Abstract
Atherosclerosis is characterized by the retention of modified lipoproteins in the arterial wall. These modified lipoproteins activate resident macrophages, and the recruitment of monocyte-derived cells, which differentiate into mononuclear phagocytes that ingest the deposited lipoproteins to become “foam cells”; a hallmark of this disease. In this part 2 of a 4-part review series covering the macrophage in cardiovascular disease, we critically review the contributions and relevant pathobiology of monocytes, macrophages and foam cells as relevant to atherosclerosis. We also review evidence that via various pathways, a failure of the resolution of inflammation is an additional key aspect of this disease process. Finally, we consider the likely role played by genomics and biologic networks in controlling the macrophage phenotype in atherosclerosis. Collectively, these data provide substantial insights on the atherosclerotic process, while concurrently offering numerous molecular and genomic candidates that appear to hold great promise for selective targeting as clinical therapies.
Keywords: Macrophage, Cardiovascular, Atherosclerosis, Inflammation, Resolution
Condensed Abstract:
Atherosclerosis is characterized by the retention of modified lipoproteins in the arterial wall. These modified lipoproteins activate resident macrophages, and the recruitment of monocyte-derived cells, which differentiate into mononuclear phagocytes that ingest the deposited lipoproteins to become “foam cells”; a hallmark of this disease. In this second of a 4-part review series we consider the contributions and pathobiology of monocytes, macrophages and foam cells as relevant to atherosclerosis. We also review evidence that failure of inflammation resolution is a key aspect of this disease, and also the role of genomics and biologic networks in controlling the macrophage phenotype in atherosclerosis.
With basic macrophage biology and phenotype reviewed in Part 1 of this series, here in Part 2 we consider one of its most critical roles in the cardiovascular system: the macrophage in atherosclerosis (Central Illustration).
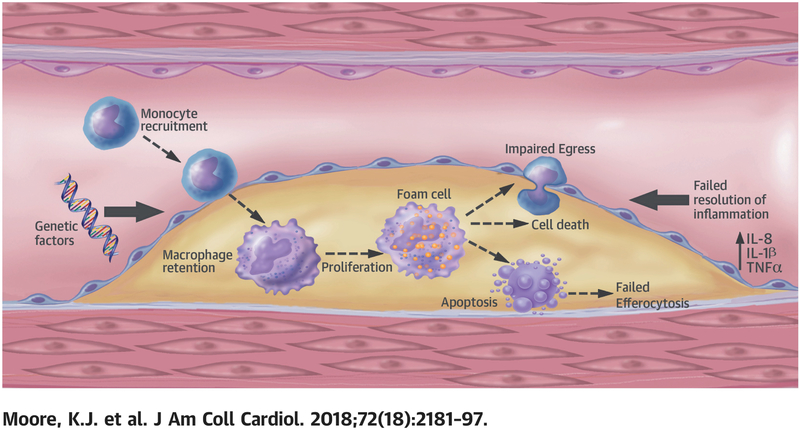
Central Illustration:
Macrophage trafficking, Role of resolution of inflammation and genetic/genomic factors in atherosclerosis.
Macrophage Trafficking in Atherosclerosis
Plaque macrophage content represents the balance between blood monocyte recruitment, their differentiation into tissue macrophages and proliferation in situ, and their retention, emigration, or death. In this section we review this important kinetic balance (Figure 1).
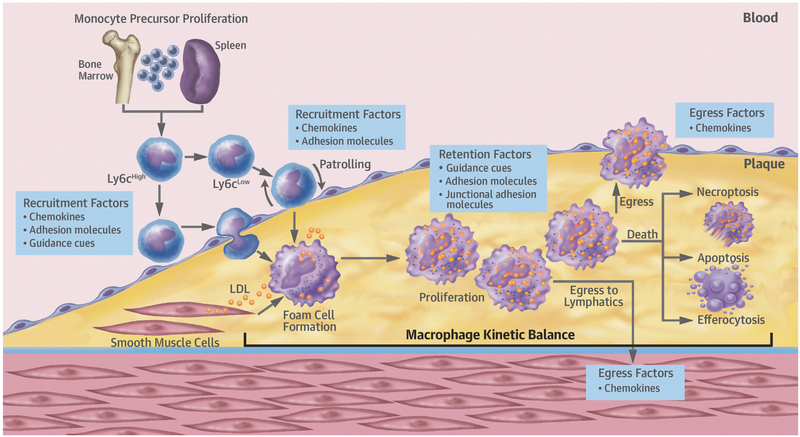
Figure 1: Macrophage dynamics during atherosclerotic plaque progression and regression.
Major kinetic processes dictating macrophage burden are recruitment of monocytes and the proliferation, retention, death, and egress of monocyte-derived macrophages. During hypercholesterolemia there is an increase in monocyte precursors in bone marrow and spleen, resulting in more circulating Ly6Chi monocytes. Some become patrolling Ly6Clow monocytes, but the majority of monocytes recruited to plaques are Ly6Chi, which transmigrate into the subendothelial space. In progression, these monocytes take up modified and retained lipoproteins transforming them into inflammatory macrophage foam cells. VSMCs can also become macrophage-appearing foam cells, but their properties and fates are largely undefined. In regression, recruited monocytes become M2, inflammation resolving, macrophages. In advanced plaques, macrophages can proliferate, and death by apoptosis and necroptosis can contribute to necrotic core formation, with falling levels of efferocytosis promoting core growth. In early plaques, reverse transmigration of macrophages may occur. This abates with progressing disease, but in regression, reverse macrophage transmigration can be restored by reduced retention and increased emigration factors.
Circulating monocytes and their recruitment into atheroma
Atherosclerotic plaque formation is initiated by the intramural retention of atherogenic lipoproteins (1). Atherosclerotic plaques tend to form at inner curvatures and branch points of arteries, where laminar flow is disturbed or insufficient to support the normal, quiescent endothelial state. In these sites, there is accumulation of extracellular matrix proteins, coincident with retention of cholesterol-rich, apolipoprotein B-containing lipoproteins. In the arterial wall microenvironment these lipoproteins are susceptible to various modifications (e.g., oxidation, enzymatic and non-enzymatic cleavage and aggregation), rendering the particles pro-inflammatory. The accumulation of these modified lipoproteins triggers resident macrophage activation and recruitment of monocyte-derived cells into the subendothelial space, where they differentiate into mononuclear phagocytes that ingest the deposited normal and modified lipoproteins. While macrophage clearance of lipoproteins is initially likely to be beneficial, there is little negative feedback of uptake and these cells become grossly engorged with lipids, transforming into “foam cells”. The foam cell is a hallmark of early atherosclerotic plaques, or so-called “fatty streaks”, where these cells persist and contribute to plaque progression.
The recruitment of circulating monocytes into plaques requires the integration of at least 3 discrete processes; their capture, rolling and transmigration. Endothelial activation by retained modified lipoproteins or endothelial damage activates the leukocyte adhesion cascade that mediates circulating monocyte arrest. Chemokines immobilized on the endothelium, particularly Chemokine (C-C motif) ligand 5 (CCL5) and CXC-chemokine ligand 1 (CXCL1), initiate monocyte capture and rolling, while vascular cell adhesion molecule 1 (VCAM1) and intercellular adhesion molecule (ICAM) 1 mediate monocyte adhesion to the endothelial surface. Transendothelial monocyte migration is then initiated by CD31 and VCAM1, as well as locally produced chemokines. Three chemokine receptor–chemokine pairs are of particular importance in this process: C-C chemokine receptor 2 (CCR2)–CCL2, CX3CR1 (CX3C chemokine receptor 1)–CX3CRL1 (CX3C chemokine receptor ligand 1) and CCR5-CCL5 (2). Targeted deletion of these chemokine axes in atheroprone ApoE−/− mice led to ~90% reduction in atherosclerotic plaque burden (3). One caveat however, is that CCR2 and CX3CR1 also participate in the extravasation and survival, respectively, of bone marrow-derived Ly6chi cells (4,5) that influence the number of circulating monocytes and thus their supply to, and lifespan in, the plaque. In another study, apoA1 (the major protein of high density lipoprotein-(HDL) cholesterol) inhibited monocyte migration and decreased their recruitment to sites of inflammation (6). This was mediated by reorganization of plasma membrane lipid rafts as a consequence of apoA1-mediated cholesterol efflux, which disrupted the signaling cascade (concentrated in the rafts) that harnesses the actin-cytoskeleton to accomplish cellular movement.
Ligands and receptors belonging to neuronal guidance molecule families are also likely involved in monocyte recruitment and retention. Members of the netrin, semaphorin and ephrin families are expressed by arterial endothelial cells and are differentially regulated by conditions that promote or protect from atherosclerosis (7). Specifically, ephrin B2 which functions as a monocyte chemoattractant (7), and sema7A (8) which interacts with β1 integrin, are upregulated in atheroprone regions. In contrast, netrin-1 and sema3A, which inhibit chemokine-directed monocyte migration, are decreased by proatherogenic factors, while their inhibition increases leukocyte adhesion to the endothelium (7). Another semaphorin family member, sema4D, is increased in infiltrating leukocytes in atherosclerotic plaques as well as its receptor plexin-B1 in neovascular plaque endothelial cells (9). In addition, EphA2 receptor expression is increased in endothelial cells and macrophages in human and mouse atherosclerotic plaques, while endothelial activation with oxidized low-density lipoprotein (LDL) or proinflammatory cytokines induces expression of EphA2 and its ligand ephrinA1 (10). Notably, the targeted deletion or repression of several of these neuroimmune guidance cues reduced atherosclerosis in mouse models, including netrin-1 (11), sema4D (9,12), sema7A (8), and EphA2 (13,14). Collectively, these studies suggest important roles for neuroimmune guidance cues in monocyte recruitment and atherosclerotic plaque progression. Interestingly, some neuronal guidance cues are also involved in plaque macrophage chemostasis (see below).
Hypercholesterolemia is associated with increased circulating monocyte numbers in mice, swine and rabbits (15,16), which is thought to accelerate monocyte recruitment into atheroprone regions. In ApoE−/− mice, circulating monocytes, particularly the Ly6chi subset, are ~50% higher than in controls (2,17). This increase likely arises from cholesterol enrichment of bone marrow (18) and splenic (19) hematopoietic stem cells that increase their proliferation and thus, the supply of monocytes that are mobilized to inflammatory sites, including atherosclerotic plaques. Hyperglycemia also increases circulating monocytes (20) through mechanisms that overlap with those in hypercholesterolemia (21). Higher levels of circulating monocytes in hyperglycemic mice led to their increased recruitment into plaques, which impaired atherosclerosis regression after lipid lowering (20,21).
In contrast, in normoglycemic mice, monocyte recruitment may be critical for disease regression and inflammation resolution (22,23). In murine plaques in which atherosclerosis regression is induced by aggressive lipid lowering or raising of HDL cholesterol levels, enrichment in M2-like macrophages was required for resolution (24-26). In the ApoE−/− aortic transplant model, the precursors of these M2-like macrophages are Ly6chi circulating monocytes recruited into plaques after the transition from a hyper- to normo-lipidemic environment (23). These findings that M2-like macrophages are atheroprotective in a regression setting are echoed by data from progression models. For example, the administration of the M2-polarizing cytokine interleukin (IL)-13 to the Ldlr−/− hypercholesterolemic mouse model was shown to drive plaque macrophages to M2-like cells and inhibit atherosclerosis progression (27).
That M2 precursors are derived from Ly6chi circulating monocytes, that were historically considered to be inflammatory, was initially surprising but resonates with the view that resolving inflammation can be impeded by a subnormal inflammatory response (22). Taken together these findings indicate that, coincident with the decline in the number of macrophages in regressing plaques, there is also a required enrichment in inflammation-resolving M2-like macrophages (20,24-26,28-30). However, the phenotypic varieties of macrophages in vivo are complex, and many subsets likely exist along the M1-M2 spectrum (see Part 1 of the review series).
Macrophage proliferation
After infiltrating the arterial intima, monocytes differentiate into macrophages, where these highly phagocytic cells internalize accumulated modified lipoproteins. Multiple means of LDL cholesterol modification facilitate macrophage cholesterol loading in vitro, but the prevailing paradigm has been that heightened oxidative stress in the artery wall promotes deleterious LDL cholesterol modifications, generating “damage” signals that are recognized by macrophage scavenger receptors. These receptors, including scavenger receptor A (SR-A), SR-B1, SREC-1, MARCO, CD36, lectin-type oxidized LDL receptor 1, and scavenger receptor for phosphatidylserine and oxidized LDL (SRPSOX; also known as CXCL16), can bind oxidized LDL cholesterol and promote foam cell formation (31), with SR-A and CD36 mediating the majority (75–90%) of oxidized LDL cholesterol degradation by macrophages in vitro (32). Engagement of scavenger receptors, particularly SR-A, may also contribute to macrophage proliferation in established atherosclerotic plaques (33).
Macrophage proliferation occurs in atherosclerotic plaques in humans, rabbits and mice (34-38). Using an array of advanced techniques, Robbins and colleagues showed that although monocyte recruitment predominates in early plaques, there is robust proliferation of macrophages in advanced lesions (33). Using parabiosis experiments in CD45.1 and CD45.2 congenic atheroprone ApoE−/− mice, the authors estimated the relative contribution of in situ macrophage proliferation was ~30% in early atherosclerosis and up to 87% in established atherosclerosis. Interestingly, macrophage proliferation in murine plaques is not dependent on granulocyte-macrophage colony-stimulating factor, but is partly dependent on SR-A. However, deficiency of SR-A alone (39,40) or both SR-A and CD36 (41,42) only partially reduces foam cell accumulation in plaques of ApoE−/− mice, suggesting that other pathways regulate macrophage proliferation in those settings, or that other kinetic processes contribute to macrophage accumulation.
The realization that macrophage proliferation contributes to advanced atherosclerosis has opened the door to therapeutic strategies targeting this process. One strategy has been nanoparticle-mediated delivery of simvastatin to plaque macrophages (43). Statins act specifically on the mevalonate pathway of cholesterol synthesis, which is critical to proliferating cells (44). Using HDL-based nanoparticles as a delivery modality, a 1 week regimen of targeted simvastatin delivery to lesional macrophages reduced plaque macrophage burden by 45% in ApoE−/− mice with advanced atherosclerosis (43). Simvastatin-HDL treatment did not reduce monocyte recruitment, but was associated with reductions in macrophage proliferation (~25%) and inflammatory gene expression. Although statins also have anti-inflammatory properties, this supports inhibiting local macrophage proliferation as a strategy for suppressing atherosclerotic inflammation.
Macrophage retention
Macrophages that accumulate in atherosclerotic plaques have diminished migratory capacity, which contributes to failure to resolve inflammation and thus to advanced, complex plaques (45). The signals regulating macrophage retention are only beginning to be defined. Cholesterol loading of macrophages increases the expression of the neuro-immune guidance cues netrin-1 and semaphorin 3E, which induce macrophage chemostasis in vitro (11,46). Netrin-1 and semaphorin 3E secreted by plaque macrophages act locally to retain macrophages by blocking their response to emigration signals. Hypoxia, which is intimately linked to atherosclerosis (46,47), can induce macrophage expression of netrin-1, that serves as a survival factor for macrophages undergoing oxidative stress (47). Studies of Ldlr−/− mice with bone marrow deficiency of netrin-1 demonstrated increased macrophage emigration from lesions and reduced atherosclerosis progression (11).
Other factors that inhibit cell movement, such as adhesion molecules (48), also likely contribute to macrophage retention. A recent study showed that upregulation of αDβ2 integrin (CD11d/CD18) on inflammatory macrophages contributes to macrophage retention in atherosclerotic plaques (49). Targeted CD11d deletion in ApoE−/− mice reduced atherosclerosis progression, despite similar recruitment of WT and Cd11d−/− monocytes to inflammatory stimuli. Using adoptive transfer experiments in which both WT and Cd11d−/− monocytes were transferred to atheroprone ApoE−/− mice, the investigators showed reduced accumulation of Cd11d−/− macrophages in the aorta, suggesting that CD11d promotes macrophage retention in atherosclerotic plaques (49). Junctional Adhesion Molecule C (JAM-C) expressed by vascular endothelial cells was also shown to block reverse-transendothelial migration of monocyte-derived cells from atheroma (50). JAM-C expression is increased in chronic inflammatory diseases, including atherosclerosis, and blocking this component of the endothelial tight junction led to increased macrophage reverse-transendothelial migration in vitro and accelerated macrophage emigration from plaques during atherosclerosis regression (50). Further study of atherosclerosis progression and regression are likely to elucidate additional signals that inhibit cell movement or impair inflammation resolution.
Macrophage death and clearance by efferocytosis
Macrophage cholesterol metabolism is regulated by the balance of cholesterol uptake, free cholesterol liberation, cholesterol esterification and storage, and free cholesterol efflux from the cell. With excessive cholesterol uptake, macrophage lipid metabolism can become dysregulated resulting in inflammatory signaling, endoplasmic reticulum (ER) stress, inflammasome activation, and ultimately, cell death.
Excess cellular cholesterol is stored in lipid droplets as cholesteryl ester, and in this form cholesterol is relatively inert. However free cholesterol, which can accumulate if cholesterol metabolism becomes dysregulated, can be toxic. Trafficking of free cholesterol out of lysosomes to the ER, where it can undergo esterification for storage in lipid droplets, becomes defective in foam cells (51). Furthermore, enrichment of ER membranes with free cholesterol or activation of ER stress responses by saturated fatty acids signaling via SR-A, and toll-like receptor-2 and −4 (52), can result in defective cholesterol esterification by acyl-CoA cholesterol acyltransferase in macrophages favoring further free cholesterol accumulation. Such dysregulation in lipid metabolism contributes to ER stress, which if prolonged and combined with other insults, can culminate in apoptotic cell death (53), which is observed in 2-4% of cells in mouse plaques, with the highest levels in advanced plaques.
Dying foam cells, if not properly cleared, can release their lipid contents and tissue factor, leading necrotic core formation. Efficient apoptotic cell clearance by surrounding macrophages (efferocytosis) requires intact lipid metabolism pathways (such as cholesterol esterification and efflux) in the engulfing cell to deal with the ingested lipids. Efficient efferocytosis coupled with inducing macrophage death may be beneficial to net changes in macrophage or foam cell content, as suggested in a recent study of regression (54). However, in late-stage plaques, the ability of macrophages to clear their dying counterparts through such receptors as Mer tyrosine kinase (MerTK) and LDL receptor-related protein 1 (LRP1) becomes compromised, which is attributed, in part, to cholesterol accumulation in the engulfing cells (55). Also implicated in defective plaque efferocytosis is the CD47 “don’t-eat-me” signal, which if expressed on the apoptotic cell can block its uptake by surrounding phagocytes (56). Interestingly, genome-wide association studies (GWAS) have identified several loci in DNA regions encoding proteins regulating efferocytosis and which are associated with cardiovascular disease risk, including the T cell immunoglobulin and mucin domain (Tim) receptor for apoptotic cells (57), calreticulin (58), and the metalloproteinase ADAM17 (59), which is implicated in MerTK cleavage (60,61). Targeting of Tim (62) or calreticulin (58) was shown to reduce efferocytosis and exacerbate atherosclerosis.
In advanced plaques, increased macrophage apoptosis combined with defective efferocytosis results in cell necrosis and the release of cellular components and lipids that form the necrotic core. This feature of advanced atherosclerotic plaques, along with thinning of the fibrous cap, may increase the vulnerability of plaques to rupture and the ensuing intravascular blood clot that underlies myocardial infarction and stroke. Although it was initially assumed that cell necrosis was a passive event, it has become clear that programmed forms of necrotic or lytic death exist, and are active in atherosclerosis (63). Pyroptosis, a caspase-dependent form of cell death associated with inflammasome activation, results from cleavage of gasdermin-D and formation of plasma membrane pores (64). Necroptosis, a caspase-independent form of programmed necrosis that depends on kinase receptor-interacting protein (RIP) 3 and phosphorylation of the multilineage kinase-domain like (MLKL) protein to form the oligomeric necrosome (65), also results in pore-formation and release of cell contents, including adenosine-triphosphate, mitochondria and damage-associated molecular patterns (DAMPs). Dissection of the roles of these forms of cell death in atherosclerosis and other diseases has proved difficult, because of overlapping signaling pathways. However, studies of RIP3 deletion (66) or inhibition (67) in mouse models reduced atherosclerotic plaque size, and importantly, necrotic core formation. The innate immune system also includes sensors of necrotic cells, such as Cecl4e (68), which participate in the recognition of DAMPs released from dying cells and may amplify plaque inflammation.
The advent of cell-specific and inducible systems for targeted cell death have allowed investigators to test whether macrophage apoptosis is beneficial in atherosclerosis. Using these tools, investigators showed that macrophage death favorably affected plaque and necrotic core size only in mice with early lesions (69). Activation of macrophage death in mice with advanced plaques had little effect on plaque size or composition (69). Conversely, strategies that inhibited macrophage death by overexpression of the survival molecule B-cell lymphoma 2 (Bcl2) exacerbated plaque development in early atherosclerosis and reduced plaque size in advanced atherosclerosis (70). These strategies highlight how targeting macrophage survival may have different outcomes at different stages of plaque development, and suggest that such an approach would not be viable in advanced lesions where efferocytosis and other components of the tissue repair process may be impaired.
Macrophage egress from plaques
The first robust evidence to support macrophage emigration from plaques was presented in pioneering pig studies from the 1970’s-80’s; interestingly, the rate of macrophage egress was found to decrease with atherosclerosis progression (71). However, for many years, the signals guiding macrophage egress via either reverse endothelial transmigration to the lumen or by migrating through the media to the adventitial lymphatics, remained poorly defined. More recent progress has come from new mouse models of atherosclerosis regression, which have established the reversibility of macrophage accumulation and activation in plaques, challenging the paradigm that failing to resolve chronic inflammation is an inevitable feature of atherosclerosis. Using cell trafficking methods, including labeling circulating monocytes by fluorescent beads (2), such studies have shown that macrophage retention can be reversed (24,25,28), leading to the identification of pathways that promote macrophage egress from the plaque. Broadly, these pathways fall into 2 groups; those that promote macrophage retention (e.g., neuroguidance and JAM molecules), and those that promote macrophage movement (e.g., chemokine-receptor pairs). In studies in which macrophage emigration from plaques was induced by normalizing the hyperlipidemic plasma profile of mice in an aortic transplant model, emigrating cells expressed several characteristic markers of both macrophages and dendritic cells (72). For example, the expression of CCR7, a regulator of immune cell homing and the receptor for CCL19 and CCL21, is upregulated in emigrating CD68+ macrophages and blocking this pathway led to their retention in the plaque (72).
Transcriptomic profiling of macrophages isolated by laser capture microdissection (72) of progressing and regressing plaques in an aortic transplantation mouse model revealed >700 differentially regulated genes (48), including downregulation of the macrophage retention factors semaphorin 3E and netrin-1, and also of adhesion molecules such as members of the cadherin family (48). In contrast, cellular motility factors were upregulated. In addition, CCR7 was expressed at low levels in plaque macrophages in progressing plaques, likely suppressed by hypercholesterolemia due to a serum response element in its promoter (73). Notably, Ccr7 transcription was upregulated in macrophages when plaques were placed in a regression environment, thereby increasing cell migratory capacity. Collectively, these data indicate that macrophage emigration from plaques is a highly regulated process, reflecting coordinated changes in macrophage retention and movement.
Smooth muscle cell transition into a macrophage-like state
It has long been appreciated that VSMCs can take up lipids and become smooth muscle foam cells (74). In 2003, it was reported that when mouse VSMCs were loaded with cholesterol in vitro, they downregulated major canonical VSMC markers (alpha-actin, calponin, etc.) and expressed macrophage-associated factors (e.g., CD68, Lgals3, ABCA1) (30). The authors predicted that if this occurred in vivo, then cells that underwent this transdifferentiation would appear as plaque macrophages (30). Using a variety of methods, including lineage marking (in mice), a proximity ligation assay (in human samples), and multiple immunohistochemical markers (both species), this was subsequently borne out (30,75-78). Depending on the lesion stage, at least 30% of macrophage marker positive cells in plaques appear to be VSMC-derived. The properties and functions of these VSMC-derived cells, particularly in vivo, remain largely unknown. In vitro, they are not particularly good at phagocytosis or efferocytosis compared to authentic macrophages (78). Based on what was discussed earlier, in advanced plaques, this would be predicted to be adverse because apoptotic cell clearance would be impaired. Conversely, they are not particularly inflammatory in vitro, and consistent with this, transcriptomic analyses showed that only a subset of macrophage-associated genes are induced, with the majority of the profile remaining VSMC-centered (78), which may be a beneficial property. The potential to have competing effects on atherosclerosis will require intensive investigation to determine the net impact of VSMC-“macrophages”, and these studies should include disease-stage specific effects, as well as the reversibility of the phenotype in a regression setting. Of course, the mechanisms by which transdifferentiation occur also need further exploration. Early results implicate miR-143/-145 (78) (known positive regulators of VSMC phenotype) and KLF4 (77), a transcriptional factor partly regulated by miR-143/-145 and with multiple interesting targets, including macrophage-associated genes.
Summary and concluding remarks on macrophage trafficking in atherosclerosis
Monocyte-macrophage kinetics are fundamental to understanding atherosclerotic plaque biology. There is optimism that therapies targeted to reduce either the content of activated macrophages (e.g., by promoting macrophage apoptosis, efferocytosis or emigration, or by reducing proliferation) or to increase the content of M2-like resolving macrophages (e.g., by use of pro-resolving mediators) may have beneficial clinical effects. However, the quantitative impact of each of the dynamic processes that regulate macrophage burden in atherosclerosis is likely to vary in different stages of disease, as would the consequences of selectively targeting them. For example, a burst of monocyte recruitment appears essential for atherosclerosis regression (23); thus, blocking this process could impair resolution of atherosclerotic inflammation. Strategies that therapeutically target macrophage death should also be approached with caution. It is expected that in early plaque development, where efferocytosis is efficient, increasing apoptosis would be beneficial, but as efferocytosis becomes impaired in advanced plaques then increasing apoptosis could expand the necrotic core and enhance its thrombogenicity. Important areas of future investigation include the regulation and quantitative impact of each of the kinetic factors that regulate plaque macrophage burden, and their effects on other immune cells. Also, the phenomenon of VSMC-derived macrophage-like cells requires considerable additional investigation.
The Macrophage and Inflammation Resolution in Atherosclerosis
Inflammation and resolution
In response to injury or infection, the immune system generates a coordinated program of acute inflammation aimed at the eradication of pathogens and subsequent repair to allow the tissue to return to homeostasis (Figure 2). During the initiation phase of the acute response, a local release of soluble mediators occurs including cytokines (e.g., IL-1β, IL-6, TNF-α), complement, bioactive lipids (prostaglandins, leukotrienes), and vasoactive amines (histamine, bradykinin), leading to increased blood flow, microvasculature permeability and tissue edema (79). In response to these signals and the upregulation of endothelial adhesion molecules, neutrophils migrate to the injury where they target and engulf pathogens. Once the initial insult has been adequately neutralized, inflammation must be dampened, and cellular debris must be cleared to limit host collateral damage and allow tissue repair. This ‘resolution’ phase was once thought to be the passive termination of the initial inflammatory response, however, we now understand that it is an active process characterized by the production of pro-resolving proteins (e.g., annexin A1 and IL-10), gases (e.g., carbon monoxide, hydrogen sulfide) and lipids (lipoxins, resolvins, protectins, and maresins) (79). In the first phase of resolution, pro-inflammatory mediator synthesis is downregulated, and these molecules are catabolized, preventing further leukocyte infiltration. Inflammatory cells are also cleared, either by rejoining the circulation or by undergoing apoptosis followed by efferocytosis, in order to prevent them from undergoing necrosis and generating an ongoing inflammatory stimulus. Macrophages, which polarize toward a pro-resolving phenotype, play an important role in apoptotic polymorphonuclear leukocyte efferocytosis and participate in tissue repair and extracellular matrix production. Finally, in the post-resolution phase, additional immune cells including T regulatory cells, memory T cells and memory B cells accumulate locally to prepare an appropriate adaptive immune response against any future inflammatory insult. Additional macrophages also accumulate, including resident macrophages and Ly6chi monocyte-derived macrophages (80). In cases where the resolution program fails, e.g., because the inflammatory stimulus persists and/or some pathological process lowers resolving mediator levels or their effectiveness, the inflammatory response is sustained and the organism will be unable to develop an appropriate secondary response. Indeed, failure to resolve inflammation is part of the etiology of many chronic inflammatory diseases including rheumatoid arthritis, asthma, chronic obstructive pulmonary disease, inflammatory bowel disease, cancers, Alzheimer’s disease and advanced atherosclerosis (22,81).
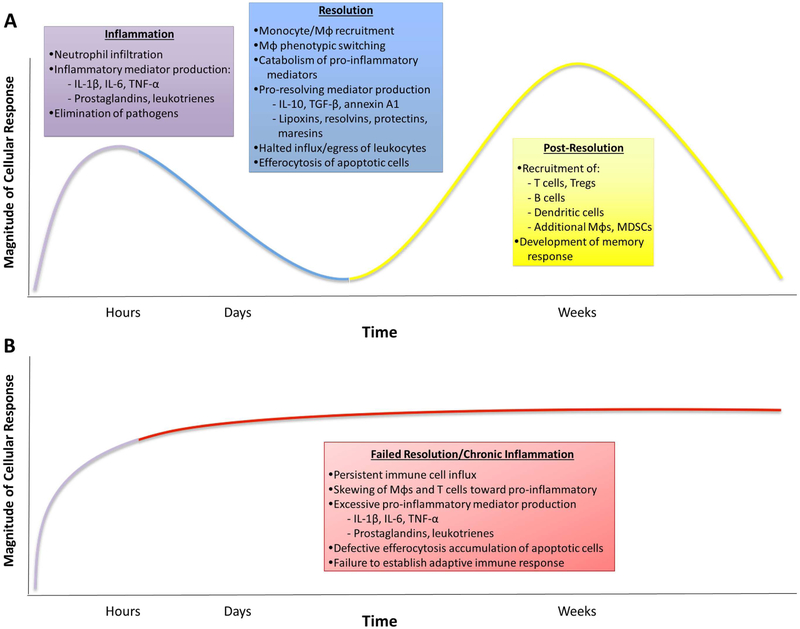
Figure 2. Inflammation and Resolution.
A generalized, healthy inflammatory response consists of three phases: inflammation, resolution and post-resolution. (A) The acute inflammatory response is characterized edema and a predominantly neutrophilic infiltrate in response to pro-inflammatory factors. These features typify the response to an acute inflammatory stimulus like trauma or infection, but are less relevant for chronic conditions like atherosclerosis. Nevertheless, this initial response activates the resolution program, which leads to lipid mediator class switching toward the production of pro-resolving factors and catabolism of pro-inflammatory factors. This response, along with increased production of additional mediators of resolution, leads to tissue repair by halting the influx of leukocytes, promoting their egress and clearing dead cells. A proper resolution response leads to post-resolution adaptive immunity, which prepares the organism for future insults. (B) If an inflammatory stimulus persists (e.g. atherosclerosis) or resolution fails, the initial inflammatory response may become chronic. This leads to a maladaptive post-resolution adaptive immune phase and impaired ability to respond to subsequent challenges. MDSC, myeloid-derived suppressor cell; Mφ, macrophage; TGF-β, transforming growth factor; TNF-α, tumor necrosis factor; Treg, T regulatory cell.
Advanced atherosclerosis results from defective resolution of inflammation
Atherosclerosis is characterized by sustained inflammation and failed inflammation resolution. The persistent sub-endothelial retention of apolipoprotein B100-containing lipoproteins provides the continued inflammatory stimulus that drives ongoing leukocyte recruitment and production of pro-inflammatory cytokines and oxidative species (82). In the presence of high levels of pro-inflammatory cytokines and modified lipoproteins, recruited macrophages polarize toward a pro-inflammatory phenotype (83). These macrophages exhibit cross-talk with VSMCs, which amplify the inflammatory cycle by producing additional pro-inflammatory cytokines and also extracellular matrix that promotes further retention of lipoproteins (82,84,85). This hyper-inflammatory microenvironment impairs the ability of VSMCs to produce the collagen necessary to develop a protective cap that isolates the thrombogenic developing plaque from the bloodstream. In addition, this persistent inflammation drives apoptosis, generating apoptotic cells that must be cleared by lesional macrophages (86). As already discussed, efferocytosis may fail in advanced plaques, leading to accumulation of debris and dead cells, which then undergo secondary necrosis with release of immunogenic antigens, more inflammation and necrotic core formation (86-88). In addition, primary necroptosis further contributes to necrotic core development (66,67). Importantly, expansive necrotic cores and thinning collagen caps are hallmarks of advanced atherosclerotic disease and are associated with clinical cardiovascular events (89,90).
Low levels of specialized pro-resolving mediators (SPMs) correlate with atherosclerosis, and the presence of CAD or peripheral arterial disease (91), while regions of human and murine atherosclerotic plaques with advanced features are characterized by impaired SPM production (92,93). Using targeted mass spectrometry of human carotid artery specimens, the relative level of SPMs in relation to the pro-inflammatory mediator leukotriene B4 (LTB4) was lower in histologically-defined areas of advanced atherosclerotic plaque compared to early areas of lesions (92). In addition, atheroprone Ldlr−/− mice had reduced levels of SPMs relative to LTB4 within the atherosclerotic aorta. When one of the most significantly reduced SPMs, resolvin D1 (RvD1), was administered to Ldlr−/− mice in levels sufficient to restore the balance of RvD1:LTB4 during plaque development, mice receiving RvD1 had smaller necrotic cores, increased lesional efferocytosis and thicker collagen caps (92). Similarly, in atherosclerotic lesions of ApoE−/− mice, high levels of the pro-inflammatory lipid mediators LTB4 and prostaglandin E2 were found in advanced lesions compared with early lesions. Concomitantly, levels of the pro-resolving mediators RvD2 and maresin 1 (MaR1) were lower in advanced lesions compared with early lesions (93). Together, these results demonstrate that the local imbalance of pro-resolving:pro-inflammatory factors likely plays a pathogenic role in both murine and human disease.
Other studies have indirectly suggested an athero-protective role for SPMs. For example, variants in the genes encoding SPM biosynthesis enzymes have been associated with atherosclerosis, stroke, and myocardial infarction (94-98). The lipoxygenases, in particular, 5-lipoxygenase (5-LOX) and 12/15-LOX, are important for the synthesis of SPMs and pro-inflammatory lipid mediators. 5-LOX expression was found to be significantly higher in human carotid atherosclerotic lesions compared with healthy arteries and correlated with clinical ischemic symptoms, suggesting that 5-LOX is associated with more severe atherosclerotic disease (99). Consistent with this, the loss of 5-LOX from atheroprone ApoE−/− mice led to a reduction in plaque size (100). The role of 12/15-LOX in atherogenesis has been somewhat more difficult to elucidate. First, humans express two forms of the enzyme, 12-LOX and 15-LOX, while mice express only one form that displays both 12- and 15-LOX activity (commonly referred to as 12/15-LOX). In humans, polymorphism in the gene encoding 15-LOX (ALOX15) is associated with higher enzyme activity in heterozygotes and was enriched in controls as compared to CAD cases, suggesting that higher levels of 15-LOX may confer atheroprotection (101). Consistent with this, hypercholesterolemic rabbits engineered to overexpress 15-LOX within macrophages were more resistant to early atherosclerosis than control rabbits (102,103). Transgenic mice overexpressing 12/15-LOX on an ApoE−/− background were found to have higher levels of pro-resolving mediator lipoxin A4 (LXA4) and reduced atherosclerotic lesion area compared to controls (104). However, if these mice received a Western diet, the protective effect of 12/15-LOX was not observed (105). When the converse studies using genetic deletion of 12/15-LOX were performed, the authors found similarly complex results. When fed a chow diet, the Alox15−/− ApoE−/− mice showed increased atherosclerotic lesion area compared to controls, however, if fed a high-fat (Western) diet for 10-14 weeks, Alox15−/− ApoE−/− mice demonstrated reduced lesion area, suggesting that diet significantly disrupts the mechanisms by which lipid mediators are atheroprotective (104,105). Although the mechanisms are not yet understood, population data suggests an interaction between dietary fatty acid precursor intake and gene variants in lipoxygenases (106). In the Los Angeles Atherosclerosis study, patients with variant 5-LOX genotypes (those lacking the common allele) exhibited increased carotid intima-media thickness. Further, the authors found an interaction between gene variation and diet which suggested that omega-6 polyunsaturated fats promote, while omega-3 fatty acids decrease, carotid intima-media thickness amongst patients carrying variant alleles (106). Finally, in addition to generating lipid mediators, 15-LOX likely plays a role in LDL cholesterol oxidation, which may partly explain its pro-atherogenic effects with a high-cholesterol Western diet (107).
Resolving mediators enhance atheroprotective mechanisms
A variety of atheroprotective mechanisms have been associated with the SPMs in vitro, including efferocytosis, reduction in inflammatory cells and oxidative stress, and repair of tissue damage. When delivered directly to atheroprone mice, SPMs have successfully ameliorated markers of plaque progression. For example, administration of aspirin-triggered lipoxin A4 (ATL), RvD1, RvD2, MaR1, IL-10 or the peptide Ac2-26 (which mimics annexin A1) have all been shown to increase collagen deposition and/or fibrous cap thickness in mouse models (Online Table 1). RvD1, IL-10, and Ac2-26 decrease lesional reactive oxygen species, while ATL, RvD1, RvD2, MaR1 and Ac2-26 all shift the balance of pro-inflammatory vs. proresolving cytokines in macrophages and other cell types known to be important in atherogenesis, particularly T cells (Online Table 1). Annexin A1 was shown to limit myeloid cell recruitment to the arterial wall by decreasing leukocyte:endothelial interactions in response to chemotactic signals, including CCL2, CCL5, CXCL1, and LTB4. In addition, RvD1, RvD2, and MaR1 prevent the differentiation of naïve CD4 into Th1 cells and enhance their differentiation into classical Treg cells both in vitro and in vivo (Online Table 1).
Efferocytosis is also a key mediator of inflammation resolution. In general, dead cell clearance is crucial to the termination of inflammation since it prevents secondary necrosis of apoptotic cells and limits necrotic core development, which otherwise augment intra-lesional inflammation (Figure 3). In addition, efferocytosis promotes several anti-inflammatory and pro-resolving pathways. Macrophages performing efferocytosis upregulate the production of antiinflammatory cytokines including IL-10 and TGF-β, while downregulating production of pro-inflammatory cytokines including IL-8, IL-1β, and TNF-α (108,109). Further, the uptake of apoptotic bodies increases macrophage synthesis of SPMs while concomitantly reducing the generation of leukotrienes (110,111). A recent paper demonstrated a link between the efferocytosis receptor, MerTK and SPM production. In response to MerTK signaling, 5-LOX translocates from the nucleus to the cytoplasm, where it drives the production of pro-resolving LXA4. When MerTK is genetically deleted from macrophages, 5-LOX is restricted to the nuclear membrane where it instead drives the production of pro-inflammatory LTB4, increasing the relative amount of LTB4:SPMs (110). Similar results were obtained when the MerTK receptor was cleaved and inactivated in response to inflammatory stimuli. In a complementary study using mice genetically engineered to express a cleavage-resistant form of MerTK on the Ldlr−/− background and fed a Western diet for 16 weeks, aortic root lesions demonstrated higher rates of efferocytosis, smaller necrotic cores and higher ratios of 5-LOX-derived SPMs:LTs (60). Interestingly, these mice also had thicker collagen caps, although the mechanism for this is not fully understood. Recent evidence suggests that alongside efferocytosis-driven SPM production, pro-resolving mediators themselves also enhance efferocytosis in a feedforward fashion. In in vitro or ex vivo models, LXA4, protectin D1 (PD1), RvD1, RvD3, and RvE1 all increased the rate of efferocytosis (111-114). Further, administration of RvD1, RvD2 or MaR1 to atheroprone mice (ApoE−/− or Ldlr−/−) reduced necrotic core size and increased lesional efferocytosis (92,93). Similarly, nanoparticle-based delivery of Ac2-26 or the pro-resolving cytokine IL-10 led to decreased necrotic core sizes, implying a likely effect on efferocytosis (115,116). Together, these studies suggest an intimate relationship between resolution and efferocytosis, which if disrupted can lead to amplification of inflammation and atheroprogression.
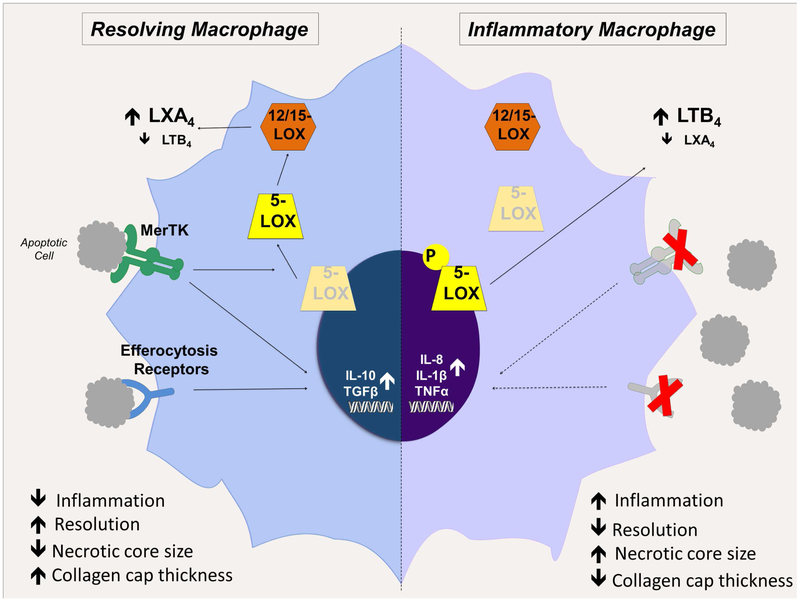
Figure 3. Efferocytosis and apoptotic cell clearance drive pro-resolving macrophage functions.
In resolving macrophages (left), MerTK signaling drives 5-LOX translocation from the nuclear membrane to the cytoplasm, where it is brought into proximity with 12/15-LOX. The coordinated activity of these enzymes favors pro-resolving LXA4 synthesis. In addition, signaling through MerTK and other efferocytosis receptors upregulates pro-resolving cytokines including IL-10 and TGF-β while inhibiting pro-inflammatory cytokine production. In inflammatory macrophages (right), as in advanced atherosclerosis, efferocytosis becomes defective. With diminished MerTK signaling, 5-LOX is phosphorylated and confined to the nuclear membrane, bringing it into proximity with LTA4 hydrolase and favoring the synthesis of the pro-inflammatory mediator LTB4. Further, in the absence of MerTK and other efferocytosis receptor signaling, there is a failure to suppress the production of pro-inflammatory cytokines or to upregulate pro-resolving cytokines. The overall effect of failed efferocytosis in advanced plaques is the accumulation of apoptotic cells that become secondarily necrotic; increased necrotic core size; increased inflammation and impaired resolution; and decreased collagen cap thickness.
Therapeutic potential of targeting inflammation and resolution in atherosclerosis
Early, mostly pilot studies focused on eliminating pro-inflammatory signals, with promising results that support the targeting of inflammation and resolution as a therapeutic strategy. Recently, the initial results of the landmark CANTOS study of canakinumab, a monoclonal antibody against IL-1β, were released. CANTOS showed that in high-risk subjects, most of whom were on statin therapy, treatment with canakinumab led to a reduction in the primary endpoint of nonfatal myocardial infarction, nonfatal stroke or cardiovascular death, without reducing lipids levels (117). There was, however, an increase in fatal infection with canakinumab, likely due to the associated immunosuppression. CANTOS marks the first clinical trial to show that explicitly targeting inflammation can reduce clinical cardiovascular events. Additional trials of anti-inflammatory targets are now underway, including the Cardiovascular Inflammation Reduction Trial (CIRT) utilizing low-dose methotrexate to determine if blocking general inflammation will lower vascular event rates (118).
On the other hand, the idea of targeting resolution is in its infancy. While the SPMs have shown promise in animal models, their clinical use is hampered by their lability and short half-lives. Despite this they make attractive candidates because, unlike therapies targeting inflammation, pro-resolving therapies are predicted to be less immunosuppressive and may, in fact, enhance host defense (119). One early approach to targeting resolution was atreleuton, a 5-LOX inhibitor. In phase II studies in patients with acute coronary syndromes, overall leukotriene production was decreased; however, vascular inflammation measured by FDG-PET imaging did not demonstrate any significant improvement (120,121). Given the dual role of 5-LOX in the generation of both leukotrienes and lipoxins, it is possible that this neutral effect was due to the loss of both the pro-inflammatory and pro-resolving downstream effects. The recent mechanistic work described above regarding the role of MerTK in the regulation of 5-LOX, and deeper mechanistic insights on SPMs in general, should provide additional rationale and therapeutic targets to move this work forward.
Another early focus of pro-resolving therapy has been dietary supplementation of omega-3 polyunsaturated fatty acids, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which are lipid mediator precursors. While in mice, supplementation of omega-3 fatty acids ameliorates atherosclerosis (122,123), human data are less clear. Population-based studies suggested that an omega-3 fatty acid rich diet may reduce the incidence of cardiovascular disease; however, randomized clinical trials in both primary and secondary prevention failed to show a benefit (124-127). These studies have been criticized for their small sample sizes, short treatment periods, low doses of supplementation (<1g/day) and overall high baseline omega-3 intake in the study populations. To address these criticisms, two new trials are currently underway. The first, REDUCE-IT, will examine the efficacy of high-dose (4g/day) EPA supplementation plus statin treatment versus statin alone in the reduction of cardiovascular events (128). A second, STRENGTH, will determine whether combined DHA/EPA supplementation plus statin therapy (versus placebo plus statin) leads to a reduction in cardiovascular events (128).
Genetics and Networks of Macrophages in Atherosclerosis and Coronary Artery Disease
Genome-wide association studies of CAD and related risk factors
To date, GWAS for CAD have identified at least 98 genetic variants passing the stringent criteria for genome-wide significance (129,130). The ensuing interpretation of these CAD loci and their mechanism(s) of effect is, however, not straightforward. Even for high priority CAD loci such as 9p21, establishing the underlying mechanisms has proven difficult. Indeed, after intense research, the causative variant at the 9p21 locus was recently proposed to act through circular ANRIL, a non-coding RNA, by regulating ribosomal RNA maturation in VSMCs and macrophages, thereby modulating apoptosis and cell proliferation (131). Although other mechanisms have been proposed, the identification of macrophage-specific molecular mechanisms for this risk variant raises the possibility that other genetic risk variants with unknown mechanisms might modulate macrophage function to promote CAD. In summary, despite the discovery of many CAD risk loci, the disease-causing mechanisms and pathways for most of these variants remain unknown (130) and in particular, the role of macrophages in mediating the effects of these risk loci is critically understudied and remains to be defined.
CAD genetics and macrophage networks in atherosclerosis
The interconnectedness of biological networks has been proposed as a possible explanation for the diversity of identified GWAS risk loci, each of which exerts only a small effect in promoting CAD. To decipher biological networks acting in CAD, co-expression networks inferred from gene expression data using algorithms such as weighted co-expression gene analysis (132,133) provide an unbiased and complimentary tool to GWAS for analyzing and annotating CAD candidate genes, including those in atherosclerosis involving macrophages. Indeed, several co-expression analyses have already begun to dissect the role of macrophages in driving atherosclerosis. For example, a co-expression network analysis of atherosclerotic tissue from mice identified a macrophage pathway acting through the transcriptional regulation of CD44 (134), while in another study a differentially co-expressed module from whole blood samples was associated with B-cell activation and inflammation (135). Yet another study used multi-tissue gene expression data from CAD patients including the atherosclerotic arterial wall and visceral fat to reveal two modules, one in each tissue, that are related to coronary stenosis (132). The modules were both enriched for genes associated with leukocyte transendothelial migration, and shared several genes, of which LIM domain-binding protein 2 (LDB2) was determined to control transcriptional regulation of a large fraction of the genes in these modules. Subsequent molecular characterization showed that the spatial distribution of LDB2 expression in arterial endothelial cells was disrupted by the development of atherosclerosis and that LDB2 was increasingly expressed in foam cells compared to macrophages and monocytes (132).
From the most extensive CAD network study to date, 171 network modules were identified acting within and across metabolic and arterial wall tissues of CAD patients (133). Several of these networks contained candidate genes previously suggested by GWAS for both CAD and also CAD risk factors. This study also applied probabilistic Bayesian algorithms using expression quantitative trait loci (eQTLs) and transcription factors to identify network genes called “key drivers” that are more essential for disease development than non-driver network genes. Interestingly, several networks of this human study were also identified in corresponding mouse tissues (136).
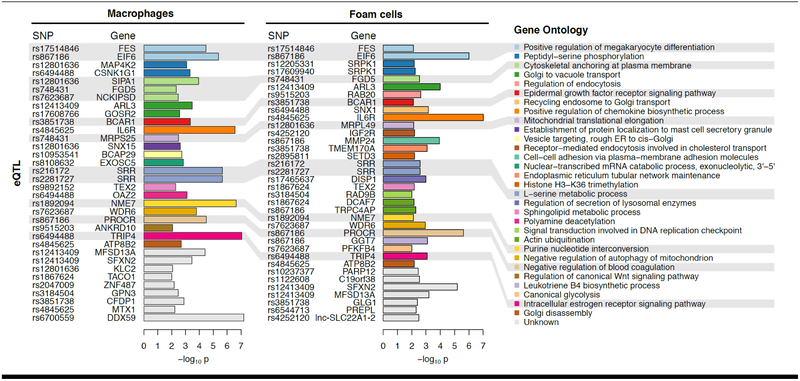
An important pathway for linking CAD risk variants to disease-causal networks has been to correlate GWAS loci with loci that control expression levels, such as gene expression (eQTL), protein expression (pQTL), or chromatin accessibility (caQTL) in specific cell types and tissues (137,138). The eQTLs for CAD-associated variants in multi-tissue gene expression data (GTEx) suggest that the causal tissues in CAD are ranked according to: artery, liver, muscle, and adipose tissues (139). Therefore, a large proportion of the genetics of CAD can be expected to exert effects in vascular tissue directly, either by modifying vascular or immune cells such as monocytes and macrophages. In the STARNET study (138), from nearly 600 patients, we performed deep RNA-sequencing and identified eQTLs on primary blood monocytes that were differentiated into macrophages in vitro (STARNET: Stockholm-Tartu Atherosclerosis Reverse Network Engineering Task). While these data are unpublished, in a preliminary directed analysis for this review, we found strong evidence for macrophage genes down-stream of as many as 29 of the 98 validated CAD genetic risk loci (129,130,140) (Figure 4 and Online Appendix). Thus, these preliminary data suggest that ~30% (29/98) of CAD GWAS risk loci may have macrophage-related mechanisms of action. Nevertheless these data remain hypothesis generating, and the full contribution of genetic/genomic modulation of macrophage functionality to promoting atherosclerosis remains to be defined.
Figure 4. Macrophage-specific association between CAD risk loci and gene expression.
We leveraged the STARNET datasets to perform a pilot analysis on the associations between CAD risk loci (single nucleotide polymorphisms [SNPs]) and gene expression in macrophages and foam cells. Thus, we investigated the 98 CAD-associated GWAS SNPs (129,130), asking whether these SNPs predict macrophage or foam cell gene expression within 1Mb; so-called cis-eQTL. We required that the eQTL had the strongest effect in gene expression from macrophages (n=95) or foam cells (n=80) compared to other tissues in STARNET. This approach identified 29 SNPs that are associated with the expression levels of 52 genes (p<0.01). Thus, 29/98 (~30%) of CAD risk loci are most strongly associated with gene expression in macrophages and/or foam cells. In particular, association with IL6R, NME7, TRIP4, PROCR, and EIF6 was the most significant and detected in both macrophages and foam cells. Candidate genes are color-coded and organized based on Gene Ontology terms according to biological processes of potential relevance to macrophage biology. Further details are provided in the Online Appendix.
Complementary approaches providing genetic evidence for macrophages in atherosclerosis
By analyzing risk variants in the context of related traits, so-called phenome-wide association scanning, newly identified CAD risk loci at RP11-664H17.1, HNF1A, CDH13, and IRS3 have been linked to well-known risk factors, including hypertension, LDL cholesterol, and diabetes (130). In addition, several newly identified loci: FN1, LOX, ITGB5, and ARHGEF26 did not associate with any other GWAS traits, suggesting that these act through unknown pathways. Specifically, functional analyses showed that the ARHGEF26 disease variant causes the formation of an abnormal ARHGEF26 protein, which leads to increased endothelial adhesion and transendothelial leukocyte migration. Collectively, this points to a genetic contribution to the excessive monocyte recruitment that arises with atherosclerosis (130).
Pathway analysis of genes from initial CAD GWAS revealed a high complexity of biochemical and physiological processes with multiple important roles for VSMCs (141). Using novel methods, additional links to angiogenesis were recently confirmed among variants detected in the UK Biobank (140). Moreover, several links to macrophage biology were identified, including impaired macrophage phagocytosis, abnormal macrophage physiology, and increased leukocyte cell number (140). These results provide further evidence that a component of the genetics of atherosclerosis involves effects on macrophages.
Concluding remarks on genetics and networks of macrophages in atherosclerosis
While we have learned a great deal about the genetic and genomic contributions to atherosclerosis and CAD, a vast amount remains to be understood. Specifically, we are just beginning our discovery of the role that genetics and biologic networks play in controlling the macrophage phenotype in atherosclerosis. Tantalizing evidence has now emerged to suggest that this role may be substantial, and that the targeting of newly identified genetic aspects of monocyte/macrophage network biology may be of clinical benefit. Novel technologies have also emerged that will assuredly facilitate this discovery such as single-cell RNA-sequencing (see Part 1 article), however this technology remains to be intersected and leveraged using genome-wide data or systems genetics approaches in the cardiovascular system. Collectively, this will be an exciting area of research and clinical opportunity for the coming years.
Supplementary Material
Acknowledgements:
Work in the laboratories of Kathryn Moore and Edward Fisher related to this review are supported by the National Institutes of Health (PO1 HL131481 and P01HL131478 [EAF, KJM]; R01 HL084312 [EAF, KJM] R01 HL129433 [EAF, KJM]; DK095684 [EAF]; R35HL135799 [KJM], and the Department of Defense (W81XWH-16-1-0374; [EAF, KJM]). Work in the laboratory of Ira Tabas related to this review is supported by the National Institutes of Health (R01HL075662, R01HL127464, and R01HL132412). Amanda Doran acknowledges research support from the American Heart Association (17FTF33660643). Jason Kovacic acknowledges research support from the National Institutes of Health (R01HL130423), the American Heart Association (14SFRN20490315; 14SFRN20840000) and The Leducq Foundation (Transatlantic Network of Excellence Award).
Abbreviations
- ATL
aspirin-triggered lipoxin A4
- CCL
chemokine (C-C motif) ligand
- CCR
C-C chemokine receptor
- CXCL
CXC-chemokine ligand
- CX3CR
CX3C chemokine receptor
- DAMP
damage-associated molecular pattern
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- eQTL
expression quantitative trait locus
- ER
endoplasmic reticulum
- FDR
false discovery rate
- GWAS
genome-wide association studies
- ICAM
intercellular adhesion molecule
- JAM-C
junctional Adhesion Molecule C
- LDB2
LIM domain-binding protein 2
- LOX
lipoxygenase
- LTB4
leukotriene B4
- LXA4
lipoxin A4
- MaR1
maresin 1
- MerTK
Mer tyrosine kinase
- PD1
protectin D1
- RIP
receptor-interacting protein
- Rv
resolvin
- SNP
single nucleotide polymorphism
- SPM
specialized pro-resolving mediators
- SR
scavenger receptor
- STARNET
Stockholm-Tartu Atherosclerosis Reverse Network Engineering Task
- Tim
T cell immunoglobulin and mucin domain
- VCAM
vascular cell adhesion molecule 1
- VSMC
vascular smooth muscle cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors declare no conflicts of interest.
References
- 1.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011;145:341–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tacke F, Alvarez D, Kaplan TJ et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest 2007;117:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Combadiere C, Potteaux S, Rodero M et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 2008;117:1649–57. [DOI] [PubMed] [Google Scholar]
- 4.Landsman L, Bar-On L, Zernecke A et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood 2009;113:963–72. [DOI] [PubMed] [Google Scholar]
- 5.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 2006;7:311–7. [DOI] [PubMed] [Google Scholar]
- 6.Iqbal AJ, Barrett TJ, Taylor L et al. Acute exposure to apolipoprotein A1 inhibits macrophage chemotaxis in vitro and monocyte recruitment in vivo. Elife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Gils JM, Ramkhelawon B, Fernandes L et al. Endothelial expression of guidance cues in vessel wall homeostasis dysregulation under proatherosclerotic conditions. Arterioscler Thromb Vasc Biol 2013;33:911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu S, Liu Y, You T et al. Vascular Semaphorin 7A Upregulation by Disturbed Flow Promotes Atherosclerosis Through Endothelial beta1 Integrin. Arterioscler Thromb Vasc Biol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yukawa K, Tanaka T, Kishino M et al. Deletion of Sema4D gene reduces intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Int J Mol Med 2010;26:39–44. [DOI] [PubMed] [Google Scholar]
- 10.Funk SD, Yurdagul A Jr., Albert P et al. EphA2 activation promotes the endothelial cell inflammatory response: a potential role in atherosclerosis. Arterioscler Thromb Vasc Biol 2012;32:686–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Gils JM, Derby MC, Fernandes LR et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol 2012;13:136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu L, Stalker TJ, Fong KP et al. Disruption of SEMA4D ameliorates platelet hypersensitivity in dyslipidemia and confers protection against the development of atherosclerosis. Arterioscler Thromb Vasc Biol 2009;29:1039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finney AC, Funk SD, Green JM et al. EphA2 Expression Regulates Inflammation and Fibroproliferative Remodeling in Atherosclerosis. Circulation 2017;136:566–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang H, Li X, Zhang X, Liu Y, Huang S, Wang X. EphA2 knockdown attenuates atherosclerotic lesion development in ApoE(−/−) mice. Cardiovasc Pathol 2014;23:169–74. [DOI] [PubMed] [Google Scholar]
- 15.Averill LE, Meagher RC, Gerrity RG. Enhanced monocyte progenitor cell proliferation in bone marrow of hyperlipemic swine. Am J Pathol 1989;135:369–77. [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman DL, Mogelesky TC, Liptak BF, Gerrity RG. Leukocytosis in rabbits with diet-induced atherosclerosis. Arterioscler Thromb 1991;11:985–94. [DOI] [PubMed] [Google Scholar]
- 17.Swirski FK, Libby P, Aikawa E et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 2007;117:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yvan-Charvet L, Pagler T, Gautier EL et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 2010;328:1689–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutta P, Courties G, Wei Y et al. Myocardial infarction accelerates atherosclerosis. Nature 2012;487:325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagareddy PR, Murphy AJ, Stirzaker RA et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab 2013;17:695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Distel E, Barrett TJ, Chung K et al. miR33 inhibition overcomes deleterious effects of diabetes mellitus on atherosclerosis plaque regression in mice. Circ Res 2014;115:759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathan C, Ding A. Nonresolving inflammation. Cell 2010;140:871–82. [DOI] [PubMed] [Google Scholar]
- 23.Rahman K, Vengrenyuk Y, Ramsey SA et al. Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression. J Clin Invest 2017;127:2904–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feig JE, Parathath S, Rong JX et al. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation 2011;123:989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feig JE, Rong JX, Shamir R et al. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A 2011;108:7166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rayner KJ, Sheedy FJ, Esau CC et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest 2011; 121:2921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardilo-Reis L, Gruber S, Schreier SM et al. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol Med 2012;4:1072–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A 2004;101:11779–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reis ED, Li J, Fayad ZA et al. Dramatic remodeling of advanced atherosclerotic plaques of the apolipoprotein E-deficient mouse in a novel transplantation model. J Vasc Surg 2001;34:541–7. [DOI] [PubMed] [Google Scholar]
- 30.Rong JX, Li J, Reis ED et al. Elevating high-density lipoprotein cholesterol in apolipoprotein E-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation 2001;104:2447–52. [DOI] [PubMed] [Google Scholar]
- 31.Kzhyshkowska J, Neyen C, Gordon S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology 2012;217:492–502. [DOI] [PubMed] [Google Scholar]
- 32.Kunjathoor VV, Febbraio M, Podrez EA et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem 2002;277:49982–8. [DOI] [PubMed] [Google Scholar]
- 33.Robbins CS, Hilgendorf I, Weber GF et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 2013;19:1166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benditt EP. Implications of the monoclonal character of human atherosclerotic plaques. Beitr Pathol 1976;158:405–16. [DOI] [PubMed] [Google Scholar]
- 35.Gordon D, Reidy MA, Benditt EP, Schwartz SM. Cell proliferation in human coronary arteries. Proc Natl Acad Sci U S A 1990;87:4600–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katsuda S, Coltrera MD, Ross R, Gown AM. Human atherosclerosis. IV. Immunocytochemical analysis of cell activation and proliferation in lesions of young adults. Am J Pathol 1993;142:1787–93. [PMC free article] [PubMed] [Google Scholar]
- 37.Pearson TA, Dillman J, Williams KJ et al. Clonal characteristics of experimentally induced "atherosclerotic" lesions in the hybrid hare. Science 1979;206:1423–5. [DOI] [PubMed] [Google Scholar]
- 38.Rosenfeld ME, Ross R. Macrophage and smooth muscle cell proliferation in atherosclerotic lesions of WHHL and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis 1990;10:680–7. [DOI] [PubMed] [Google Scholar]
- 39.Babaev VR, Gleaves LA, Carter KJ et al. Reduced atherosclerotic lesions in mice deficient for total or macrophage-specific expression of scavenger receptor-A. Arterioscler Thromb Vasc Biol 2000;20:2593–9. [DOI] [PubMed] [Google Scholar]
- 40.de Winther MP, Gijbels MJ, van Dijk KW et al. Scavenger receptor deficiency leads to more complex atherosclerotic lesions in APOE3Leiden transgenic mice. Atherosclerosis 1999;144:315–21. [DOI] [PubMed] [Google Scholar]
- 41.Kuchibhotla S, Vanegas D, Kennedy DJ et al. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc Res 2008;78:185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manning-Tobin JJ, Moore KJ, Seimon TA et al. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol 2009;29:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang J, Lobatto ME, Hassing L et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci Adv 2015;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soma MR, Corsini A, Paoletti R. Cholesterol and mevalonic acid modulation in cell metabolism and multiplication. Toxicol Lett 1992;64–65 Spec No:1–15. [DOI] [PubMed] [Google Scholar]
- 45.Randolph GJ. Emigration of monocyte-derived cells to lymph nodes during resolution of inflammation and its failure in atherosclerosis. Curr Opin Lipidol 2008;19:462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wanschel A, Seibert T, Hewing B et al. Neuroimmune guidance cue Semaphorin 3E is expressed in atherosclerotic plaques and regulates macrophage retention. Arterioscler Thromb Vasc Biol 2013;33:886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramkhelawon B, Yang Y, van Gils JM et al. Hypoxia induces netrin-1 and Unc5b in atherosclerotic plaques: mechanism for macrophage retention and survival. Arterioscler Thromb Vasc Biol 2013;33:1180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feig JE, Vengrenyuk Y, Reiser V et al. Regression of atherosclerosis is characterized by broad changes in the plaque macrophage transcriptome. PLoS One 2012;7:e39790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aziz MH, Cui K, Das M et al. The Upregulation of Integrin alphaDbeta2 (CD11d/CD18) on Inflammatory Macrophages Promotes Macrophage Retention in Vascular Lesions and Development of Atherosclerosis. J Immunol 2017;198:4855–4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bradfield PF, Menon A, Miljkovic-Licina M et al. Divergent JAM-C Expression Accelerates Monocyte-Derived Cell Exit from Atherosclerotic Plaques. PLoS One 2016;11:e0159679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jerome WG. Advanced atherosclerotic foam cell formation has features of an acquired lysosomal storage disorder. Rejuvenation Res 2006;9:245–55. [DOI] [PubMed] [Google Scholar]
- 52.Seimon TA, Nadolski MJ, Liao X et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab 2010;12:467–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng B, Yao PM, Li Y et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol 2003;5:781–92. [DOI] [PubMed] [Google Scholar]
- 54.Potteaux S, Gautier EL, Hutchison SB et al. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J Clin Invest 2011;121:2025–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yvan-Charvet L, Pagler TA, Seimon TA et al. ABCA1 and ABCG1 protect against oxidative stress-induced macrophage apoptosis during efferocytosis. Circ Res 2010;106:1861–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kojima Y, Volkmer JP, McKenna K et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 2016;536:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Do R, Willer CJ, Schmidt EM et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet 2013;45:1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kojima Y, Downing K, Kundu R et al. Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. J Clin Invest 2014;124:1083–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Satoh M, Ishikawa Y, Itoh T, Minami Y, Takahashi Y, Nakamura M. The expression of TNF-alpha converting enzyme at the site of ruptured plaques in patients with acute myocardial infarction. Eur J Clin Invest 2008;38:97–105. [DOI] [PubMed] [Google Scholar]
- 60.Cai B, Thorp EB, Doran AC et al. MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J Clin Invest 2017;127:564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thorp E, Vaisar T, Subramanian M, Mautner L, Blobel C, Tabas I. Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cdelta, and p38 mitogen-activated protein kinase (MAPK). J Biol Chem 2011;286:33335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foks AC, Engelbertsen D, Kuperwaser F et al. Blockade of Tim-1 and Tim-4 Enhances Atherosclerosis in Low-Density Lipoprotein Receptor-Deficient Mice. Arterioscler Thromb Vasc Biol 2016;36:456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rayner KJ. Cell Death in the Vessel Wall: The Good, the Bad, the Ugly. Arterioscler Thromb Vasc Biol 2017;37:e75–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi J, Zhao Y, Wang K et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015;526:660–5. [DOI] [PubMed] [Google Scholar]
- 65.Sun L, Wang H, Wang Z et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012;148:213–27. [DOI] [PubMed] [Google Scholar]
- 66.Lin J, Li H, Yang M et al. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep 2013;3:200–10. [DOI] [PubMed] [Google Scholar]
- 67.Karunakaran D, Geoffrion M, Wei L et al. Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Sci Adv 2016;2:e1600224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clement M, Basatemur G, Masters L et al. Necrotic Cell Sensor Clec4e Promotes a Proatherogenic Macrophage Phenotype Through Activation of the Unfolded Protein Response. Circulation 2016;134:1039–1051. [DOI] [PubMed] [Google Scholar]
- 69.Stoneman V, Braganza D, Figg N et al. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res 2007;100:884–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gautier EL, Huby T, Witztum JL et al. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation 2009;119:1795–804. [DOI] [PubMed] [Google Scholar]
- 71.Gerrity RG, Naito HK. Lipid clearance from fatty streak lesions by foam cell migration. Artery 1980;8:215–9. [PubMed] [Google Scholar]
- 72.Trogan E, Choudhury RP, Dansky HM, Rong JX, Breslow JL, Fisher EA. Laser capture microdissection analysis of gene expression in macrophages from atherosclerotic lesions of apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A 2002;99:2234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feig JE, Shang Y, Rotllan N et al. Statins promote the regression of atherosclerosis via activation of the CCR7-dependent emigration pathway in macrophages. PLoS One 2011;6:e28534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolfbauer G, Glick JM, Minor LK, Rothblat GH. Development of the smooth muscle foam cell: uptake of macrophage lipid inclusions. Proc Natl Acad Sci U S A 1986;83:7760–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 2014;129:1551–9. [DOI] [PubMed] [Google Scholar]
- 76.Feil S, Fehrenbacher B, Lukowski R et al. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res 2014;115:662–7. [DOI] [PubMed] [Google Scholar]
- 77.Shankman LS, Gomez D, Cherepanova OA et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 2015;21:628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vengrenyuk Y, Nishi H, Long X et al. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler Thromb Vasc Biol 2015;35:535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fredman G, Tabas I. Boosting Inflammation Resolution in Atherosclerosis: The Next Frontier for Therapy. Am J Pathol 2017;187:1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov 2016;15:551–67. [DOI] [PubMed] [Google Scholar]
- 81.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science 2013;339:166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 1995;15:551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gordon S, Pluddemann A. Tissue macrophages: heterogeneity and functions. BMC Biol 2017;15:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edwards IJ, Wagner WD, Owens RT. Macrophage secretory products selectively stimulate dermatan sulfate proteoglycan production in cultured arterial smooth muscle cells. Am J Pathol 1990;136:609–21. [PMC free article] [PubMed] [Google Scholar]
- 85.Ikeda K, Souma Y, Akakabe Y et al. Macrophages play a unique role in the plaque calcification by enhancing the osteogenic signals exerted by vascular smooth muscle cells. Biochem Biophys Res Commun 2012;425:39–44. [DOI] [PubMed] [Google Scholar]
- 86.Tabas I Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol 2010;10:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Linton MF, Babaev VR, Huang J, Linton EF, Tao H, Yancey PG. Macrophage Apoptosis and Efferocytosis in the Pathogenesis of Atherosclerosis. Circ J 2016;80:2259–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol 2005;25:1256–61. [DOI] [PubMed] [Google Scholar]
- 89.Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. J Intern Med 2015;278:483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Libby P, Tabas I, Fredman G, Fisher EA. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res 2014;114:1867–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elajami TK, Colas RA, Dalli J, Chiang N, Serhan CN, Welty FK. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J 2016;30:2792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fredman G, Hellmann J, Proto JD et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun 2016;7:12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Viola JR, Lemnitzer P, Jansen Y et al. Resolving Lipid Mediators Maresin 1 and Resolvin D2 Prevent Atheroprogression in Mice. Circ Res 2016;119:1030–1038. [DOI] [PubMed] [Google Scholar]
- 94.Helgadottir A, Manolescu A, Helgason A et al. A variant of the gene encoding leukotriene A4 hydrolase confers ethnicity-specific risk of myocardial infarction. Nat Genet 2006;38:68–74. [DOI] [PubMed] [Google Scholar]
- 95.Helgadottir A, Manolescu A, Thorleifsson G et al. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet 2004;36:233–9. [DOI] [PubMed] [Google Scholar]
- 96.Kajimoto K, Shioji K, Ishida C et al. Validation of the association between the gene encoding 5-lipoxygenase-activating protein and myocardial infarction in a Japanese population. Circ J 2005;69:1029–34. [DOI] [PubMed] [Google Scholar]
- 97.Wang G, Wang Y, Sun H et al. Variants of the arachidonate 5-lipoxygenase-activating protein (ALOX5AP) gene and risk of ischemic stroke in Han Chinese of eastern China. J Biomed Res 2011;25:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zintzaras E, Rodopoulou P, Sakellaridis N. Variants of the arachidonate 5-lipoxygenase-activating protein (ALOX5AP) gene and risk of stroke: a HuGE gene-disease association review and meta-analysis. Am J Epidemiol 2009;169:523–32. [DOI] [PubMed] [Google Scholar]
- 99.Qiu H, Gabrielsen A, Agardh HE et al. Expression of 5-lipoxygenase and leukotriene A4 hydrolase in human atherosclerotic lesions correlates with symptoms of plaque instability. Proc Natl Acad Sci U S A 2006;103:8161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mehrabian M, Allayee H, Wong J et al. Identification of 5-lipoxygenase as a major gene contributing to atherosclerosis susceptibility in mice. Circ Res 2002;91: 120–6. [DOI] [PubMed] [Google Scholar]
- 101.Wittwer J, Bayer M, Mosandl A, Muntwyler J, Hersberger M. The c.−292C>T promoter polymorphism increases reticulocyte-type 15-lipoxygenase-1 activity and could be atheroprotective. Clin Chem Lab Med 2007;45:487–92. [DOI] [PubMed] [Google Scholar]
- 102.Serhan CN, Jain A, Marleau S et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol 2003;171:6856–65. [DOI] [PubMed] [Google Scholar]
- 103.Shen J, Herderick E, Cornhill JF et al. Macrophage-mediated 15-lipoxygenase expression protects against atherosclerosis development. J Clin Invest 1996;98:2201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J 2008;22:3595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Merched AJ, Serhan CN, Chan L. Nutrigenetic disruption of inflammation-resolution homeostasis and atherogenesis. J Nutrigenet Nutrigenomics 2011;4:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dwyer JH, Allayee H, Dwyer KM et al. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med 2004;350:29–37. [DOI] [PubMed] [Google Scholar]
- 107.Poeckel D, Funk CD. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovasc Res 2010;86:243–53. [DOI] [PubMed] [Google Scholar]
- 108.Alciato F, Sainaghi PP, Sola D, Castello L, Avanzi GC. TNF-alpha, IL-6, and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. J Leukoc Biol 2010;87:869–75. [DOI] [PubMed] [Google Scholar]
- 109.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 1998;101:890–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cai B, Thorp EB, Doran AC et al. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc Natl Acad Sci U S A 2016;113:6526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 2012;120:e60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krishnamoorthy S, Recchiuti A, Chiang N et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A 2010;107:1660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maderna P, Cottell DC, Toivonen T et al. FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis. FASEB J 2010;24:4240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007;447:869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fredman G, Kamaly N, Spolitu S et al. Targeted nanoparticles containing the proresolving peptide Ac2–26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci Transl Med 2015;7:275ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kamaly N, Fredman G, Fojas JJ et al. Targeted Interleukin-10 Nanotherapeutics Developed with a Microfluidic Chip Enhance Resolution of Inflammation in Advanced Atherosclerosis. ACS Nano 2016;10:5280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ridker PM, Everett BM, Thuren T et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 118.Everett BM, Pradhan AD, Solomon DH et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J 2013;166:199–207 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014;510:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gaztanaga J, Farkouh M, Rudd JH et al. A phase 2 randomized, double-blind, placebo-controlled study of the effect of VIA-2291, a 5-lipoxygenase inhibitor, on vascular inflammation in patients after an acute coronary syndrome. Atherosclerosis 2015;240:53–60. [DOI] [PubMed] [Google Scholar]
- 121.Tardif JC, L'Allier PL, Ibrahim R et al. Treatment with 5-lipoxygenase inhibitor VIA-2291 (Atreleuton) in patients with recent acute coronary syndrome. Circ Cardiovasc Imaging 2010;3:298–307. [DOI] [PubMed] [Google Scholar]
- 122.Brown AL, Zhu X, Rong S et al. Omega-3 fatty acids ameliorate atherosclerosis by favorably altering monocyte subsets and limiting monocyte recruitment to aortic lesions. Arterioscler Thromb Vasc Biol 2012;32:2122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Van Noolen L, Back M, Arnaud C et al. Docosahexaenoic acid supplementation modifies fatty acid incorporation in tissues and prevents hypoxia induced-atherosclerosis progression in apolipoprotein-E deficient mice. Prostaglandins Leukot Essent Fatty Acids 2014;91:111–7. [DOI] [PubMed] [Google Scholar]
- 124.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet 1999;354:447–55. [PubMed] [Google Scholar]
- 125.Bosch J, Gerstein HC, Dagenais GR et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med 2012;367:309–18. [DOI] [PubMed] [Google Scholar]
- 126.Oikawa S, Yokoyama M, Origasa H et al. Suppressive effect of EPA on the incidence of coronary events in hypercholesterolemia with impaired glucose metabolism: Sub-analysis of the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis 2009;206:535–9. [DOI] [PubMed] [Google Scholar]
- 127.Roncaglioni MC, Tombesi M, Avanzini F et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med 2013;368:1800–8. [DOI] [PubMed] [Google Scholar]
- 128.Sperling LS, Nelson JR. History and future of omega-3 fatty acids in cardiovascular disease. Curr Med Res Opin 2016;32:301–11. [DOI] [PubMed] [Google Scholar]
- 129.Howson JMM, Zhao W, Barnes DR et al. Fifteen new risk loci for coronary artery disease highlight arterial-wall-specific mechanisms. Nat Genet 2017;49:1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Klarin D, Zhu QM, Emdin CA et al. Genetic analysis in UK Biobank links insulin resistance and transendothelial migration pathways to coronary artery disease. Nat Genet 2017;49:1392–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Holdt LM, Stahringer A, Sass K et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun 2016;7:12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hagg S, Skogsberg J, Lundstrom J et al. Multi-organ expression profiling uncovers a gene module in coronary artery disease involving transendothelial migration of leukocytes and LIM domain binding 2: the Stockholm Atherosclerosis Gene Expression (STAGE) study. PLoS Genet 2009;5:e1000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Talukdar HA, Foroughi Asl H, Jain RK et al. Cross-Tissue Regulatory Gene Networks in Coronary Artery Disease. Cell Syst 2016;2:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Albright J, Quizon PM, Lusis AJ, Bennett BJ. Genetic network identifies novel pathways contributing to atherosclerosis susceptibility in the innominate artery. BMC Med Genomics 2014;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huan T, Zhang B, Wang Z et al. A systems biology framework identifies molecular underpinnings of coronary heart disease. Arterioscler Thromb Vasc Biol 2013;33:1427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bennett BJ, Farber CR, Orozco L et al. A high-resolution association mapping panel for the dissection of complex traits in mice. Genome Res 2010;20:281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Foroughi Asl H, Talukdar HA, Kindt AS et al. Expression quantitative trait Loci acting across multiple tissues are enriched in inherited risk for coronary artery disease. Circ Cardiovasc Genet 2015;8:305–15. [DOI] [PubMed] [Google Scholar]
- 138.Franzen O, Ermel R, Cohain A et al. Cardiometabolic risk loci share downstream cis- and trans-gene regulation across tissues and diseases. Science 2016;353:827–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ongen H, Brown AA, Delaneau O et al. Estimating the causal tissues for complex traits and diseases. Nat Genet 2017;49:1676–1683. [DOI] [PubMed] [Google Scholar]
- 140.Nelson CP, Goel A, Butterworth AS et al. Association analyses based on false discovery rate implicate new loci for coronary artery disease. Nat Genet 2017;49:1385–1391. [DOI] [PubMed] [Google Scholar]
- 141.Torkamani A, Topol EJ, Schork NJ. Pathway analysis of seven common diseases assessed by genome-wide association. Genomics 2008;92:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.