Abstract
Methamphetamine (Meth) is a potent and commonly abused psychostimulant. Meth alters neuron and astrocyte activity; yet the underlying mechanism(s) is not fully understood. Here we assessed the impact of acute Meth on human fetal astrocytes (HFAs) using whole-cell patch-clamping. We found that HFAs displayed a large voltage-gated K+ efflux (IKv) through Kv/Kv- like channels during membrane depolarization, and a smaller K+ influx (Ikir) via inward-rectifying Kir/Kir-like channels during membrane hyperpolarization. Meth at a ‘recreational’ (20 μM) or toxic/fatal (100 μM) concentration depolarized resting membrane potential (RMP) and suppressed IKv/Kv-like. These changes were associated with a decreased time constant (T), and mimicked by blocking the two-pore domain K+ (K2P)/K2P- like and Kv/Kv-like channels, respectively. Meth also diminished IKir/Kir-like, but only at toxic/fatal levels. Given that Meth is a potent agonist for the trace amine-associated receptor type-1 (TAAR1), and TAAR1-coupled cAMP/cAMP-activated protein kinase (PKA) cascade, we further evaluated whether the Meth impact on K+ efflux was mediated by this pathway. We found that antagonizing TAAR1 with N-(3-Ethoxyphenyl)-4-(1-pyrrolidinyl)-3-(trifluoromethyl)benzamide (EPPTB) reversed Meth-induced suppression of IKv/Kv-like; and inhibiting PKA activity by H89 abolished Meth effects on suppressing IKv/Kv- like. Antagonizing TAAR1 might also attenuate Meth-induced RMP depolarization. Voltage-gated Ca2+ currents were not detected in HFAs. These novel findings demonstrate that Meth suppresses IKv/Kv-like by facilitating the TAAR1/Gs/cAMP/PKA cascade and altering the kinetics of Kv/Kv-like channel gating, but reduces K2P/K2P-like channel activity through other pathway(s), in HFAs. Given that Meth-induced decrease in astrocytic K+ efflux through K2P/K2P-like and Kv/Kv-like channels reduces extracellular K+ levels, such reduction could consequently contribute to a decreased excitability of surrounding neurons.
Keywords: astrocyte, calcium channel, electrophysiology, methamphetamine, potassium channel, TAAR1
Methamphetamine (Meth) is a psychostimulant that is highly addictive and widely abused in the USA (Panenka et al. 2013). Meth alters cognition and motivation-driven behaviors in humans, which are associated with neurodegeneration, microgliosis, and astrocytosis (Cadet and Krasnova 2009; Krasnova and Cadet 2009; Parsegian et al. 2011). Animal studies also indicate that Meth self-administration (Meth-SA) decreases neuronal excitability in the nucleus accumbens (NAc) (Graves et al. 2015) and medial prefrontal cortex (Chen et al. 2012), two major regulators of cognition and addiction. Other studies suggest that Meth-induced neuronal dysregulation is functionally affected by glia (Narita et al. 2006; Zhang et al. 2006; Beardsley et al. 2010; Fujita et al. 2012; Snider et al. 2012, 2013). However, little is known about the mechanism(s) by which Meth suppresses neuronal firing; and how Meth, besides disturbing catecholamine neurotransmission and glutamate uptake, alters astrocyte function (Cisneros and Ghorpade 2014), thereby altering excitability of surrounding neurons.
Astrocytes play a crucial role in the CNS, including, but not limited to, maintenance of extracellular levels of glutamate and K+ ([K+]o) (Beardsley and Hauser 2014; Cheung et al. 2015; Kadala et al. 2015). Previous studies reveal that astrocytes functionally express various K+ channel subtypes; while Meth alters neuronal and nonneuronal cell activity by altering K+ channels. For example, Meth decreases the expression of alternatively spliced variants of Ca2+-activated bulk-conductance K+ channels in human frontal cortex neurons (Tatro et al. 2013), and reduces K+ currents through them in mouse neurons (Wang et al. 2013). Meth also suppresses the expression of voltagegated K+ (Kv) channels and inwardly rectifying K+ (Kir) channels (Qu et al. 2014), thereby inhibiting IKir and IKv, respectively, in rat cardiac myocytes (Liang et al. 2010). Meth-SA also diminishes K+ efflux via G protein-coupled inwardly rectifying K+ channels in rat dopaminergic neurons (Sharpe et al. 2015). However, very little is known about how and to what extent Meth alters K+ channel activity in astrocytes.
Besides dysregulating the catecholamine neurotransmission, Meth also disrupts activity of K+ channels by affecting trace amine-associated receptor type-1 (TAAR1)-coupled signaling. Meth is a potent agonist for TAAR1 (Bunzow et al. 2001; Cotter et al. 2015), and expressed widely in neurons (Xie and Miller 2009; Miller 2011; Liberles 2015) and astrocytes (Cisneros and Ghorpade 2014). TAAR1 mediates dopamine neurotransmission and astrocyte glutamate uptake by facilitating the TAAR1/Gs/cAMP/cAMP-activated protein kinase (PKA) signaling (Jing and Li 2015). Meth binds to TAAR1, which promotes the Gs protein-coupled PKA signaling, and Gq protein-coupled phospholipase C/protein kinase C (PKC) signaling (Miller et al. 2005; Xie et al. 2007; Xie and Miller 2009; Lin et al. 2010; Panas et al. 2012). Meth-induced TAAR1 activation also increases intracellular cAMP levels in primary human astrocytes (Cisneros and Ghorpade 2014). PKA-induced phosphorylation reduces Kv channel activity (Dong and White 2003), while reduced PKA activity is associated with decreased IKir, in cortical pyramidal neurons (Dong et al. 2004). Moreover, PKC-induced phosphorylation also inhibits activity of the two-pore K+ (K2P) channel that controls K+ efflux at resting status to maintain RMP (Veale et al. 2007). PKC-induced phosphorylation also inhibits Kir channel activity in cardiac cells (Scherer et al. 2016). Together, these findings strongly suggest that Meth alters astrocyte-mediated K+ homeostasis by disturbing the TAAR1-mediated PKA and PKC signaling pathway.
Our recent studies have been focusing on how acute Meth in vitro dysregulates activity and signaling pathway(s) in human fetal astrocytes (HFAs) (Sharma et al. 2011; Yu et al. 2017a), additional to that mediated by PKA and PKC. Such Meth dysregulation also contributes to the mechanism(s) by which Meth alters neuronal activity, and exacerbates the neuropathogenesis induced by comorbid HIV infection (Al-Harthi 2012; Narasipura et al. 2012). To advance our understanding for the Meth impact on K+ channel activity and its underlying mechanism(s) in HFAs, we performed electrophysiological studies using whole-cell voltage-clamp recording of HFAs following acute Meth exposure (20 and 100 μM) in vitro. We selected such concentrations based on that the blood levels of Meth in ‘recreational’ users are reported at 0.2–17 lM (Melega et al. 2007); while higher Meth blood levels (~ 58–400 μM) could be toxic/fatal (Takekawa et al. 2007; Kiely et al. 2009). These clinical studies provide us the opportunity to suggest and use such ‘recreational’ and ‘fatal/toxic’ doses of Meth in the present study. Furthermore, using various K+ channel blockers, TAAR1 antagonist, and PKA inhibitor, we also evaluated the mechanism(s) by which Meth affects astrocyte function.
The present study not only determines the acute effects of Meth in vitro on HFAs, but also defines a novel mechanism, by which acute Meth exposure alters astrocyte activity through altering K+ channel function by interrupting the TAAR1-mediated signaling pathways. Importantly, that in turn could functionally disrupt astrocyte-neuron communication in the brain.
Materials and Methods
Cell culture and treatments
Primary HFAs (catalogue #CC-2565) were purchased from Lonza, Inc (Allendale, NJ, USA), and grown in clonetics astrocyte basal medium (CABM) supplemented with Astrocyte growth media Bullet-Kit (catalogue # CC-3186) (both from Lonza, Inc) in a 37°C incubator with 95% O2 and 5% CO2. HFAs (called Normal Human Astrocytes by Lonza) are not a cell line, which differ significantly from many cell lines. They are primary cells (not glioblastoma), and do not replicate. Using such HFAs, we avoided possible influences from other cells, especially those that can replicate and possibly take-over a dish. HFAs (up to the fourth splitting) were plated on petri dishes (~ 5000–8000 cells/dish) coated with polyornithine and fibronectin. Cells were fed with CABM 1 day after plating, and every 3–4 days thereafter. Prior to electrophysiological assessment, HFAs were treated in culture medium or bath with Meth, selective K+ channel blockers, TAAR1 inhibitor, and/or compounds that affect related signaling (see Selective blocker and inhibitor section below). Cultured HFAs were used for electrophysiological studies within 2–5 days after plating.
In Meth studies, CABM containing Meth (20 or 100 μM) was used to treat cells acutely for 3–6 h prior to assessment. We chose this exposure time period based on the fact that peak plasma Meth concentrations are achieved in approximately 3–6 h after drug ingestion (Schep et al. 2010), during which brain astrocyte/neuron activity are altered. Our previous studies demonstrate that Meth exposure in vitro (10–1000 μM) for 5 days significantly alters HFA function, and repeated Meth administrations in 1 day also alter astrocyte function, and induces neuronal toxicity in vivo (Yu et al. 2017b). Our pilot data also showed that 20 μM Meth induced similar suppression on astrocytic K+ currents after a 2-day exposure (data not shown) compared to a 3–6-h exposure. These findings encourage us to use 3–6-h Meth exposure regimen to evaluate acute effects of Meth on affecting functional activity of HFAs. Phosphate- buffered saline was used as a vehicle control. Before assessment, the media was replaced by the bath solution (see below in Electrophysiology section) containing the same concentration of Meth in pretreatment.
Electrophysiology
Whole-cell voltage-clamp mode was used for patch-clamping study. HFAs were visualized using a Nikon Diaphot 300 upright microscope (Nikon instruments, Melville, NY, USA). All HFAs were in isolation by visual inspection, in which neither the soma nor any process was physically contacted with any others. Healthy HFAs were chosen and studied using the following criteria: (i) had smooth edges with a more transparent appearance compared to dead cells; (ii) had a higher profile than the flat counterparts (that were extremely difficult to record); and (iii) HFAs were distinguished from neurons by their morphology and size in coculture. Specifically, HFAs had more than two processes and a flatter appearance, while Lund human mesencephalic neurons neurons were smaller (less than 17 pF). A Digidata 1440A digitizer and Axon 200B or 700A amplifier (Molecular Devices, Sunnyvale, CA, USA) were used for recording. Electrodes (4–10 MΩ) were pulled from borosilicate pipettes using a pipette puller P-97 (Sutter Instruments Co. Novato, CA, USA), and filled with pipette solution. The pipette solution contained (in mM): K-gluconate 120, HEPES 20, EGTA 0.1, KCl 10, MgCl2 1, Na2ATP 4 (pH 7.29–7.31, 270–280 mOsm). For assessing the ‘basal’ condition, the bath solution contained (in mM): NaCl 135, MgCl2 1, CaCl2 2, HEPES 5, KCl 4, glucose 5 (pH 7.37–7.43, 280–290 mOsm); but no ion channel blockers. For assessing the ‘control’ condition of K+ channel activity, bath solution also contained blockers for the voltage-gated Na+ channel (tetrodotoxin, TTX, 500 nM) (Abcam, Cambridge, MA, USA) and Ca2+ channel (CdCl2, 2 μM) (Hu et al. 2004, 2005; Nasif et al. 2005a,b; Khodr et al. 2016).
For studying voltage-gated Ca2+ currents (VGCCs) in HFAs, all other non-Ca2+ ionic currents were blocked to ensure accurate evaluation of possible VGCCs in HFAs. In Ca2+ current (ICa) studies, the pipette solution contained (in mM): Cs-gluconate 140, HEPES 10, MgCl2 2, Na2ATP, and NaGTP 0.3 (pH 7.29–7.31, 270–280 mOsm). Assessments were performed with blockade of (i) the voltage-gated Na+ channels (TTX, 500 nM), (ii) voltage-gated chloride channels (4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid, 200 μM) (Tocris Biosciences, Bristol, UK) (Olsen et al. 2003), (iii) the hyperpolarization-activated (Ih) channels (2 mM CsCl), and (iv) various types of K+ channels [using tetraethyl-ammonium (TEA), 20 mM]; and 4-aminopyridine (1–4 mM, a blocker for voltagesensitive A-type Kv channels) (Calbiochem Inc, San Diego, CA, USA), and BaCl2 (2 mM; this concentration blocked not only Kir channels, but also Kv and K2P channels) (Hille 2001; Hu et al. 2004, 2005; Nasif et al. 2005a,b; Khodr et al. 2016).
Extremely gentle suction (−0.1 to −1 psi) was applied to form a gig-ohm seal, aided with a Pyle PDMM 15 Manometer (Pyle Instruments, Brooklyn, NY, USA). The experimenter waited 1.5 to 3 min before breaking in to form whole-cell configuration. To allow time for solution equilibration, the first recording was performed at least 5 min after formation of the whole-cell configuration. Stable RMP was measured with the amplifier in I = 0 mode. For assessment of K+ channel activities, the membrane potential (Vm) was initially held at −70 mV level, and currents were assessed from −50 to +100 mV with a 10 mV increment for 250 ms (unless specified). We chose this Vm range because we found that under our experimental condition, the reversal potential of HFAs was around −50 mV. For Ca2+ channel studies, voltages were tested in the range of −70 to +50 mV with 5 mV increments for 100 ms (cocultured), or 250 ms (cultured alone). Multiple recordings were performed and consistency was assessed.
pClamp 10 software (Molecular Devices) was used for acquisition and analysis. All the currents were measured at the steady state time point 10 ms prior to the end of each step. The current density (pA/pF) was compared via dividing the actual current magnitude by membrane capacitance (Cm). Input resistance (Rin) was calculated as 10 mV/ΔI, where ΔI = the difference between the steady state K+ currents at the two voltage steps encompassing 0 pA. Given that moderately high series resistances were common (the mean of control cells was approximately 35 MΩ), Channellab software (Synaptosoft Inc, Atlanta, GA, USA) was used to correct the series resistance errors off-line and to achieve 100% correction (Traynelis 1998), while the correction and prediction in pClamp were set to zero during recording.
Ion blockers and inhibitors
All drugs were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise specified. During recording, various ion channel blockers were added in bath solution for at least 10 min (unless specified). Quinidine (VWR, Radnor, PA, USA) is a blocker for a variety of K+ channels, including human K2P channel subtypes (TASK-1, TREK-1, and TREK-2) (Lotshaw 2007); and was used here to exam acute Meth effects on IK2p, a ‘leaking’ K+ efflux that maintains RMP and is mediated mainly by K2P channels (Hille 2001). Given that K2P channels are commonly expressed in astrocytes (Kettenmann and Ransom 2013), and the IC50 of quinidine was ~ 100 μM in blocking human K2P channels (Lotshaw 2007), 1 mM quinidine was used to ensure its maximal effects on blocking IK2P. Another K+ blocker, TEA, was used to determine if it could mimic and confirm the direct, acute effects of Meth on suppressing voltage-sensitive K+ channels during Vm depolarization in HFAs in vitro.
The TAAR1 antagonist EPPTB (25 nM) (Tocris Biosciences, Bristol, UK) was used to block or reverse acute Meth effects on TAAR1 (Bradaia et al. 2009). It is worth noting that EPPTB also acts as an inverse agonist, which could have an opposite effect on TAAR1 compared to a TAAR1 agonist (Bradaia et al. 2009). On the day of electrophysiological assessment, cells were pre-treated with CABM containing EPPTB for 30 min, followed by treatment with CABM-containing EPPTB (25 nM) + Meth (20 μM) for 3–6 h, prior to recording. H89 (Tocris Biosciences, Bristol, UK), a PKA inhibitor (Lochner and Moolman 2006; Murray 2008), was also used to determine if Meth exerted its acute effects on K+ channel activity through the cAMP/PKA cascade. During recordings, HFA currents were assessed prior to H89 application, and then were treated with H89 (10 μM) for 15–20 min before a further assessment.
Measurement of Time Constant
The time constant (T) was assessed at all Vm levels in HFAs to determine if there was any significant change in the kinetics of voltage-sensitive K+ channel gating induced by Meth, which could be reflected partly by altered activation of K+ currents through these channels. The time courses of IKv traces were fitted over a 100 ms time period using a two term exponential function (Levenburg–Marquardt search and Sum-of-Squares minimization method) in Clampfit 10.7. This measurement obtained two T: the fast T that reflects the kinetics of K+ channel gating in the initial period of the activation prior to the steady state; and the slow T that reflects the kinetics of K+ channel gating mainly in an early period of the steady state; and this would normally include an influence from the inactivation of some A-type K+ channels (which are more quickly activated, and then inactivated, than other subtypes of Kv channel, a.k.a. the delayed rectifier). Individual points of the T were excluded if they did not fit properly (e.g., the regression fails to fit, or the value of the T was over 5000 ms). The outlier cells (which had two or more Vm levels excessing the ± twofold SD of the mean) were excluded (also see the Results section for more detail).
Statistical Analysis
Student’s t-tests were used for the comparison of Rin, Cm, and RMP from basal control versus vehicle-treated control cells. One-way ANOVA was used for the comparison of RMP in K2P channel studies. In this study, replication refers to the same cell before and after treatment or at different time points. Two-way ANOVA with repeated measurement (rm) was used to assess the effects of Meth, inhibitors or blockers on K+ currents in HFAs, with the exception of the TEA and Meth effects which was analyzed first using three-way ANOVA. When there was no significant difference in the three-way interactions, but a significance difference in the main factors and/or two- way interaction, data analysis was further performed using the two- way rmANOVA. All ANOVA were followed by post hoc tests as specified. Cells were excluded if they were found to be the outliers (which had a value that exceeded the mean ± 2 × standard deviation, SD) in any category, including Rin, RMP, Cm, and series resistance. All values were reported as the mean standard error (SE). The ‘n’ in the figure legend indicates the number of cells. Statistical analysis was conducted accordingly using Prism 7 (GraphPad Software Inc., La Jolla, CA, USA) and considered significant when p ≤ 0.05. Some raw data were used two or three times for statistical data analysis. Under such experimental conditions, the Bonferroni correction was applied and α = 0.025 or 0.0167 (instead of 0.05) was used appropriately. For example, the RMP measurement from all the HFAs under the control condition (n = 41; e.g., with blockade of voltage-gated Ca2+ and Na+ channels) were used to compare with the basal condition (without blockade of voltage-gated Ca2+ and Na+ channels). Among which, a subset of control cells (n = 37) was used again for assessing the acute effects of Meth (with different doses, Fig. 1c) on RMP and the effect of EPPTB on RMP (Fig. 1e); and the rest of that group of data (n = 4; Fig. 1d) were used to access the effects of Meth and quinidine on RMP. The data of Kv currents from the control cells and the Meth-treated cells were also used twice for assessing the effects of Meth (with different doses; Fig. 2), and the effects of Meth plus EPPTB (Fig. 4) on Kv currents. This study was not preregistered. No blinding, randomization, or sample size calculation was performed in this study.
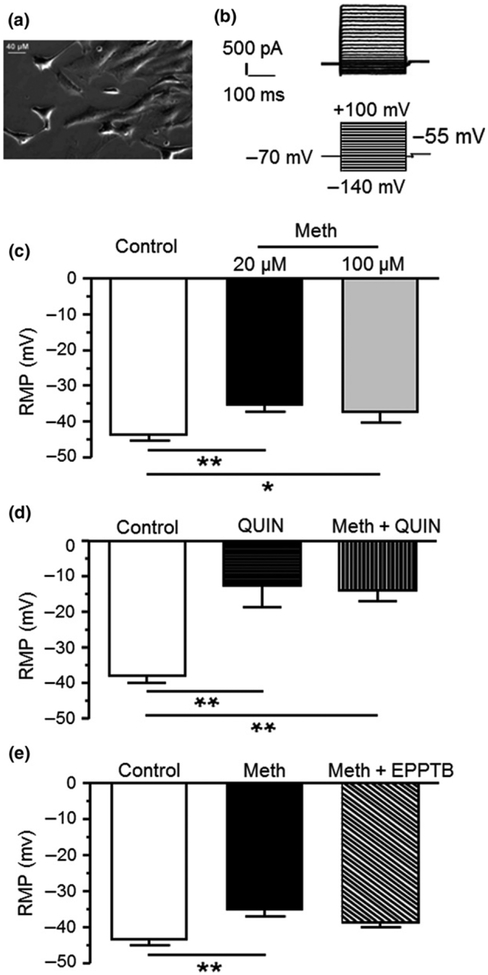
Fig. 1.
(a) Cultured human fetal astrocytes (HFAs) in a phase-contrast image. (b) The top panel shows representative current traces from an untreated HFA in response to membrane hyperpolarization (downward) or depolarization (upward). The lower panel shows the recording protocol (Vm clamping) used for assessing IK in HFAs. (c) Resting membrane potential (RMP) was depolarized in response to 20 and 100 μM methamphetamine (Meth) compared to vehicle-treated control (Control vs. 20 and 100 μM Meth, n = 37 vs. 17 and 12 HFAs (cells) from 29, 14, and 8 independent experiments, respectively; One-way ANOVA: F(2,63) = 6.119, p = 0.004; with Newman–Keuls post hoc test: *,**p < 0.05 or 0.01). (d) K2P channel blockade (QUIN, 1 mM) also induced RMP depolarization in control cells. There was no further RMP depolarization in HFAs treated with Meth + quinidine (n = 4/4 cells/independent experiments for each group; One-way ANOVA: F(2,9) = 13.1, p = 0.002; with Newman–Keuls post hoc test: **p < 0.01). (e) Meth-induced RMP depolarization was partially attenuated by combined treatment with TAAR1 antagonist N-(3-Ethoxyphenyl)-4-(1-pyrrolidinyl)-3-(trifluoromethyl)benzamide (EPPTB) (25 nM) (control vs. Meth, and Meth + EPPTB, n = 37/29 vs. 17/14 and 9/4 cells/independent experiments, respectively; One-way ANOVA: F(2,60) = 6.598, p = 0.003; with New-man Keuls post hoc test, **p < 0.01). There was no significant difference in RMP between control cells and those treated with Meth plus TAAR1 antagonist (p = 0.181).
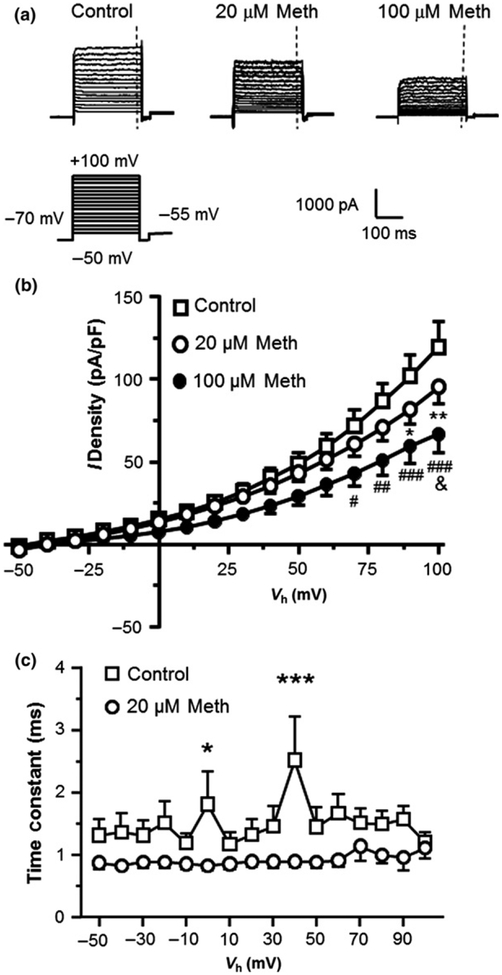
Fig. 2.
Voltage-sensitive outflowing IKv during Vm depolarization were significantly reduced in response to acute methamphetamine (Meth) exposure. (a) Sample traces of IKv at control condition, and in response to 20, and 100 μM Meth. The vertical dashed lines indicate the time points at which the currents were measured. (b) Acute Meth exposure in vitro induced a significant decrease in the voltage-sensitive outflowing IKv (Control vs. 20 and 100 μM Meth, n = 22/18 vs. 19/12 and 9/7 cells/independent experiments, respectively; Two-way rmANOVA: treatment: F(2,47) = 2.775, p = 0.0726; voltage: F(15,705) = 106.7, p < 0.001; interaction: F(30,705) = 2.784, p < 0.001. Tukey’s post hoctest: *,**p < 0.05 or 0.01 for Control vs. Meth 20 μM, #,##,###p < 0.05, 0.01, or 0.001 for Control vs. 100 μM Meth, and &p < 0.05 for 20 μM Meth vs. 100 μM Meth). (c) Acute Meth significantly reduced the time constant (T) of IKv during the activation of Kv/Kv-like K+ channels in human fetal astrocytes compared to controls (Control: n = 20/18 vs. Meth: n = 14/10 cells/independent experiments; Two-way rmANOVA: treatment: F(1,32) = 5.377, p = 0.027; voltage: F(15,467) = 1.028, p > 0.05; interaction: F(15,467) = 1.143, p > 0.05. Newman–Keuls post hoc test: *,***p < 0.05 or 0.001).
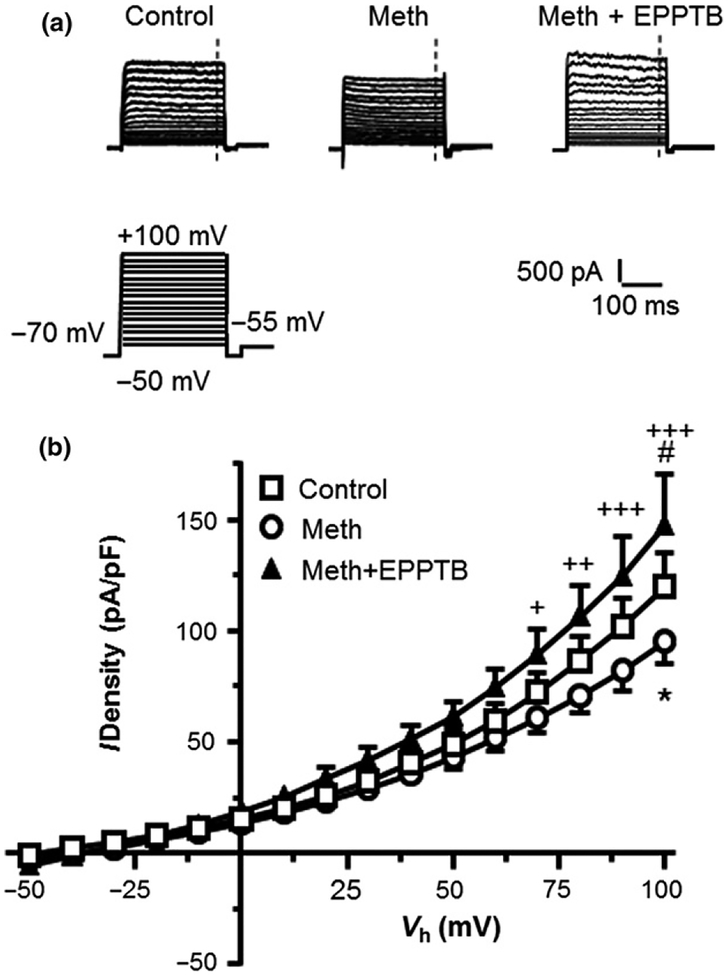
Fig. 4.
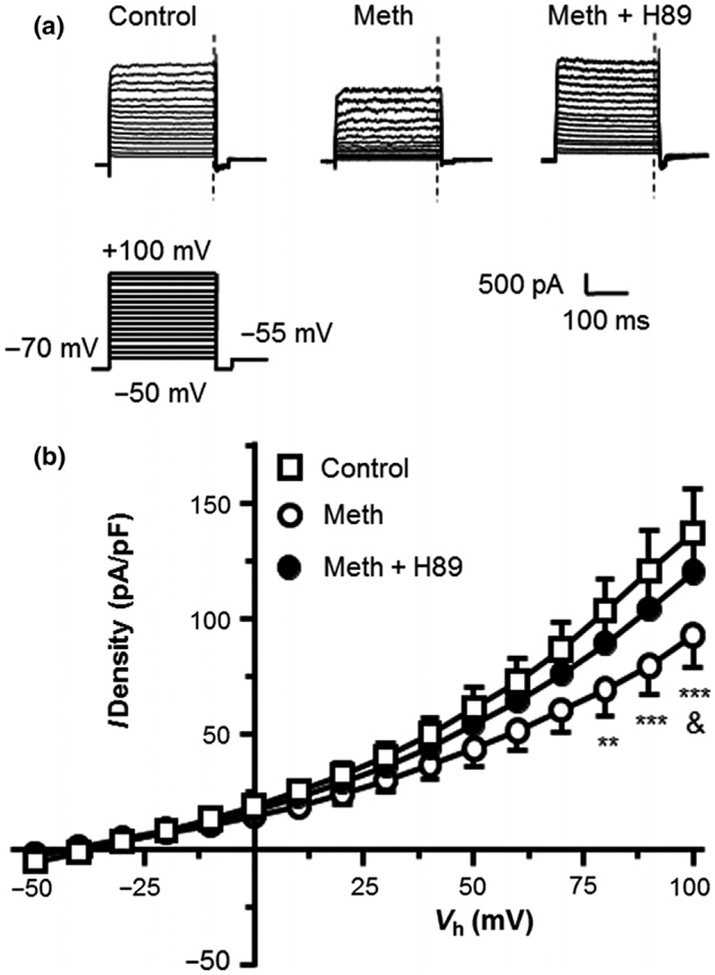
Antagonism of TAAR1 by N-(3-Ethoxyphenyl)-4-(1-pyrrolidinyl)-3-(trifluoromethyl)benzamide (EPPTB) reversed acute effects of methamphetamine (Meth) on suppressing IKv. (a) Sample traces from human fetal astrocytes (from the left to right) at control condition, treated with Meth (20 μM), or with Meth (20 μM) plus EPPTB (25 nM). The vertical dashed lines indicate the time points at which the currents were measured. (b) The I-V curves show that combined treatment of EPPTB and Meth reversed the Meth-induced decrease in IKv (Control vs. Meth and Meth + EPPTB: n = 22/18 vs. 19/12 and 9/4 cells/independent experiments, respectively; Two-way rmANOVA: treatment: F(2,47) = 1.786, p = 1.786; voltage: F(15,705) = 155.7, p < 0.001; interaction: F(30,705) = 2.112, p < 0.001; with Tukey’s post hoc test: Control vs. Meth, *p < 0.05; Control vs. Meth + EPPTB, #p < 0.05; Meth vs. Meth + EPPTB: +,++,+++p < 0.05, 0.01 or 0.001).
Results
Electrophysiological properties of HFAs
We conducted voltage-clamp recordings to assess the membrane properties of cultured primary HFAs (Fig. 1a), including RMP, Rin, and Cm. We found that RMP was −42.0 ± 6.8 mV (n = 5, range of −27 to −63 mV) in HFAs at a basal condition (without blockade of any type of membrane ion channels). This result is consistent with previous studies of cultured astrocytes, showing a more depolarized RMP in immature astrocytes from the neocortex of prenatal rats (−22 to −82 mV) (McKhann et al. 1997), or in freshly isolated astrocytes from the hippocampus of postnatal rats (−25 to −85 mV) (Zhou and Kimelberg 2000) (also see more detail regarding this issue in the Discussion section). We also found that Rin was 181.5 ± 67.2 MΩ, and Cm was 18.9 ± 5.9 pF in HFAs (n = 5). There was no any type of voltage sensitive spike in HFAs in response to membrane depolarization (Fig. 1b).
We further assessed the membrane properties of HFAs, with or without blockade of voltage-gated Na+ channels (with TTX, 500 nM) and Ca2+ channels (with CdCl2, 2 μM). We found that Na+ and Ca2+ channel blockade did not significantly affect the HFA membrane properties, including RMP (without blockers: n = 5, −42.0 ± 6.8 mV; with blockers: n = 41, −43.3 ± 1.3 mV; t44 = 0.287, p = 0.775), Rin (without blockers: n = 5, 181.5 ± 67.2 MΩ; with block-ers: n = 37, 275.4 ± 34.4 MΩ; t40 = 0.964, p = 0.341), and Cm (without blockers: n = 5, 18.9 ± 5.9 pF; with blockers n = 37, 31.2 ± 2.3 pF; t40 = 1.822, p = 0.076). Given that astrocytes play a critical role in K+ buffering; and their RMP depends upon K+ channel activity, these findings suggest that the activity of HFAs also relays on normal function of K+ channels. Thus, the following experiments throughout the present study were conducted and focused on K+ channel activity under blockade of voltage-sensitive Na+/Ca2+ currents at RMP and during Vm changes.
Meth depolarized RMP of HFAs
RMP of cells is established by K+ efflux at resting states (e.g., IK2P), which is typically regulated by K2P channels (Kim 2005), and some K2P-like Kir channels in mature astrocytes (Olsen et al. 2006; Olsen and Sontheimer 2008; Kettenmann and Ransom 2013). To determine Meth effects on RMP of HFAs, we assessed RMP that was exposed to 20 or 100 μM Meth in vitro. We found that both 20 μM Meth (n = 17, −35.6 ± 1.8 mV) that mimicked non-lethal blood levels of Meth in Meth users (Melega et al. 2007), and 100 μM Meth (n = 12, −37.4 ± 2.9 mV) that was similar lethal Meth levels (Takekawa et al. 2007; Kiely et al. 2009), induced a significant RMP depolarization in HFAs compared to those in the control condition (n = 37, −43.8 ± 1.4 mV; One-way ANOVA: F(2,63) = 6.119,p = 0.004) (Fig. 1c). There was no significant difference between RMP depolarization induced by 20 or 100 μM Meth, suggesting that 20 μM Meth exerted a maximal effect on suppressing IK2P/IK2P-like that led to RMP depolarization in HFAs.
To determine whether Meth-induced RMP depolarization resulted from reduced activity of K2P/K2P-like channels, we assessed the effects of K2P/K2P-like channel blockade by quinidine (1 mM, a general blocker for various K+ channels including human K2P channels) on RMP (Lotshaw 2007); and then compared that with the effects of quinidine + Meth (20 μM) on RMP. We found that quinidine blockade also significantly depolarized RMP compared to HFAs in control condition. Combined quinidine + Meth treatment did not induce further depolarization compared to that induced by quinidine alone (n = 4/ea; Control: −38.2 ± 1.9 mV; quinidine: −12.7 ± 5.9 mV; quinidine + Meth: −14.0 ± 2.9 mV; One-way ANOVA: F(2,9) = 13.1, p = 0.002) (Fig. 1d). Together, these findings suggest that Meth depolarizes RMP by suppressing K2P or K2P-like channels that conduct outflowing K+ at the rest.
We then determined whether Meth-induced RMP depolarization was mediated by TAAR1 using the TAAR1 antagonist EPPTB (25 nM; which also is an inverse agonist of TAAR1) (Bradaia et al. 2009), and H89 (10 μM), an inhibitor for PKA which is a downstream player in the TAAR1-mediated signaling pathway (Cisneros and Ghorpade 2014). We found that neither EPPTB (Control: n = 37, −43.8 ± 1.4 mV; Meth: n = 17, 35.6 ± 1.8 mV; Meth + EPPTB: n = 9, 38.5 ± 1.5 mV; One-way ANOVA: F(2,60) = 6.598, p = 0.003) (Fig. 1e), nor H89 (data not shown), blocked Meth-induced RMP depolarization (p > 0.05). Intriguingly, there was also no significant difference (p > 0.05) in RMP between control cells and those treated by Meth + TAAR1 antagonist (Fig. 1e). Collectively, these results suggest that, although acute Meth effect on depolarizing RMP appears to be PKA-independent, we can’t rule out the possibility that Meth reduces K2P/K2P-like channel activity by activating TAAR1 through other mechanisms (e.g., the Gq/phospholipase C (PLC)/protein kinase C (PKC)/K2P channel signaling pathway). Future investigation is needed to clarify this issue.
Meth reduced voltage-sensitive K+ efflux (IKv) in HFAs
Kv channels (including, but not limited to, the delayed rectifier) play a key role in astrocyte activation (MacFarlane and Sontheimer 1997). By conducting K+ efflux, Kv channels regulate the extracellular K+ homeostasis (Somjen et al. 2008); and in-turn modulates neuronal activity. To examine the Meth effect (20 or 100 μM) on Kv/Kv-like channels (Olsen and Sontheimer 2008), we assessed IKv. We found that HFAs displayed a large voltage-dependent IKv (i.e., indicated by the upward traces) (Fig. 2a, Control). The current-voltage relationships (I-V curves) show that astrocytic IKv was activated by Vm depolarization from a holding potential Vh = −70 mV to −50 mV and above up to +100 mV 2b, Control). Given that other types of voltage-gated ion channels were blocked; and that the reversal potential (approximately −50 mV) for this K+ efflux was near the Vm at which Kv channels were normally activated, this finding suggest that the evoked K+ efflux (IKv) was mediated mainly by Kv channels.
We also found that acute exposure to Meth (20 μM, n = 19; and 100 μM, n = 9) induced a significant reduction in IKv compared to vehicle-treated controls (n = 22; Two- way rmANOVA: interaction, F(30,705) = 2.784, p < 0.001) (Fig. 2a and b). IKv was further suppressed by 100 μM Meth compared to 20 μM Meth. The X-axis and the Y-axis indicate the Vm and current density (pA/pF), respectively. The different Y intersects in the I-V plots indicate different K+ current densities measured at the point of zero voltage level under blockade of other voltage-sensitive ion channels. Given that the ‘conventional’ Kv channel (a.k.a. the delayed rectifier) is activated at Vm around −60 mV level, whether these K+ currents out-flowing at Vm = 0 mV level are actually through such Kv channels, or via other Kv-like channel(s), needs to be identified in future studies.
We also assessed Meth effects on the T (which partly reflects the alterations in the kinetics of Kv/Kv-like channel gating). We found that the fast T was significantly decreased in HFAs following Meth treatment compared to controls (control n = 20, Meth n = 14, Two-way rmANOVA: treatment: F(1,32) = 5.377, p = 0.027) (Fig. 2c). In contrast, there was no significant change in the slow T (p > 0.05). Together, these results suggest that, in a dose-dependent manner, Meth reduces IKv by suppressing Kv/Kv-like channel activity; and this IKv reduction is associated with alterations in the kinetics of Kv/Kv-like K+ channel gating, including but might not be limited to, a significantly decreased fast T during an early period of the activation of Kv/Kv-like channels, but not in their steady state. Future investigations are needed to reveal whether and how the activation, and inactivation, of Kv/Kv- like channels in mature astrocytes are altered after chronic exposure to Meth in vivo.
Meth-induced IKv reduction was mimicked by blockade of KV channels
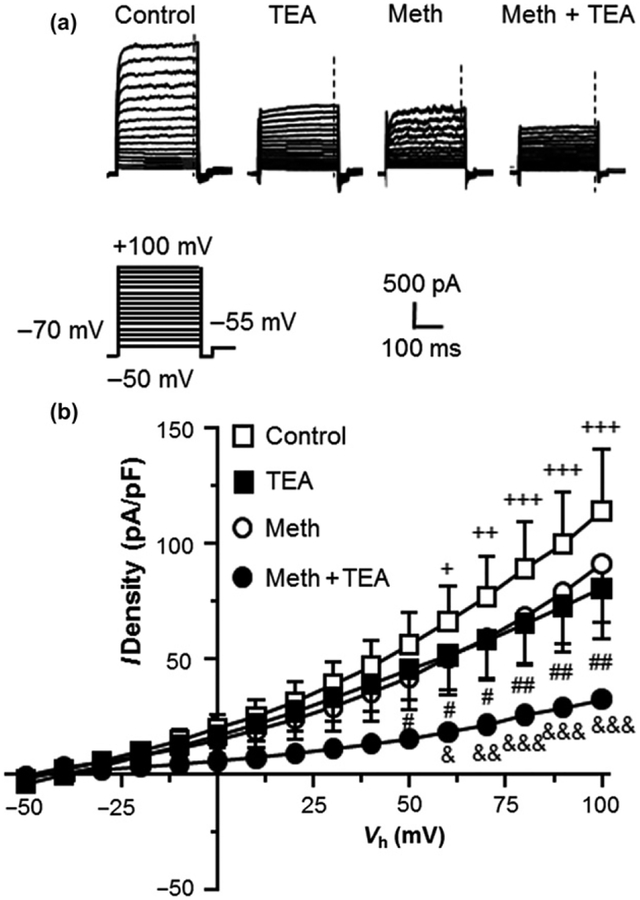
To determine whether Meth-induced reduction of K+ efflux during Vm depolarization was mediated by decreased Kv channel activity, we mimicked this Meth effect by blocking IKv with TEA (20 mM) (Fig. 3a). We found that blockade of Kv/Kv-like channel activity with TEA during Vm depolarization significantly reduced IKv in the control group (n = 6/ea.; Two-way rmANOVA: interaction: F(15,75) = 6.397, p < 0.001) (Fig. 3b). This TEA-induced IKv reduction was similar to that induced by Meth (n = 4) (Fig. 3b). Combined Meth + TEA treatment (n = 4) induced a further reduction in IKv compared to either one alone (Two-way rmANOVA: Meth vs. Meth + TEA: interaction: F(15,45) = 4.308, p < 0.001; TEA vs. Meth + TEA: interaction: F(15,120) = 3.027, p < 0.001) (Fig. 3b). These results indicate that Meth suppresses astrocytic IKv, in part by reducing Kv channel activity. However, combined Meth/TEA treatment induced a significantly greater decrease in IKv. Although the mechanism underlying this additional decrease is unknown, this result suggests that Meth and TEA might affect different subtypes of Kv (or Kv-like) channels.
Fig. 3.
IKv efflux through Kv channels was significantly reduced by methamphetamine (Meth) and tetraethyl-ammonium (TEA). (a) Sample traces of IKv in human fetal astrocytes, with or without Meth (20 μM) and/or TEA. The vertical dashed lines indicate the time points at which the currents were measured. (b) The I-V curves show that both TEA and Meth decreased IKv by blocking Kv channels; and that a significantly greater reduction was induced by combined exposure to TEA and Meth (Control and TEA, n = 6/5 cells/independent experiments; Meth and Meth + TEA, n = 4/3 cells/independent experiments; Two-way rmANOVA; Control vs. TEA: treatment: F(1,5) = 11.01, p = 0.021; voltage: F(15,75) = 16.81, p < 0.001; interaction: F(15,75) = 7.088, p < 0.001; with Newman–Keuls post hoc test: +,++,+++p < 0.05, 0.01 or 0.001; Meth vs. Meth + TEA: treatment: F(1,3) = 3.519, p = 0.157; voltage: F(15,45) = 27.83, p < 0.001; interaction: F(15,45) = 4.308, p < 0.001; with Newman–Keuls post hoc test: &,&&,&&&p < 0.05, 0.01 or 0.001; TEA vs. Meth + TEA: treatment: F(1,8) = 2.947, p = 0.124; voltage: F(15,120) = 15.47, p < 0.001; interaction: F(15,120) = 3.027, p < 0.001; with Newman–Keuls post hoc test: #,##p < 0.05 or 0.01).
Antagonism of TAAR1 abolished the acute effects of Meth on suppressing IKv in HFAs
To determine if Meth suppressed IKv by activating TAAR1 in HFAs, we antagonized TAAR1 with EPPTB, and assessed IKv that was activated by Vm depolarization from −50 mV to +100 mV in HFAs treated with vehicle (control, n = 22), Meth (20 μM, n = 19), or combined Meth (20 μM) + EPPTB (25 nM, n = 9) (Fig. 4a). We found that Meth decreased IKv, but combined EPPTB/Meth treatment completely reversed Meth-induced suppression of IKv in HFAs (Two-way rmANOVA: interaction: F(30,705) = 2.112, p < 0.001) (Fig. 4b). Antagonizing TAAR1s by EPPTB alone did not induce any significant change in IKv compared to control HFAs or Meth + EPPTB; and there was no significant difference in the IKv density between EPPTB + Meth and EPPTB alone (both p > 0.05; data not shown). Together, these findings suggest that Meth reduces IKv by suppressing Kv channel activity; and that is mediated by TAAR1-coupled signaling in HFAs.
Inhibition of PKA-like protein kinases abolished acute Meth effects on suppressing IKv in HFAs
PKA is a downstream substrate in the TAAR1-mediated signaling pathway (Cisneros and Ghorpade 2014). To evaluate the role of PKA-like protein kinases on the dynamic activity of Kv channels in HFAs, we assessed the effects of H89, a PKA inhibitor, on Meth-induced decrease of IKv. We found that Meth-induced suppression of IKv was completely abolished by concurrent application of H89 plus Meth (n = 5/ea; Two-way rmANOVA: interaction: F(30,180) = 2.178, p < 0.001) (Fig. 5a and b). There was no significant difference in the IKv density between H89 + Meth and H89 alone (p > 0.05; data not shown). These results suggest that inhibition of PKA-related protein kinases blocks the acute Meth effects on suppressing IKv via Kv/Kv-like K+ channels in HFAs, which is mediated by facilitating the TAAR1-mediated cAMP/PKA cascading.
Fig. 5.
PKA inhibition by H89 abolished acute effects of methamphetamine (Meth) on IKv. (a) Sample traces from human fetal astrocytes at control condition, treated with Meth (20 μM), or with Meth (20 μM) plus H89 (10 μM). The vertical dashed lines indicate the time points at which the currents were measured. (b) The I-V curves show that combined treatment of H89 and Meth reversed the Meth-induced reduction in IKv (Control vs. Meth & Meth + H89: n = 5/4, 5/5, 5/5 cells/independent experiments, respectively; Two-way rmANOVA: treatment: F(2,12) = 1.343, p > 0.05; voltage: F(15,180) = 157.6, p < 0.001; interaction: F(30,180) = 2.178, p < 0.001; with Tukey’s post hoc test: Control vs. Meth: **,***p < 0.01, or 0.001; Meth vs. Meth + H89: &p < 0.05).
HFAs displayed inwardly rectifying K+ influx (IKir) in response to Vm hyperpolarization; and that was not affected by lower level of Meth
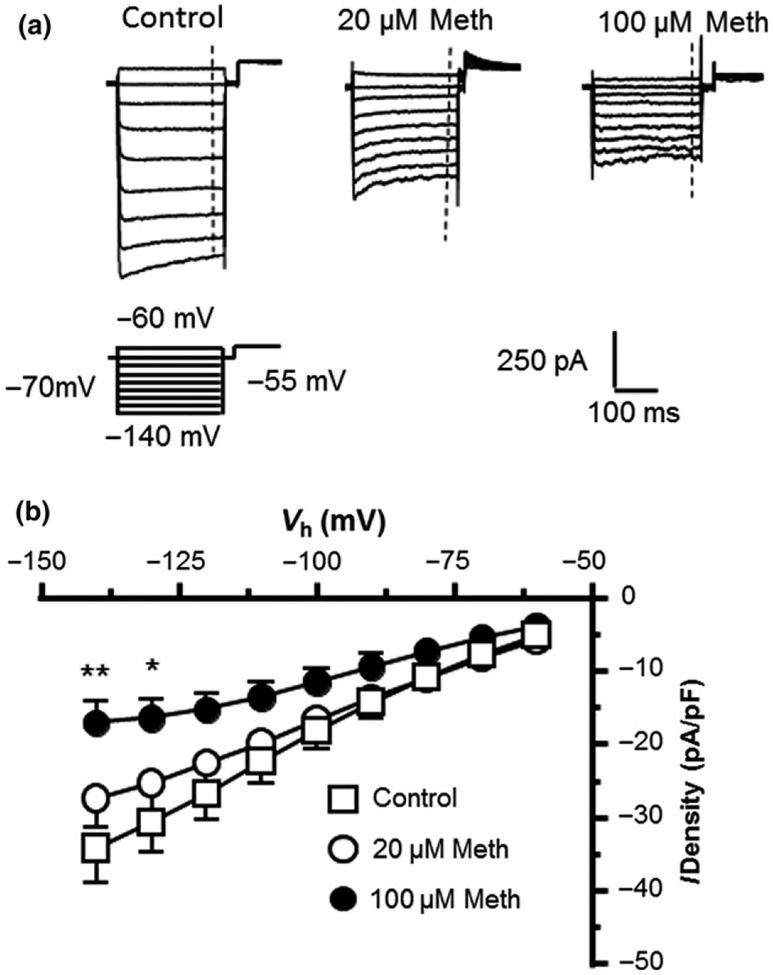
In contrast to a large IKv, we found that HFAs displayed a small IKir, which was mainly mediated by activated Kir channels in response to Vm hyperpolarization (Vm = −60 to −140 mV levels; the downward traces in Fig. 1b). These Kir channels were activated at Vm levels more hyperpolarized than −50 mV that induced K+ influx. Typically they are expressed at very low levels in immature astrocytes as reported by previous studies (Kressin et al. 1995; Olsen et al. 2006; Olsen and Sontheimer 2008; Montiel-Herrera and Garcia-Colunga 2010). Thus, IKir was relatively small compared to IKv during Vm depolarization (the upward traces in Fig. 1b). Previous studies show that Meth decreases IKir in cardiac myocytes (Liang et al. 2010; Qu et al. 2014), and suppresses activity of G protein-coupled inwardly rectifying K+ channels in dopamine neurons (Sharpe et al. 2015); but its effect on Kir channels in HFAs is unknown. Here we evaluated the Meth effect on IKir. We found that 20 μM Meth did not significantly affect IKir; but 100 μM Meth significantly increased IKir (Fig. 6). These results suggest that some Kir channels are functionally expressed in HFAs; but they appeared to be less sensitive to acute effects of Meth at 20 μM level compared to K2P and Kv channels. Chronic Meth effects in vivo on dysregulating Kir activity of mature astrocytes need to be investigated in future studies.
Fig. 6.
Methamphetamine (Meth) at higher (but not lower) concentration increased inwardly rectifying K+ currents in human fetal astrocytes (HFAs). (a) Sample traces of inwardly rectifying K+ currents (IKir) indicate that 100 μM (but not 20 μM) Meth increased IKir in HFAs. (b) The I-V relationships reveal that IKir was significantly increased by 100 μM Meth compared to controls (Control vs. 20 and 100 μM Meth, n = 32/26 vs. 17/12 and 9/7 cells/independent experiments; Two-Way rmANOVA: Treatment effect: F(2,55) = 1.41, p > 0.05; Voltage effect: F(8,440) = 60.91, p < 0.001; interaction: F(16,440) = 2.889, p = 0.0002; with Newman–Keuls post hoc test: *,**p < 0.05 or 0.01).
Voltage-gated Ca2+ currents were not detected in HFAs
We also assessed whether there was a ICa through VGCCs in HFAs, which were studied under physiological concentration of 2 mM [Ca2+]o. In the Ca2+-related study, voltage-sensitive Na+, K+, Ih, and CLC-3 (a voltage-gated chloride channel that conducts outflowing Cl− currents) were all blocked. We found that when Vm was depolarized from −70 to +50 mV levels, there was no detectable inflowing Ca2+ current through VGCCs in HFAs (p > 0.05; Figure S1).
Discussion
The present study determined the electrophysiological characteristics of HFAs and acute Meth effects on HFAs. Our findings indicate that (i) HFAs express functional K2P, Kv, and Kir channels, (ii) Meth suppresses IKv, which is blocked by antagonizing TAAR1, or by inhibiting PKA activity, (iii) Meth at 20 and 100 μM depolarizes RMP of HFAs; and this Meth effect is mimicked and exceeded by blocking K2P/K2P-like channels, (iv) activity of Kir/Kir-like channel is relatively weak and not affected by 20 μM Meth (but reduced by 100 μM of this drug), and (v) there is no functional expression of VGCCs in HFAs. To our knowledge, these novel findings, for the first time in the field, elucidate a mechanism by which acute Meth exposure in vitro alters astrocytic K+ efflux/[K+]o by affecting dynamic activity of K+ channels, which could consequently alter the excitability of surrounding neurons.
The major finding of the present study was that Meth reduced IKv through Kv/Kv-like channels in HFAs during Vm depolarization. This Meth effect was mimicked by blocking Kv channels with TEA. Importantly, these findings shed light on the underlying mechanism by which Meth suppresses IKv in HFAs. Specifically, we demonstrate that Meth-induced TAAR1 activation mediates the reduced Kv channel activity, which is reversed by antagonizing TAAR1. It is well established that Meth is a potent TAAR1 agonist (Xie and Miller 2009; Miller 2011; Reese et al. 2014; Cotter et al. 2015; Liberles 2015). TAAR1 activation promotes the TAAR1/Gs/cAMP/PKA signaling (Jing and Li 2015), which is associated with Meth-induced behavioral alterations (Cotter et al. 2015). Phosphorylation of Kv channels by PKA in the cAMP/PKA cascade decreases Kv channel activity in rat cortical neurons (Dong and White 2003; Dong et al. 2004), and cardiac myocytes (Walsh and Kass 1988). In agreement with these previous studies, our study reveals that inhibition of PKA activity by antagonizing TAAR1 abolished Meth- induced decrease of IKv through Kv channels in HFAs. This novel finding indicates that Meth-induced IKv reduction in HFAs is mediated by the TAAR1-coupled cAMP/PKA signaling. The finding that H89 significantly attenuates Meth-induced IKv reduction confirms the effect of PKA activation on suppressing Kv/Kv-like channel activity.
Meth-induced reduction in IKv efflux in HFAs was associated with a reduced time constant (T) during IKv activation, suggesting that Meth alters the kinetics of Kv channel gating. Interestingly, the T appears to be stabilized in relatively negative Vm levels in HFAs. This may result from the following reasons. First, in mature astrocytes, the voltage-dependence of T occurs from RMP (or holding potentials) to Vm levels at which Kv channels begin to activate (~ −60 mV levels). They could achieve their 1/2 activation (V1/2) at Vm at ~ −50 mV levels (Zagotta et al. 1994; Hille 2001). IKv depends upon the density, opening probability, and opening time of activated KA/KD-like channels, which achieves a steady-status at more depolarized Vm levels (Fig. 2c), at which the T should not be significantly altered by further Vm change. Second, the immaturity of HFAs could affect the property of KA/KD channels, including their T (and cause some inconsistency in the V-T relationship). Such immaturity-related variety in RMP mediated by K+ channels is reported by many previous studies and in the present study. Third, a potential difference in the channel density between KA channels (which activate/inactivate very fast) and KD channels (which activate/inactivate slower than KA channels; or even don’t inactivate), could also affect the T. Whether, how, and to what extent these KA/KD channels influence the T in HFAs (either during activation or inactivation) are not fully understood. Fourth, a lack of the expression of Kir4.1 channels in immature astrocytes likely also affects the T. The Kir4.1 channel is a unique subtype of the inwardly rectifying K+ channel that is not fully expressed in astrocytes until p15–21 day; but it is the pre-dominant Kir channel subtype in mature astrocytes. Importantly, these unique Kir channels are activated not only during Vm hyperpolarization (like other Kir channels), but also during Vm depolarization (like other Kv channels). Therefore, further investigation is needed to better understand the electrophysiological characteristics of HFAs ex vivo, mature astrocytes in the brain, and their responses to the impact of drugs of abuse in vivo.
We also found a Meth-induced RMP depolarization, which was mimicked by blocking IK2P. Combined Meth exposure/K2P channel blockade did not induce further RMP depolarization, while inhibiting PKA activity did not affect Meth-induced RMP depolarization. However, there was no significant difference in RMP between control HFAs and those treated by Meth + TAAR1 antagonist, suggesting an involvement of TAAR1. Thus, it is likely that Meth depolarizes RMP by activating TAAR1 and inhibiting K2P channel activity through a mechanism other than the cAMP/PKA cascade in HFAs. Together, these findings suggest that Meth-induced RMP depolarization results from a decreased K2P channel activity and increased intracellular K+; but such changes are not mediated by the TAAR1-coupled cAMP/PKA signaling. Given that K2P channel activity can be reduced by TAAR1-mediated promotion of PKC activation (Fig. 7), future investigation is also needed to determine if acute Meth effects on depolarizing RMP of HFAs are mediated by the TAAR1-coupled Gq/PLC/PKC signaling pathway.
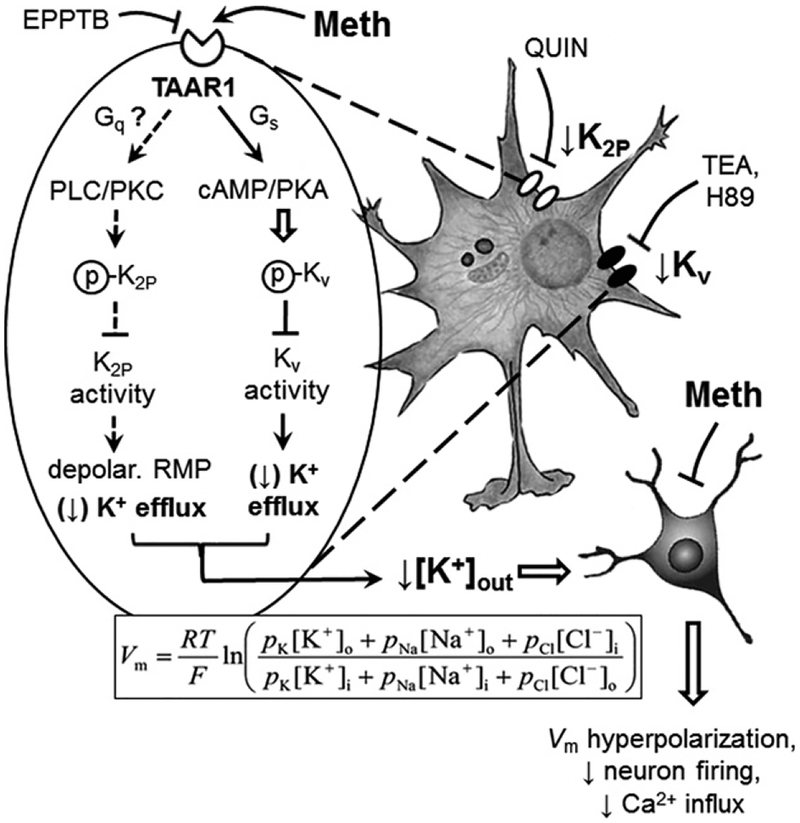
Fig. 7.
Acute methamphetamine (Meth) effects on altering astrocyte activity are mediated by TAAR1-coupled signaling and K+ channel dysfunction. Astrocytes play a key role in regulating extracellular K+ homeostasis in the brain. Meth alters this dynamic function of astrocytes in regulating K+ homeostasis by suppressing K+ efflux through dysfunctional Kv, K2P and Kir channels. Acute Meth effects on altering K+ efflux through Kv channels are mediated by disrupting the TAAR1-coupled cAMP/PKA signaling pathway. The mechanism underlying acute Meth effects on suppressing K2P channel activity [leading to resting membrane potential (RMP) depolarization] could be mediated by dysfunction of the Gs/cAMP/PKA and/or Gq/phospholipase C (PLC)/protein kinase C (PKC) signaling pathway. Meth is a potent agonist for TAAR1. Direct activation of TAAR1 by Meth promotes the TAAR1/Gs/cAMP/PKA signaling pathway, as well as the TAAR1/Gq/PLC/PKC signaling pathway, thereby enhancing PKA/PKC-induced phosphorylation of Kv/K2P channels, respectively. Enhanced phosphorylation of Kv/K2P channels reduces their activity to conduct outflowing K+. Reducing K+ efflux from astrocytes via Kv/K2P channels decreases extracellular K+ levels ([K+]o). The Goldman-Hodgkin Katz equation indicates that reduced [K+]o results in Vm hyperpolarization of surrounding neurons; and therefore decreases their excitability. The mechanism, by which toxic/fatal level of Meth suppresses Kir channel activity, remains to be identified. These findings suggest that Meth disturbs functional and dynamic activity of astrocytes by altering the functional activity of Kv and K2P channels through the cAMP/PKA and likely PLC/PKC signaling pathway, respectively; and that could affect their interactions with neurons in the brain.
The present study supports the perspective regarding the heterogeneity of astrocyte RMP, which is associated with the immaturity of HFAs. Indeed, the gap junction and some ion channel expression are developmentally regulated (Sontheimer et al. 1992; Kressin et al. 1995; Giaume and McCarthy 1996; Ben-Ari 2008). This also includes K2P-like channels that play a critical role in regulating RMP in mature astrocytes (Olsen et al. 2006; Olsen and Sontheimer 2008). Therefore, RMP of HFAs is much more depolarized in a wider Vm range than those in mature hippocampal, spinal cord, and cortical astrocytes from humans or rats (e.g., RMP = −70 to −80 mV) (Schroder et al. 2000; Olsen et al. 2006; our unpublished data).
Meth-induced dysfunction alters a variety of K+ channels, which differs from each other, depending on dosage/blood levels of Meth, cell type, exposure/withdrawal time, and in vivo/in vitro exposure (with or without synaptic activity and neurotransmission). These differences can increase or decrease K+ channels’ function. For example, besides suppressing K+ efflux via Kv/Kv-like and K2P/K2P-like channels in HFAs (the present study), Meth also decreases K+ efflux by inhibiting Ca2+-activated K+ channel activity in mouse neuroblastoma/rat glioma cells (Wang et al. 2013). Repeated Meth exposure reduces Kv1.4/1.7/3.4/4.2 and Kir2.1/2.2/2.3/2.4 channels in ventricular myocytes of rats (Qu et al. 2014). In contrast, exposure to Meth (≥ 24 h) increases K+ efflux via Kv1.3 channels in cultured rat microglia (Wang et al. 2014); while higher Meth levels (100–1000 μM) cause dose-dependent microglial cell damage and death). Kv1.1/Kv1.2 and their accessory protein KCNAB1 are also upregulated in the NAc of non-addictive rats compared to addictive/compulsive rats following chronic Meth-SA (Cadet et al. 2016). Therefore, further studies are needed to confirm or modify the ‘recreational’ and ‘toxic/lethal levels of Meth in the blood of drug users. It will also be interesting to assess the blood levels of Meth in rats that self-administer the drug.
The importance of Meth-induced alterations in astrocytic K+ channels results from its influence on the extracellular K+ homeostasis, and consequently on neuronal activity. Meth-induced decrease in astrocytic K2P/Kv channel function will reduce IK outflowing from HFAs; and therefore lower [K+]o. Based on the Goldmann-Hodgkin Katz voltage equation (Hille 2001), decreased K+ efflux/[K+]o facilitates Vm hyperpolarization of surrounding neurons, and consequently reduce their firing (Fig. 7). Thus, it is very likely that such an altered astrocyte/neuron interaction in the brain will contribute to the underlying mechanism of Meth neuropatho-physiology. In agreement with this hypothesis, our previous studies demonstrate a significant decrease of neuronal activity in the rat medial prefrontal cortex and NAc following chronic Meth-SA (Chen et al. 2012; Graves et al. 2015), or repeated Meth subcutaneous injections (White et al. 1995; Hu et al. 2002), respectively.
The present study also revealed the functional expression of Kir channels in HFAs. Compared to IKv, astrocyte IKir was relatively small, which is in agreement with other studies of immature astrocytes (Kressin et al. 1995; Olsen et al. 2006; Olsen and Sontheimer 2008; Montiel-Herrera and Garcia-Colunga 2010). Intriguingly, unlike RMP and IKv, IKir in HFAs was not affected by 20 μM Meth; but reduced by toxic/fatal concentrations of Meth (100 μM). This finding suggests that Kir/Kir-like channels are less sensitive, but still vulnerable, to the acute Meth compared to K2P/K2P-like and Kv/Kv- like channels in HFAs. However, we also acknowledge that the Meth effects (including its selectivity) on astrocytes (as well as their K+ channel activity), either in vitro or in vivo, are not fully understood. The present study alone will not be able to address/answer all the questions regarding the impact of high concentrations of Meth on all subtypes of K+ channels. Given that this study is the first one of its kind in the field (up to the time of submission), we hope that our novel findings will inspire more investigations to define the mechanism(s) by which drugs of abuse alter the functional activity of astrocytes and neurons in vivo, in laboratory animals and humans. Intriguingly, in our previous studies (Nasif et al. 2005b; Wayman et al. 2015), we have also demonstrated a lack of selectivity regarding cocaine-induced alterations in various K+ channel subtypes in rat cortical neurons.
In summary, the present study demonstrates that the HFA activity is dynamically regulated by a variety of K+ channel subtypes at different Vm levels. Meth at a recreational level reduces astrocytic K+ efflux through K2P/K2P-like and Kv/Kv-like channels. Such Kv/Kv-like channel dysfunction is mediated by the TAAR1-coulped cAMP/PKA cascade; while K2P/K2P-like channel dysfunction may also be mediated by other mechanisms. Together, these Meth-induced alterations in K+ channels disturb astrocyte regulation of [K+]o, which could consequently decrease excitability of surrounding neurons in the brain regions that are vulnerable to Meth.
Supplementary Material
Figure S1. There was no functional expression of voltage-gated Ca2+ currents (VGCCs).
Acknowledgments and conflict of interest disclosure
We thank Dr. Wesley N. Wayman for technical support. This work was supported by NIH NS084817, DA044552 (X-TH), and DA033966 (LA). The authors declare no conflict of interest.
Abbreviations used:
- [K+]o
extracellular K+ level
- CABM
clonetics astrocyte basal medium
- cAMP
cyclic adenosine monophosphate
- Cm
membrane capacitance
- CNS
central nervous system
- EPPTB
N-(3-Ethoxyphenyl)-4-(1-pyrrolidinyl)-3-(trifluoromethyl)benzamide
- Gq
Gq protein
- Gs
Gs protein
- HFA
human fetal astrocyte
- Ih
the hyperpolarization-activated channel current
- IK2P
the ‘leaking’ K+ current
- IKir
the inwardly rectifying K+ current
- Ikv
the voltage-sensitive K+ current
- K2P
channel, the two-pore domain K+ channel
- Kir
channel, the inwardly rectifying K+ channel
- Kv channel
the voltage-gated K+ channel
- LUHMES
Lund human mesencephalic neurons
- Meth
methamphetamine
- Meth-SA
Meth self-administration
- mPFC
medial prefrontal cortex
- ms
millisecond
- NAc
nucleus accumbens
- PKA
cAMP-activated protein kinase
- PKC
protein kinase C
- PLC
phospholipase C
- QUIN
quinidine
- Rin
input resistance
- RMP
resting membrane potential
- RM
repeated measurement
- RRID
research resource identifier
- SA
self-administration
- TAAR1
the trace amine-associated receptor type-1
- TEA
tetraethyl-ammonium
- TTX
tetrodotoxin
- VGCC
voltage-gated Ca2+ current
- Vh
holding potential
- Vm
membrane potential
Footnotes
Open science badges
This article has received a badge for *Open Materials* because it provided all relevant information to reproduce the study in the manuscript. The complete Open Science Disclosure form for this article can be found at the end of the article. More information about the Open Practices badges can be found at https://cos.io/our-services/open-science-badges/.
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- Al-Harthi L (2012) Interplay between Wnt/beta-catenin signaling and HIV: virologic and biologic consequences in the CNS. J. Neuroimmune Pharmacol 7, 731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM and Hauser KF (2014) Glial modulators as potential treatments of psychostimulant abuse. Adv. Pharmacol 69, 1–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Shelton KL, Hendrick E and Johnson KW (2010) The glial cell modulator and phosphodiesterase inhibitor, AV411 (ibudilast), attenuates prime- and stress-induced methamphetamine relapse. Eur. J. Pharmacol 637, 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y (2008) Neuro-archaeology: pre-symptomatic architecture and signature of neurological disorders. Trends Neurosci 31, 626–636. [DOI] [PubMed] [Google Scholar]
- Bradaia A, Trube G, Stalder H et al. (2009) The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc. Natl Acad. Sci. USA 106, 20081–20086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzow JR, Sonders MS, Arttamangkul S et al. (2001) Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharmacol 60, 1181–1188. [DOI] [PubMed] [Google Scholar]
- Cadet JL and Krasnova IN (2009) Molecular bases of methamphetamine-induced neurodegeneration. Int. Rev. Neurobiol 88, 101–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Brannock C, Krasnova IN, Jayanthi S, Ladenheim B, McCoy MT, Walther D, Godino A, Pirooznia M and Lee RS (2016) Genome-wide DNA hydroxymethylation identifies potassium channels in the nucleus accumbens as discriminators of methamphetamine addiction and abstinence. Mol. Psychiatry 22, 1196–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Graves SM, Napier TC and Hu X-T (2012) Excitability of medial prefrontal cortex pyramidal neurons was decreased in rats trained to self-administer methamphetamine followed by a short-term withdrawal
- Cheung G, Sibille J, Zapata J and Rouach N (2015) Activity-dependent plasticity of astroglial potassium and glutamate clearance. Neural. Plast 2015, 109106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisneros IE and Ghorpade A (2014) Methamphetamine and HIV-1-induced neurotoxicity: role of trace amine associated receptor 1 cAMP signaling in astrocytes. Neuropharmacology 85, 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter R, Pei Y, Mus L, Harmeier A, Gainetdinov RR, Hoener MC and Canales JJ (2015) The trace amine-associated receptor 1 modulates methamphetamine’s neurochemical and behavioral effects. Front. Neurosci 9, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y and White FJ (2003) Dopamine D1-class receptors selectively modulate a slowly inactivating potassium current in rat medial prefrontal cortex pyramidal neurons. J. Neurosci 23, 2686–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Cooper D, Nasif F, Hu XT and White FJ (2004) Dopamine modulates inwardly rectifying potassium currents in medial prefrontal cortex pyramidal neurons. J. Neurosci 24, 3077–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Kunitachi S, Iyo M and Hashimoto K (2012) The antibiotic minocycline prevents methamphetamine-induced rewarding effects in mice. Pharmacol. Biochem. Behav 101, 303–306. [DOI] [PubMed] [Google Scholar]
- Giaume C and McCarthy KD (1996) Control of gap-junctional communication in astrocytic networks. Trends Neurosci 19, 319–325. [DOI] [PubMed] [Google Scholar]
- Graves SM, Clark MJ, Traynor JR, Hu XT and Napier TC (2015) Nucleus accumbens shell excitability is decreased by methamphetamine self-administration and increased by 5-HT2C receptor inverse agonism and agonism. Neuropharmacology 89, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B (2001) Ionic Channels of Excitable Membranes Skyscrape Inc, New York. [Google Scholar]
- Hu X-T, Koeltzow TE, Cooper DC, Robertson GS, White FJ and Vezina P (2002) Repeated ventral tegmental area amphetamine administration alters dopamine D1 receptor signaling in the nucleus accumbens. Synapse 45, 159–170. [DOI] [PubMed] [Google Scholar]
- Hu X-T, Basu S and White FJ (2004) Repeated cocaine administration suppresses HVA-Ca2+ potentials and enhances activity of K+ channels in rat nucleus accumbens neurons. J. Neurophysiol 92, 1597–1607. [DOI] [PubMed] [Google Scholar]
- Hu X-T, Ford K and White FJ (2005) Repeated cocaine administration decreases calcineurin (PP2B) but enhances DARPP-32 modulation of sodium currents in rat nucleus accumbens neurons. Neuropsychopharmacology 30, 916–926. [DOI] [PubMed] [Google Scholar]
- Jing L and Li JX (2015) Trace amine-associated receptor 1: a promising target for the treatment of psychostimulant addiction. Eur. J. Pharmacol 761, 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadala A, Verdier D, Morquette P and Kolta A (2015) Ion homeostasis in rhythmogenesis: the interplay between neurons and astroglia. Physiology (Bethesda) 30, 371–388. [DOI] [PubMed] [Google Scholar]
- Kettenmann H and Ransom BR (2013) Neuroglia Oxford University Press, New York, NY. [Google Scholar]
- Khodr CE, Chen L, Dave S, Al-Harthi L and Hu XT (2016) Combined chronic blockade of hyper-active L-type calcium channels and NMDA receptors ameliorates HIV-1 associated hyper-excitability of mPFC pyramidal neurons. Neurobiol. Dis 94, 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely E, Lee CJ and Marinetti L (2009) A fatality from an oral ingestion of methamphetamine. J. Anal. Toxicol 33, 557–560. [DOI] [PubMed] [Google Scholar]
- Kim D (2005) Physiology and pharmacology of two-pore domain potassium channels. Curr. Pharm. Des 11, 2717–2736. [DOI] [PubMed] [Google Scholar]
- Krasnova IN and Cadet JL (2009) Methamphetamine toxicity and messengers of death. Brain Res. Rev 60, 379–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressin K, Kuprijanova E, Jabs R, Seifert G and Steinhauser C (1995) Developmental regulation of Na+ and K+ conductances in glial cells of mouse hippocampal brain slices. Glia 15, 173–187. [DOI] [PubMed] [Google Scholar]
- Liang R, Zhou Y, Wu F, Zhou C, Zhao X, Zhang M, Tian X and Zhu B (2010) Effect of methamphetamine on potassium and L-type calcium currents in rat ventricular myocytes. Toxicol. Mech. Methods 20, 458–465. [DOI] [PubMed] [Google Scholar]
- Liberles SD (2015) Trace amine-associated receptors: ligands, neural circuits, and behaviors. Curr. Opin. Neurobiol 34C, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PL, Tsai MC, Lu GL, LU DY, Chuang CM, Yang HY, Huang SS and Chen YH (2010) Ecstasy and methamphetamine elicit action potential bursts via different mechanisms in a central snail neuron. Neurotoxicology 31, 26–41. [DOI] [PubMed] [Google Scholar]
- Lochner A and Moolman JA (2006) The many faces of H89: a review. Cardiovasc. Drug Rev 24, 261–274. [DOI] [PubMed] [Google Scholar]
- Lotshaw DP (2007) Biophysical, pharmacological, and functional characteristics of cloned and native mammalian two-pore domain K+ channels. Cell Biochem. Biophys 47, 209–256. [DOI] [PubMed] [Google Scholar]
- MacFarlane SN and Sontheimer H (1997) Electrophysiological changes that accompany reactive gliosis in vitro. J. Neurosci 17, 7316–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, D’Ambrosio R and Janigro D (1997) Heterogeneity of astrocyte resting membrane potentials and intercellular coupling revealed by whole-cell and gramicidin-perforated patch recordings from cultured neocortical and hippocampal slice astrocytes. J. Neurosci 17, 6850–6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melega WP, Cho AK, Harvey D and Lacan G (2007) Methamphetamine blood concentrations in human abusers: application to pharmacokinetic modeling. Synapse 61, 216–220. [DOI] [PubMed] [Google Scholar]
- Miller GM (2011) The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J. Neurochem 116, 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GM, Verrico CD, Jassen A, Konar M, Yang H, Panas H, Bahn M, Johnson R and Madras BK (2005) Primate trace amine receptor 1 modulation by the dopamine transporter. J. Pharmacol. Exp. Ther 313, 983–994. [DOI] [PubMed] [Google Scholar]
- Montiel-Herrera M and Garcia-Colunga J (2010) Current profiles of astrocytes from the corpus callosum of newborn and 28-day-old rats. Neurosci. Lett 485, 189–193. [DOI] [PubMed] [Google Scholar]
- Murray AJ (2008) Pharmacological PKA inhibition: all may not be what it seems. Sci. Signal 1, re4. [DOI] [PubMed] [Google Scholar]
- Narasipura SD, Henderson LJ, Fu SW, Chen L, Kashanchi F and Al-Harthi L (2012) Role of beta-catenin and TCF/LEF family members in transcriptional activity of HIV in astrocytes. J. Virol 86, 1911–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Narita M, Shibasaki M, Shindo K, Nakamura A, Kuzumaki N, Nagumo Y and Suzuki T (2006) Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology 31, 2476–2488. [DOI] [PubMed] [Google Scholar]
- Nasif FJ, Hu XT and White FJ (2005a) Repeated cocaine administration increases voltage-sensitive calcium currents in response to membrane depolarization in medial prefrontal cortex pyramidal neurons. J. Neurosci 25, 3674–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasif FJ, Sidiropoulou K, Hu XT and White FJ (2005b) Repeated cocaine administration increases membrane excitability of pyramidal neurons in the rat medial prefrontal cortex. J. Pharmacol. Exp. Ther 312, 1305–1313. [DOI] [PubMed] [Google Scholar]
- Olsen ML and Sontheimer H (2008) Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J. Neurochem 107, 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Schade S, Lyons SA, Amaral MD and Sontheimer H (2003) Expression of voltage-gated chloride channels in human glioma cells. J. Neurosci 23, 5572–5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Higashimori H, Campbell SL, Hablitz JJ and Sontheimer H (2006) Functional expression of Kir4.1 channels in spinal cord astrocytes. Glia 53, 516–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panas MW, Xie Z, Panas HN, Hoener MC, Vallender EJ and Miller GM (2012) Trace amine associated receptor 1 signaling in activated lymphocytes. J. Neuroimmune Pharmacol 7, 866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panenka WJ, Procyshyn RM, Lecomte T, Macewan GW, Flynn SW, Honer WG and Barr AM (2013) Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend 129, 167–179. [DOI] [PubMed] [Google Scholar]
- Parsegian A, Glen WB Jr, Lavin A and See RE (2011) Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol. Psychiatry 69, 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu YH, Leung KP, Qiao DF, Li DR, Liu C, Yue X and Wang HJ (2014) Remodeling of ion channel expression may contribute to electrophysiological consequences caused by methamphetamine in vitro and in vivo. Biochem. Biophys. Res. Commun 443, 441–446. [DOI] [PubMed] [Google Scholar]
- Reese EA, Norimatsu Y, Grandy MS, Suchland KL, Bunzow JR and Grandy DK (2014) Exploring the determinants of trace amine-associated receptor 1′s functional selectivity for the stereoisomers of amphetamine and methamphetamine. J. Med. Chem 57, 378–390. [DOI] [PubMed] [Google Scholar]
- Schep LJ, Slaughter RJ and Beasley DM (2010) The clinical toxicology of metamfetamine. Clin. Toxicol. (Phila) 48, 675–694. [DOI] [PubMed] [Google Scholar]
- Scherer D, Seyler C, Xynogalos P et al. (2016) Inhibition of cardiac kir current (IK1) by protein kinase C critically depends on PKCbeta and Kir2.2. PLoS ONE 11, e0156181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder W, Hinterkeuser S, Seifert G, Schramm J, Jabs R, Wilkin GP and Steinhauser C (2000) Functional and molecular properties of human astrocytes in acute hippocampal slices obtained from patients with temporal lobe epilepsy. Epilepsia 41(Suppl 6), S181–S184. [DOI] [PubMed] [Google Scholar]
- Sharma A, Hu XT, Napier TC and Al-Harthi L (2011) Methamphetamine and HIV-1 Tat down regulate beta-catenin signaling: implications for methampetamine abuse and HIV-1 co-morbidity. J. Neuroimmune Pharmacol 6, 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AL, Varela E, Bettinger L and Beckstead MJ (2015) Methamphetamine self-administration in mice decreases GIRK channel-mediated currents in midbrain dopamine neurons. Int. J. Neuropsychopharmacol 18, pyu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, Vunck SA, van den Oord EJ, Adkins DE, McClay JL and Beardsley PM (2012) The glial cell modulators, ibudilast and its amino analog, AV1013, attenuate methamphetamine locomotor activity and its sensitization in mice. Eur. J. Pharmacol 679, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, Hendrick ES and Beardsley PM (2013) Glial cell modulators attenuate methamphetamine self-administration in the rat. Eur. J. Pharmacol 701, 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somjen GG, Kager H and Wadman WJ (2008) Computer simulations of neuron-glia interactions mediated by ion flux. J. Comput. Neurosci 25, 349–365. [DOI] [PubMed] [Google Scholar]
- Sontheimer H, Black JA, Ransom BR and Waxman SG (1992) Ion channels in spinal cord astrocytes in vitro. I. Transient expression of high levels of Na+ and K+ channels. J. Neurophysiol 68, 985–1000. [DOI] [PubMed] [Google Scholar]
- Takekawa K, Ohmori T, Kido A and Oya M (2007) Methamphetamine body packer: acute poisoning death due to massive leaking of methamphetamine. J. Forensic Sci 52, 1219–1222. [DOI] [PubMed] [Google Scholar]
- Tatro ET, Hefler S, Shumaker-Armstrong S, Soontornniyomkij B, Yang M, Yermanos A, Wren N, Moore DJ and Achim CL (2013) Modulation of BK channel by MicroRNA-9 in neurons after exposure to HIV and methamphetamine. J. Neuroimmune Pharmacol 8, 1210–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF (1998) Software-based correction of single compartment series resistance errors. J. Neurosci. Methods 86, 25–34. [DOI] [PubMed] [Google Scholar]
- Veale EL, Kennard LE, Sutton GL, MacKenzie G, Sandu C and Mathie A (2007) G(alpha)q-mediated regulation of TASK3 two-pore domain potassium channels: the role of protein kinase C. Mol. Pharmacol 71, 1666–1675. [DOI] [PubMed] [Google Scholar]
- Walsh KB and Kass RS (1988) Regulation of a heart potassium channel by protein kinase A and C. Science 242, 67–69. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Chan MH and Chen HH (2013) Methamphetamine inhibits voltage-gated potassium currents in NG108–15 cells: possible contribution of large-conductance calcium-activated potassium channels. Toxicol. Lett 223, 139–145. [DOI] [PubMed] [Google Scholar]
- Wang J, Qian W, Liu J, Zhao J, Yu P, Jiang L, Zhou J, Gao R and Xiao H (2014) Effect of methamphetamine on the microglial damage: role of potassium channel Kv1.3. PLoS ONE 9, e88642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman WN, Chen L, Napier TC and Hu XT (2015) Cocaine self-administration enhances excitatory responses of pyramidal neurons in the rat medial prefrontal cortex to human immunodeficiency virus-1 Tat. Eur. J. Neurosci 41, 1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ, Hu X-T, Zhang X-F and Wolf ME (1995) Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J. Pharmacol. Exp. Ther 273, 445–454. [PubMed] [Google Scholar]
- Xie Z and Miller GM (2009) A receptor mechanism for methamphetamine action in dopamine transporter regulation in brain. J. Pharmacol. Exp. Ther 330, 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Westmoreland SV, Bahn ME, Chen GL, Yang H, Vallender EJ, Yao WD, Madras BK and Miller GM (2007) Rhesus monkey trace amine-associated receptor 1 signaling: enhancement by monoamine transporters and attenuation by the D2 autoreceptor in vitro. J. Pharmacol. Exp. Ther 321, 116–127. [DOI] [PubMed] [Google Scholar]
- Yu C, Narasipura SD, Richards M, Hu X-T, Yamamoto B and Al-Harti L (2017a) HIV and drug abuse mediate astrocyte senescence in a b-catenin-dependent manner leading to neuronal toxicity. Aging Cell 16, 956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Narasipura SD, Richards MH, Hu XT, Yamamoto B and Al-Harthi L (2017b) HIV and drug abuse mediate astrocyte senescence in a beta-catenin-dependent manner leading to neuronal toxicity. Aging Cell 16, 956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta WN, Hoshi T, Dittman J and Aldrich RW (1994) Shaker potassium channel gating. II: transitions in the activation pathway. J. Gen. Physiol 103, 279–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Kitaichi K, Fujimoto Y, Nakayama H, Shimizu E, Iyo M and Hashimoto K (2006) Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine. Prog. Neuropsychopharmacol. Biol. Psychiatry 30, 1381–1393. [DOI] [PubMed] [Google Scholar]
- Zhou M and Kimelberg HK (2000) Freshly isolated astrocytes from rat hippocampus show two distinct current patterns and different [K(+)](o) uptake capabilities. J. Neurophysiol 84, 2746–2757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. There was no functional expression of voltage-gated Ca2+ currents (VGCCs).