Abstract
Interleukin-1β (IL-1 β) is a cytokine involved in atherothrombosis and is known to depress cardiac function. We hypothesized that blocking IL-1 β in patients with symptomatic systolic heart failure (HF) would improve their cardiorespiratory fitness. The purpose of the study was to measure changes in peak oxygen consumption (VO2) in 30 patients with prior myocardial infarction, high-sensitivity C-reactive protein ≥ 2 mg/l and HF with left ventricular ejection fraction (LVEF) < 50% enrolled in the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) in an independent single center substudy. We measured peak VO2 before and after 3 and 12 months of treatment with Canakinumab every 3 months (50, 150, or 300 mg subcutaneously) or placebo, and measured LVEF before and after 12 months. In December 2013, the CANTOS study announced early termination of enrollment, halting enrollment for this substudy after only 15 patients, of which 3 were assigned to placebo and 12 to Canakinumab (50 mg [1; 7%], 150 mg [5; 33%], 300 mg [6; 40%]). Patients treated with Canakinumab had a significant improvement in peak VO2, from 19.2 to 22.8 ml/kg/min at 3 months (p = 0.023 within-group changes, p = 0.026 for time_x_group interaction versus placebo [primary end point]), and an improvement in LVEF 38% (33–43) to 44% (38–52) at 12 months (p = 0.012 for within-group changes). No significant changes were seen in the placebo group. In conclusion, the findings of this small prespecified secondary analysis of the CANTOS trial support the positive results of the overall study, and confirm IL-1 as a potential therapeutic target in HF. https://clinicaltrials.gov/ct2/show/NCT01900600
Keywords: Inflammation, Translational Studies, Heart Failure
Interleukin-1β (IL-1 β) is the prototypical inflammatory cytokine that is known to modulate cardiac contractility, behaving as a “soluble myocardial depressant factor”.1 In early phase studies of stable systolic heart failure2 and recently decompensated heart failure (HF), IL-1 receptor blockade with anakinra improved peak aerobic capacity.3 The Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) clinical trial4 tested Canakinumab, a humanized IL-1 β blocking antibody, to prevent recurrent atherothrombotic events in patients with previous myocardial infarction (MI) and elevated C-reactive protein (high sensitivity C-reactive protein [hs] CRP) ≥ 2 mg/l. The current study was designed as an independent investigator-initiated single-center substudy to determine whether Canakinumab improves peak oxygen consumption (VO2) in systolic HF (left ventricular ejection fraction [LVEF] < 50%).
Methods
The study design of the CANTOS trial4 and the main results5 have been published elsewhere. Briefly, 10,061 patients with prior MI and hsCRP level ≥ 2 mg/l were randomized to three different doses of Canakinumab or placebo. Exclusion criteria have been described.4 Randomization was not stratified by site. The Western Institutional Review Board approved both the main CANTOS trial and substudy, which required patients to be also symptomatic with NYHA class II III, Stage C HF and LVEF < 50%.6 All participants gave written informed consent. The substudy was registered as a separate study on clinicaltrials.gov (NCT01900600).
All patients underwent a maximal cardiopulmonary exercise test using a metabolic cart interfaced with a treadmill at baseline (during the screening visit or initial visit but before treatment) and 3 months to measure interval changes in peak VO2 with Canakinumab versus placebo (primary end point), and again at 12 months.3 For the test to be considered suitable for analysis, all the following conditions were needed to be met: (1) patient was limited by shortness of breath or fatigue; (2) no evidence of ischemia, arrhythmias, or uncontrolled hypertension limiting exercise; and (3) a respiratory exchange ratio > 1.0 at peak exercise.3 Interval changes in LVEF, another surrogate for systolic HF,7 were measured with transthoracic echocardiography at baseline, 3-months, and 12-months.3 Simpson method was used to measure LVEF in 4- and 2-chamber apical views. All images and loops were recorded and analyzed off-line at the end of the study by two experienced investigators unaware of treatment allocation: the values obtained by the two readers were averaged if the differences were <5% or reviewed jointly and remeasured for values differing ≥5%.
We hypothesized that IL-1β blockade with Canakinumab on top of standard of care treatment would improve cardiorespiratory fitness, measured by peak VO2, in patients with systolic HF due to ischemic cardiomyopathy. Given an expected average peak VO2 of 13 ± 3 ml/ kg/min for patients with symptomatic systolic HF, we planned to enroll 30 subjects (assuming 2:1 randomization) to provide a >90% power to detect a difference of 3.5 ml/kg/min (standard deviation of 2 ml/kg/min) between the interval change in peak VO2 between Canakinumab (all three doses combined) and placebo at 3 months, and >80% power to detect a similar difference between each individual Canakinumab dose and placebo at 3 months. We considered that an improvement in peak VO2 of 3.5 ml/kg/min, equaling 1-metabolic equivalent, would be both clinically significant since it has been associated with a reduction in mortality,8 and achievable as already seen in the first proof-of-concept study of IL-1 blockade with anakinra in stable systolic HF patients completed in 2011.2 Data was collected at the Virginia Commonwealth University and sent to a core analysis laboratory at the University of Illinois at Chicago to an investigator blinded to treatment allocation (R.A.) for analysis and database creation. Here the data are presented as median and interquartile range. Changes within the group were assessed with the Wilcoxon or the Friedman tests. We used the repeated measures analysis of variance (ANOVA)(time_x_group interaction) to compare paired changes in peak VO2 comparing Canakinumab and placebo at 3 months. No missing data imputation was used for instances in which subjects did not complete a follow-up test. In consideration that only 3 patients were in the placebo group, the data regarding placebo is presented as individual data point with the absolute range, rather than median and interquartile range, as a distribution in quartiles would have been misrepresentative. We therefore present individual data points. All analyses regarding the placebo-treated subjects are presented, but the limited statistical power needs to be acknowledged, and results need to be considered subject to error. The effects of Canakinumab on the outcomes of interest are presented as within-group analysis, but also as between-group analysis as prespecified, albeit significantly under-powered due to small size of the placebo group.
Results
Patients were enrolled in the substudy between April 2013 and February 2014 and randomly assigned to Canakinumab or matching placebo administered subcutaneously every 3 months. In December 2013, the main CANTOS study announced early termination of enrollment at the request of the sponsor, since power recalculations predicted that a sample size of 10,000 (instead of 17,200) would be sufficient to test the hypothesis.9 Overall 10,061 patients were enrolled in the CANTOS study, and enrollment for this substudy was unexpectedly stopped after 15 patients were enrolled at Virginia Commonwealth University (VCU): 12 patients were assigned to Canakinumab (50 mg [1; 7%], 150 mg [5; 33%] or 300 mg [6; 40%]) and 3 patients (20%) to placebo (Table 1).
Table 1.
Additional cardiopulmonary exercise test and Doppler echocardiography parameters.
| Baseline |
3 months |
12 months |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject (#) | Age (yrs) | Gender | Ethnicity | Exe time | Peak VO2 (mL/kg/min) | VE/VCO2 Slope |
OUES | RER | LVEDV (mL) | LVEF (%) | Exe time (sec) | Peak VO2 (mL/kg/min) | VE/ VCO2 | OUES | RER | Exe time | Peak VO2 (mL/kg/min) | VE/ VCO2 | OUES | RER | LVEDV (mL) | LVEF (%) | |
| Canakinumab 50 mg | 1 | 47 | M | C | 6.4 | 14.3 | 38.0 | 2.25 | 1.01 | 168 | 30 | 8.1 | 14.4 | 30.5 | 2.52 | 1.07 | 8.2 | 14.7 | 37.5 | 2.29 | 1.23 | 205 | 38 |
| 2 | 53 | M | C | 9.3 | 22.4 | 25.6 | 2.85 | 1.11 | 155 | 39 | 11.0 | 24.4 | 25.9 | 3.84 | 1.18 | 10.5 | 23.4 | 25.3 | 2.87 | 1.09 | 147 | 51 | |
| 3 | 56 | F | C | 6.0 | 13.3 | 30.8 | 1.81 | 1.05 | 71 | 43 | 6.4 | 15.0 | 32.3 | 2.68 | 1.10 | 6.5 | 15.3 | 30.6 | 2.18 | 0.98 | 71 | 46 | |
| Canakinumab 150 mg | 4 | 61 | M | AA | 7.5 | 20.9 | 33.5 | 1.93 | 1.13 | 170 | 38 | 8.1 | 24.4 | 32.7 | 2.20 | 1.10 | 8.3 | 16.9 | 36.8 | 1.94 | 1.02 | 132 | 39 |
| 5 | 65 | M | C | 7.2 | 13.8 | 54.8 | 1.15 | 1.13 | 180 | 24 | 9.0 | 18.7 | 48.5 | 1.19 | 1.05 | 8.0 | 14.5 | 55.0 | 1.37 | 1.22 | 170 | 44 | |
| 6 | 68 | M | C | 11.0 | 18.4 | 29.3 | 2.00 | 1.14 | 103 | 50 | 12.3 | 22.5 | 29.4 | 2.01 | 1.06 | 12.5 | 24.8 | 31.8 | 2.22 | 1.12 | 92 | 63 | |
| 7 | 44 | M | H | 15.2 | 29.2 | 21.6 | 3.80 | 1.11 | 154 | 36 | 14.5 | 27.8 | 25.0 | 3.00 | 1.15 | 16.2 | 34.1 | 26.9 | 3.70 | 0.92 | 200 | 44 | |
| 8 | 52 | M | AA | 9.1 | 20.0 | 29.1 | 2.11 | 1.02 | 152 | 38 | 10.4 | 23.0 | 28.6 | 2.57 | 1.11 | 9.4 | 19.8 | 31.4 | 2.17 | 1.02 | 134 | 40 | |
| 9 | 53 | M | AA | 12.0 | 29.8 | 26.1 | 3.33 | 1.26 | 211 | 32 | 14.0 | 29.3 | 33.9 | 2.70 | 1.23 | n/a | n/a | n/a | n/a | n/a | 210 | 35 | |
| Canakinumab 300 mg | 10 | 58 | M | C | 6.5 | 17.5 | 31.0 | 2.54 | 1.11 | 169 | 36 | 7.5 | 16.9 | 29.1 | 3.16 | 1.00 | 5.1 | 15.6 | 33.7 | 3.13 | 0.91 | 198 | 28 |
| 11 | 59 | M | AA | 6.2 | 16.1 | 27.4 | 2.36 | 1.10 | 93 | 43 | 8.0 | 16.8 | 29.7 | 2.33 | 1.13 | 7.3 | 13.8 | 34.0 | 2.00 | 1.00 | 117 | 52 | |
| 12 | 62 | M | C | 10.4 | 24.4 | 28.9 | 2.71 | 1.20 | 100 | 45 | 11.1 | 26.0 | 31.3 | 2.50 | 1.17 | 11.3 | 24.8 | 31.6 | 2.45 | 1.21 | 100 | 56 | |
| 1 | 43 | M | C | 8.4 | 15.7 | 23.1 | 3.02 | 1.09 | 196 | 36 | 8.4 | 25.6 | 25.9 | 4.42 | 1.15 | n/a | n/a | n/a | n/a | n/a | 218 | 16 | |
| Placebo | 2 | 52 | F | AA | 3.3 | 9.0 | 43.4 | 1.61 | 1.15 | 146 | 34 | 3.1 | 11.8 | 42.1 | 1.26 | 1.16 | 3.3 | 9.6 | 36.3 | 1.28 | 1.23 | 131 | 37 |
| 3 | 54 | M | AA | 10.5 | 22.6 | 25.2 | 2.92 | 1.05 | 100 | 36 | 11.5 | 26.3 | 25.6 | 3.38 | 1.13 | n/a | n/a | n/a | n/a | n/a | 76 | 54 | |
Abbreviations: AA = African-American; BNP = Brain natriuretic peptide (pg/ml); C = Caucasian; E/Eˊ = ratio of transmitral Doppler early filling velocity to tissue Doppler early diastolic mitral annular velocity; F = female; hs-CRP = high sensitivity C-reactive protein (mg/l); LVEDV = left ventricular end-diastolic volume (ml); LVEF = left ventricular ejection fraction (%); M = male; n/a = not available; OUES = oxygen-uptake efficiency slope; Peak VO2 = maximal oxygen consumption during cardiopulmonary exercise testing (ml.kg.min−1); VE/VCO2 = minute ventilation to carbon dioxide production ratio; Y = yes.
Of the 15 subjects, 13 (87%) were male, 8 (53%) were Caucasian, 6 (40%) were African-American, and 1 was (7%) Hispanic. Time from acute MI was 34 (12 114) months. All patients were on a stable (>3 months) regimen of maximally-tolerated guideline-directed medications for HF. Seven (47%) patients had New York Heart Association class II and 8 (53%) class III symptoms. No significant changes in hsCRP were seen in the placebo group [5.3, 5.4, and 8.2 mg/l at baseline, 2.7, 6.0, and 6.2 mg/dl at 3 months, and 5.6, 6.6, and 11.6 mg/dl at 12 months, p = 0.10 for within group changes]. Treatment with Canakinumab significantly reduced hsCRP levels from 5.3 mg/l (3.0 11.2) to 2.0 (0.9 5.2) at 3 months (p = 0.028), and to 1.0 (0.7 2.6) at 12 months (p = 0.002 for within-group changes, p = 0.042 for between-group difference).
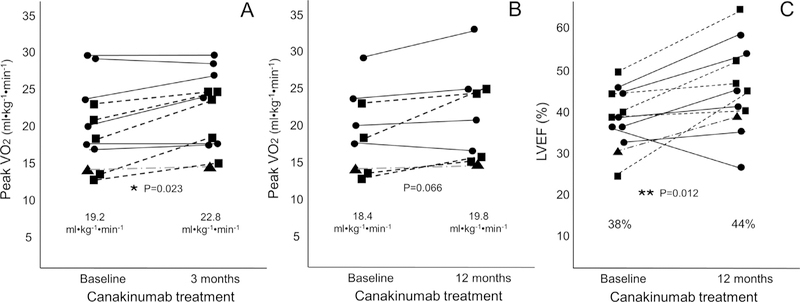
Treatment with Canakinumab significantly increased peak VO2 from 19.2 to 22.8 ml/kg/min at 3 months (p = 0.023 within-group changes, p = 0.026 between group changes [primary end point] with a median interval change of +1.6 [–0.4/+3.4] ml/kg/min) (Figure 1, Table 1). Of the 3 doses, only Canakinumab 150 mg was independently associated with a significant increase in peak VO2 at 3 months (from 18.4 [13.6 21.6] to 22.5 [16.8 24.4], with a median increase of +3.5 [+1.8/+4.5] ml/kg/min, uncorrected p = 0.043) (Figure 1). No significant effect of placebo was detected on peak VO2 at 3 months (Table 1, Figure 2).Only 9 of the 12 Canakinumab-treated patients and 1 placebo-treated patient had repeat peak VO2 assessed at 12 months; peak VO2 showed a trend toward improvement with Canakinumab in a within-group analysis [from 18.4 (14.0 23.4) to 19.8 (15.0 24.8), p = 0.066](a time_-x_group interaction analysis could not be performed as only 1 placebo-treated patient had peak VO2 measured at 12 months)(Figures 1 and 2).
Figure 1. Interval changes in peak oxygen consumption with Canakinumab.
Individual patient values are shown according to Canakinumab dose (triangle = 50 mg [n = 1]; squares = 150 mg [n = 5]; circles = 300 mg [n= 6]). Panel A shows changes in peak oxygen consumption (VO2) at 3 months, *p = 0.023 for within group changes versus baseline, and p = 0.026 versus placebo at time_x_group interaction at 3 months. Of the 3 doses, only Canakinumab 150 mg (squares) was associated with a significant increase in peak VO2 at 3 months (from 18.4 [13.6—21.6] to 22.5 [16.8—24.4], p = 0.043 with a median increase of +3.5 [+1.8/+4.5] ml/kg/min). Panel B shows changes in VO2 at 12 months. Panel C shows changes in left ventricular ejection fraction (LVEF) at 12 months. **p = 0.012 for within group changes versus baseline, and p = 0.30 versus placebo at time_x_group interaction. Of the 3 doses, only Canakinumab 150 mg was associated with a significant increase in LVEF at 12 months (from 38% [31—46] to 46% [42—57], p = 0.043 with a median increase of +12% [+3/+17]).
Figure 2.
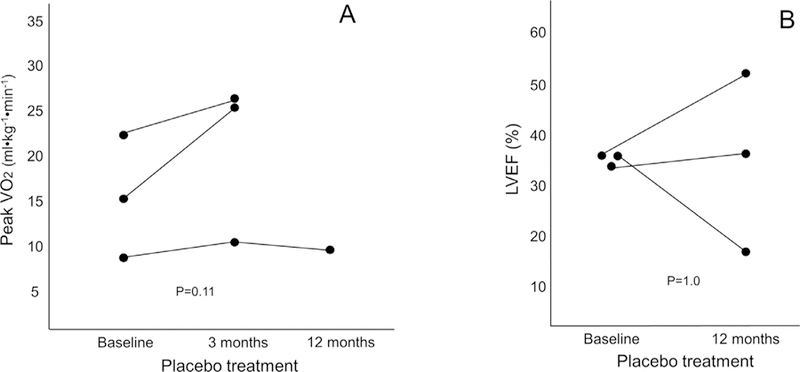
Interval changes in peak oxygen consumption in patients treated with placebo are shown (circles). Panel A shows changes in peak oxygen consumption (VO2) at 3 months and 12 months (Of the 3 patients, only 1 patient had peak VO2 measured at 12 months). Panel B shows changes in left ventricular ejection fraction in the 3 patients at echocardiography at 12 months.
Paired echocardiograms were available in all 15 substudy patients. Canakinumab-treated patients experienced an improvement in LVEF from 38% (33 43) to 44% (38 52) at 12 months (p = 0.012 for within-group changes, with median change of +7% [+2/+12]) which, however, did not reach statistical significance for time_x_group interaction versus placebo (p = 0.30) (Table 1, Figures 1 and 2). Of the 3 doses, only Canakinumab 150 mg was associated with a significant increase in LVEF at 12 months (from 38% [31 46] to 46% [42 57], with a median increase of +12% [+3/+17], uncorrected p = 0.043). There were no significant changes in peak VO2 or LVEF in the placebo group (n = 3) at any time point (Figure 2).
In addition to the above variables, changes in other cardiopulmonary exercise test and Doppler echocardiographic are provided in the Table 1. There were 2 HF admissions within the first 12 months: one in the placebo (1, 33%) and 1 in the low dose (50 mg) Canakinumab group (1, 8%). One patient in the placebo group (33%) was hospitalized for critical limb ischemia, and 4 (25%) Canakinumabtreated patients were hospitalized for noncardiac reasons. Reasons for hospitalization among Canakinumab-treated patients included pneumonia/sepsis (n = 1), pulmonary embolism (n = 1), and noncardiac chest pain (n = 2). None of the patients discontinued treatment.
Discussion
The findings of improved peak VO2 and LVEF in patients with systolic HF treated with Canakinumab supports the hypothesis that IL-1b can modulate cardiac function by acting as a soluble cardiodepressant factor1 and exercise capacity,10 and is consistent with the effects of anakinra, a recombinant IL-1 receptor antagonist, in patients with stable or with recently decompensated systolic HF.2,3 Peak VO2 and LVEF are two strong and independent predictors of outcomes in patients with systolic heart failure.7,11 Peak VO2 is a highly reliable and reproducible measure of cardiorespiratory fitness that integrates cardiac function as well as oxygen exchange, transport, and delivery. When the patient provides a maximal effort and is limited by cardiac output (as evidenced by gas exchange), peak VO2 provides a reliable and valid measure of cardiac function.12 LVEF is also a measure of cardiac function and predicts outcome, but is sensitive to changes in pre- and afterload and therefore may reflect a direct or indirect improvement in cardiac function. Nevertheless, the improvement in both peak VO2 and LVEF with Canakinumab is encouraging and in-line with the proposed mechanism by which IL-1β impairs cardiac function by depressing contractility.1
The limitations of this small substudy include the small sample size representing a very small part of the patients in the CANTOS trial with HF at a single site, even smaller than the planned 30 patients. Moreover, this substudy represents the results of a randomization not stratified by site, thus providing an imbalance in groups with only included 3 placebo-treated patients and only 1 patient treated with low dose Canakinumab. Many of the patients also had peak VO2 levels which were higher than expected, a feature which may have limited the ability to detect further improvement and responsiveness to IL-1 blockade, as previously described.13 Despite the smaller-than-planned sample size and the imbalance between the doses, Canakinumab led to a significant increase in peak VO2 at 3 months, which was the predefined end point. The overall change was less than predicted, but increases in peak VO2 >1 ml/kg/min have been associated with improved prognosis.14 The Canakinumab 150 mg dose was associated with a significant median increase in peak VO2 of 3.5 ml/kg/min, as predicted in the sample size analysis, although this was without adjusting for multiple group comparisons. Of note, Canakinumab 150 mg was also the only dose meeting the predefined significance criteria in terms of reduction of primary clinical outcome in the CANTOS trial.5 The current findings support the expanded therapeutic potential of Canakinumab (specifically) and IL-1 blockers (in general), as a potential means to improve exercise capacity in patients with systolic HF; however, due to the above limitations should be considered exploratory. Additional analysis on hard outcomes of the entire cohort of CANTOS patients with systolic heart failure may further explore whether functional improvement with IL-1 blockade may translate into improved clinical outcomes as suggested by the pilot phase II clinical trials.3,15
Acknowledgments
Disclosure
The study was completed as part of an Investigator Initiated Study supported by Novartis (Basel, Switzerland).
Footnotes
See page 1370 for disclosure information.
References
- 1.Van Tassell BW, Toldo S, Mezzaroma E, Abbate A. Targeting interleukin-1 in heart disease. Circulation 2013;128:1910–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Tassell BW, Arena RA, Toldo S, Mezzaroma E, Azam T, Seropian IM, Shah K, Canada J, Voelkel NF, Dinarello CA, Abbate A. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS One 2012;7:e33438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Tassell BW, Canada J, Carbone S, Trankle C, Buckley L, Oddi Erdle C, Abouzaki NA, Dixon D, Kadariya D, Christopher S, Schatz A, Regan J, Viscusi M, Del Buono M, Melchior R, Mankad P, Lu J, Sculthorpe R, Biondi-Zoccai G, Lesnefsky E, Arena R, Abbate A. Interleukin-1 blockade in recently decompensated systolic heart failure: results from REDHART (recently decompensated heart failure anakinra response trial). Circ Hear Fail 2017;10:e004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1b inhibition and the prevention of recurrent cardiovascular events: Rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J 2011;162:597–605. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 7.Breathett K, Allen LA, Udelson J, Davis G, Bristow M. Changes in left ventricular ejection fraction predict survival and hospitalization in heart failure with reduced ejection fraction. Circ Hear Fail 2016;9:e002962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women. J Am Med Assoc 2009;301:2024–2035. [DOI] [PubMed] [Google Scholar]
- 9.Novartis Pharmaceuticals. Cardiovascular Risk Reduction Study (Reduction in Recurrent Major CV Disease Events) (CANTOS) 2017. Available at: https://clinicaltrials.gov/ct2/show/NCT01327846.
- 10.Canada JM, Fronk DT, Cei LF, Carbone S, Erdle CO, Abouzaki NA, Melchior RD, Thomas CS, Christopher S, Turlington JS, Trankle CR, Thurber CJ, Evans RK, Dixon DL, Van Tassell BW, Arena R, Abbate A. Usefulness of C-reactive protein plasma levels to predict exercise intolerance in patients with chronic systolic heart failure. Am J Cardiol 2016;117:116–120. [DOI] [PubMed] [Google Scholar]
- 11.De Groote P, Dagorn J, Soudan B, Lamblin N, McFadden E, Bauters C. B-type natriuretic peptide and peak exercise oxygen consumption provide independent information for risk stratification in patients with stable congestive heart failure. J Am Coll Cardiol 2004;43:1584–1589. [DOI] [PubMed] [Google Scholar]
- 12.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, MacKo R, Mancini D, Milani RV. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 2010;122:191–225. [DOI] [PubMed] [Google Scholar]
- 13.Canada JM, Van Tassell BW, Christopher S, Oddi C, Abouzaki NA, Gambill ML, Mueller G, Melchior R, Shah KB, Dinarello CA, Abbate A, Arena R. Clinical predictors of response to Anakinra in patients with heart failure. Int J Cardiol 2014;173:537–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keteyian SJ, Brawner CA, Savage PD, Ehrman JK, Schairer J, Divine G, Aldred H, Ophaug K, Ades PA. Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am Heart J 2008;156:292–300. [DOI] [PubMed] [Google Scholar]
- 15.Abbate A, Kontos MC, Abouzaki NA, Melchior RD, Thomas C, Van Tassell BW, Oddi C, Carbone S, Trankle CR, Roberts CS, Mueller GH, Gambill ML, Christopher S, Markley R, Vetrovec GW, Dinarello CA, Biondi-Zoccai G. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Am J Cardiol 2015;115:288–292. [DOI] [PubMed] [Google Scholar]