless, the exact diagnosis of EED is not easy, as it requires an invasive gastrointestinal tissue biopsy.44
Exposure to toxic substances in the environment, including pesticides, leads to oxidative stress,49 which results in increased energy expenditure for activation of the immune system. Therefore, less energy will remain to be spent for the maintenance, reproduction, growth and thermoregulation.50 The term used for this wasteful use of energy is “Barrel Model.”51 Research on juvenile Japanese quails proved that exposure to the subchronic exposure to chlorpyrifos, one of the most widely used pesticides in agricultural areas, disrupts energy expenditure and the ability to detoxify toxic materials.52
The prevalence of anemia was almost equal in cases and controls (Table 2). This might be due to no differences between both groups in terms of level of nutrient intakes like iron and protein (serum albumin), essential elements for hemoglobin synthesis.
Based on its etiology, stunting can be divided into two categories—those with GH deficiency and idiopathic.3,53 Children born and raised in agricultural areas have the potential to be exposed to pesticides since they are in utero, making them at risk for various health problems including growth disorders. Growth disorders caused by exposure to pesticides can work through several mechanisms, such as the disruption of the hormone system that plays a role in the growth process. Several types of pesticides, including organophosphates and carbamates, which are widely used in agricultural activities, are classified as endocrine-disrupting chemicals (EDCs), chemicals in the environment that can interfere with synthesis, secretion, transport, metabolism, binding action, and elimination of hormones in the body that keep homeostasis, reproduction, and the process of growth and development.20 Thyroid hormone and IGF-1 are the hormones that are necessary for the process of children growth. Several studies showed that exposure to pesticides is a risk factor for hypothyroidism.12,54-56 Thyroid hormone deficiency (hypothyroidism) will cause metabolic disorders with resultant growth and developmental disorders.57 The thyroid dysfunction caused by pesticides exposure works through some mechanisms that disrupts the TSH receptor on the thyroid gland,58 for the similarity of the pesticide chemical structure with thyroid hormone,58 leading to a decrease in D1 (deiodinase type 1) enzyme activity,60 and stimulating the D3 enzyme.58 Exposure to pesticides, especially organochlorines, can also disrupt the IGF-1 function.13,14 Research in Spain shows that the mean IGF-1 levels in the women in whom DDT metabolites were detected, were lower than other women.13
We found that children with low levels of IGF-1 had 8.35 times higher risk for stunting compared with those with normal IGF-1 levels. IGF-1 exerts its role in growth as a mitogen and cell proliferation stimulator and plays an important role in tissue repair/regeneration.60 IGF-1 also mediates protein anabolic processes and increases GH activity.35,61 Some other chemicals such as lead,53,62 phthalate,63 have been shown to disrupt the function of IGF-1. Lead is a toxic substance widely available in the environment, in paints, toys, cookware, battery, electronic equipment, etc. Lead exposure in children can occur through several routes, ie, oral (contaminated food/drink) and inhalation.64 Phthalate is a chemical exist in raw plastic materials used in toys and building materials, such as paints. Children are thus potentially exposed to phthalate in their daily activities.65 Other chemicals, eg, dioxins and polychlorinated biphenyls (PCBs), have also been shown to interfere in child growth.65
In this study, we could not eliminate unmeasured confounders, especially exposure to other EDCs (eg, phthalates, dioxins, and PCBs). However, we examined the potential for some known confounding factors including levels of TSH, IGF-1, zinc, hemoglobin, and nutrient intake. The results of measurements for these potentially confounding variables except for IGF-1 level, were homogenous between the studied groups. Notwithstanding, the level of nutrient intake in the case group was better than that of the control group. These results indicated the possibility of any contribution of the environmental toxicants on children's growth disorders. Another limitation in our study was that in data collection for pesticide exposure; we only used an interview. There could be information bias. Further studies are better to measure pesticide metabolites in the urine of the participants as a surrogate for their level of exposure to pesticides.
In conclusion, we found an association between pesticide exposure and stunting among children living in agricultural areas. Pesticide exposure could be a risk factor for the occurrence of stunting. In future studies, potential confounders such as lead, phthalate, dioxins and PCBs levels should also be considered. These results provide strong support for taking action in the prevention or reduction of pesticide exposure among pregnant women and children, eg, by reducing or limiting their involvements in agricultural activities.
Acknowledgments
The authors are grateful to the Directorate of Research and Community Services, Directorate General of Research and Development, Ministry of Research, Technology, and Higher Education of the Republic of Indonesia for providing funding of doctoral research. We also would like to thank the principals and teachers in fours Elementary Schools in Brebes District, Indonesia for their support to this study; and we are grateful for the support of all subjects.
Conflicts of Interest
None declared.
Cite this article as: Kartini A, Subagio HW, Hadisaputro S, et al. Pesticide exposure and stunting among children in agricultural areas. Int J Occup Environ Med 2019;1:17-29. doi: 10.15171/ijoem.2019.1428
References
- 1. Gibson R. Principles of Nutritional Assessment. 2nd ed. New York, Oxford University Press, Inc, 2005.
- 2.Mikhail W, Sobhy H, El-sayed H. et al. Effect of Nutritional Status on Growth Pattern of Stunted Preschool Children in Egypt National Nutrition Institute (NNI), Cairo, Egypt. Acad J Nutr. 2013;2:1–9. [Google Scholar]
- 3. Nicol L, Allen D, Czernichow P, Zeitler P. Normal growth and growth disorders. In: Kappy M, Allen D, Geffner M, eds. Pediatric Practice Endocrinology. New York, The McGraw-Hill Companies, Inc, 2010:23-79.
- 4.Candra A, Puruhita N, Susanto J. Risk factors of stunting among 1-2 years old children in Semarang City. Media Medica Indonesa. 2011;45:206–12. [Google Scholar]
- 5.Paudel R, Pradhan B, Wagle RR. et al. Risk Factors for Stunting Among Children: A Community Based Case Control Study in Nepal. Kathmandu Univ Med J. 2012;39:18–24. doi: 10.3126/kumj.v10i3.8012. [DOI] [PubMed] [Google Scholar]
- 6.Vogiatzi M, Copeland K. The Short Child. Pediatrics in Review. 1998;19:92–9. doi: 10.1542/pir.19-3-92. [DOI] [PubMed] [Google Scholar]
- 7.Kar B, Rao S, Chandramouli B. Cognitive development in children with chronic protein energy malnutrition. Behav Brain Funct. 2008;4:31. doi: 10.1186/1744-9081-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sokolovic N, Selvam S, Srinivasan K. et al. Catch-up growth does not associate with cognitive development in Indian school-age children. Eur J Clin Nutr. 2014;68:14–8. doi: 10.1038/ejcn.2013.208. [DOI] [PubMed] [Google Scholar]
- 9.Brito G, Onis M de. Growth status and academic performance in Brazilian school age children: growth retardation impairs mathematical, but not reading and spelling abilities TT - Crescimento e desempenho acadêmico em escolares brasileiros: retardo no crescimento interfere co. Arq Neuropsiquiatr. 2006;64:921–5. doi: 10.1590/s0004-282x2006000600006. [DOI] [PubMed] [Google Scholar]
- 10.Prendergast A, Humphrey J. The stunting syndrome in developing countries. Paediatr Int Child Health. 2014;34:250–65. doi: 10.1179/2046905514Y.0000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Indonesian Health Ministry. National Survey on Primary Health Report. 2013.
- 12.Suhartono S, Kartini A, Subagio HW. et al. Pesticide exposure and thyroid function in elementary school children living in an agricultural area, Brebes district, Indonesia. Int J Occup Environ Med. 2018;9:137–44. doi: 10.15171/ijoem.2018.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boada L, Lara P, Alvarez-León E. et al. Serum levels of insulin-like growth factor-I in relation to organochlorine pesticides exposure. Growth Horm IGF Res. 2007;17:506–11. doi: 10.1016/j.ghir.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Zumbado M, Luzardo O, Lara P. et al. Insulin-like growth factor-I (IGF-I) serum concentrations in healthy children and adolescents: Relationship to level of contamination by DDT-derivative pesticides. Growth Horm IGF Res. 2010;20:63–67. doi: 10.1016/j.ghir.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Idohou-Dossou N, Wade S, Guiro A. et al. Nutritional status of preschool Senegalese children: long-term effects of early severe malnutrition. Br J Nutr. 2003;90:1123. doi: 10.1079/bjn2003990. [DOI] [PubMed] [Google Scholar]
- 16.Rauh V, Garfinkel R, Perera F. et al. Impact of Prenatal Chlorpyrifos Exposure on Neurodevelopment in the First 3 Years of Life Among Inner-City Children. Pediatrics. 2006;118:e1845–59. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouchard M, Bellinger D, Wright R, Weisskopf M. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics. 2010;125:e1270–7. doi: 10.1542/peds.2009-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budiyono Budiyono, Suhartono Suhartono, Kartini A. et al. Pesticide Metabolites, Anti-Thyroid Peroxidase and Thyroid Stimulating Hormone Status in School Children: A Preliminary Study in Agriculture Areas in Indonesia. Int J Sci Basic Appl Res. 2015;22:1–12. [Google Scholar]
- 19.Kartini A, Suhartono, Pangestuti D. et al. Goiter and hypothyroidism among elementary school children in lowland agricultural area, Brebes District Indonesia. Indian J Public Heal Res Dev. 2018;9:120–5. [Google Scholar]
- 20.Diamanti-Kandarakis E, Bourguignon J, Giudice L. et al. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stone MB, Wallace RB. Medicare Coverage of Routine Screening for Thyroid Dysfunction. Washington DC, The National Academic Press, 2003. [PubMed]
- 22. Lemeshow S, Hosmer Jr D, Klar J, Lwanga S. Adequacy of Sample Size in Health Studies. New York, John Wiley & Sons Ltd, 1993.
- 23. World Health Organization. WHO Child Growth Standards. Geneva: WHO Press, 2006.
- 24. Schlesselman J, Stolley P. Case-Control Studies. Design, Conduct, Analysis. New York, Oxford University Press, 1982.
- 25.Youden W. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Habibzadeh F, Habibzadeh P, Yadollahie M. On determining the most appropriate test cut-off value: the case of tests with continuous results. Biochem Medica. 2016;26:297–307. doi: 10.11613/BM.2016.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. 2011:1–6. [Google Scholar]
- 28.Dehghani S, Katibeh P, Haghighat M. et al. Prevalence of Zinc Deficiency in 3-18 Years Old Children in Shiraz-Iran. Iran Red Crescent Med J. 2011;13:4–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Abdullah SF, Ahmed FE, Lutfi MF. Serum Albumin Level in Sudanese Children with Edematous and Non-Edematous Malnutrition. Asian Journal of Biomedical and Pharmaceutical Sciences. 2014;4:47–9. [Google Scholar]
- 30.Yasmin G, Kustiyah L, Dwiriani C. Risk factors of stunting among school-aged children from eight provinces in Indonesia. Pak J Nutr. 2014;13:557–66. [Google Scholar]
- 31.World Health Organization. Catalogue of health indicators. 1996:37–8. [Google Scholar]
- 32.de Onis M, Branca F. Childhood stunting: a global perspective. Matern Child Nutr. 2016;12:12–26. doi: 10.1111/mcn.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umeta M, West C, Verhoef H. et al. Factors Associated with Stunting in Infants Aged 5 – 11 Months in the Dodota-Sire District , Rural Ethiopia. J Nutr. 2003;133:1064–9. doi: 10.1093/jn/133.4.1064. [DOI] [PubMed] [Google Scholar]
- 34.Esfivariz C, Ziegler T. Nutrition and the Insulin-Like Growth Factor System. Endocrine. 1997;7:65–71. doi: 10.1007/BF02778066. [DOI] [PubMed] [Google Scholar]
- 35.Skottner A. Biosynthesis of Growth Hormone and Insulin-Like Growth Factor-I and the Regulation of their Secretion. Open Endocrinol J. 2012;6(Sup1:M2) [Google Scholar]
- 36.Gibson R, Manger M, Krittaphol W. et al. Does zinc deficiency play a role in stunting among primary school children in NE Thailand? British Journal of Nutrition. 2007;97:167–75. doi: 10.1017/S0007114507250445. [DOI] [PubMed] [Google Scholar]
- 37.Ninh N, Thissen J, Maiter D. et al. Reduced liver insulin-like growth factor-I gene expression in young zinc-deprived rats is associated with a decrease in liver growth hormone (GH) receptors and serum GH-binding protein. J Endocrinol. 1995;144:449–56. doi: 10.1677/joe.0.1440449. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi M. Role of zinc in bone metabolism and preventive effect on bone disorder. Biomed Res Trace Elem. 2007;18:346–66. [Google Scholar]
- 39.Salgueiro M, Zubillaga M, Lysionek A. et al. The Role of Zinc in the Growth and Development of Children. Nutrition. 2002;18:510–9. doi: 10.1016/s0899-9007(01)00812-7. [DOI] [PubMed] [Google Scholar]
- 40.Rivera M, De Souza A, Araujo-Jorge T. et al. Trace elements, innate immune response and parsitespdf. Clin Chem Lab Med. 2003;41:1020–5. doi: 10.1515/CCLM.2003.156. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe M, Higashiyama A, Kokubo Y. et al. Protein Intakes and Serum Albumin Levels in a Japanese General Population: NIPPON DATA90. J Epidemiol. 2010;2010 20(Sup3):S531–6. doi: 10.2188/jea.JE20090221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crane R, Jones K, Berkley J. Environmental enteric dysfunction: An overview. Food Nutr Bull. 2015;36:S76–87. doi: 10.1177/15648265150361S113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller G, Jones D. The Nature of Nurture : Refining the Definition of the Exposome. Toxicol Sci. 2014;137:1–2. doi: 10.1093/toxsci/kft251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin A, Arnold B, Afreen S. et al. Household Environmental Conditions Are Associated with Enteropathy and Impaired Growth in Rural Bangladesh. Am J Trop Med Hyg. 2013;89:130–7. doi: 10.4269/ajtmh.12-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prendergast A, Kelly P. Review : Enteropathies in the Developing World: Neglected Effects on Global Health. Am J Trop Med Hyg. 2012;86:756–63. doi: 10.4269/ajtmh.2012.11-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mapesa J, Maxwell A, Ryan E. An Exposome Perspective on Environmental Enteric Dysfunction. Environ Health Perspect. 2016;124:1121–6. doi: 10.1289/ehp.1510459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Owino V, Ahmed T, Freemark M, Kelly P. Environmental Enteric Dysfunction and Growth Failure / Stunting in Global Child Health. Pediatrics. 2016;138:1–10. doi: 10.1542/peds.2016-0641. [DOI] [PubMed] [Google Scholar]
- 48.Prendergast AJ, Rukobo S, Chasekwa B. et al. Stunting is characterized by chronic inflammation in Zimbabwean infants. PLoS One. 2014;9:e86928. doi: 10.1371/journal.pone.0086928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lukaszewicz-hussain A. Role of oxidative stress in organophosphate insecticide toxicity – Short review. Pestic Biochem Physiol. 2010;98:145–50. [Google Scholar]
- 50. Degen A. Effect of macroparasites on the energy budget of small mammals. In: Morand S, Krasnov Y, Poulin R, eds. In: Morand S, Krasnov Y, Poulin R, eds. Micromammals and Macroparasites: From Evolutionary Ecology to Management. Tokyo, Springer-Verlag, 2006:371-400.
- 51.Weiner J. Physiological limits to Energy Budgets Sustainable in Birds and Mammals: Ecological Implications. Trends Ecol Evol. 1992;7:384–8. doi: 10.1016/0169-5347(92)90009-Z. [DOI] [PubMed] [Google Scholar]
- 52.Narváez C, Manuel J, Píriz G. et al. Subchronic exposure to chlorpyrifos affects energy expenditure and detoxification capacity in juvenile Japanese quails. Chemosphere. 2016;144:775–84. doi: 10.1016/j.chemosphere.2015.09.060. [DOI] [PubMed] [Google Scholar]
- 53.Xu Y, Liu M, Wang P. et al. Correlation between serum IGF-1 and blood lead level in short stature children and adolescent with growth hormone deficiency. Int J Clin Exp Med. 2014;7:856–64. [PMC free article] [PubMed] [Google Scholar]
- 54.Goldner W, Sandler D, Yu F. et al. Pesticide use and thyroid disease among women in the agricultural health study. Am J Epidemiol. 2010;171:455–64. doi: 10.1093/aje/kwp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farokhi F, Taravati A. Pesticide exposure and thyroid function in adult male sprayers. Int J Med Invest. 2014;3:127–32. [Google Scholar]
- 56.Piccoli C, Cremonese C, Koifman R. et al. Pesticide exposure and thyroid function in an agricultural population in Brazil. Environ Res. 2016;151:389–98. doi: 10.1016/j.envres.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 57.Setian N. Hypothyroidism in children: diagnosis and treatment. J Pediatr (Rio J) 2007;83:S209–16. doi: 10.2223/JPED.1716. [DOI] [PubMed] [Google Scholar]
- 58.Boas M, Feldt-Rasmussen U, Skakkebæk N, Main K. Environmental chemicals and thyroid function. Eur J Endocrinol. 2006;154:599–611. doi: 10.1530/eje.1.02128. [DOI] [PubMed] [Google Scholar]
- 59.Wade M, Parent S, Finnson K. et al. Thyroid toxicity due to subchronic exposure to a complex mixture of 16 organochlorines, lead, and cadmium. Toxicol Sci. 2002;67:207–18. doi: 10.1093/toxsci/67.2.207. [DOI] [PubMed] [Google Scholar]
- 60.Guntur A, Rosen C. IGF-1 regulation of key signaling pathways in bone. Bonekey Rep. 2013;2:1–6. doi: 10.1038/bonekey.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laron Z. Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol Pathol. 2001;54:311–6. doi: 10.1136/mp.54.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fleisch AF, Burns JS, Williams PL. et al. Blood lead levels and serum insulin-like growth factor 1 concentrations in peripubertal boys. Environ Health Perspect. 2013;121:854–8. doi: 10.1289/ehp.1206105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boas M, Frederiksen H, Feldt-Rasmussen U. et al. Childhood exposure to phthalates: Associations with thyroid function, insulin-like growth factor I, and growth. Environ Health Perspect. 2010;118:1458–64. doi: 10.1289/ehp.0901331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu J, Goyer R, Waalkes P. Toxic effects of metals. In: Shanahan J, Naglieri C, eds. Casarett and Doull's Toxicology: The Basic Science of Poisons. 7th ed. New York, The McGraw-Hill Companies, Inc, 2008:931-79.
- 65.Ejaredar M, Nyanza E, Eycke K, Dewey D. Phthalate exposure and childrens neurodevelopment: A systematic review. Environ Res. 2015;142:51–60. doi: 10.1016/j.envres.2015.06.014. [DOI] [PubMed] [Google Scholar]