Abstract
We have shown previously that γ-glutamyl transpeptidase (GGT) activity is essential for the nephrotoxicity of cisplatin. In this study we asked whether GGT activity was necessary for the antitumor activity of cisplatin. GGT was transfected into PC3 cells, a human prostate tumor cell line. Two independent GGT-positive cell lines were isolated and characterized. GGT cleaves extracellular glutathione providing the cells with access to additional cysteine. Expression of GGT had no effect on the growth rate of the cells in vitro where the culture medium contains high levels of cysteine. However, when the cells were injected into nude mice the GGT-positive tumors grew at more than twice the rate of the GGT-negative tumors. Weekly treatment with cisplatin was toxic to both GGT-positive and negative tumors. The GGT-positive tumors were significantly more resistant to the toxicity of cisplatin than the GGT-negative tumors. Therefore, expression of GGT is required for the nephrotoxicity of cisplatin, but diminishes the tumor toxicity of the drug. These results indicate that the nephrotoxicity and the tumor toxicity of cisplatin are via two distinct pathways.
Introduction
Cisplatin is one of the most widely used chemotherapy drugs. Platinum-based chemotherapy regimens are used in the treatment of germ cell tumors, ovarian and bladder carcinomas, squamous cell tumors of the head and neck and non-small cell lung tumors (1).
Acute nephrotoxicity is the primary dose-limiting side effect of cisplatin. We discovered that expression of γ-glutamyl transpeptidase (GGT) is essential for the nephrotoxic effects of cisplatin. GGT is a cell surface enzyme that cleaves the γ-glutamyl bond of extracellular glutathione and glutathione conjugates (2). Cleavage of extracellular glutathione makes available additional cysteine that can be used for protein synthesis and synthesis of intracellular glutathione (3). Cleavage of glutathione-conjugated compounds by GGT on the surface of the proximal tubule cells in the kidney is the first step in the formation of mercapturic acids (4). Inhibition of GGT activity completely blocks the nephrotoxicity of cisplatin (5). It is unclear whether GGT expression alters the sensitivity of tumors to cisplatin.
Results from in vitro studies on the effect of GGT expression and cisplatin sensitivity vary. No correlation was observed between GGT expression and cisplatin sensitivity among the human cell lines used in the National Cancer Institute Screening Program (6). However, selection of a human ovarian tumor cell line for resistance to cisplatin yielded a series of resistant cell sublines with elevated levels of GGT mRNA (7). Transfection of GGT cDNA into a human prostate tumor cell line did not alter its sensitivity to cisplatin (8).
In this study we asked whether the expression of GGT alters the sensitivity of tumors to cisplatin in vivo. If GGT activity is essential for the tumor response to cisplatin, as it is for the nephrotoxicity, this would provide new insight into factors that affect the in vivo sensitivity. If GGT activity only affects the nephrotoxicity of the drug this would provide the first evidence that the mechanism by which cisplatin kills the cells of the kidney is distinct from its therapeutic mechanism of action.
For this study the GGT-negative, human prostate tumor cell line PC3 was transfected with GGT. Two independent clones of GGT-positive cells and two independent clones of negative-cells were isolated and characterized. The cells were transplanted into nude mice where they formed tumors. The mice were treated weekly with cisplatin. The growth of the tumors derived from the GGT-positive and negative cells was monitored. At the termination of the experiment the tumors were removed, fixed and immunostained for GGT.
Materials and methods
Cell culture
PC3, a human prostate carcinoma cell line (ATCC CRL 1435) was obtained from the American Type Culture Collection (Rockville, MD). The PC3 cells were maintained in RPMI1640 media (Gibco BRL, Grand Island, NY), with 5% fetal calf serum (HyClone Laboratories, Logan, UT) and penicillin–streptomycin (Gibco BRL).
Transfection of PC3 cells
A full length cDNA clone for human GGT was generously provided to us by Dr Henry Pitot (9). The cDNA was inserted into two different transfection vectors. The first vector was pLEN-PT as previously described (3). The plasmid consists of a full length human GGT cDNA inserted into a pUC8-based vector with an SV40 origin of replication, SV40 enhancer sequences, human metallothionein II promoter, a polylinker region, an SV40 poly(A) addition signal and poly(A) tract (9). The pLEN-PT vector does not contain a selectable marker for mammalian cells that necessitates co-transfection with another plasmid such as pWLneo, which contains a neomycin resistance gene. For the second set of experiments GGT cDNA was inserted into pcDNA3.1(+) vector (Invitrogen, San Diego, CA), which contains a neomycin resistance gene. The pcDNA3.1 vector uses the human cytomegalovirus immediate-late promoter rather than the human metallothionein II promoter.
For transfection, the PC3 cells were cultured in a 1:1 mixture of F12:Dulbecco’s minimum essential medium (DMEM) (Gibco BRL), enriched with 10% fetal calf serum (HyClone Laboratories), and 25 µg/ml gentamicin (Gibco BRL). The plasmids were transfected by CaPO4 precipitation as previously described (3). For the first transfection, PC3 cells were transfected with GGT/pLEN-PT and co-transfected with pWLneo, a plasmid containing a G418 resistance marker. Control cells were transfected with pWLneo alone. For the second transfection, PC3 cells were transfected with GGT/pcDNA3.1 plasmid and control cells were transfected with pcDNA3.1 vector. For both transfections stable transfectants were selected by adding 500 µg/ml of G418 to the culture media. Individual colonies were picked and grown into cell lines in RPMI1640 media supplemented with 5% fetal calf serum, penicillin–streptomycin and 150 to 200 µg/ml of G418. The GGT-positive cell line derived from the GGT/pLEN-PT transfectants was named PC3/GGT1 and the control line PC3/C1. The GGT-positive cell line derived from GGT/pcDNA3.1 was named PC3/GGT2 and the control line PC3/C2.
Animals
Male athymic Swiss nude mice (defined flora), 5–8 weeks old, were obtained from Taconic Farms (New York, NY). Animals were housed under pathogen-free conditions at the Animal Resource Facility of the University of Virginia. Food and water were provided ad libitum.
In vivo tumor experiments
At 24 h prior to harvesting the cells for injection the medium was changed to fresh medium that did not contain G418. For the injections, the cells were suspended in RPMI1640, mixed 1:1 in Matrigel (Collaborative Biomedical Products, Bedford, MA) and injected subcutaneously into the flanks. Each mouse received one injection in each flank. Each injection contained 2.5×106 PC3 cells. An aliquot of both GGT-positive and -negative cells was stained histochemically for GGT on the day of the injection (10).
In experiment 1, 25 mice were injected with PC3/GGT1 and 25 mice were injected with PC3/C1 cells. One week after the cells were injected both sets of mice were divided into three groups of eight to nine mice each. Group 1 received weekly injections of saline, group 2 received weekly injections of 2.5 mg/kg cisplatin (Platinol-AQ; Bristol-Myers Squibb, Princeton, NJ) and group 3 received weekly injections of 5 mg/kg cisplatin. The mice were weighed weekly. Tumor size was measured weekly with vernier calipers. Nine weeks after the tumor cells were injected, and 1 week after the final injection of cisplatin or saline, the animals were killed and the tumors excised. The tumors were weighed, fixed in Bouin’s fixative, embedded in paraffin and sectioned at 4 µm (11). The tissue sections were stained with an antibody directed against human GGT as previously described (12).
In experiment 2, 18 mice were injected with PC3/GGT2 cells and 17 mice were injected with PC3/C2 cells. One week after the cells were injected both sets of mice were divided into two groups of eight to nine mice each. Group 1 received weekly injections of saline, group 2 received weekly injections of 5 mg/kg cisplatin. The animals were weighed and tumor size was measured weekly. The animals were killed 10 weeks after the tumor cells were injected and 1 week after the final cisplatin or saline injection. Tumors were removed, weighed and one tumor from each mouse was immediately frozen in liquid nitrogen and stored at –80°C. The other tumor was fixed, processed and stained as described above.
Glutathione assay
The tumor tissue, while still frozen, was pulverized with a Bessman Tissue Pulverizer (Fisher Scientific, Pittsburgh, PA) then immediately homogenized in ice cold 4.31% sulfosalicylic acid with a Tissue Tearor (Fisher Scientific). The homogenate was centrifuged at 8000 g for 15 min at 4°C. The supernatant was assayed for glutathione by the method of Tietze (13).
GGT assays
GGT activity was determined biochemically by the method of Tateishi et al. (14) and histochemically by the method of Rutenburg et al. (10). Protein was determined with the BCA protein assay (Pierce, Rockford, IL). The level of GGT activity in the transfected clones was measured in cells that were being maintained in vitro under G418 selection while the in vivo tumor experiments were in progress.
Data analysis
The tumor volumes were calculated according to the formula:, where a is the length of the longest diameter and b the length of the shortest diameter (15). The number of tumors that grew out were reported as the number of cell injection sites in which tumors grew over the total number of cell injection sites. Statistically significant differences between the groups were detected by a two-tailed version of Fisher’s exact test (16). Mean values and standard deviation for tumor doubling time and animal weight were computed for each group. These data were analyzed for statistically significant differences between groups with Student’s t-test corrected for unequal sample size when only two groups were compared and Dunnett’s test when two treatment groups were compared with the control (16).
Results
GGT-positive PC3 cell lines
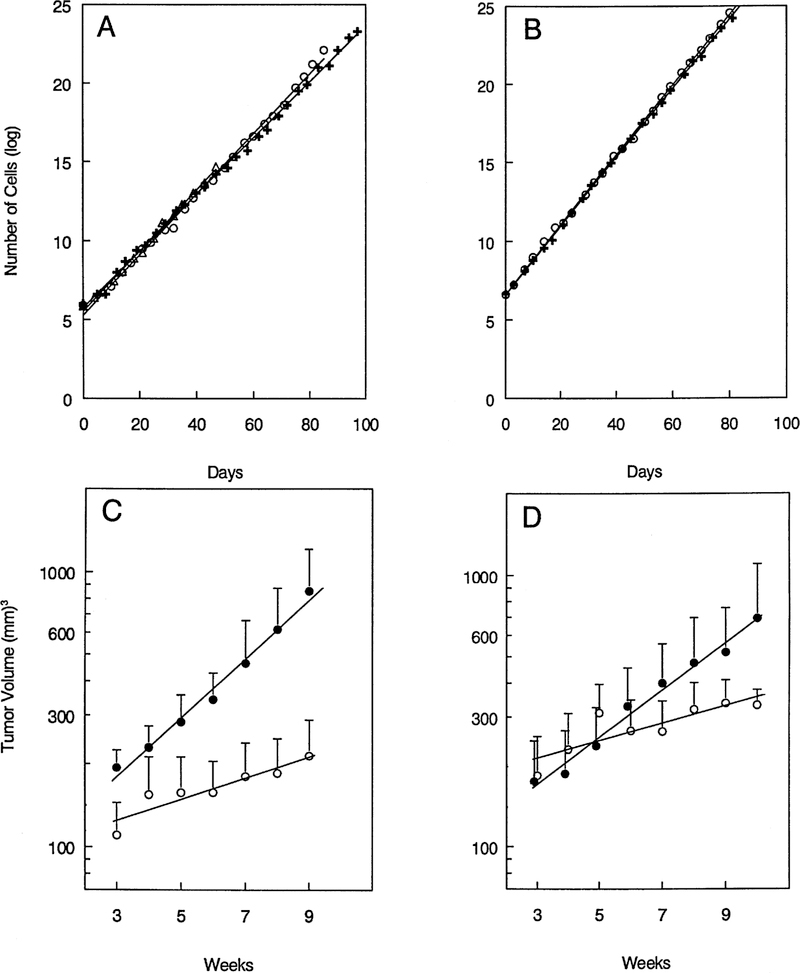
No GGT activity was detected in the parental PC3 cell line with either the biochemical or histochemical assay. We inserted a full length human GGT cDNA into two transfection vectors, pLEN-PT and pcDNA3.1. Transfection of PC3 cells with either of the GGT-containing vectors gave rise to PC3 cells that expressed functionally active GGT. The PC3/GGT1 cell line was isolated from PC3 cells transfected with the pLEN-PT/GGT plus pWLneo. PC3/GGT1 cells expressed 0.290 ± 0.004 U GGT activity/mg protein. PC3/C1 was a control cell line isolated from cells transfected with pWLneo alone. No GGT activity was detected in these cells with either the biochemical or histochemical assay. Expression of GGT did not affect the growth rate of the cells in culture (Figure 1A). In the absence of G418, the doubling times of the PC3/GGT1 and PC3/C1 lines were equivalent to the doubling time of the parent PC3 cell line (doubling times 38.5 ± 1.5 h).
Fig. 1.
Growth of GGT-positive and -negative PC3 cells in vitro and in vivo. Growth in vitro of GGT-positive, PC3 cells (+), GGT-negative, PC3 cells (○) and parental PC3 cells (△) in experiment 1 (A) and experiment 2 (B). Growth of the same PC3 GGT-positive cells (●) and GGT-negative cells (s) when injected into nude mice, experiment 1 (C); experiment 2 (D).
The PC3/GGT2 cell line was isolated from the PC3 cells transfected with pcDNA3.1/GGT plasmid. The PC3/GGT2 cells expressed 0.126 ± 0.007 U GGT activity/mg protein. The control cell line PC3/C2 transfected with the pcDNA3.1 vector was negative for GGT. The doubling time of the PC3/GGT2 and PC3/C2 cells in vitro were equivalent (Figure 1B).
Tumor growth in vivo
The tumor cells were injected s.c. into the right and left flanks of nude mice. In experiment 1 tumors arose at 15/16 sites that were injected with the GGT-positive PC3/GGT1 cells and at 12/16 sites injected with GGT-negative PC3/C1 cells (Table I). Similar results were obtained in experiment 2 in which tumors arose at 17/18 sites injected with GGT-positive PC3/GGT2 cells and at 12/16 sites injected with GGT-negative PC3/C2 cells. The difference in the tumor take between the GGT-positive and -negative cells was not statistically significant in either experiment. Analysis of the growth rate of the GGT-positive and -negative tumors shows that the tumors that arose from the GGT-positive cells grew more rapidly than the tumors from the GGT-negative cells (Figure 1C and D). The doubling time of the GGT-positive tumors was 24 days in experiment 1 and 35 days in experiment 2 (Table II). In contrast, the doubling times of the GGT-negative tumors were 65 and 72 days for experiments 1 and 2, respectively. In both experiments the GGT-positive tumors were growing more than twice as fast as the GGT-negative tumors. The difference in doubling time between the GGT-positive and -negative tumors was statistically significant (P < 0.01) in both experiments. Expression of GGT does provide a growth advantage to tumor cells in vivo.
Table I.
Outgrowth of GGT-positive and -negative tumors during treatment with cisplatin
| No. tumors/no. injection sites |
Tumor response, positive versus negative | ||
|---|---|---|---|
| Cisplatin (mg/kg) | GGT-positive tumors | GGT-negative tumors | |
| Experiment 1 | |||
| 0 | 15/16 | 12/16 | NS |
| 2.5 | 14/16 | 6/16 | P < 0.01 |
| 5.0 | 8/18**,a | 0/18*** | P < 0.01 |
| Experiment 2 | |||
| 0 | 17/18 | 12/16 | NS |
| 5.0 | 8/18** | 0/18*** | P < 0.01 |
NS, no significant difference between GGT-positive and -negative tumors.
Statistically significant differences from saline treated controls were detected by Fisher’s exact test;
P < 0.01
P < 0.001.
Table II.
Growth of GGT-positive and -negative tumors during treatment with cisplatin
| Doubling time (days) |
||
|---|---|---|
| Cisplatin (mg/kg) | GGT-positive tumors | GGT-negative tumors |
| Experiment 1 | ||
| 0 | 24 ± 9 | 65 ± 31 |
| 2.5 | 58 ± 58 | 120 ± 47** |
| 5.0 | 188 ± 205** | − |
| Experiment 2 | ||
| 0 | 35 ± 28 | 72 ± 32 |
| 5.0 | 330 ± 293*** | − |
Data are significantly different from untreated control
P < 0.01
P < 0.001.
Cisplatin toxicity
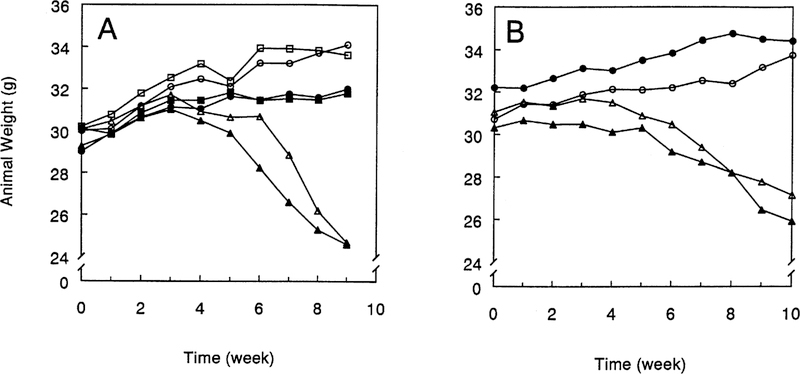
In both experiments tumor-bearing mice treated with saline gained weight throughout the course of the experiment (Figure 2). Weekly treatment with 2.5 mg/kg cisplatin did not significantly affect the amount of weight that the mice gained over the course of the experiment. One mouse treated with 2.5 mg/kg cisplatin continued to gain weight over the course of the experiment but died of unknown causes during the 9th week of the experiment. Mice treated weekly with 5.0 mg/kg cisplatin began to lose weight within 2–3 weeks. In experiment 1 four mice treated with 5.0 mg/kg cisplatin died prior to the termination of the experiment. Two mice with GGT-negative tumors died during the 7th week. Two mice with GGT-positive tumors died, one during the 8th week and one during the 9th. None of the animals in experiment 2 died prior to the termination of the experiment. The toxicity of cisplatin lead to the decision to terminate experiment 1 at 9 weeks, which was 1 week earlier than experiment 2.
Fig. 2.
Weight of mice during treatment with cisplatin. Mice bearing GGT-positive, PC3 tumors (closed symbols) and GGT-negative PC3 tumors (open symbols) were weighed weekly prior to injection with saline (circles), 2.5 mg/kg cisplatin (squares) or 5.0 mg/kg cisplatin (triangles) in experiment 1 (A) and experiment 2 (B).
Response of tumors to cisplatin
GGT-positive tumors were less sensitive than GGT-negative tumors to the toxicity of cisplatin (Table I). As noted above, there was no statistically significant difference in the percentage of GGT-positive and -negative tumors that grew in the saline treated controls. In experiment 1 a weekly dose of 2.5 mg/kg cisplatin was significantly more toxic to the GGT-negative tumors than the positive tumors (P<0.01). In both experiments treatment of mice with 5.0 mg/kg cisplatin was more toxic to the GGT-negative tumors (P < 0.01). A dose of 5.0 mg/kg cisplatin reduced the percentage of GGT-positive tumors that grew from 15/16 or 17/18 (94%) to 8/18 (44%, P < 0.01). Treatment of mice bearing GGT-negative tumors with 5.0 mg/kg cisplatin reduced the percentage of tumors that grew from 12/16 (75%) to zero (P < 0.001).
Analysis of the doubling time of the tumors that did grow out during treatment also showed that the GGT-positive tumors were less sensitive to cisplatin than the GGT-negative tumors (Table II). In experiment 1 the lower dose of cisplatin (2.5 mg/kg) did not significantly affect the doubling time of the GGT-positive tumors but did increase the doubling time of the GGT-negative tumors from 65 to 120 days (P < 0.01).
GGT expression in tumors
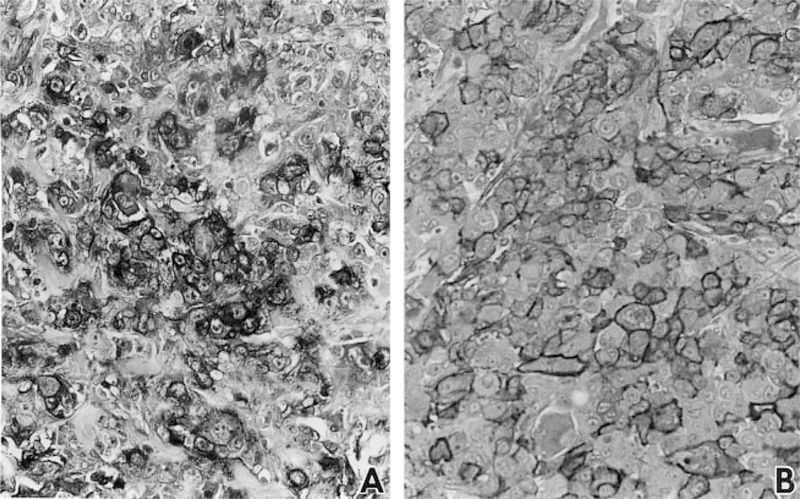
Tumors were fixed and stained immunohistochemically with GGT129, an antibody directed against human GGT. The GGTcDNA that was used to transfect the PC3 cells was a human cDNA, therefore, the GGT protein that was expressed was recognized by the antibody. Immunohistochemical staining showed that the tumors derived from the two control cell lines, PC3/C1 and PC3/C2, were uniformly GGT-negative. Whereas, all of the tumors that arose from the GGT transfected cells, PC3/GGT1 and PC3/GGT2, were GGT-positive. In the PC3/GGT1-derived tumors both cytoplasmic and membrane staining were observed (Figure 3A). The cytoplasmic staining may reflect GGT protein that is being synthesized and processed within the cell. In the PC3/GGT2-derived tumors the antibody staining was strongly localized to the cell membrane (Figure 3B).
Fig. 3.
Immunohistochemical staining of tumors derived from PC3/GGT1 cells (A) and PC3/GGT2 cells (B). Tumors were stained with GGT129, an antibody directed against a 20 amino acid sequence in the human GGT protein. Antibody staining in the controls, PC3/C1 and PC3/C2 derived tumors, was negative (data not shown).
Some of the PC3/GGT1 tumors had areas of GGT-negative tumor cells within them. The mixture of GGT-positive and -negative cells that arose from the injection of 100% GGT-positive PC3/GGT1 cells suggested that some of the PC3/GGT1 cells were losing the transfected cDNA when not under constant selection with G418. This was confirmed in culture. Histochemical staining showed that when PC3/GGT1 cells were maintained in culture without G418 for 12 weeks, the percentage of cells that were GGT-positive decreased from 100 to 61%. Addition of 150 µg/ml of G418 to the culture medium was toxic to the GGT-negative cells and resulted in the restoration of the cell line to 100% GGT-positive within 14 days. Tumors derived from PC3/GGT2 were uniformly GGT-positive. The stability of GGT expression in PC3/GGT2 cells was also noted in culture. PC3/GGT2 cells grown in vitro without G418 for 12 weeks showed no reduction in the percentage of GGT-positive cells.
The presence of GGT-negative areas in the PC3/GGT1-derived tumors indicates that not only does GGT provide a growth advantage for the cells that express the enzyme but it also provides a growth advantage for cells surrounding the GGT-positive cells. Areas of GGT-negative cells were more commonly observed in the saline-treated animals than in the animals treated with 5.0 mg/kg cisplatin. The regional effect of GGT appears to be stronger in promoting growth than in reducing cisplatin toxicity.
Glutathione in tumors
Glutathione levels were measured in the tumor tissue from experiment 2 that had been stored frozen. The number of tumors that could be assayed for glutathione was limited because only half of the tumors were frozen and some of the tumors were too small to obtain accurate glutathione levels. The results showed that in five GGT-positive tumors from saline-treated mice and two GGT-positive tumors from cisplatin-treated mice the glutathione levels were 1.47 ± 0.48 and 1.39 ± 0.63 µmol glutathione/g tumor, respectively. In six GGT-negative tumors from saline-treated mice and one GGT-negative tumor from a cisplatin-treated mouse the glutathione levels were 1.36 ± 0.34 and 1.42 µmol glutathione/g tumor. Analysis of the data showed that there was no significant difference between the four groups. The glutathione levels in the GGT-positive tumors were similar to those in the GGT-negative tumors. The mice were killed 1 week after the final injection of cisplatin or saline. No difference in glutathione levels was detected between the cisplatin-and saline-treated groups.
Discussion
Prior studies demonstrated that the nephrotoxicity of cisplatin could be blocked by administering an inhibitor of GGT activity prior to treatment with cisplatin (5). The current studies were undertaken to determine whether expression of GGT affects the sensitivity of tumors to cisplatin toxicity. The data showed that expression of GGT did not affect the growth rate of cells in culture but did increase the growth rate when the cells were injected into nude mice. In contrast to the nephrotoxicity of cisplatin, which requires GGT, the tumor toxicity was reduced by GGT expression. These results suggest that the nephrotoxicity and tumor toxicity of cisplatin are mediated by two different mechanisms.
GGT is a cell surface enzyme that cleaves γ-glutamyl bonds (17). It is localized to the luminal surface of ducts and glands throughout the body (12). In these tissues GGT prevents the excretion of glutathione by initiating the cleavage of glutathione into its constituent amino acids, cysteine, glycine and glutamic acid, which can then be reabsorbed (3). The proximal tubule cells of the kidney express high levels of GGT. The enzyme cleaves glutathione in the glomerular filtrate and its constituent amino acids are reabsorbed. In GGT knockout mice, glutathione remains intact as it flows through the kidney. Large amounts of glutathione are excreted in the urine and as a result the knockout mice suffer from a severe cysteine deficiency (18).
The level of GGT activity expressed in the transfectants (0.126 and 0.290 U GGT/mg protein) is similar to the levels expressed in human, mouse and bovine kidney (0.103, 0.275 and 0.405 U GGT/mg protein, respectively) (12,14). Comparison between these levels and GGT expression in human tumor cell lines is difficult because most studies of human tumor cell lines have been based on relative amounts of GGT mRNA expression (6,7). The level of GGT activity in our transfected cell lines is higher than the levels reported in chemically induced rat liver tumors and preneoplastic lesions (0.076 and 0.037 U GGT/mg protein) (14,19).
Unlike normal cells that express GGT and are strongly polarized in vivo, the cells in poorly differentiated tumors are not polarized and the enzyme is present over the entire cell surface (20,21). In GGT-positive tumors, the enzyme is in contact with the interstitial fluid and can cleave glutathione present in the fluid. Hockwald et al. (22) showed that as blood circulates through a GGT-positive tumor the glutathione levels in the blood decrease more rapidly than they do in the systemic circulation. The cysteine liberated from the extracellular glutathione by GGT serves as a secondary source of cysteine for the tumor. In vitro studies with the mouse hepatoma cell line Hepa 1–6 showed that at physiological concentrations cysteine can becoming limiting for tumor cell growth (23). The GGT-positive PC3 cells in this study had access to the cysteine in extracellular glutathione and grew more rapidly in vivo than the GGT-negative PC3 cells.
In this study, immunostaining showed differences in the localization of GGT in the two experiments. In the first experiment, the staining appeared to be both membranous and cytoplasmic whereas, in the second, most of the staining was localized to the cell membrane. This is probably an indirect result of the different level of GGT expression in the cell lines used in the two experiments. We have seen previously in both normal and neoplastic tissue that the higher the level of GGT expression the more common it is to see antibody staining that appears to be cytoplasmic in addition to the membrane staining (12,20). This observation may be the result of immunolocalization of GGT protein that is being synthesized and processed intracellularly. The peptide should be recognized by the antibody as soon as the 20 amino acids at the C-terminus of the heavy subunit are synthesized (12). There is only one report of a cell line in which GGT appears to be localized intracellularly, the ARL-16T2 tumor cell line, which is derived from a non-tumorigenic liver epithelial cell line (24).
In this study, GGT did not provide a growth advantage in vitro. Tissue culture media contains cysteine at concentrations 3-to 4-fold higher than the concentration in serum. Therefore, cysteine is not rate-limiting for cell growth in vitro and additional cysteine made available by GGT does not further increase cell growth. Warren et al. (25) obtained similar data with a mouse epidermal cell line transfected with GGT. In rapidly growing tumors cysteine can become limiting for cell growth in vivo; but, access to additional cysteine has no effect on growth when cells are grown in tissue culture media that is cysteine-rich.
In contrast to the experimental animal models, a recent clinical study showed no statistically significant correlation between GGT expression and mitotic rate among 79 cases of infiltrating mammary carcinoma (20). One explanation for this discrepancy between clinical tumors and experimental tumors is that most primary tumors grow more slowly than experimental tumors derived from cell lines. As shown in this study and others, expression of GGT will only provide a selective growth advantage to tumors growing so rapidly that access to cysteine is limiting for growth.
Cisplatin is a planar molecule that contains a central platinum atom surrounded by two chloride atoms in the cis configuration and two ammonia moieties. Chloride ions dissociate from the platinum at low salt concentrations equivalent to the intracellular concentration of chloride (26). This dissociation creates an electrophilic form of platinum that will bind to cellular nucleophiles such as DNA, RNA and negatively charged groups on proteins. DNA damage caused by the formation of platinum–DNA adducts is thought to be the mechanism by which cisplatin kills dividing cells (27). Expression of GGT in the PC3 cells resulted in increased resistance to the toxicity of cisplatin. It is of interest that cisplatin was more toxic to the slower growing GGT-negative PC3 tumors in comparison with the more rapidly dividing GGT-positive PC3 tumors. This finding is contrary to the general observation that the higher the rate of growth the more sensitive a tumor is to the toxicity of most chemotherapy agents.
Glutathione is a nucleophile that can bind to the electrophilic form of platinum. Intracellular platinum–glutathione conjugates have been isolated from tissue culture cells treated with cisplatin (28,29). In vitro studies have shown a correlation between increased intracellular glutathione and resistance to cisplatin (7,30), although a study done in vivo failed to show a correlation (31). In this study the steady state glutathione levels were the same in GGT-positive and -negative tumors. Seven days after treatment with cisplatin there was no difference between the glutathione levels in cisplatin-treated and control tumors. The resistance of the GGT-positive tumors to cisplatin may result from the ability of GGT-positive tumors to replenish intracellular glutathione levels more rapidly than GGT-negative tumors because of the cysteine made available by the cleavage of extracellular glutathione (3). Cisplatin– glutathione conjugates have been shown to be formed intracellularly and actively pumped out of the cell by the MRP/GS-X pump (28,32). Resistance of tumor cells to cisplatin is associated with induction of intracellular glutathione and the MRP/GS-X pump (32).
Our data, which show that expression of GGT in the PC3 cells protects the cell from the toxicity of cisplatin, is in direct contrast to our studies of the nephrotoxicity of cisplatin, which show that expression of GGT increases the nephrotoxicity of cisplatin. These data indicate that the antitumor activity of cisplatin and nephrotoxicity of cisplatin are via a distinct mechanism. In tumor cells, cisplatin is conjugated to glutathione and pumped out of the cell. Our hypothesis is that in the kidney, GGT on the surface of the proximal tubule cells cleaves extracellular glutathione–cisplatin conjugates, which initiates their metabolism through the mercapturic acid or betalyase pathways that are unique to the kidney (5). Several potent nephrotoxins have been shown to be conjugated to glutathione and activated to toxins through these GGT-mediated pathways in the proximal tubule cells of the kidney (4).
The results from this study show that in vivo GGT increases the growth of PC3 cells and increases the resistance of the tumor cells to the toxicity of cisplatin. These data show for the first time that in vivo the same enzyme that is essential for the nephrotoxicity of cisplatin inhibits the tumor toxicity of cisplatin. These observations strongly support the hypothesis that the pathway by which cisplatin is metabolized to a nephrotoxin is distinct from the pathway by which it kills tumor cells.
Acknowledgements
We gratefully acknowledge the staff of the University of Virginia Cell Science Core Facility for embedding and sectioning the tumor tissue and the staff of the Molecular Biology Core for plasmid preparation. Both core facilities are supported by NIH P30-HD28934, Center for Cellular and Molecular Studies in Reproduction. These studies were supported by grant RO1 CA 57530 from the National Cancer Institute.
Abbreviations:
- DMEM
Dulbecco’s minimum essential medium
- GGT
γ-glutamyl transpeptidase.
References
- 1.Colvin OM (1997) Alkylating agents and platinum antitumor compounds. In Holland JF, Bast RC Jr, Morton DL, Frei E III, Kufe DW and Weichselbaum RR (eds) Cancer Medicine Williams and Williams, Baltimore, MD, pp. 949–975. [Google Scholar]
- 2.Hanigan MH (1998) Gamma-glutamyl transpeptidase, a glutathionease: its expression and function in carcinogenesis. Chem. Biol. Interact, 111–112, 333–342. [DOI] [PubMed] [Google Scholar]
- 3.Hanigan MH and Ricketts WA (1993) Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase. Biochemistry, 32, 6302–6306. [DOI] [PubMed] [Google Scholar]
- 4.Elfarra AA and Anders MW (1984) Renal processing of glutathione conjugates, role in nephrotoxicity. Biochem. Pharmacol, 33, 3729–3732. [DOI] [PubMed] [Google Scholar]
- 5.Hanigan MH, Gallagher BC, Taylor PT Jr and Large MK (1994) Inhibition of gamma-glutamyl transpeptidase activity by acivicin in vivo protects the kidney from cisplatin-induced toxicity. Cancer Res, 54, 5925–5929. [PubMed] [Google Scholar]
- 6.Tew KD, Monks A, Barone L, Rosser D, Akerman G, Montali JA, Wheatley JB and Schmidt DE Jr (1996) Glutathione-associated enzymes in the human cell lines of the National Cancer Institute Drug Screening Program. Mol. Pharmacol, 50, 149–159. [PubMed] [Google Scholar]
- 7.Godwin AK, Meister A, O’Dwyer PJ, Huang CS, Hamilton TC and Anderson ME (1992) High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc. Natl Acad. Sci. USA, 89, 3070–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey HH, Gipp JJ and Mulcahy RT (1994) Increased expression of gamma-glutamyl transpeptidase in transfected tumor cells and its relationship to drug sensitivity. Cancer Lett, 87, 163–170. [DOI] [PubMed] [Google Scholar]
- 9.Goodspeed DC, Dunn TJ, Miller CD and Pitot HC (1989) Human gamma-glutamyl transpeptidase cDNA: comparison of hepatoma and kidney mRNA in the human and rat. Gene, 76, 1–9. [DOI] [PubMed] [Google Scholar]
- 10.Rutenburg AM, Kim H, Fischbein JW, Hanker JS, Wasserkrug HL and Seligman AM (1969) Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J. Histochem. Cytochem, 17, 517–526. [DOI] [PubMed] [Google Scholar]
- 11.Davenport HA (1960) Histological and Histochemical Technics W.B.Saunders Publishers, Philadelphia, PA. [Google Scholar]
- 12.Hanigan MH and Frierson HF Jr (1996) Immunohistochemical detection of gamma-glutamyl transpeptidase in normal human tissue. J. Histochem. Cytochem, 44, 1101–1108. [DOI] [PubMed] [Google Scholar]
- 13.Tietze F (1969) Enzymatic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal. Biochem, 27, 502–522. [DOI] [PubMed] [Google Scholar]
- 14.Tateishi N, Higashi T, Nomura T, Naruse A, Nakashima K, Shiozaki H and Sakamoto Y (1976) Higher transpeptidation activity and broad acceptor specificity of gamma-glutamyltransferase of tumors. Gann, 67, 215–222. [PubMed] [Google Scholar]
- 15.Goldin A, Venditti JM, MacDonald JS, Muggia FM, Henney JE and DeVita VTJr (1981) Current results of the screening program at the division of cancer treatment, National Cancer Institute. Eur. J. Cancer, 17, 129–142. [DOI] [PubMed] [Google Scholar]
- 16.Glantz SA (1992) Primer of Bio-statics McGraw-Hill, New York, NY. [Google Scholar]
- 17.Hanigan MH and Pitot HC (1985) Gamma-glutamyl transpeptidase—its role in hepatocarcinogenesis. Carcinogenesis, 6, 165–172. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman MW, Wiseman AL, Shi Z et al. (1996) Growth retardation and cysteine deficiency in gamma-glutamyl transpeptidase-deficient mice. Proc. Natl Acad. Sci. USA, 93, 7923–7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanigan MH and Pitot HC (1985) Activities of benzphetamine N-demethylase and aryl hydrocarbon hydroxylase in cells isolated from gamma-glutamyl transpeptidase-positive foci and surrounding liver. J. Natl Cancer Inst, 75, 1107–1112. [PubMed] [Google Scholar]
- 20.Durham JR, Frierson HF Jr and Hanigan MH (1997) Gamma-glutamyl transpeptidase immunoreactivity in benign and malignant breast tissue. Breast Cancer Res. Treat, 45, 55–62. [DOI] [PubMed] [Google Scholar]
- 21.Hanigan MH, Frierson HF Jr, Brown JE, Lovell MA and Taylor PT (1994) Human ovarian tumors express gamma-glutamyl transpeptidase. Cancer Res, 54, 286–290. [PubMed] [Google Scholar]
- 22.Hochwald SN, Harrison LE, Rose DM, Anderson M and Burt ME (1996) Gamma-glutamyl transpeptidase mediation of tumor glutathione utilization in vivo. J. Natl Cancer Inst, 88, 193–197. [DOI] [PubMed] [Google Scholar]
- 23.Hanigan MH (1995) Expression of gamma-glutamyl transpeptidase provides tumor cells with a selective growth advantage at physiologic concentrations of cyst(e)ine. Carcinogenesis, 16, 181–185. [DOI] [PubMed] [Google Scholar]
- 24.Meredith MJ and Williams GM (1986) Intracellular glutathione cycling by τ-glutamyl transpeptidase in tumorigenic and non-tumorigenic cultured rat liver cells. J. Biol. Chem, 261, 4986–4992. [PubMed] [Google Scholar]
- 25.Warren BS, Naylor MF, Winberg LD, Yoshimi N, Volpe JPG, Gimenez-Conti I and Slaga TJ (1993) Induction and inhibition of tumor progression. Proc. Soc. Exp. Biol. Med, 202, 9–15. [DOI] [PubMed] [Google Scholar]
- 26.Borch RF (1987) The platinum antitumour drugs. In Powis G and Prough RA (eds) Metabolism and Action of Anti-cancer Drugs Taylor and Francis, New York, NY, pp. 163–193. [Google Scholar]
- 27.Zamble DB and Lippard SJ (1995) Cisplatin and DNA repair in cancer chemotherapy. Trends Biochem. Sci, 20, 435–439. [DOI] [PubMed] [Google Scholar]
- 28.Ishikawa T and Ali-Osman F (1993) Glutathione-associated cis-diamminedichloroplatinum (II) metabolism and ATP-dependent efflux from leukemia cells. J. Biol. Chem, 268, 20116–20125. [PubMed] [Google Scholar]
- 29.Mistry P, Loh SY, Kelland LR and Harrap KR (1993) Effect of buthionine sulfoximine on PtII and PtIV drug accumulation and the formation of glutathione conjugates in human ovarian-carcinoma cell lines. Int. J. Cancer, 55, 848–856. [DOI] [PubMed] [Google Scholar]
- 30.Chen G, Hutter KJ and Zeller WJ (1995) Positive correlation between cellular glutathione and acquired cisplatin resistance in human ovarian cancer cells. Cell. Biol. Toxicol, 11, 273–281. [DOI] [PubMed] [Google Scholar]
- 31.Goddard P, Valenti M and Kelland LR (1994) The role of glutathione in determining sensitivity to platinum drugs in vivo in platinum-sensitive and -resistant murine leukaemia and plasmacytoma and human ovarian carcinoma xenographs. Anticancer Res, 14, 1065–1070. [PubMed] [Google Scholar]
- 32.Ishikawa T, Bao JJ, Yamane Y, Akimaru K, Frindrich K, Wright CD and Kuo MT (1996) Coordinated induction of MRP/GS-X pump and gamma-glutamylcysteine synthetase by heavy metals in human leukemia cells. J. Biol. Chem, 271, 14981–14988. [DOI] [PubMed] [Google Scholar]