Abstract
Purpose
The aim of this registry study was to analyze the long-term safety and effectiveness of recombinant human growth hormone (rhGH) in South Korean pediatric patients (≥2 years of age) with growth hormone deficiency GHD) of idiopathic or organic etiology, idiopathic short stature, Turner syndrome, small for gestational age and chronic renal failure.
Methods
The study patients were followed-up till two years after the epiphyseal closure, with visits scheduled every six months. The outcome measures included the incidence of adverse events (AEs, in particular, neoplasia, glucose intolerance and hypothyroidism), as well as height standard deviation score (Ht SDS) and annual height velocity. The results of the interim analysis of a 5-year accumulated data for 2,024 patients (7,342 patient-years, PY) are presented.
Results
A total of 14 neoplasms were diagnosed (191/100,000 PY); 7 out of 9 malignancies were recurrent craniopharyngioma found in patients with organic GHD. Seven cases of glucose intolerance (95/100,000 PY) and 22 cases of hypothyroidism (300/100,000 PY) were detected; about half of the cases (4 and 10 cases each) were considered to be related with rhGH treatment. Most of the growth-retarded patients showed continuous improvement in Ht SDS, with the most prominent effect observed within a year of treatment initiation. The beneficial effect of rhGH on Ht SDS gain was maintained for 2–4 years.
Conclusions
The incidence of AEs of interest in rhGH-treated patients was low, and most of the neoplasms were benign and/or non-related to rhGH. Most patients benefited from the therapy in terms of height increment.
Introduction
Nowadays, indications for treatment with recombinant human growth hormone (rhGH) include not only growth hormone deficiency (GHD) but also a number of other conditions, such as Turner syndrome (TS), Prader-Willi syndrome (PWS), small for gestational age (SGA), chronic renal failure (CRF) and idiopathic short stature (ISS) [1–5]. Although rhGH therapy is generally considered to be safe, still there are safety issues that need to be addressed, i.e., the risk of neoplasia and cardiovascular mortality [6–8]. Another important question is the long-term effectiveness of rhGH in various indications. This subject is rarely explored in clinical trials conducted for a purpose of product registration, which usually have relatively short follow-up periods.
To overcome these knowledge gaps, several large-scale registry studies of rhGH-treated patients have been initiated [9–11]. In addition, pharmaceutical companies carried out research on the safety and effectiveness of their products in various populations [12–15]. However, the efficacy and safety of rhGH have not been investigated in a large number of Asian patients. Thus, we designed a registry study (LG Growth Study, LGS) to analyze the long-term safety and effectiveness of rhGH (Eutropin inj., Eutropin AQ inj., Eutropin Pen inj. and Eutropin Plus inj., LG Chem, Ltd., Korea) in patients with GHD, ISS, TS, SGA and CRF. Detailed descriptions of the individual rhGH products and study background are provided in the previous publication introducing the study protocol and cohort characteristics [16]. In this paper, we present the results of the interim analysis of a 5-year accumulated data from the LGS.
Methods
Patients and study design
LGS is a multi-center (total 73 sites), non-interventional registry study to evaluate the long-term safety and effectiveness of four rhGH products: a weekly rhGH (Eutropin Plus inj.) and three daily rhGHs (Eutropin inj., Eutropin AQ inj. and Eutropin Pen inj.) in patients ≥2 years of age with GHD, ISS, TS, SGA, or CRF. Patients treated with one of the aforementioned products were prospectively followed-up till two years after the epiphyseal closure. If patients who had been already on treatment were registered, the preregistration data were collected retrospectively. A set of minimal eligibility criteria was applied for each indication to register eligible patients, and approved product leaflets were referenced for choosing the initial dosage of rhGH [16]. When GH stimulation test was indicated, the investigator chose the appropriate test(s) among commonly used methods in the clinic, such as insulin tolerance test, L-dopa test, clonidine test, or glucagon test. No interventional element was applied under the study protocol. All treatment-related decisions were left at an investigator’s discretion to collect the data from real-life clinical practice.
Measurements of height, bone age, insulin-like growth factor I (IGF-I), IGF binding protein 3 (IGFBP-3), thyroid stimulating hormone (TSH), total thyroxine (T4), free T4 (fT4), hemoglobin A1c (HbA1c) and serum glucose levels were recorded at 6-month intervals. No central laboratory was used; all laboratory analyses were carried out according to local standard procedures of each site. IGF-1 SDS and IGFBP-3 SDS were calculated based on normative data for Korean population [17]. Adverse events (AEs), including AEs of interest (such as neoplasia, glucose intolerance and hypothyroidism), were collected through interviews and/or chart reviews. To maintain the real-life setting, all decisions regarding choice of rhGH product, its dosage and titration were solely at the investigator’s discretion, whereas the methodology of data collection was standardized to guarantee the integrity of the results. The study was conducted in accordance with the principles expressed in the Declaration of Helsinki and applicable regulations. The study protocol and consent form were reviewed by the institutional review board of each medical center, when required, and the list of institutional review boards that approved the study is provided in S1 Table. Written informed consent was obtained from the patients and/or their parents/legal representatives. The study was registered at ClinicalTrials.gov (identifier: NCT01604395).
Statistical analysis
All patients treated at least once with one of the target products were included in the safety analysis. The incidence of AEs was expressed per 100,000 patient-years (PY). To calculate total PY, each patient’s time was summed up from the first date of rhGH treatment to the earliest date of the last follow-up, withdrawal, death or data cut-off. AEs were coded using MedDRA software ver. 17.0 (International Federation of Pharmaceutical Manufacturers Associations, http://www.meddra.org). Adverse drug reactions (ADRs) were classified as AEs with definite, probable, possible or unassessable causative relationship with rhGH.
Patients who did not satisfy the indication criteria, received rhGH for off-label treatment or had incomplete information for baseline or post-treatment height measurements were excluded from the effectiveness analysis. Height standard deviation score (Ht SDS) and annual height velocity (HV) were calculated from height records at protocol defined timepoints (with ± 1-month window period). To calculate Ht SDS, the following equation was used referencing the Box-Cox transformation (L), median (M), and coefficient of variation (S) values from growth standard for Korean children and adolescents [18]: height SDS = [Power (measured height/M, L)– 1] / L×S.
The data cut-off point was 22 March 2017. The study data and the results of the interim analysis were evaluated by the dedicated Observational Study Monitoring Board. The results for CRF cohort (n = 9) were included in the whole dataset, but are not presented separately in the effectiveness aspect due to a small sample size. Missing data were not substituted. Results with the P-values <0.05 were considered statistically significant. All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patients characteristics
A total of 2,277 patients were registered between November 2011 and March 2017. The safety data were available for 2,024 patients, which corresponds to 7,342 PY. Other 253 patients were excluded from the safety analysis because of missing treatment record or indication information, lack of consent or registration error (Fig 1). Median age at the time of registration was 8.49 years (range 2.01 to 19.23 years); 82.9% (male: 89.9%, female: 78.5%) patients were prepubertal (Tanner stage I). Other baseline characteristics are presented in Table 1. The most common indication for rhGH treatment was GHD (64.1%); the vast majority of GHD patients had idiopathic GHD (IGHD) (n = 1,189, 91.7%). Median Ht SDS in the study population was -2.26. Median Ht SDS at the baseline was the lowest in TS cohort (-2.58), and the most severe bone age (BA) delay (BA–chronological age) was observed in GHD cohort (median -1.71 years).
Fig 1. Disposition of patients.
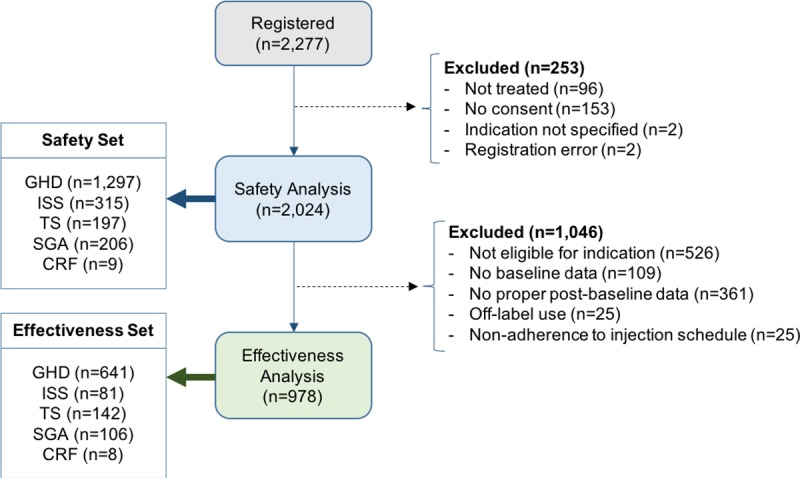
Table 1. Baseline demographic characteristics of patients.
| Variable | Total (n = 2,024) |
GHD (n = 1,297) |
TS (n = 197) |
SGA (n = 206) |
ISS (n = 315) |
CRF (n = 9) |
|---|---|---|---|---|---|---|
| Sex: | ||||||
| male | 1048 (51.8%) |
771 (59.4%) |
0 (0.0%) |
104 (50.5%) |
167 (53.0%) |
6 (66.7%) |
| female | 976 (48.2%) |
526 (40.6%) |
197 (100.0%) |
102 (49.5%) |
148 (47.0%) |
3 (33.3%) |
| Age (years) | 8.49 (2.01, 19.23) |
8.42 (2.10, 19.23) |
9.24 (2.01, 18.58) |
6.66 (3.04, 14.18) |
9.05 (2.38, 16.10) |
8.58 (2.48, 15.88) |
| Pubertya | ||||||
| pre-pubertal | 793 (82.9%) |
502 (87.3%) |
107 (83.0%) |
77 (83.7%) |
105 (66.0%) |
2 (100.0%) |
| pubertal | 164 (17.1%) |
73 (12.7%) |
22 (17.1%) |
15 (16.3%) |
54 (34.0%) |
0 (0.0%) |
| BA (years) | 6.8 (0.6, 17) |
6.0 (0.6, 17) |
8.5 (1.0, 14) |
5.5 (1.5, 13.5) |
8.4 (2.0, 15.6) |
8.8 (1.5, 13.5) |
| BA-CA (years) | -1.46 (-8.83, 4.49) |
-1.71 (-8.83, 2.71) |
-0.87 (-5.75, 2.53) |
-1.05 (-4.67, 2.51) |
-0.98 (-5.30, 4.49) |
-1.69 (-4.87, 0.30) |
| Height SDS | -2.26 (-6.97, 6.23) |
-2.25 (-6.97, 6.23) |
-2.58 (-6.34, -0.66) |
-2.23 (-5.14, -0.18) |
-2.17 (-5.31, 1.54) |
-2.43 (-3.54, 0.00) |
| BMI SDS | -0.27 (-16.88, 3.47) |
-0.23 (-16.88, 2.88) |
0.51 (-1.92, 3.47) |
-0.81 (-5.60, 2.09) |
-0.58 (-4.60, 2.69) |
-0.89 (-2.89, 1.61) |
a Some patients’ puberty data are missing and only available data are accounted.
Data show numbers (%) or medians (min, max).
BA bone age, BMI body mass index, CA chronological age, CRF chronic renal failure, GHD growth hormone deficiency, ISS idiopathic short stature, SDS standard deviation score, SGA small for gestational age, TS Turner syndrome.
Out of 2,024 patients included in the safety set, 1,046 were excluded from the effectiveness analysis because of non-eligibility for indications, missing baseline or follow-up data, off-label use and non-adherence (Fig 1). Demographic characteristics of the remaining 978 patients included in the effectiveness set resembled those of the safety set (S2 Table). The upper quartile for Ht SDS was -2.07, which implies that >75% of patients were severely growth-retarded.
Use of rhGH products
Approximately 80% of the study patients were initially treated with a daily rhGH (n = 1,626, Eutropin inj. or Eutropin AQ inj.) whereas others were treated with a weekly rhGH (n = 398, Eutropin Plus inj.). Median (IQR) on-treatment follow-up period was 3.03 years (1.67, 4.65): 2.87 years (1.56, 4.51) for Eutropin inj., 2.29 years (0.9, 4.02) for Eutropin Plus inj. and 0.89 years (0.51, 1.52) for Eutropin AQ inj.. No significant changes were noted in the yearly descriptive statistics for rhGH doses in each indication. The 5-year overall median (IQR) dose of Eutropin inj. for GHD, TS, SGA, ISS and CRF cohorts was 0.24 mg/kg/week (0.21, 0.27), 0.31 mg/kg/week (0.27, 0.33), 0.27 mg/kg/week (0.22, 0.31), 0.26 mg/kg/week (0.21, 0.29) and 0.29 mg/kg/week (0.25, 0.31), respectively. Median (IQR) dose of Eutropin Plus inj. for GHD cohort was 0.60 mg/kg/week (0.53, 0.73). Statistical characteristics of rhGH dose for the effectiveness set were similar. Median (IQR) on-treatment follow-up period for the effectiveness set was 3.60 years (2.08, 5.10): 3.71 years (2.35, 5.14), 2.32 years (1.27, 3.82), 4.84 years (3.53, 7.08) and 1.99 years (1.44, 2.59) years for GHD, ISS, TS and SGA cohorts, respectively.
Adverse events during on-treatment follow-up period
During on-treatment follow-up period (6,898 PY), 954 AEs (13,831 per 100,000 PY) were reported in 458 patients (22.6%). Proportions of patients experiencing AEs, ADRs, SAEs, and serious ADRs are provided in the Table 2. The following results are based on the number of events (cases). Most AEs (97.7%) were mild (772 cases) or moderate (160 cases) in severity, and 80.4% resolved. The most frequent AEs were upper respiratory tract infections (1,305 per 100,000 PY), typically unrelated to rhGH treatment. Out of 954 AEs, 119 (12.5%) were ADRs (1,725 per 100,000 PY). The most common type of ADRs were reactions in the injection site (25 cases, 362 per 100,000 PY), and among them, injection site pain (13 cases, 188 per 100,000 PY). Other common ADRs were headache (15 cases, 217 per 100,000 PY), hypothyroidism (10 cases, 145 per 100,000 PY) and arthralgia (7 cases, 101 per 100,000 PY). The highest incidence of AEs (per 100,000 PY) was documented in ISS cohort (18,745), followed by TS (17,885), SGA (15,179) and GHD (11,796) cohorts, and the highest incidence of ADRs (per 100,000 PY) in ISS cohort (2,734), followed by SGA (2,039), GHD (1,605) and TS (1,346) cohorts. Approximately 10% of AEs (94 cases) were serious AEs (SAEs), and 8 of them (116 per 100,000 PY) were regarded as rhGH-related, autoimmune thyroiditis, hypothyroidism, diabetes mellitus, hematuria, intervertebral disc protrusion, supraventricular tachycardia (1 case each) and craniopharyngioma (2 cases). No mortality was reported during the on-treatment follow-up period.
Table 2. Incidence of adverse events during on-treatment follow-up and whole study period.
| Variable | Total (n = 2,024)a |
IGHD (n = 1,189) |
OGHD (n = 107) |
TS (n = 197) |
SGA (n = 206) |
ISS (n = 315) |
CRF (n = 9) |
|---|---|---|---|---|---|---|---|
| On-treatment follow-up period: | |||||||
| AEs | 458 (22.6%) |
231 (19.4%) |
45 (42.1%) |
72 (36.6%) |
35 (17.0%) |
70 (22.2%) |
5 (55.6%) |
| ADRs | 93 (4.6%) |
43 (3.6%) |
13 (12.2%) |
11 (5.6%) |
8 (3.9%) |
17 (5.4%) |
1 (11.1%) |
| SAEs | 66 (3.3%) |
31 (2.6%) |
12 (11.2%) |
13 (6.6%) |
4 (1.9%) |
6 (1.9%) |
- |
| Serious ADRs | 7 (0.4%) |
4 (0.3%) |
2 (1.9%) |
1 (0.5%) |
- | - | - |
| Whole study period: | |||||||
| AEs | 462 (22.8%) |
232 (19.5%) |
46 (43.0%) |
73 (37.1%) |
35 (17.0%) |
70 (22.2%) |
6 (66.7%) |
| ADRs | 94 (4.6%) |
43 (3.6%) |
14 (13.1%) |
11 (5.6%) |
8 (3.9%) |
17 (5.4%) |
1 (11.1%) |
| SAEs | 68 (3.4%) |
31 (2.6%) |
13 (12.2%) |
13 (6.6%) |
4 (1.9%) |
6 (1.9%) |
1 (11.1%) |
| Serious ADRs* | 8 (0.4%) |
4 (0.3%) |
3 (2.8%) |
1 (0.5%) |
- | - | |
a Addition of each subgroup number does not sum up to 2,024 as one patient with GHD could not be classified as either IGHD or OGHD. The patient with non-specified GHD etiology did not experience any adverse event. Data show numbers (%) of patients with events.
*Total of nine serious ADRs were reported in eight patients during whole study period: autoimmune thyroiditis, hypothyroidism, diabetes mellitus, hematuria, intervertebral disc protrusion and supraventricular tachycardia (n = 1 each) and craniopharyngioma recurrence (n = 3). Except one case of craniopharyngioma recurrence, all occurred during on-treatment follow-up period.
ADR adverse drug reaction, AE adverse event, CRF chronic renal failure, GHD growth hormone deficiency, IGHD idiopathic growth hormone deficiency, ISS idiopathic short stature, OGHD organic growth hormone deficiency, SAE serious AE, SGA small for gestational age, TS Turner syndrome.
Adverse events during off-treatment follow-up period
Off-treatment follow-up has been completed or ongoing in 335 patients. Median off-treatment follow-up duration was 1.0 year (range 0 to 9.55 years). During this period, 26 additional AEs (25 non-related to rhGH and 1 ADR) occurred including 5 SAEs. One SAE (craniopharyngioma recurrence) was evaluated as rhGH-related. A 12-year-old male patient from IGHD cohort, with unremarkable medical history, was diagnosed with medulloblastoma during the rhGH treatment (approximately one year after the start of the rhGH treatment). At the baseline, the patient’s IGF-I (SDS) and IGFBP-3 (SDS) were 161 ng/mL (-0.84) and 5,280 ng/mL (3.83), respectively, and the initial dosage was 0.28 mg/kg/week with Eutropin inj. About 6 months later, IGF-I (SDS) and IGFBP-3 (SDS) were 257 ng/mL (-0.51) and 3,291 ng/mL (0.40), respectively. The treatment was discontinued immediately after the diagnosis of medulloblastoma. Approximately 1.2 years later, death of the patient was confirmed during the off-treatment follow-up process.
Adverse events of interest
The incidence of AEs of interest was analyzed for the whole study period. Fourteen neoplasms (191 per 100,000 PY) were diagnosed in 11 patients (6 females, 5 males): 5 benign (68 per 100,000 PY) and 9 malignant (123 per 100,000 PY) (Table 3). Seven cases out of total 9 malignant neoplasms were recurrence cases in craniopharyngioma, diagnosed solely in OGHD patients, 2–6 years after the diagnosis of primary tumors and 8 months to 5 years after rhGH treatment initiation. Four neoplasms, three malignant and one benign, were considered as ADRs (Table 4). Ovarian dysgerminoma was reported in 9 years old TS (45,X/46,XY) patient during the rhGH treatment (approximately one year after the start of the rhGH treatment). The treatment was discontinued immediately after the diagnosis and she recovered after receiving chemotherapy.
Table 3. Incidence of adverse events of interest during whole study period.
| Variable | Total (n = 2,024)a |
IGHD (n = 1,189) | OGHD (n = 107) | TS (n = 197) |
SGA (n = 206) |
ISS (n = 315) |
CRF (n = 9) |
|---|---|---|---|---|---|---|---|
| Death | 1 (14) | 1 (23) | - | - | - | - | - |
| All neoplasms | 14 (191) | 4 (93) | 7 (1289) | 3 (255) | - | - | - |
| Malignancy | 9 (123) | 1 (23) | 7 (1289) | 1 (85) | - | - | - |
| Benign | 5 (68) | 3 (70) | 2 (170) | - | - | - | |
| Hypothyroidism | 22 (300) | 6 (140) | 3 (552) | 11 (937) | - | 1 (118) | 1† |
| Glucose intolerance* | 7 (95) | 2 (47) | 3 (552) | - | 1 (218) | 1 (118) | - |
| Scoliosis | 11 (150) | 7 (163) | 1 (184) | 2 (170) | 1 (218) | - | - |
| Benign intracranial hypertension | 1 (14) | 1 (23) | - | - | - | - | - |
| Pancreatitis | 1 (14) | 1 (23) | - | - | - | - | - |
| Fluid retention | 1 (14) | 1 (23) | - | - | - | - | - |
| Gynecomastia | 1 (14) | - | - | - | - | 1 (118) | - |
| Sleep apnea syndrome | 1 (14) | 1 (23) | - | - | - | - | - |
a Addition of each subgroup number does not sum up to 2,024 as one patient with GHD could not be classified as either IGHD or OGHD. The patient with non-specified GHD etiology did not experience any adverse event. Data show absolute numbers (rates per 100,000 patient-years).
*Includes diabetes mellitus (n = 3), hyperglycemia (n = 3) and glucose tolerance impaired (n = 1),
†Due to small sample size, rates per 100,000 patient-years of chronic renal failure cohort was not analyzed separately.
CRF chronic renal failure, GHD growth hormone deficiency, IGHD idiopathic growth hormone deficiency, ISS idiopathic short stature, OGHD organic growth hormone deficiency, SGA small for gestational age, TS Turner syndrome.
Table 4. Neoplasms reported during whole study period.
| Indication for rhGH treatment | Sex | Age at the baseline (years) | Age at neoplasm diagnosis (years) | Neoplasm type | rhGH at the time of diagnosis | Relationship with rhGH | Action undertaken | Outcome |
|---|---|---|---|---|---|---|---|---|
| Malignant | ||||||||
| Organic GHD | female | 19 | 21 | craniopharyngioma recurrence*,† | yes | possible | rhGH stopped | ongoing |
| Organic GHD | male | 9 | 14 | craniopharyngioma recurrence† | yes | unlikely | rhGH interrupted | resolved |
| 17 | craniopharyngioma recurrence†,‡ | yes | unlikely | none | resolved | |||
| Organic GHD | female | 13 | 14 | craniopharyngioma recurrence*,† | yes | possible | rhGH stopped | resolved |
| 15 | craniopharyngioma recurrence† | yes | unlikely | none | resolved | |||
| 17 | craniopharyngioma recurrence†,§ | off-treatment | unlikely | none | resolved | |||
| Organic GHD | male | 7 | 8 | craniopharyngioma recurrence*,† | off-treatment | possible | rhGH stopped | resolved |
| Idiopathic GHD | male | 12 | 13 | medulloblastoma† | yes | unlikely | rhGH stopped | death |
| TS | female | 8 | 9 | ovarian dysgerminoma stage unspecified† | yes | not related | rhGH interrupted | resolved |
| Benign | ||||||||
| Idiopathic GHD | female | 9 | 9 | skin papilloma* | yes | possible | none | resolved |
| Idiopathic GHD | male | 6 | 8 | skin papilloma | yes | unlikely | none | resolved |
| Idiopathic GHD | male | 3 | 11 | skin papilloma | yes | not related | none | resolved |
| TS | female | 4 | 11 | neurofibroma | yes | not related | none | ongoing |
| TS | female | 10 | 13 | osteochondroma | yes | not related | none | ongoing |
*Evaluated as related to rhGH treatment,
†reported as SAE,
‡Epilepsy was reported as AE approximately 2 years after this was resolved (ongoing).
§CSF leak was reported as AE 6 days after this was resolved (resolved after 9 days).
GHD growth hormone deficiency, rhGH recombinant human growth hormone, TS Turner syndrome
Hypothyroidism (22 cases, 300 per 100,000 PY) was reported primarily in TS (11 cases) and GHD cohorts (9 cases; 6 patients with IGHD and 3 with OGHD), typically during treatment (21 cases) and mostly in female patients (19 cases). Median time elapsed from GH treatment onset -to hypothyroidism diagnosis was 1.9 (IQR: 0.8, 2.7) years. More than 50% of the cases were considered to be unrelated to rhGH, and 10 (136 per 100,000 PY) were considered as ADRs.
There were seven cases (95 per 100,000 PY) of glucose intolerance (Table 3). Of these, four cases were regarded as ADRs, including type 2 diabetes mellitus (T2DM, 2 cases), glucose tolerance impaired (1 case), and hyperglycemia (1 case). In case of new-onset T2DM, three cases were detected during the whole follow-up period (Table 5). In 3 out of 7 glucose intolerance cases, hyperglycemia (2 cases) and glucose tolerance impaired (1 case), have been resolved.
Table 5. New-onset diabetes cases reported during whole study period.
| Type of DM | Indication for rhGH treatment | Age at the baseline (years) | Age at DM diagnosis (years) | Additional risk factors | BMI (SDS) at the baseline | rhGH at the time of diagnosis | Relationship with rhGH | Action undertaken | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Type 2 | Idiopathic GHD | 9 | 11 | Family history (+) | 1.98 | yes | possible | none | ongoing |
| Type 2 | Organic GHD | 16 | 17 | - | 2.27 | yes | not related | none | ongoing |
| Type 2 | Organic GHD | 11 | 15 | - | 1.47 | yes | probable | rhGH interrupted | ongoing |
DM Diabetes mellitus, BMI body mass index, GHD growth hormone deficiency, rhGH recombinant human growth hormone
Scoliosis (11 cases, 150 per 100,000 PY) was reported in SGA (1 case), TS (2 cases) and GHD cohorts (8 cases; 7 patients with IGHD and 1 with OGHD) during treatment. Median time elapsed from GH treatment onset -to scoliosis diagnosis was 2.3 (IQR: 1.1, 3.2) years. Five cases (68 per 100,000 PY) were considered as ADRs.
Laboratory results
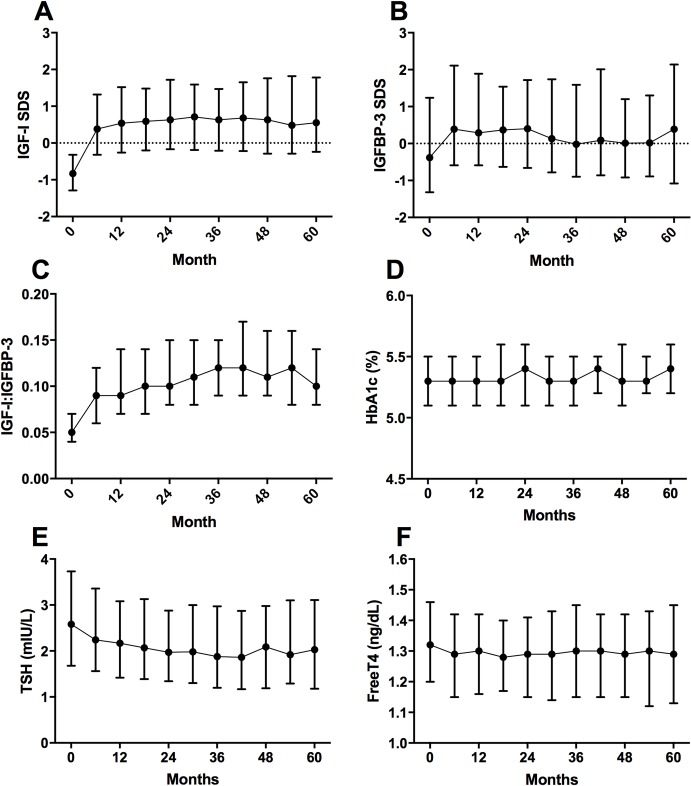
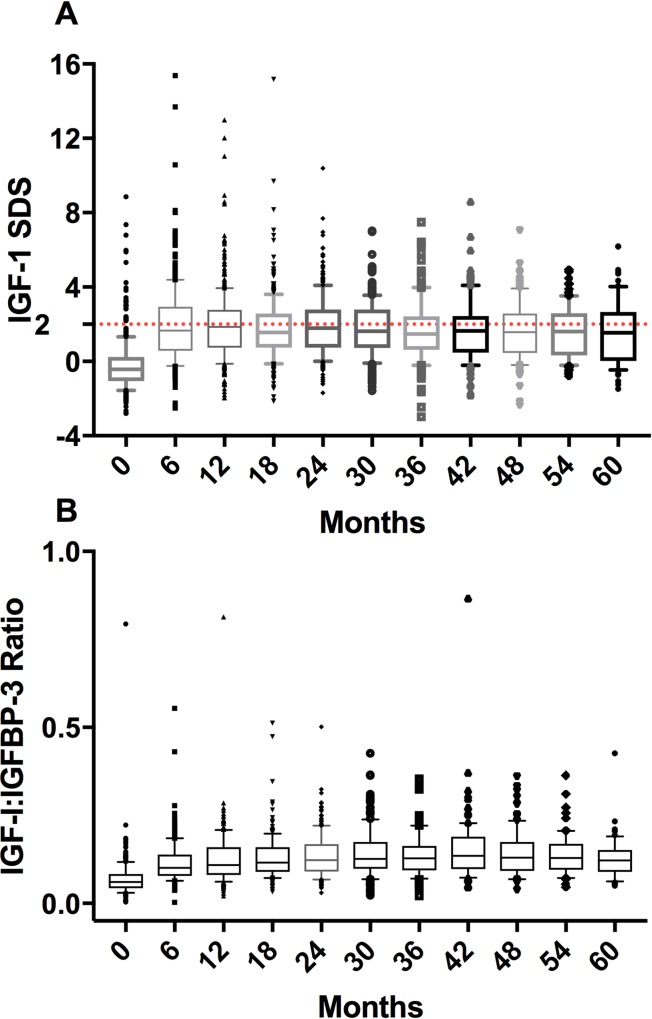
Irrespective of the cohort, IGF-I SDS and IGFBP-3 SDS significantly increased at six months after rhGH treatment (p<0.001), and their levels remained at a plateau thereafter (Fig 2). Supraphysiological levels of IGF-I (IGF-I SDS >+2) were found at least once in 508 patients (25.1%), more frequently within one year of treatment and in TS cohort (82/197 patients; Fig 3). Median IGF-I to IGFBP-3 ratio remained at a stable level of 0.1. The subgroup with at least one IGF-I SDS value >+2 did not differ from the other patients in terms of AE (15,027 vs. 29,197 per 100,000 PY) and SAE frequencies (1,940 vs. 2,527 per 100,000 PY), but more often presented with serious ADRs (226 vs. 181 per 100,000 PY).
Fig 2. Laboratory tests for IGF-I, IGFBP-3, HbA1c, TSH, and free T4 (safety set).
The symbols represent median values and the vertical bars indicate interquartile range.
Fig 3. IGF-I SDS.
(A) and IGF-I to IGFBP-3 ratio (B) in a subgroup of patients with supra-physiological level of IGF-I. The boxes represent interquartile ranges and the whiskers represent 10th and 90th percentiles. (A) In the subgroup of patients having supra-physiological level of IGF-I, some were observed to have extremely high SD scores of IGF-I since the initiation of rhGH treatment; however, such extreme values tended to disappear as treatment progressed. (B) IGF-I to IGFBP-3 ratio stayed relatively stable since 6 months of treatment.
Other laboratory findings included a slight decrease in TSH levels, observed until 42 months of treatment. No significant changes were found in fT4 and HbA1c levels (Fig 2).
Effectiveness results
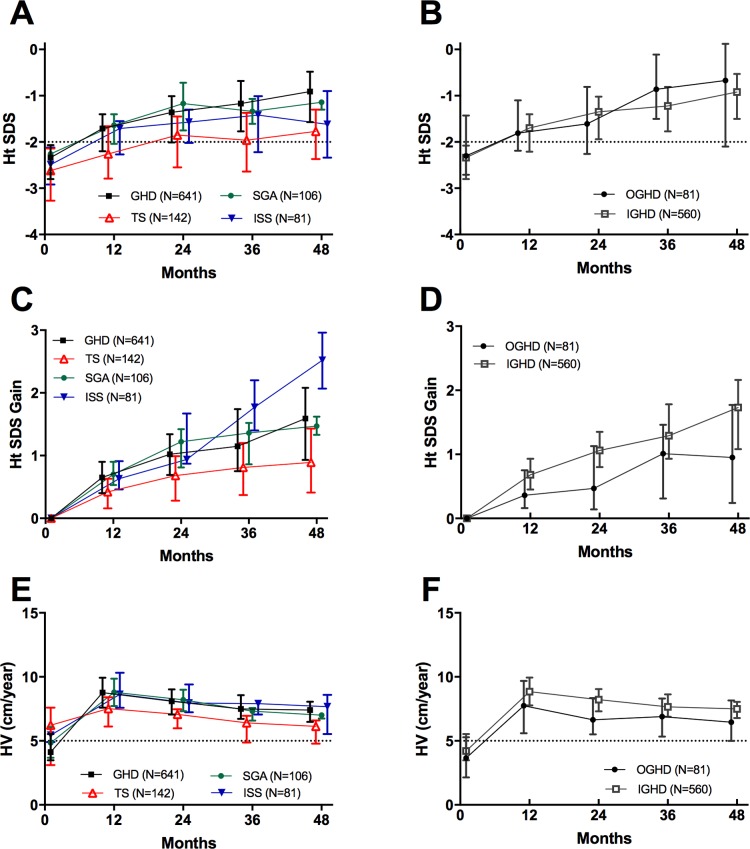
Median (IQR) height SDS for patients included in the effectiveness set was -2.37 (-2.89, -2.07, n = 976) at the baseline, and -1.39 (-2.05, -0.87, n = 243) and -1.30 (-1.99, -0.62, n = 148) at 36 and 48 months of treatment, respectively. Ht SDS at six months of treatment was significantly higher (p<0.001) than at the baseline in GHD, ISS, TS and SGA cohorts. In GHD cohort, an increase in height SDS continued until the fourth year, and in the other cohorts, until the second year (Fig 4). After 4 years of GH treatment, mean changes (95% CI) in Ht SDS in GHD (n = 95), TS (n = 44) and SGA cohorts (n = 5) were 1.52 (1.32, 1.73), 0.86 (0.64, 1.08) and 1.27 (0.53, 2.00), respectively. In GHD subgroups, mean Ht SDS gain (95% CI) at 4 years was 1.68 (1.49, 1.87) in IGHD (n = 71) and 1.06 (0.48, 1.64) in OGHD (n = 24).
Fig 4. Auxological measurements through the fourth year.
The symbols indicate the median values and the vertical bars indicate interquartile range. IGHD was confirmed by at least two GH stimulation tests (peak responses from both tests <10 ng/mL).
At six months of treatment, mean (95% CI) HV was 9.20 (9.02, 9.38), being nearly twice as high as at the baseline. Most patients achieved the highest HV during the first year of treatment (Fig 4). At 12 months, the highest HV was documented in ISS cohort (mean: 8.81, 95% CI: 8.31, 9.32) and the lowest in TS cohort (mean: 7.27; 95% CI: 6.94, 7.60). In GHD, SGA and ISS cohorts, mean HV was maintained above 7 cm per year until the second year and then decreased gradually, down to 5.30–5.74 cm annually at four years. Irrespective of the study timepoint, HV for TS cohort was lower than for other cohorts (Fig 4).
Discussion
According to a general consensus, rhGH can be safely administered for patients without known risk factors of malignant neoplasia, but the available evidence in this matter is still inconclusive. Recently, SAGhE study demonstrated a higher incidence of bone and bladder neoplasms in the rhGH-treated cohort, but without enough evidence for a causal role of the agent [19]. In our study, 9 malignant neoplasms were diagnosed in 6 patients (crude incidence: 0.3%, 123 per 100,000 PY). After excluding seven cases of craniopharyngioma recurrence, primary malignant neoplasia incidence was 0.1% (27 per 100,000 PY); in the investigators’ causality assessment, none were rhGH-related. The incidence of malignant neoplasms was slightly higher than in the previous studies, KIGS and NCGS (16.4 and 5.8 per 100,000 PY, respectively) [20,21]. However, the latter two studies were markedly larger than LGS (58,603 and 33,161 patients participated in KIGS and NCGS, respectively). Furthermore, it should be emphasized that the neoplasia risk in rhGH-treated patients is known to decrease with follow-up time, and both KIGS and NCGS were based on more than 20-year accumulated data, as compared with only a 5-year dataset in our study. Hence, conclusions about cancer risk in the South Korean pediatric population treated with rhGH should not be formulated earlier than at the end of the designated follow-up period for all targeted patients.
One patient died during off-treatment follow-up period after being diagnosed with medulloblastoma (mortality: 0.05%, 14 per 100,000 PY). A contribution of rhGH therapy was considered unlikely in this case. Medulloblastoma is one of the most common pediatric cancers [22], and previous studies found no correlation between rhGH treatment and progression or recurrence of brain tumors [6,23,24].
The incidence of AEs associated with glucose intolerance in LGS patients was 0.3% (95 per 100,000 PY), and T2DM was diagnosed in 0.15% of the study participants. While the risk of glucose intolerance and T2DM in rhGH-treated patients with GHD and ISS is low, those who were treated with rhGH for TS or SGA are more prone to metabolic diseases than individuals from the general population [6,25]. However, it is unclear whether the increased metabolic risk in this group is directly related to the treatment, or is rather a consequence of underlying conditions. In a previous study, 199 SGA patients treated with rhGH were followed-up for 5 years; both insulin resistance and beta cell dysfunction caused by rhGH have been resolved within 6 months post-treatment, and at the end of the follow-up period, body composition, insulin sensitivity and beta-cell function did not differ between the study group and the untreated controls [26]. This implies that metabolic AEs related to rhGH treatment are reversible and probably clinically irrelevant.
Our cohort included 22 patients who developed hypothyroidism during rhGH treatment (incidence: 1.1%, 300 per 100,000 PY). Most patients who developed hypothyroidism received rhGH due to TS (n = 11) or GHD (n = 9). Hypothyroidism is a common comorbidity in TS patients, occurring regardless of rhGH treatment [27]; indeed, in 8 out of 11 patients with TS included in LGS study, the investigators evaluated hypothyroidism as not related to rhGH therapy.
Scoliosis has been observed in 11 patients (incidence: 0.5%, 150 per 100,000 PY). Among them, two were patients with TS, in which condition scoliosis is known to be more prevalent even without rhGH treatment [6,28]. As the rapid growth during rhGH treatment may accelerate the progression of scoliosis, regular radiographic check-up is recommended for those receiving rhGH therapy [1,6].
One of the concerns related to administration of rhGH at higher doses is potential risk associated with the supraphysiological level of IGF-I. In our study, 25% of patients at least once had IGF-I SDS >+2, which corresponds to the supraphysiological level of this compound. However, in most of these patients, the elevated levels of IGF-I were observed temporarily, usually at early stages of the treatment, and then remained within the recommended range. Although serious ADR rate in patients with IGF-I SDS >+2 was slightly higher than in those with lower IGF-I SDS values, none of them was withdrawn from the study prematurely. The increase in IGF-I SDS was associated with a concomitant increase in IGFBP-3 SDS and therefore, IGF-I to IGFBP-3 ratio remained at a relatively stable level. Thus, it may be presumed that the increase in IGF-I level was counterbalanced by IGFBP-3. The increase in IGF-I level is generally known to be proportional to rhGH dose. The TS cohort, in which median dose of rhGH was the highest, contained also the largest proportion of patients with IGF-I SDS >+2. In International Outcome Study, including 13,843 patients receiving Norditropin, no significant correlation was found between the rhGH dose and AE rate [29], which is consistent with our findings.
While the treatment outcomes were most favorable in patients with complete GHD, individuals with TS seemed not to benefit fully from rhGH therapy. In up to 61% of patients for TS cohort, Ht SDS at one-year follow-up was no greater than 0.5. A previous study demonstrated that the outcomes of rhGH treatment are predicted by its dose, age at the therapy onset, mid-parental height and growth response within the first year [30]. The 5-year overall median dose of rhGH in our TS cohort was 0.31 mg/kg/week; this shows more than a half of the patients received rhGH at a slightly lower dose than recommended (0.33 mg/kg/week). Another potential reason for less satisfactory growth outcomes in TS patients might be their relatively older age at treatment initiation (median 9.49 years). Although no strict guideline exists in this matter, it is generally accepted that the earlier the rhGH treatment implemented, the better its outcome.
More than 50% of patients considered in the safety set were excluded from the effectiveness analysis. The largest proportion of the excluded patients did not satisfy the indication requirements, especially for IGHD (25.8%) and ISS (55.9%). This implies that regardless of the reimbursement policies and commonly accepted clinical guidelines, there might be a parental pressure to prescribe rhGH products; indeed, such a tendency was documented in one study conducted in the South Korean population [31]. The rationale for the implementation of rhGH treatment in all ISS patients raises controversies and hence, the decision to start the therapy in this group should be based on comprehensive evaluation of several factors, among them quality of life-related issues, economic burden and functional impact, rather than solely on height benefit and parental request [32].
The results presented above may have a few limitations inherent to each registry study. This study is an observational study without an untreated control group. Moreover, laboratory parameters, such as IGF-I and IGFBP-3, were measured at each site, which might contribute to an interlaboratory measurement bias. To overcome these limitations and to minimize the risk of bias due to missing data, we used a systematic approach to data collection. Many registry studies are based on voluntary reporting of AEs, and/or only ADRs are registered. Meanwhile, our patients were seen at participating centers every six months, to collect all AE data and physical measurements in a standardized manner.
Conclusions
The spectrum of AEs present in rhGH-treated Korean patients was similar to previously reported for other populations. The incidence of AEs, including adverse events of interest, such as neoplasia, glucose intolerance and hypothyroidism, was low. No alarming safety signal was identified at the time of the interim analysis. Most of the growth-retarded patients showed continuous improvement in their Ht SDS during the follow-up period, with the most prominent effect observed within a year of treatment initiation. The beneficial effect of rhGH on Ht SDS gain was maintained for up to four years.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
The authors would like to thank all the principal investigators of “LG Growth Study.”
The following are the principle investigators of LGS (in alphabetical order): Byung Ho Choi (Department of Pediatrics, Hanyoung Children's Hospital, Daegu, Korea); Byung Kyu Suh (Department of Pediatrics, The Catholic University of Korea, Seoul St. Mary’s Hospital, Seoul, Korea); Chan Jong Kim (Department of Pediatrics, Chonnam National University Hospital, Gwangju, Korea); Cheol Woo Ko (Department of Pediatrics, Kyungpook National University Children’s Hospital, Daegu, Korea); Dae-Yeol Lee (Department of Pediatrics, Chonbuk National University Medical School, Jeonju, Korea); Dong Whan Lee (Department of Pediatrics, College of Medicine, Soonchunhyang University Hospital, Seoul, Korea); Dong-Kyu Jin (Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea); Duk Hee Kim (Department of Pediatrics, Sowha Children’s Hospital, Seoul, Korea); Eun Byoul Lee (Department of Pediatrics, The Catholic Kwandong University International St. Mary's Hospital, Incheon, Korea); Eun Young Kim (Department of Pediatrics, Kwangju Christian Hospital, Gwangju, Korea); Eun Young Kim (Department of Pediatrics, College of Medicine, Chosun University, Gwang-ju, Korea); Eun-Gyong Yoo (Department of Pediatrics, CHA University School of Medicine, CHA Bundang Medical Center, Seongnam, Korea); Eun-Mi Cho (Department of Pediatrics, Hanyoung Children's Hospital, Daegu, Korea); Gyung Min Lee (Department of Pediatrics, Konyang University Hospital, Daejeon, Korea); Hae Soon Kim (Department of Pediatrics, Ewha Womans University, College of Medicine, Seoul, Korea); Han Hyuk Lim (Department of Pediatrics, Chungnam National University College of Medicine, Daejeon, Korea); Han-Wook Yoo (Department of Pediatrics, Asan Medical Center, Seoul, Korea); Heon-Seok Han (Department of Pediatrics, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea); Heung-Sik Kim (Department of Pediatrics, Keimyung University, Dongsan Medical Center, Daegu, Korea); Hwa Young Kim (Department of Pediatrics, Kangwon National University Hospital, Chuncheon, Korea); Hye Jin Lee (Department of pediatrics, College of Medicine, Hallym University Kangnam Sacred Heart Hospital, Seoul, Korea); Hye Jung Shin (Department of Pediatrics, National Medical Center, Seoul, Korea); Hye Sook Kim (Department of Pediatrics, Deagu Fatima Hospital, Deagu, Korea); Hye Young Jin (Department of Pediatrics, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea); Hyo-Kyoung Nam (Department of Pediatrics, Korea University Guro Hospital, Seoul, Korea); In Kyu Lee (Department of Pediatrics, Ejin Childrens Hospital, Cheonan, Korea); Jaehong Yu (Department of Pediatrics, Joey Hospital, Daejeon, Korea); Jeesuk Yu (Department of Pediatrics, Dankook University College of Medicine, Dankook University Hospital, Cheonan, Korea); Ji Eun Lee (Division of Pediatric Endocrinology, Department of Pediatrics, Inha University College of Medicine, Inha University Hospital, Incheon, Korea); Ji Hyun Kim (Department of Pediatrics, Dongguk University Ilsan hospital, Goyang, Korea); Jieun Lee (Department of Pediatrics, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea); Jin Kyung Kim (Department of Pediatrics, School of Medicine, Catholic University of Daegu, Daegu, Korea); Jin Soon Hwang (Department of Pediatrics, Ajou University Hospital, Suwon, Korea); Ji-Young Seo (Department of Pediatrics, Nowon Eulji Medical Center, Eulji University, Seoul, Korea); Jong Duk Kim (Department of Pediatrics, Wonkwang University School of Medicine, Iksan, Korea); Joon Woo Baek (Department of pediatrics, College of Medicine, Hallym University Chuncheon Sacred Heart Hospital, Gangwon-do, Korea); Ju Hyung Kang (Department of Pediatrics, Eulji University Hospital, Daejeon, Korea); Jung-Hyun Lee (Department of Pediatrics, Kosin University Gospel Hospital, Busan, Korea); Kee-Hyoung Lee (Department of Pediatrics, Korea University Anam Hospital, Seoul, Korea); Kye Shik Shim (Department of Pediatrics, Kyung Hee University Hospital at Gangdong, Seoul, Korea); Kyoung Soon Cho (Department of Pediatrics, The Catholic University Of Korea, Bucheon St. Mary’s Hospital, Bucheon, Korea); Kyung Hee Yi (Department of Pediatrics, Wonkwang University Sanbon Medical Center, Gunpo, Korea); Mi Jung Park (Department of Pediatrics, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea); Min Ho Jung (Department of Pediatrics, The Catholic University Of Korea, Yeouido St. Mary’s Hospital, Seoul, Korea); Min Jae Kang (Department of Pediatrics, Hallym University Sacred Heart Hospital, Anyang, Korea); Min Jung Kwak (Department of Pediatrics, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea); Se Young Kim (Department of Pediatrics, Bundang Jeseang General Hospital, Seongnam, Korea); Seong Yong Lee (Department of Pediatrics, Seoul metropolitan government- Seoul national university Boramae medical center, Seoul, Korea); So Eun Park (Department of Pediatrics, CHA University School of Medicine, CHA Gangnam Medical Center, Seoul, Korea); Su Jin Kim (Department of Pediatrics, Myongji Hospital, Goyang, Korea); Sung Yeon Ahn (Department of Pediatrics, Ulsan University hospital, Ulsan, Korea); Sung Yoon Cho (Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea); SungWon Park (Department of Pediatrics, Dankook University College of Medicine, Cheil General Hospital & Woman's Health care Center, Seoul, Korea); Su-Yung Kim (Pediatric Department, Pusan National University Yangsan Hospital, Pusan, Korea); Won Kyoung Cho (Department of Pediatrics, The Catholic University Of Korea, St. Vincent`S Hospital, Suwon, Korea); Woo Yeong Chung (Department of Pediatrics, Inje University Busan Paik Hospital, Busan, Korea); Yong Hyuk Kim (Department of Pediatrics, Wonju College of Medicine, Yonsei University, Wonju, Korea); Young Suk Shim (Department of pediatrics, College of Medicine, Hallym University Dongtan Sacred Heart Hospital, Hwaseong-si, Korea); Young-Lim Shin (Department of Pediatrics, College of Medicine, Soonchunhyang University Bucheon Hospital, Bucheon, Korea)
Data Availability
Raw data of this paper can be found in the following address: https://doi.org/10.5061/dryad.3d01n50.
Funding Statement
This cohort study was sponsored by LG Chem, Ltd (http://www.lgchem.com/global/main). LG Chem, Ltd provided support in the form of salary for author EYK and involved with the study design, interpretation of data, decision to publish, and preparation of the manuscript. The specific roles of the authors are articulated in the ‘author contributions’ section.
References
- 1.Grimberg A, DiVall SA, Polychronakos C, Allen DB, Cohen LE, Quintos JB, et al. Guidelines for Growth Hormone and Insulin-Like Growth Factor-I Treatment in Children and Adolescents: Growth Hormone Deficiency, Idiopathic Short Stature, and Primary Insulin-Like Growth Factor-I Deficiency. Horm Res Paediatr. 2016;86(6):361–397. 10.1159/000452150 [DOI] [PubMed] [Google Scholar]
- 2.Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, et al. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol. 2017;177(3):G1–G70. 10.1530/EJE-17-0430 [DOI] [PubMed] [Google Scholar]
- 3.Deal CL, Tony M, Hoybye C, Allen DB, Tauber M, Christiansen JS, et al. GrowthHormone Research Society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab. 2013;98(6):E1072–1087. 10.1210/jc.2012-3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clayton PE, Cianfarani S, Czernichow P, Johannsson G, Rapaport R, Rogol A. Management of the child born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J Clin Endocrinol Metab. 2007;92(3):804–810. 10.1210/jc.2006-2017 [DOI] [PubMed] [Google Scholar]
- 5.Mahan JD, Warady BA; Consensus Committee. Assessment and treatment of short stature in pediatric patients with chronic kidney disease: a consensus statement. Pediatr Nephrol (Berlin, Germany). 2006;21(7):917–930. 10.1007/s00467-006-0020-y [DOI] [PubMed] [Google Scholar]
- 6.Allen DB, Backeljauw P, Bidlingmaier M, Biller BM, Boguszewski M, Burman P, et al. GH safety workshop position paper: a critical appraisal of recombinant human GH therapy in children and adults. Eur J Endocrinol. 2016;174(2):P1–9. 10.1530/EJE-15-0873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raman S, Grimberg A, Waguespack SG, Miller BS, Sklar CA, Meacham LR, et al. Risk of Neoplasia in Pediatric Patients Receiving Growth Hormone Therapy—A Report From the Pediatric Endocrine Society Drug and Therapeutics Committee. J Clin Endocrinol Metab. 2015;100(6):2192–2203. 10.1210/jc.2015-1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pekic S, Stojanovic M, Popovic V. Controversies in the risk of neoplasia in GH deficiency. Best Pract Res Clin Endocrinol Metab. 2017;31(1):35–47. 10.1016/j.beem.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 9.Swerdlow AJ, Cooke R, Albertsson-Wikland K, Borgstrom B, Butler G, Cianfarani S, et al. Description of the SAGhE Cohort: A Large European Study of Mortality and Cancer Incidence Risks after Childhood Treatment with Recombinant Growth Hormone. Horm Res Paediatr. 2015;84(3):172–183. 10.1159/000435856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libruder C, Blumenfeld O, Dichtiar R, Laron Z, Zadik Z, Shohat T, et al. Mortality and cancer incidence among patients treated with recombinant growth hormone during childhood in Israel. Clin Endocrinol. 2016;85(5):813–818. 10.1111/cen.13131 [DOI] [PubMed] [Google Scholar]
- 11.Albertsson-Wikland K, Martensson A, Savendahl L, Niklasson A, Bang P, Dahlgren J, et al. Mortality Is Not Increased in Recombinant Human Growth Hormone-treated Patients When Adjusting for Birth Characteristics. J Clin Endocrinol Metab. 2016;101 (5):2149–2159. 10.1210/jc.2015-3951 [DOI] [PubMed] [Google Scholar]
- 12.Wyatt D. Lessons from the national cooperative growth study. Eur J Endocrinol. 2004;151 Suppl 1:S55–59. [DOI] [PubMed] [Google Scholar]
- 13.Hoybye C, Savendahl L, Christesen HT, Lee P, Pedersen BT, Schlumpf M, et al. The NordiNet(R) International Outcome Study and NovoNet(R) ANSWER Program(R): rationale, design, and methodology of two international pharmacoepidemiological registry-based studies monitoring long-term clinical and safety outcomes of growth hormone therapy (Norditropin(R)). Clin Epidemiol. 2013;5:119–127. 10.2147/CLEP.S42602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Child CJ, Zimmermann AG, Jia N, Robison LL, Bramswig JH, Blum WF. Assessment of Primary Cancer Incidence in Growth Hormone-Treated Children: Comparison of a Multinational Prospective Observational Study with Population Databases. Horm Res Paediatr. 2016;85(3):198–206. 10.1159/000444124 [DOI] [PubMed] [Google Scholar]
- 15.Ranke MB, Lindberg A, Tanaka T, Camacho-Hubner C, Dunger DB, Geffner ME. Baseline Characteristics and Gender Differences in Prepubertal Children Treated with Growth Hormone in Europe, USA, and Japan: 25 Years' KIGS(R) Experience (1987–2012) and Review. Horm Res Paediatr. 2017;87(1):30–41. 10.1159/000452887 [DOI] [PubMed] [Google Scholar]
- 16.Chung S, Yoo JH, Choi JH, Rhie YJ, Chae HW, Kim JH, et al. Design of the long-term observational cohort study with recombinant human growth hormone in Korean children: LG Growth Study. Ann Pediatr Endocrinol Metab. 2018;23 (1):43–50. 10.6065/apem.2018.23.1.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyun SE, Lee BC, Suh BK, Chung SC, Ko CW, Kim HS, et al. Reference values for serum levels of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in Korean children and adolescents. Clin Biochem. 2012;45(1–2):16–21. 10.1016/j.clinbiochem.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 18.Korea Center for Disease Control and Prevention, The Korean Pediatric Society, The Committee for the Development of Growth Standard for Korean Children and Adolescents [Internet]. Korean children and adolescents growth standard (commentary for the development of 2007 growth chart). c2007 [cited 2015 Apr 01]. Available from: http://cdc.go.kr/.
- 19.Swerdlow AJ, Cooke R, Beckers D, Borgstrom B, Butler G, Carel JC, et al. Cancer Risks in Patients Treated With Growth Hormone in Childhood: The SAGhE European Cohort Study. J Clin Endocrinol Metab. 2017;102(5):1661–1672. 10.1210/jc.2016-2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilton P, Mattsson AF, Darendeliler F. Growth hormone treatment in children is not associated with an increase in the incidence of cancer: experience from KIGS (Pfizer International Growth Database). J Pediatr. 2010;157(2):265–270. 10.1016/j.jpeds.2010.02.028 [DOI] [PubMed] [Google Scholar]
- 21.Maneatis T, Baptista J, Connelly K, Blethen S. Growth hormone safety update from the National Cooperative Growth Study. J Pediatr Endocrinol Metab. 2000;13 Suppl 2:1035–1044. [PubMed] [Google Scholar]
- 22.Packer RJ, Cogen P, Vezina G, Rorke LB. Medulloblastoma: clinical and biologic aspects. Neuro Oncol. 1999;1(3):232–250. 10.1093/neuonc/1.3.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen L, Sun CM, Li XT, Liu CJ, Zhou YX. Growth hormone therapy and risk of recurrence/progression in intracranial tumors: a meta-analysis. Neurol Sci. 2015;36(10):1859–1867. 10.1007/s10072-015-2269-z [DOI] [PubMed] [Google Scholar]
- 24.Indini A, Schiavello E, Biassoni V, Bergamaschi L, Magni MC, Puma N, et al. Long-term safety of growth hormone replacement therapy after childhood medulloblastoma and PNET: it is time to set aside old concerns. J Neurooncol. 2017;131(2):349–357. 10.1007/s11060-016-2306-7 [DOI] [PubMed] [Google Scholar]
- 25.Bell J, Parker KL, Swinford RD, Hoffman AR, Maneatis T, Lippe B. Long-term safety of recombinant human growth hormone in children. J Clin Endocrinol Metab. 2010;95(1):167–177. 10.1210/jc.2009-0178 [DOI] [PubMed] [Google Scholar]
- 26.van der Steen M, Smeets CC, Kerkhof GF, Hokken-Koelega AC. Metabolic health of young adults who were born small for gestational age and treated with growth hormone, after cessation of growth hormone treatment: a 5-year longitudinal study. Lancet Diabetes Endocrinol. 2017;5(2):106–116. 10.1016/S2213-8587(16)30422-3 [DOI] [PubMed] [Google Scholar]
- 27.El-Mansoury M, Bryman I, Berntorp K, Hanson C, Wilhelmsen L, Landin-Wilhelmsen K. Hypothyroidism is common in turner syndrome: results of a five-year follow-up. J Clin Endocrinol Metab. 2005;90(4):2131–2135. 10.1210/jc.2004-1262 [DOI] [PubMed] [Google Scholar]
- 28.Kim JY, Rosenfeld SR, Keyak JH. Increased prevalence of scoliosis in Turner syndrome. J Pediatr Orthop. 2001;21(6):765–766. [PubMed] [Google Scholar]
- 29.Savendahl L, Pournara E, Pedersen BT, Blankenstein O. Is safety of childhood growth hormone therapy related to dose? Data from a large observational study. Eur J Endocrinol. 2016;174(5):681–691. 10.1530/EJE-15-1017 [DOI] [PubMed] [Google Scholar]
- 30.Ranke MB, Lindberg A, Chatelain P, Wilton P, Cutfield W, Albertsson-Wikland K, et al. Prediction of long-term response to recombinant human growth hormone in Turner syndrome: development and validation of mathematical models. KIGS International Board. Kabi International Growth Study. J Clin Endocrinol Metab. 2000;85(11):4212–4218. 10.1210/jcem.85.11.6976 [DOI] [PubMed] [Google Scholar]
- 31.Hwang JW, Seo JY. Parents' perception about child's height and psychopathology in community children with relatively short stature. Ann Pediatr Endocrinol Metab. 2015;20(2):79–85. 10.6065/apem.2015.20.2.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen DB. Growth Promotion Ethics and the Challenge to Resist Cosmetic Endocrinology Horm Res Paediatr. 2017;87(3):145–152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
Raw data of this paper can be found in the following address: https://doi.org/10.5061/dryad.3d01n50.