Abstract
Purpose:
Nanotheranostic platforms, i.e., the combination of both therapeutic and diagnostic agents on a single platform, are emerging as an interesting tool for the personalized cancer medicine. Therefore, the aim of this work was to evaluate the in vivo properties of a Tc-99m-labeled nanostructured lipid carrier (NLC) formulation, co-loaded with doxorubicin (DOX) and docosahexaenoic acid (DHA), for theranostic applications.
Procedures:
NLC-DHA-DOX were prepared busing the hot melting homogenization method using an emulsification-ultrasound and were radiolabeled with Tc-99m. Biodistribution studies, scintigraphic images, and antitumor activity were performed in 4T1 tumor-bearing mice.
Results:
NCL was successfully radiolabeled with Tc-99m. Blood clearance showed a relatively long half-life, with blood levels decaying in a biphasic manner (T1/2α = 38.7 min; T1/2β = 516.5 min). The biodistribution profile and scintigraphic images showed higher tumor uptake compared to contralateral muscle in all time-points investigated. Antitumor activity studies showed a substantial tumor growth inhibition ratio for NLC-DHA-DOX formulation. In addition, the formulation showed more favorable toxicity profiles when compared to equivalent doses of free administered drugs, being able to reduce heart and liver damage.
Conclusions:
Therefore, NLC-DHA-DOX formulation demonstrated feasibility in breast cancer treatment and diagnosis/monitoring, leading to a new possibility of a theranostic platform.
Keywords: Docosahexaenoic acid, Doxorubicin, Nanostructured lipid carrier, Tc-99m, Theranostic
Introduction
Cancer is a major health issue worldwide. In 2012, there were 14.1 million new cases and 8.6 million people died due of cancer [1]. Breast cancer is the most common type of tumor among women, representing about 20 % of the new cases reported every year.
Doxorubicin (DOX) is an anthracycline with a broadspectrum of anticancer activity, and is widely used in cancer therapy. However, DOX has low “therapeutic index,” mainly due to myelosuppression and cardiotoxicity [2, 3], limiting its use. Moreover, its low tumor penetration and limited distribution within solid tumors are the main causes of treatment failures [4–6]. The use of anthracycline-based combination chemotherapy regimens has shown improvements in activity compared to a single drug. In clinics, doxorubicin is already used combined with cyclophosphamide, cisplatin and fluorouracil. However, despite the greater activity, these regimens are also more toxic than single-drug approaches [2, 7].
Among dual-drug strategies, the combination of docosahexaenoic acid (DHA) and doxorubicin has been described as an alternative to improve antitumor efficacy compared to doxorubicin alone [8–10]. DHA is a long-chain (C22) polyunsaturated fatty acid (Omega 3) that enhances oxidative stress and subsequent lipid peroxidation of tumor cells [11]. Previous studies have reported the effect of DHA in enhancing antitumor activity of doxorubicin in rodents [9, 12]. However, variable drug administration schedules have complicated management of the pharmacokinetic and pharmacodynamic profiles, and achieving uniform temporal and spatial co-delivery remains challenging. Thus, the combination of drugs co-delivered in a single drug delivery system has emerged as an attractive alternative [13]. Furthermore, these systems are capable of raising the therapeutic index, reducing toxicity, and thereby increasing the effectiveness of the drug [14].
Among the drug delivery systems, nanostructured lipid carrier (NLC) has emerged as an exquisite strategy due to their advantages and benefits such as easy translation to large-scale production, absence of organic solvents in its composition, and relatively low toxicity. In addition, more synchronized, controlled pharmacokinetics of the drugs and improved drug bioavailability can be achieved [15, 16].
Theranostic platforms are emerging as interesting tools in the clinical setting. These systems are the combination of both therapeutic and diagnostic agents on a single platform [17], in other words, by using theranostic approaches it is possible to diagnosis, treat and monitoring the efficacy of therapeutic regimens [18]. Recent advances in nanomedicine and imaging modalities enable the design of nanotheranostic agents. It is well known that nanoparticulate systems might prolong blood circulation leading to high accumulation in tumor tissue, with the ability to co-deliver therapeutic and imaging functions [19–21]. For theranostic applications, several imaging modalities are possible. Tc-99m is widely used for SPECT images in clinics, due to its suitable physical and chemical characteristics, large availability in nuclear medicine laboratories, and low isotope cost [22, 23].
Recently, our research group demonstrated the promising cytotoxic effect of NLC loaded with doxorubicin and DHA in spheroid tumor models, when compared to both free and liposomal doxorubicin [23]. The aim of this work was to evaluate the in vivo properties of this NLC formulation for theranostic applications. To achieve this purpose, the NLC was radiolabeled with Tc-99m for scintigraphic images and biodistribution studies in tumor-bearing mice. Furthermore, antitumor activity of free and encapsulated DOX was evaluated against 4T1 breast cancer murine cells in BALB/c mice.
Materials and Methods
Material
A description of the material, animals and cells used in this study is detailed in Electronic supplementary material (ESM).
Preparation of NLC
NLC were prepared by the hot melting homogenization method using an emulsification-ultrasound. The composition of NLC was previously described by Mussi et al. [23, 24] and more details are shown in ESM.
Characterization of NLC
The mean particle diameter and polydispersity index (PDI) were measured by dynamic light scattering (DLS) using a Zetasizer Nano ZS90 (Malvern Instruments, UK). Zeta potential measurements were carried out by DLS associated with electrophoretic mobility, at a fixed angle of 90° and 25 °C. The encapsulation efficiency (EE) of doxorubicin into NLC was determined by ultrafiltration method using passivated centrifugal devices (Amicon® Ultra - 0.5 ml 100 k; Millipore, USA), as previously described [23, 24].
Radiolabeling of NLC-DHA-DOX
One milligram of SnCl2·2H2O in an acid solution (HCl 0.25 M) was added to a sealed vial containing 1.0 ml of the NLC suspension. The pH was adjusted to 7. Then, vacuum was applied to the vial and an aliquot of 0.1 ml of Na[99mTc]O4 (37 MBq) was added to the mixture reaction. The solution was stirred by vortexing for 2 min and kept at room temperature (r.t.) for 10 min.
Radiolabeling of DOX
Doxorubicin was labeled with Tc-99m as previously described by Fernandes et al. [25]. More details are shown in ESM.
Radiochemical Purity Evaluation of [99mTc]DOX and [99mTc]NLC-DHA-DOX
Radiochemical purity analyses were performed by thin-layer chromatography (TLC) on silica gel (TLC-SG; Merck, Darmstadt, Germany) to determine the amount of free [99mTc]O4−, and both solutions were purified from [99mTc]O2 using a 0.22-μm syringe filter. More details are given in ESM.
Characterization of [99mTc]NLC-DHA-DOX
The radiolabeled complex was characterized as the mean diameter, PDI, zeta potential and EE, as previous described.
In Vitro Stability
Tests in saline 0.9 % (w/v) and in mouse plasma were performed to evaluate the stability of [99mTc]NLC-DHA-DOX. More details see in ESM.
Blood Clearance
The [99mTc]DOX or [99mTc]NLC-DHA-DOX complexes were administrated to each mouse (n = 6 for each complex) through the tail vein, and blood samples (~ 20 μl each) were collected, from the tail vein, at pre-determined times. Each sample was weighted, and the radioactivity was measured with an automatic scintillation counter.
Tumor Cell Inoculation
Aliquots (100 μl) of 2.5 × 106 4T1 cells were injected subcutaneously into the right thigh of female BALB/c mice (18–22 g). Tumor cells were allowed to grow in vivo for 7 days, leading to tumors with a diameter of no more than 10 mm. Breast tumor-bearing BALB/c mice were used for biodistribution studies, scintigraphic images and in vivo antitumor activity.
Scintigraphic Images
Aliquots (100 μl) of 18 MBq of [99mTc]DOX or [99mTc]NLC-DHA-DOX were injected intravenously into tumor-bearing BALB/c mice (n = 6). Anesthetized mice were horizontally placed under a gamma camera (Mediso, Budapest, Hungary) coupled with a low-energy high-resolution collimator. Images were acquired at 1, 4, and 8 and 24 h after injection using a 256 × 256 × 16 matrix size, with a 20 % energy window set at 140 keV for a period of 300 s each.
Biodistribution Studies
Aliquots (100 μl) of 3.7 MBq of [99mTc]DOX or [99mTc]NLC-DHA-DOX were injected intravenously into tumor-bearing BALB/c mice (n = 6). At 1, 4, and 8 and 24 h after injection, mice were anesthetized with a mixture of xylazine (15 mg/kg) and ketamine (80 mg/kg). Liver, spleen, kidneys, stomach, heart, lungs, muscle, thyroid, intestine, and tumor were removed, and placed in pre-weighed plastic test tubes. The radioactivity was measured using an automatic scintillation counter. Results were expressed as the percentage of injected dose per gram of tissue (%ID/g).
In vivo Antitumor Activity
On the 7th day after 4T1 cell inoculation, once the tumor volume reached 100 mm3, the mice were randomly assigned to four groups (n = 6 for each group), named group 1: blank-NLC (control group); group 2: free DOX; group 3: NLC-DOX; and group 4: NLC-DHA-DOX. For all treatments the dose of DOX was 4 mg/kg/day, in a total of 4 administrations, every 2 days, injected by the tail vein. Throughout the study, body weight and tumor volumes (measured with caliper) were obtained every 2 days. Ten days after the beginning of the treatment (D10), mice were euthanized and the tumors, heart, liver, spleen, lungs, and kidneys were collected for histology. Tumor volumes, relative tumor volume (RTV) and inhibition ratio (IR) were calculated (ESM).
Tumor Histology
On D10, after mice euthanize, tumors were collected and washed with NaCl 0.9 % (w/v) solution and set in 10 % (v/v) buffered formalin for further analyses. More details see in ESM.
Toxicity Evaluation
The body weight of the mice was monitored at D0, D2, D4, D6, D8, and D10 after application, and their body weight variation was expressed as the difference between the body weight at D10 and the initial body weight. In addition, the kidneys, heart, lungs, liver, and spleen were also removed for histological analysis (ESM). A scheme of our experimental procedure is resumed in Fig. 1.
Fig. 1.
Scheme of our experimental protocol.
Statistical Analysis
Statistical analyses were performed using GraphPad PRISM, version 5.00 software (GraphPad Software Inc., La Jolla, CA, USA). The difference among experimental groups was tested using the one-way analysis of variance (ANOVA), followed by Tukey’s test. Student’s t test was performed to compare data of the physicochemical characteristics of NLC and blood clearance. The regression model estimates were used at time intervals for tumor growth investigations. Differences were considered statistically significant when P values were < 0.05.
Results
Radiolabeling of NLC-DHA-DOX, Characterization and In Vitro Stability
Radiochemical purity of [99mTc]DOX reaching values of 98.6 ± 0.4 % (n = 5). This finding is in agreement with purity reported by our group [25], in which DOX was radiolabeled with high efficiency and high stability.
For [99mTc]NLC-DHA-DOX, radiochemical purity was 95.3 ± 0.9 % (n = 5), and these results were highly reproducible during the experiments. The NLC and the radiolabeled complex were characterized according to their mean particle diameter, PDI, zeta potential and EE, and the results are shown in Table 1.
Table 1.
Mean diameter, PDI, zeta potential and EE for the NLC before and after the radiolabeling with Tc-99m
| NLC-DHA-DOX | [99mTc]NLC-DHA-DOX | |
|---|---|---|
| Mean particle diameter (nm) | 64.4 ± 3.6 | 72.5 ± 2.6* |
| PDI | 0.174 ± 0.01 | 0.139 ± 0.006* |
| Zeta potential (mV) | − 31.6 ± 1.4 | − 21.7 ± 2.2* |
| Doxorubicin EE (%) | 96.9 ± 0.7 | 95.7 ± 3.3 |
Represents statistical differeneces between NLC-DHA-DOX and [99mTc]NLC-DHA-DOX (p<0.05)
The average size of NLC was 64.4 ± 3.6 nm, and PDI was lower than 0.3 (0.176 ± 0.01), which indicates homogeneity. NLC exhibited a negative zeta potential (− 31.6 ± 1.4 mV), which can be attributed to the presence of ionized oleic acid in the formulation. The high value of EE (96.9 ± 0.7 %) is also due to the oleic acid, a lipophilic anion that forms an ion pair with doxorubicin, increasing their affinity for the lipid matrix [23]. After radiolabeling, it is possible to observe a significant increase in mean particle diameter (72.5 ± 2.6 nm), and a significant reduce of PDI (0.139 ± 0.006) and zeta potential (− 21.7 ± 2.2 mV).
[99mTc]NLC-DHA-DOX showed an excellent stability, even over long periods of time (> 85 % up to 24 h after radiolabeling), was observed, which allows further in vivo studies The radiochemical stability curve for the complex is demonstrated in ESM Fig. S1.
Blood Clearance
Pharmacokinetic parameters for [99mTc]NLC-DHA-DOX and [99mTc]DOX are represented on Table 2. Both complexes, after injection into healthy BALB/c female mice, showed a relatively long clearance, with blood levels decaying in a biphasic manner. Noteworthy is the longer T1/2α (~ 9-fold) and T1/2β (~ 2-fold) for [99mTc]NLC-DHA-DOX in comparison to [99mTc]DOX, indicating that long circulating was achieved after encapsulating DOX into NLC.
Table 2.
Pharmacokinetic parameters for [99mTc]NLC-DHA-DOX and [99mTc]DOX
| [99mTc]NLC-DHA-DOX | [99mTc]DOX | |
|---|---|---|
| T1/2 α (min) | 38.7 | 4.5 |
| T1/2 β (min) | 516.5 | 277.2 |
| AUC (%ID min−1) | 5796 | 2633 |
Biodistribution Studies and Scintigraphic Images for [99mTc]NLC-DHA-DOX
Fig. 2 shows scintigraphic images of [99mTc]NLC-DHA-DOX administered intravenously in tumor-bearing mice. High uptake was observed in organs from mononuclear phagocyte system (MPS), such as the liver and spleen. In addition, no significant uptake was observed in other organs, mainly the stomach and thyroid, which corroborates the in vitro stability data, since the stomach and thyroid showed low uptake in all time frames. It is well known that free technetium are, preferentially, taken up by these organs [26]. Regarding the tumor uptake (arrows on Fig. 2), it could be observed higher uptake compared to non-targeted tissues, such as the muscle, in all investigated time points, especially in the longest investigated point.
Fig. 2.
Scintigraphic images obtained at a 1, b 4, c 8, and d 24 h after intravenous injection of [99mTc]NLC-DHA-DOX in tumor-bearing mice (n = 6). Arrows indicate tumor lesion.
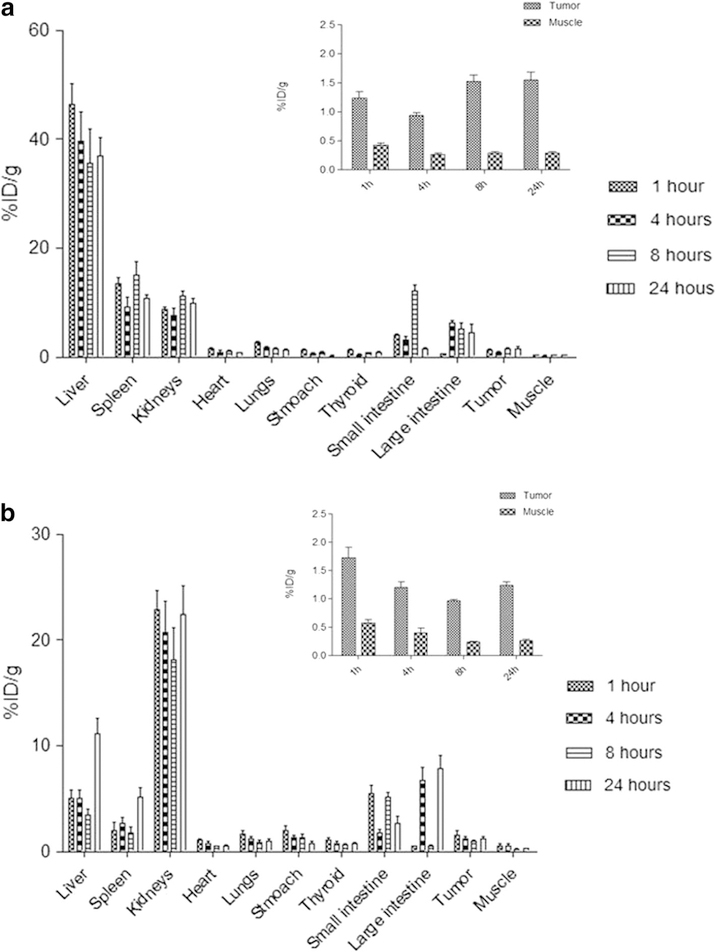
These data are similar to those obtained by biodistribution studies (Fig. 3). [99mTc]NLC-DHA-DOX and [99mTc]DOX biodistribution profilesare shown in Fig. 3a, b, respectively. As previously described [25], [99mTc]DOX shows a dual-elimination pathway (renal and hepatobiliary), which clearly observed in Fig. 3b. In addition, there was higher tumor uptake compared with control tissue, in all time-points investigated.
Fig. 3.
Biodistribution profile of a [99mTc]NLC-DHA-DOX and b [99mTc]DOX following intravenous tail-vein administration in tumor-bearing BALB/c mice (n = 6) (inset: tumor and muscle uptake at 1, 4, 8, and 24 h after injection). All data are the mean percentage of the injected dose per gram of organ, ± SD of the mean.
Target-to-Non-target Ratios
Target-to-non-target ratios were obtained from biodistribution and scintigraphic images (Fig. 4). A gradual increase over time for [99mTc]NLC-DHA-DOX ratios was observed, suggesting higher accumulation of the NLC in the tumor area. Meanwhile, for [99mTc]DOX, after 4 h, no further increase in the ratios was evidenced. Interestingly, at 24 h post-injection [99mTc]NLC-DHA-DOX ratios were significantly higher than those for [99mTc]DOX, suggesting that longer circulation time presented by NLC might favor tumor accumulation. It is also worth mentioning that tumor-to-blood ratios for [99mTc]NLC-DHA-DOX, after 24 h, was 2, suggesting that tumor accumulation was not only by the hypervascularization typically expected in this type of tumor.
Fig. 4.
Target-to-non target ratios for [99mTc]NLC-DHA-DOX and [99mTc]DOX, 1, 4, 8, and 24 h after administration: a tumor-to-muscle ratio (biodistribution studies), b tumor-to-blood ratio, and c target-to-non target ratio (scintigraphic image). Asterisks indicate statistically significant differences between the complexes at the same time point (P < 0.05).
In vivo Antitumor Activity
The antitumor activity of NLC-DHA-DOX was evaluated in 4T1 tumor-bearing BALB/c mice. As shown in Fig. 5, the tumor growth was significantly lower after NLC-DOX or NLC-DHA-DOX treatments compared to blank-NLC and free DOX (p < 0.05). In addition, NLC-DHA-DOX significantly inhibited 4T1 growth compared to NLC-DOX treatment (p < 0.05).
Fig. 5.
Antitumor effect of blank-NLC, free DOX, NLC-DOX and NLC-DHA-DOX on the growth of 4T1 tumor-bearing BALB/c mice (female, n = 6). Each treatment was intravenously administered four times, every 2 days, at a dose of 4 mg/kg. The total dose of DOX in all treatment groups was 16 mg/kg.
Regression analysis was performed to detect the changes in the growth delay after treatment within the different groups. The best-fit model for each group, together with their respective determination coefficients, is shown in Table 3. There was significant statistical difference between the free DOX and NLC-DOX, since they fit on different mathematical models. For the NLC-DHA-DOX treatment group, the tumor volume was significantly suppressed compared with the other groups with no tumor volume increment throughout the experiment, suggesting that DHA plays a substantial role in the antitumor effect. As a result, any mathematical model fits to this group as indicated in Table 3. In addition, statistical differences were observed after the D4 by analyzing each time point individually.
Table 3.
Regression analysis of the data of antitumor activity
| Treatment | Regression model | r2 |
|---|---|---|
| NLC | y = 8.52x2 + 34.85x + 39.37 | 0.9899 |
| DOX | y = 52.1x + 1.26 | 0.9250 |
| NLC-DOX | y = 2.03x2 − 0.196x + 8.91 | 0.9100 |
| NLC-DHA-DOX | N.A | N.A |
Relative tumor volume (RTV) and tumor growth inhibition ratio (IR) data are shown in Table 4 and corroborate with the regression analysis findings. NLC-DHA-DOX treatment group was able to inhibit tumor growth by over 90 %; hence, RTV remained almost constant over time. Nevertheless, free doxorubicin and NLC-DOX were able to inhibit tumor growth in only 56 and 68 %, respectively.
Table 4.
Relative tumor volume (RTV) and tumor growth inhibition ratio (IR) after administration of free DOX, NLC-DOX and NLC-DHA-DOX
| Treatment | RTV (mean ± sd) | IR (%) |
|---|---|---|
| NLC | 10.57 ± 2.71a | – |
| NLC-DHA-DOX | 1.03 ± 0.43b | 90.3 |
| NCL-DOX | 3.34 ± 0.28c | 68.4 |
| DOX | 4.63 ± 0.43d | 56.2 |
Different letters represent statitical differences among groups
Tumor Histology
Histological sections of tumor tissue were evaluated after different treatments. 4T1 tumors are characterized by epithelial cells with pleomorphism, evident nucleolus and mitosis figures [27], which were observed in all groups. Additionally, for the treated groups, it is possible to observe necrosis areas, characterized by pyknotic nuclei and eosinophilic regions. Interestingly, free DOX group showed less intense necrosis area, indicating that NLC formulations were more effective than free drug. Histological sections of tumor tissue are demonstrated in ESM Fig. S2.
Toxicity Evaluation
Body Weight
Loss of body weight is an important toxicological parameter of antitumor therapy that may help to predict side effects from drugs. In this study, mice weight changes after treatments are illustrated in Fig. 6. Animals from the control group showed a positive weight variation after treatment protocol. For the treated groups, animals which received free DOX showed a severe weight loss, reaching a maximum loss on D8 of 25 % of body weight, which led to the animals’ death. The animals treated with the NLC formulations showed a slight body weight loss (under 10 %), with no significant difference between them. These data suggest that the treatment with the encapsulated drug was able to alleviate the inherent toxic effects of the free drug.
Fig. 6.
Body weight alteration, in grams, between D0 andD10, for animals treated with blank-NLC, free DOX, NLC-DOX and NLC-DHA-DOX. The total dose of DOX in all treatment groups was 16 mg/kg.
Histological Analysis
The kidneys, heart, lungs, liver, and spleen were collected from animals for histological analysis. No histological changes were observed for the lungs, kidneys, and spleen, for all groups (data not shown). All evaluated groups showed preserved tissues with their typical architecture.
For all the animals treated with free DOX, it could be observed multifocal areas of hydropic degeneration in liver, with vacuolized tissue, and necrotic areas. For NLC formulations, a protective effect in the liver tissue was observed since only 30 % (2/6) of the mice showed points of hydropic degeneration. Important to mention that the livers from animals treated with NLC formulations did not show any necrotic area, indicating the capability of this nanocarrier in effectively diminish the toxic effect of doxorubicin. Liver histological sections are demonstrated in ESM Fig. S3.
It is well known that anthracyclines, including DOX, are potentially cardiotoxic often limiting their use in the clinics. For this reason, the hearts were also collected and evaluated after the different treatments employed. As observed for all the other organs evaluated, no histological change was observed for the control group. However, for all treated groups, there were focal areas of hyaline degeneration, characterized by thick and strongly eosinophilic cardiac fibers. Similar to the results described in the liver analysis, the extent and the frequency of the tissue damage were different for free and encapsulated drug. Meanwhile, the hearts from free DOX group showed multifocal areas of hyaline degeneration in 100 % of the animals, while for the NLC-DHA-DOX group this change was observed in only 30 % (2/6) of the animals. Once again, indicating the protective effect of the formulation over the free drug. Heart histological sections are demonstrated in ESM Fig. S3.
Discussion
Combination therapies have gained much attention as an alternative to improve cancer treatment [2, 7]. In this scenario, improved antitumor efficacy have described by the combination of doxorubicin and DHA [9–13]. Nanocarriers have become a tool to overcome chemotherapy limitations, such as drug resistance, bioavailability, adverse effects, besides provides a uniform temporal and spatial codelivery of the two encapsulated drugs [13–16].
The [99mTc]NLC-DHA-DOX was characterized as mean diameter, PDI, zeta potential and EE (Table 1). The significant increase in mean diameter and the reduction of the zeta potential are, probably, due to an incorporation of Tc-99m, which is a positive metal. The significant PDI decrease might be a consequence of the filtration step, which removes particle populations larger than 200 nm along with radiocolloids. Importantly, although a slight increase in the particle size was evidenced after radiolabeling procedure, the final mean diameter and PDI remain under the envisioned size range for in vivo applications [28–30]. In addition, upon the labeling protocol used it was possible to efficiently radiolabeled NLC with minimum effect on their physical-chemical features. Therefore, this technique could be a possibility for labeling other particles for bioimaging approaches without using a chelating agent to stabilize the metal complex.
[99mTc]NLC-DHA-DOX showed excellent radiochemical stability over time (Fig. S1). This data is in agreement with previous studies, which have related high stability for SLN [31] radiolabeled with Tc-99m. The choice in labeling the surface of NLC is due to the fact that doxorubicin, as itself, is a hydrophilic drug not being incorporated into the hydrophobic core of NLC. As proposed by our group, an ion-pair between oleic acid and DOX must be prepared to encapsulate efficiently the drug. However, the amino group of the doxorubicin is responsible for both complexation with technetium, and formation of ion-pair with oleic acid [23, 25]. Therefore, the encapsulation of [99mTc]DOX complex into NLC was not possible. It is worthy to mention that previous release studies have demonstrated a controlled release of doxorubicin; therefore, the drug is stable into NLC even after long periods [23, 24]. These data indicate that in vivo assay carried out with [99mTc]NLC-DHA-DOX will reflect the fate of the encapsulated drug.
The use of a radiolabeled nanoparticles carrying antitumor drug might be an alternative for the future of the personalized medicine, taking advantage from the high sensitivity of nuclear medicine imaging associate with potentialities of nanomedicine for antitumor therapies [17–20].
[99mTc]NLC-DHA-DOX exhibited longer blood circulation time compared to the free drug (Table 2). The small particle size of NLC (~ 70 nm), along with the presence of hydrophilic surfactant (Tween 80), might contribute to the prolonged circulation time. Polyethylene glycol chains (PEG) in the Tween 80 may provide “stealth” properties to nanoparticles [32, 33].
The target-to-non target ratios (Fig. 3) demonstrate the higher accumulation of [99mTc]NLC-DHA-DOX in the tumor tissue when compared to [99mTc]DOX. The longer blood circulation time shown by the [99mTc]NLC-DHA-DOX allows that nanoparticles pass though the tumor area more times than the free drug favoring their accumulation by the enhanced permeability and retention effect (EPR effect). This effect is a well-established phenomenon in which the endothelial lining of the blood vessel wall of tumors becomes more permeable than in the normal state. As a result, in such areas, particles ranging from 10 to 500 nm in size can leave the vascular bed and accumulate inside the interstitial space. Moreover, the compromised lymphatic filtration impair a proper drainage resulting in their accumulation. Unlike these nanoparticles, low-molecular-weight pharmaceutical agents, like doxorubicin, are not retained in tumors because they freely diffuse from tumors and return to the circulation [28, 29, 34–38].
Tumor volume data corroborate the findings in the biodistribution and scintigraphic images, where the higher tumor accumulation of NLC-DHA-DOX leads to a better antitumor activity. The treatment groups that received doxorubicin-loaded nanoparticles (Fig. 4, Table 4) had a slower tumor growth, and increased necrosis areas in the histological sections (Fig. S2), showing that nanocarriers are an interesting approach to delivery drugs to the tumor site. There are several studies reporting the advantages in using different types of nanoparticles loaded with doxorubicin for the antitumor activity [39–41].
With regard to the NLC-DHA-DOX, the small particle size, along with the EPR effect and the controlled release of doxorubicin from the particle [23], contributes to the increase of antitumor effectiveness. Besides that, it is possible to observe the substantial contribution of DHA in the antitumor activity. DHA might contribute to enhance anticancer therapy by different mechanisms, such as improving sensitization of cells to anticancer drugs, favoring uptake of chemotherapeutic agents, overcoming drug resistance, and reducing inherent drug-associated side effects [10]. Furthermore, as DHA is a highly polyunsaturated fatty acid, cell membrane might be more susceptible to lipid peroxidation leading to membrane damage [42]. Results obtained by in vivo distribution and antitumor activity accredit NLC-DHA-DOX, in combination with technetium-99m, a potential theranostic tool for breast tumor therapy.
The search for new therapeutic alternatives in cancer treatment aims to develop potentially effective formulations, but above all with low toxicity. Concerning to the weight variation, it is possible to suggest the safety of the blank formulation, once the group that received blank-NLC was the only group that gained weight over time. These data were expected, since only biocompatible lipids and standard surfactants for parenteral administration, such as Tween 80, were used [15, 43]. The histological analysis also confirms the reduction of toxicity compared to free drug. It is already known that nanocarriers are extensively uptaken by MPS, leading to higher uptake in the liver and spleen [30, 44]. Liver evaluation shows that, despite the presence of degeneration areas in the tissue of animals treated with the formulations, the encapsulation into nanocarrier offers a protective effect, since the extent and frequency of areas of degeneration were significantly reduced after encapsulation. Additionally, heart tissue histological evaluation also indicated a potential cardio-protective effect for NLC-DHA-DOX group and these findings are in agreement with previous studies using nanocarriers loaded with doxorubicin [45, 46].
Conclusions
In summary, from labeling NLC-DHA-DOX surface with technetium-99m, it was possible to evaluate their biodistribution profile and to confirm the preferential accumulation in the tumor. The longer circulation time contribute to the tumor accumulation in a high payload leading to a greater antitumor effectiveness. Furthermore, data showed the antitumor activity increment by using DHA. In addition, NLC-DHA-DOX was able to reduce the toxicity compared to the free drug. Therefore, NLC-DHA-DOX formulation has shown great potential for using in breast cancer treatment and diagnosis/monitoring, leading to a new possibility of a theranostic platform.
Supplementary Material
Acknowledgments
Financial Support. The authors thank Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG-Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brazil) for their financial support and fellowships.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11307-017-1133-3) contains supplementary material, which is available to authorized users.
References
- 1.Stewart BW, Wild CP (2014) World cancer report 2014 latest world cancer statistics. International Agency for Research on Cancer, Lyon [Google Scholar]
- 2.Livi L, Meattini I, de Cardillo CL et al. (2009) Non-pegylated liposomal doxorubicin in combination with cyclophosphamide or docetaxel as first-line therapy in metastatic breast cancer: a retrospective analysis. Tumori 95:422–426 [DOI] [PubMed] [Google Scholar]
- 3.Shi Y, Moon M, Dawood S et al. (2011) Mechanisms and management of doxorubicin cardiotoxicity. Herz 36:296–305 [DOI] [PubMed] [Google Scholar]
- 4.Primeau AJ, Rendon A, Hedley D et al. (2005) The distribution of the anticancer drug doxorubicin in relation to blood vessels in solid tumors. Clin Cancer Res 27:8782–8788 [DOI] [PubMed] [Google Scholar]
- 5.Trédan O, Galmarini CM, Patel K, Tannock IF (2007) Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 99:1441–1454 [DOI] [PubMed] [Google Scholar]
- 6.Carvalho C, Santos RX, Cardoso S et al. (2009) Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem 16:3267–3285 [DOI] [PubMed] [Google Scholar]
- 7.Jassem J, Pieńkowski T, Płuzańska A et al. (2001) Doxorubicin and paclitaxel versus fluorouracil, doxorubicin, and cyclophosphamide as first-line therapy for women with metastatic breast cancer: final results of a randomized phase III multicenter trial. J Clin Oncol 19:1707–1715 [DOI] [PubMed] [Google Scholar]
- 8.Germain E, Chajès V, Cognault S et al. (1998) Enhancement of doxorubicin cytotoxicity by polyunsaturated fatty acids in the human breast tumor cell line MDA-MB-231: relationship to lipid peroxidation. Int J Cancer 75:578–583 [DOI] [PubMed] [Google Scholar]
- 9.Colas S, Mahéo K, Denis F et al. (2006) Sensitization by dietary docosahexaenoic acid of rat mammary carcinoma to anthracycline: a role for tumor vascularization. Clin Cancer Res 12:5879–5886 [DOI] [PubMed] [Google Scholar]
- 10.Siddiqui RA, Harvey KA, Xu Z et al. (2011) Docosahexaenoic acid: a natural powerful adjuvant that improves efficacy for anticancer treatment with no adverse effects. Biofactors 37:399–412 [DOI] [PubMed] [Google Scholar]
- 11.Mahéo K, Vibet S, Steghens JP et al. (2005) Differential sensitization of cancer cells to doxorubicin by DHA: a role for lipoperoxidation. Free Radic Biol Med 39:742–751 [DOI] [PubMed] [Google Scholar]
- 12.Hardman WE, Avula CP, Fernandes G, Cameron IL (2001) Three percent dietary fish oil concentrate increased efficacy of doxorubicin against MDA-MB 231 breast cancer xenografts. Clin Cancer Res 7:2041–2049 [PubMed] [Google Scholar]
- 13.Parhi P, Mohanty C, Sahoo SK (2012) Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov Today 17:1044–1052 [DOI] [PubMed] [Google Scholar]
- 14.Mamot C, Drummond DC, Hong K et al. (2003) Liposome-based approaches to overcome anticancer drug resistance. Drug Resist Update 6:271–279 [DOI] [PubMed] [Google Scholar]
- 15.Muller RH, Mader K, Gohla D (2000) Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur J Pharm Biopharm 50:161–177 [DOI] [PubMed] [Google Scholar]
- 16.Mehnert W, Mader K (2001) Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev 47:165–196 [DOI] [PubMed] [Google Scholar]
- 17.Janib SM, Moses AS, MacKay JA (2010) Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev 62:1052–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jo SD, SH K, Won Y-Y et al. (2016) Targeted nanotheranostics for future personalized medicine: recent progress in cancer therapy. Theranostics 6:1362–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi KY, Liu G, Lee S, Chen X (2012) Theranosticnanoplatforms for simultaneous cancer imaging and therapy: current approaches and future perspectives. Nano 4:330–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie J, Chen X (2010) Nanoparticle-based theranostic agents. Adv Drug Deliv Rev 62:1064–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang DJ, Kim C, Schechter NR et al. (2003) Imaging with 99mTc-ECDG targeted at the multifunctional glucose system: feasibility studies with rodents. Radiology 226:465–473 [DOI] [PubMed] [Google Scholar]
- 22.Saha GB (2010) Radiopharmaceuticals and methods of radiolabeling In: Fundamentals of nuclear pharmacy. Springer, New York, pp 83–113 [Google Scholar]
- 23.Mussi SV, Sawant R, Perche F et al. (2014) Novel nanostructured lipid carrier co-loaded with doxorubicin and docosahexaenoic acid demonstrates enhanced in vitro activity and overcomes drug resistance in MCF-7/Adr cells. Pharm Res 8:1882–1890 [DOI] [PubMed] [Google Scholar]
- 24.Mussi SV, Silva RC, Oliveira MC et al. (2013) New approach to improve encapsulation and antitumor activity of doxorubicin loaded in solid lipid nanoparticles. Eur J Pharm Sci 48:282–290 [DOI] [PubMed] [Google Scholar]
- 25.Fernandes RS, Silva JO, Lopes SCA et al. (2016) Technetium-99m-labeled doxorubicin as an imaging probe for murine breast tumor (4T1 cell line) identification. Nucl Med Commun 37:307–312 [DOI] [PubMed] [Google Scholar]
- 26.Thrall JH, Ziessman HA (2003) Medicina Nuclear, 2nd edn. Guanabara Koogan, Rio de Janeiro [Google Scholar]
- 27.Souza CM, Carvalho LF, Vieira TS et al. (2012) Thalidomide attenuates mammary cancer associated-inflammation, angiogenesis and tumor growth in mice. Biomed Pharmacother 66:491–498 [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Wang J, Wientjes MG, JL A (2012) Delivery of nanomedicines to extracellular and intracellular compartments of a solid tumor. Adv Drug Deliv Rev 64:29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda H, Wu J, Sawa J, Matsumura Y, Hori K (2000) Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 65:271–284 [DOI] [PubMed] [Google Scholar]
- 30.Torchilin V (2011) Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev 63:131–135 [DOI] [PubMed] [Google Scholar]
- 31.Reddy LH, Sharma LK, Chuttani K et al. (2005) Influence of administration route on tumor uptake and biodistribution of etoposide loaded solid lipid nanoparticles in Dalton’s lymphoma tumor bearing mice. J Control Release 105:185–198 [DOI] [PubMed] [Google Scholar]
- 32.Beloqui A, Solinis MA, Delgado A et al. (2013) Biodistribution of nnostructured lipid carriers (NLCs) after intravenous administration to rats: influence of technological fators. Eur J Pharm Biopharm 84:309–314 [DOI] [PubMed] [Google Scholar]
- 33.Torchilin V (2009) Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur J Pharm Biopharm 71:431–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan F, Dellian M, Fukumura D et al. (1995) Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res 55:3752–3756 [PubMed] [Google Scholar]
- 35.Leu AJ, Berk DA, Lymboussaki A et al. (2000) Absence of functional lymphatics within murine sarcoma: molecular and functional evaluation. Cancer Res 60:4324–4327 [PubMed] [Google Scholar]
- 36.Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407:246–257 [DOI] [PubMed] [Google Scholar]
- 37.Munn II (2003) Aberrant vascular architecture in tumors and its importance in drug-based therapies. Drug Discov Today 8:396–403 [DOI] [PubMed] [Google Scholar]
- 38.Gosh K, Thodeti CK, Dudley AC et al. (2008) Tumor-derived endothelial cells exhibit aberrant Rhon-mediated mechanosensing and abnormal angiogenesis in vitro. Proc Natl Acad Sci U S A 105:11305–11310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mastria EM, Chen M, McDaniel JR (2015) Doxorubicin-conjugated polypeptide nanoparticles inhibit metastasis in two murine models of carcinoma. J Control Release 118:52–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun L, Deng X, Yang X et al. (2014) Co-delivery of doxorubicin and curcumin by polymeric micelles for improving antitumor efficacy on breast carcinoma. RSC Adv 4:46737–46750 [Google Scholar]
- 41.Wang J, Ma W, Tu W (2015) Synergistically improved anti-tumor efficacy by co-delivery doxorubicin and curcumin polymeric micelles. Macromol Biosci 15:1252–1261 [DOI] [PubMed] [Google Scholar]
- 42.Guffy MM, North JA, Burns CP (1984) Effect of cellular fatty acid alteration on adriamycin sensitivity in cultured L1210 murine leukemia cells. Cancer Res 44:1863–1866 [PubMed] [Google Scholar]
- 43.Wissing SA, Kayser O, Muller RH (2004) Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev 56:1257–1272 [DOI] [PubMed] [Google Scholar]
- 44.Haley B, Frenkel E (2008) Nanoparticles for drug delivery in cancer treatment. Urol Oncol 26:57–64 [DOI] [PubMed] [Google Scholar]
- 45.O’Brien MER, Wigler N, Inbar M et al. (2004) Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX™/Doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 26:440–449 [DOI] [PubMed] [Google Scholar]
- 46.Shuhendler AJ, Prasad P, Zhang RX et al. (2014) Synergistic nanoparticulate drug combination overcomes multidrug resistance, increases efficacy, and reduces cardiotoxicity in a nonimmuno compromised breast tumor model. Mol Pharmaceutics 14:2659–2674 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.