Abstract
Human ovarian carcinoma cells (C70 and C200) made resistant to cisplatin from A2780 cells demonstrated an ~20-fold resistance to the drug. These same cell lines showed no collateral resistance (as compared with the wild-type) to a novel glutathione S-transferase π-activated prodrug {γ-glutamyl-α-amino-β[2-ethyl-β N,N,N′,N′-tetrakis (2-chloroethyl) phosphorodiamidate]-sulfonyl-propionyl-(R)-(—) phenylglycine; TLK286}. Previous results have shown a direct correlation between levels of GST π expression and cytotoxicity for TLK286 (L. A. Rosario et al., Mol. Pharmacol., 58: 167–174, 2000.). However, protein levels of the isozyme were identical in wild-type C70 and C200 cell lines. In analyzing the DNA repair capacity of C70 and C200, an altered expression of the DNA-dependent protein kinase (DNA-PK) complex (catalytic subunit DNA-PKcs, and the heterodimers Ku70 and Ku80) was found. In C70 and C200 cells, DNA-PKcs was overexpressed at both the transcript and protein levels, whereas amounts of Ku70 and Ku80 were higher only at the level of protein expression. TLK286 in either its parent or activated form inhibited the catalytic kinase activity of purified DNA-PK with an IC50 value of ~ µm. Coimmunoprecipitation of Ku70 after TLK286 treatment of purified DNA-PK and C70 cells showed a drug-induced destabilization of the protein-protein interaction between the catalytic subunit and the Ku heterodimer. Overall, these results implicate inhibition of DNA-PK as a component of TLK286 cytotoxicity and provide a rationale for its use in the clinical management of cisplatin-resistant ovarian cancer.
Introduction
Cisplatin is one of the most important drugs in the front line treatment of surgically resected ovarian cancer. The antitumor activity of cisplatin is attributed to the formation of a variety of DNA adducts, including monoadducts, and intrastrand and interstrand cross-links (1). A major obstacle in the successful treatment of the disease is the development of drug resistance to the chemotherapeutic agent (2). Identifying mechanisms leading to refractory disease can provide insight into the development of drugs that may subvert collateral resistance with platinum-based therapy. Our laboratory has shown previously that enhanced expression of a serine/threonine kinase, DNA-PK,4 is associated with resistance to cisplatin and Adriamycin in various ovarian cancer cell lines (3). Other investigators report a similar correlation of DNA-PK expression and cisplatin resistance in a murine leukemic cell line (4). Tumor cell lines resistant to other DNA damaging agents, such as nitrogen mustards (5) and bleomycin (6), have also been shown to have enhanced DNA-PK expression.
DNA-PK, a member of the phosphatidylinositol 3′-kinase family, plays a role in V(D)J recombination and DNA double-strand break repair (7, 8), and is composed of a large catalytic subunit (Mr 460,000) and a heterodimeric autoantigen, Ku 70 and Ku 80, which participates in the enzyme complex and plays a role in DNA damage recognition. DNA-PK is potentially a key enzyme in determining the response to DNA-damaging agents through the capacity to repair damaged DNA.
TLK286, is a novel prodrug activated by GSTπ (9). An advantageous therapeutic index for TLK286 was predicted on the requirement for activation through a β-elimination reaction that cleaves the drug into a phosphorodiamidate (the eventual alkylating moiety) and a glutathione analogue (Fig. 1). Drug design ensured that this reaction was preferentially catalyzed by the tyrosine 7 in the active site of GSTπ (9). Because numerous solid tumors and a number of drug-resistant cell lines express high levels of GSTπ (10), selectivity should be enhanced. The successful completion of Phase I studies with TLK286 has provided a platform for the subsequent initiation of Phase II trials, which include ovarian cancer patients (11). Primary ovarian cancer biopsies (12) and cell lines established from drug-resistant cancer patients (13) have been shown to express high levels of GSTπ. In addition, a number of clinical trials in ovarian cancer have analyzed, in a retrospective fashion, patient response and GSTπ expression. Of these, 8 trials have reported a significant inverse correlation between high levels of GSTπ expression and disease response to chemotherapy. Seven other trials found no correlation. The present study was designed to establish the efficacy of TLK286 and potential mechanism of action in platinum-resistant ovarian cancer cell lines to provide a rationale platform for the implementation of Phase II trials in platinum-resistant patients.
Fig. 1.
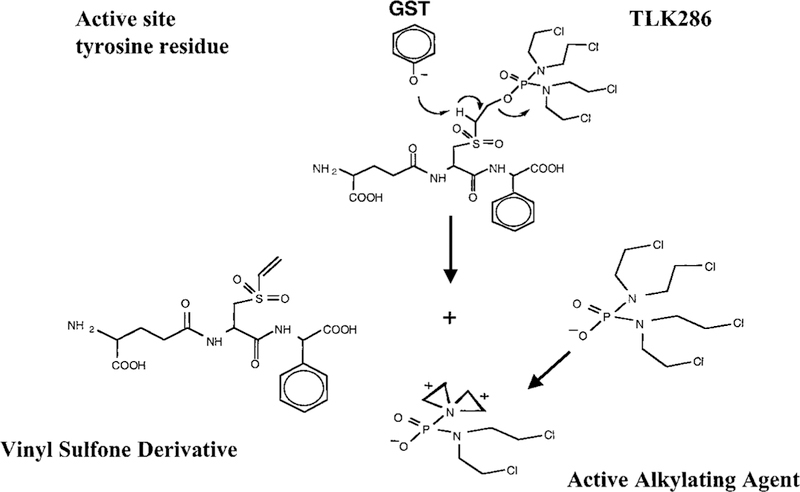
Structure and activation of TLK286.
Materials and Methods
Cell Lines and Preparation of Cell Lysates.
Human ovarian cells (C70 and C200) made resistant to cisplatin from A2780 cells were obtained from the laboratory of Johnson et al. (14). Cells were cultured in RPMI 1640 with 10% fetal bovine serum and 0.3 units/ml insulin (porcine). The cells were harvested at 90% confluency and centrifuged at 400 × g for 10 min. The cell pellet was washed twice in ice-cold PBS before suspension for 30 min at 4°C in lysis buffer [10 mm Tris HCl (pH 7.4) and 250 mm sucrose] containing protease inhibitors (0.2 mm phenylmethylsulfonyl fluoride, 0.2 mm leupeptin, 0.3 mm aprotinin, 0.5 mm EDTA, and 0.1 mm pepstatin). After incubation, samples were sonicated and centrifuged at 55,000 × g at 4°C for 25 min. The resulting supernatants were used in subsequent experiments.
Reverse Transcription-PCR Analysis.
Various drug-resistant cell lines generated by stepwise selection were obtained from this laboratory and others (3, 14, 15). Total RNA was isolated according to methods described previously (16). The reverse transcriptase reaction mixture contained 50 mm Tris-HCl (pH 8.3), 75 mm KCl, 3 mm MgCl2, 200 µm of each deoxynucleotide triphosphate, 100 units Super-Script II RNase H— Reverse Transcriptase (Life Technologies, Inc., Bethesda, MD), 1 µg RNA, and 2.5 µm random primers (Pharmacia, Piscataway, NJ). The reaction mixture was performed for 10 min at 25°C, 30 min at 42°C, 5 min at 95°C, and 5 min at 4°C. The PCR reaction mixture contained 20 mm Tris-HCl, 50 mm KCl, 1.5 mm MgCl2,1 µm of each primer, 2.5 units Taq DNA polymerase (Life Technologies, Inc.), and 2 µl of reverse transcription product. The primers used for PCR were selected from the published cDNA sequence of the catalytic subunit of DNA-PK near the NH2 terminus (17). The sense primer was 5′AGG GAA GAA GAG TCT CTG GTG G 3′. The antisense primer was 5′ ATT AGG GGA TCT GTT GCC TGG C 3′. The reaction was carried out with 30 cycles of 94°C for 30 s, 63°C for 30 s, and 72°C for 45 s. The amplification was followed by a 5-min incubation at 72°C. To normalize loading conditions equal amounts of RNA were used to synthesize cDNA, and 18S-RNA was used as a loading control. NIH imaging software, version 1.57, was used to quantify the intensity of the PCR products on an agarose gel.
SDS-PAGE and Immunoblot Analysis.
Protein concentrations of cell lysates were measured using the Bio-Rad protein Assay (Bio-Rad, Hercules, CA). Seventy µg of protein were loaded onto a 7.5% acrylamide mini-gel for analysis of DNA-PK. Fifty µg of protein were loaded onto a 10% acrylamide mini-gel for analysis of Ku70 and Ku80. After SDS-PAGE, proteins were transferred to nitrocellulose membranes and probed with monoclonal antibodies against DNA-PKcs, Ku70, or Ku80 (Kamiya, Seattle, WA). Proteins were visualized using an enhanced chemiluminescence detection kit (Amersham, Buckinghamshire, England). To assess equivalent loading, blots were probed with a monoclonal antiactin antibody (Calbiochem, La Jolla, CA).
DNA-PK Enzyme Activity Assay.
DNA-PK activity was measured using the Signa TECT DNA-PK Assay System (Promega, Madison, WI). Samples contained 0.1 mm ATP with 0.5 µCi of [γ-32P]ATP (30 Ci/mmol) and TLK286 (Telik, San Francisco, CA) at a final concentration of 10–104 nm, and were started by the addition of commercially available DNA-PK (Promega). Reactions were at 30°C for 10 min. Aliquots of each sample were spotted onto the SAM2 Biotin Capture membrane. After washing, incorporated radioactivity was counted. Activity assays were repeated three times with triplicate samples processed in each experiment.
Cell Survival Assay.
Drug sensitivities were determined by the Cell Titer 96 aqueous cell proliferation assay using MTS (Promega). Cells were plated onto 96-well plates in culture medium (3000 cells/well). After attachment, cells were treated with various concentrations of either cisplatin (Sigma, St. Louis, MO) or TLK286 for 72 h. After treatment, cell survival was measured (3).
HPLC.
TLK286 activation via GSTπ was analyzed using a Beckman HPLC System with 126 pump and 166UV Detector with the detector focused at 220 nm. A Rainin Microsorb MV # R0086200D5 column (4.6 cm × 150 mm identifier sequence), 5-µm particle size, was used. The mobile phase (A) was 95% 0.05 m NH4H2P04, 5% Acetonitrile; mobile phase (B) was 70% Acetonitrile, 30% 0.05 m NH4H2P04. The incubation mixtures were analyzed with a 22-min total run time with the following gradient: 100% A for 0.5 min, then 85% B for 7 min, 100% A for 1 min and equilibrated for 4.5 min in 100% A. The mobile phase was degassed in an ultrasonic bath. The flow rate was 1.5 ml/min.
Immunoprecipitation Assay.
Cells were grown to 90% confluency, treated with 150 µm TLK286 for 1 h, harvested by trypsin treatment and centrifugation at 1,200 × g for 10 min, and washed twice with ice cold PBS. The cell pellet was lysed in 100 µl of radioimmunoprecipitation assay buffer with freshly added protease inhibitors (2 mm phenylmethylsulfonyl fluoride, 10 µg/ml leupeptin, 1 µg/ml aprotinin, and 0.7 µg/ml pepstatin) and sonicated six times for 15 s. After ultracentrifugation (55,000 × g) for 30 min, protein concentrations were determined using the Bio-Rad assay. Purified DNA-PK was incubated with 150 nm TLK286 for 30 min to 1 h before immunoprecipitation. Immunoprecipitation experiments were performed overnight at 4°C with 2 mg of protein incubated with 2 µg of anti-Ku70 conjugated to agarose beads (Santa Cruz Biotechnology Inc., Santa Cruz, CA) in a total volume of 500 µl. After washing, beads were resuspended in sample buffer, heated at 100°C for 5 min, and spun at full speed in a microcentrifuge. The supernatant was resolved on SDS-7.5% PAGE gels and transferred to a nitrocellulose membrane. Immunoblotting was performed using DNA-PKcs, Ku70, and Ku80 antibodies. Bands were visualized using enhanced chemiluminescence (Amersham).
Results
Because many drug-resistant cell lines have been shown to overexpress GSTπ, an immunoblot analysis of the wild-type human ovarian carcinoma cell line A2780 and two derived cisplatin-resistant populations, C70 and C200, was undertaken. Equivalent GSTπ protein levels were detected in each of the cell lines (Fig. 2), indicating that selection by chronic cisplatin exposure did not increase GSTπ expression in this case. Thus, activation of TLK286 via GSTπ should be comparable in each of the lines.
Fig. 2.
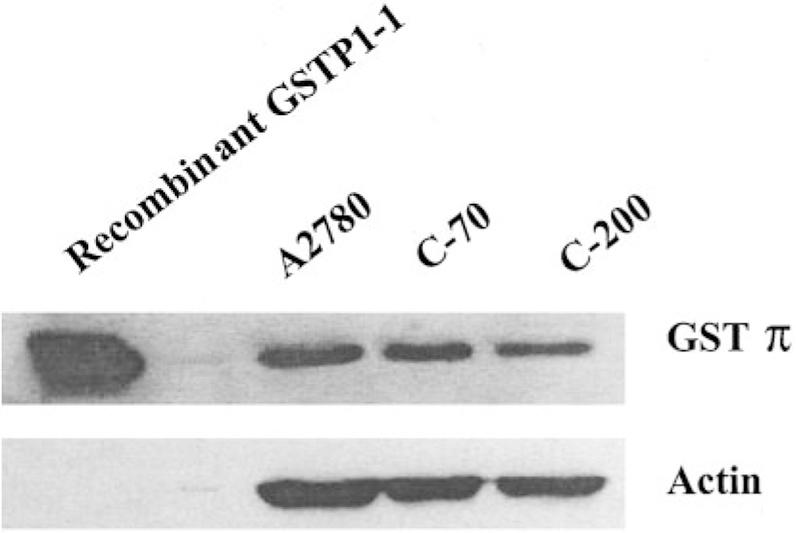
GSTπ expression does not differ in wild-type and cisplatin-resistant cell lines. GSTπ levels were determined by immunoblot analysis using 70 µg of protein from whole cell lysates. The blot is a representative example of three experiments.
The cisplatin response profile of A2780, C70, and C200 is shown in Fig. 3A. The approximate IC50 value for the wild-type cell line was 10 µm. The equivalent IC50 values for C200 and C70 were ~200 µm and 250 µm, respectively. Thus, both of these cell lines were ~20-fold resistant to cisplatin compared with the wild-type (14). The cisplatin-resistant cell lines are cross-resistant to melphalan, a nitrogen mustard with some structural similarity to the active moiety of TLK286. The approximate IC50 value for A2780 cells was 50 µm versus 100 µm for C70 cells (data not shown). In contrast, Fig. 3B shows that TLK286 was equitoxic toward A2780, C70, and C200. The approximate IC50 values for the cell lines were 100–120 µm suggesting that collateral resistance between TLK286 and cisplatin does not occur.
Fig. 3.
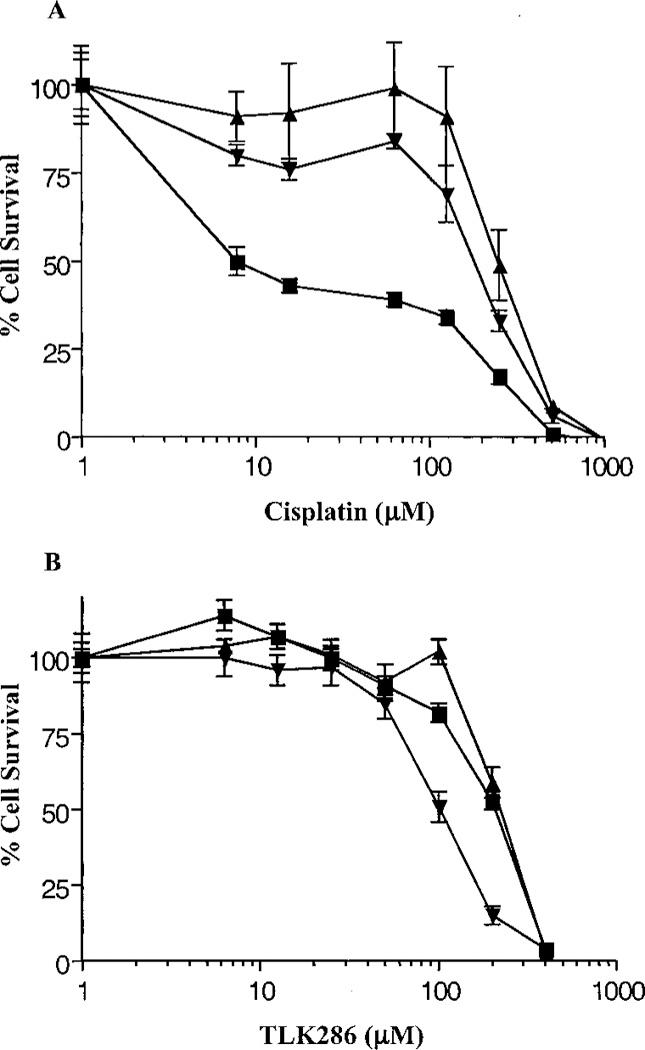
Cytotoxic effect of cisplatin (A) and TLK286 (B) on platinum-sensitive and resistant ovarian cancer cell lines. A2780 (■), C70 (▲), and C200 (●) were seeded at 5 × 103 cells/100 µl into wells of 96-well plates. After incubation for 72 h in the presence of indicated concentrations of cisplatin or TLK286, cell viability was determined using MTS reagents. Data are the mean of three experiments; bars, ±SD.
Prior studies in our laboratory have shown that an increased DNA repair capacity because of elevated levels of DNA-PK contributes to Adriamycin resistance (3). In those studies, a human leukemia cell line (HL60) was selected for resistance via long-term exposure to Adriamycin (HL60-ADR). Using that model system, we analyzed the cytotoxicity of cisplatin, TLK286, and Adriamycin. The approximate IC50 value for Adriamycin in the wild-type cell line was 0.10 µm versus 3 µm in HL60-ADR (Fig. 4A). Collateral resistance was observed with cisplatin, where the IC50 value in the wild-type cell line was 2 µm verses 4 µm in HL60-ADR (Fig. 4B). In contrast, TLK286 was equitoxic with an approximate IC50 value of 30 µm in both cell lines (Fig. 4C). These data suggest that the determinants of resistance to Adriamycin are not the same as those for TLK286.
Fig. 4.
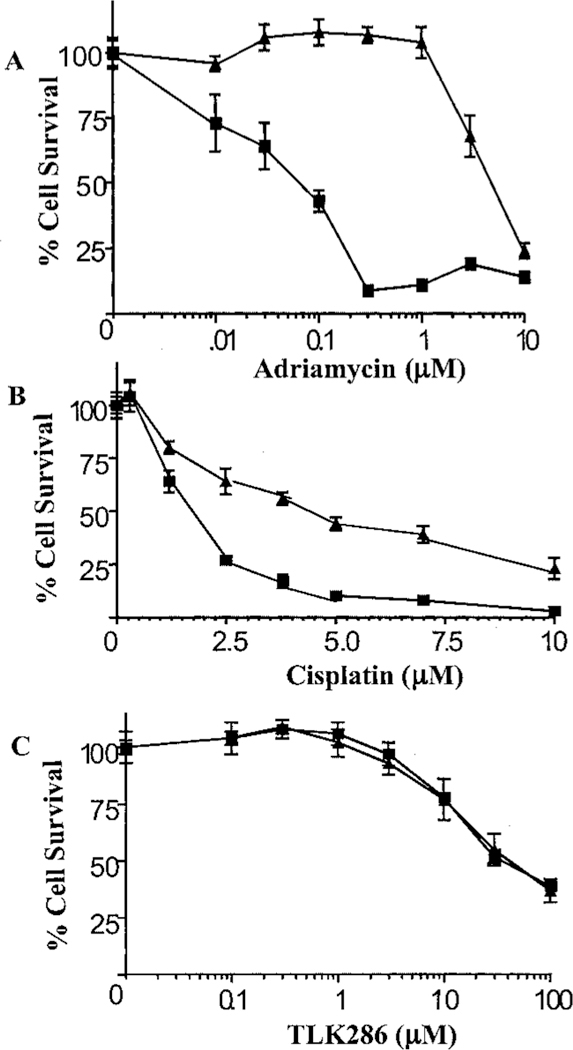
Cytotoxic effect of Adriamycin (A), cisplatin (B), and TLK286 (C) on Adriamycin-sensitive (■) and resistant human leukemia cell lines (▲). Five × 103 cells/100 µl were dispensed into 96-well plates. After incubation for 72 h in the presence of indicated concentrations of Adriamycin, cisplatin, or TLK286, cell viability was determined using MTS reagents. Data are the mean of three experiments; bars, ±SD.
An analysis of the expression of the DNA-PK holoenzyme complex is shown in Table 1 (transcript levels) and Fig. 5 (protein expression). As measured by reverse transcription-PCR, the two cisplatin-resistant ovarian cell lines C30 and C70 had elevated levels of DNA-PKcs (Table 1). Transcript levels for both Ku70 and Ku80 were unchanged (data not shown). However, at the protein level, increased expression of DNA-PKcs together with Ku70 and Ku80 was detected by immunoblot (Fig. 5). In a cell line permitted to revert to cisplatin sensitivity (A2870/revertant), this increased expression disappeared (Table 1). Analysis of a panel of resistant cell lines showed that selecting drugs that can cause DNA damage (cisplatin, 4-OH cyclophosphamide, and Adriamycin) and not antimicrotubule drugs (estramustine, Taxol, and colchicine) caused increased expression of DNA-PKcs (Table 1). Thus, DNA-PK overexpression correlates with resistance to DNA-damaging agents in tumor cell lines.
Table 1.
Comparative expression of DNA-PK transcript levels in drug-resistant cancer cell lines
| Cell line | Resistant to | DNA-PKcs expression ratio (resistant:wild-type) |
|---|---|---|
| A2780/C30 | Cisplatin | 3.0 |
| A2780/C70 | Cisplatin | 5.0 |
| A2780/revertant | Reverted from cisplatin | 1.0 |
| MCF7/CDDP | Cisplatin | 2.0 |
| HL60/ADR | Adriamycin | 15.0 |
| MCF7/HC | 4-OH cyclophosphamide | 2.4 |
| MCF7/ADR | Adriamycin | 3.0 |
| SKOV3/EM | Estramustine | 1.0 |
| SKOV3/TAX | Taxol | 1.0 |
| KB3–1 | Colchicine | 1.0 |
Fig. 5.
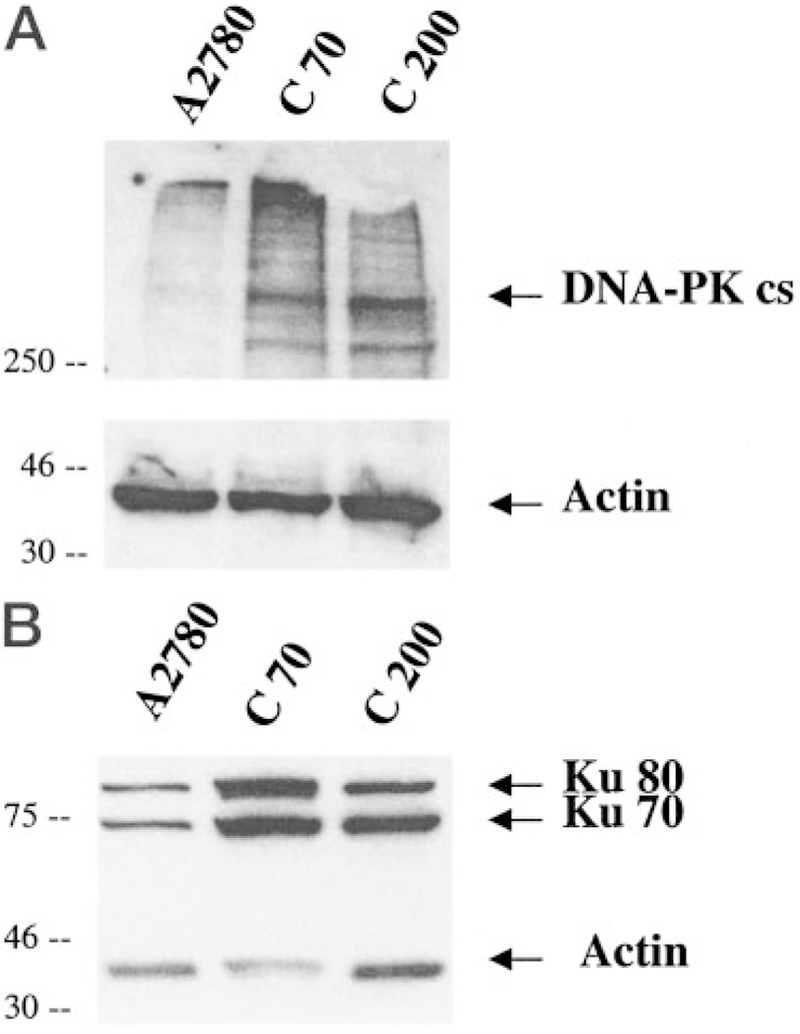
DNA-PKcs expression is elevated in cisplatin-resistant cell lines C70 and C200 compared with wild-type (A2780). DNA-PKcs (A), Ku 70 and Ku 80 (B) expression levels were determined by immunoblot analysis using 70 µg of protein from whole cell lysates. Densitometry was performed with NIH image analysis using actin as loading control. The blot is a representative example of three experiments.
We have shown previously that glutathione conjugates inhibit DNA-PK activity by binding to the catalytic subunit and disrupting the protein-protein interactions of DNA-PKcs with Ku70 and Ku80 (18). Initially, to ascertain whether TLK286 had an impact on DNA-PK, an in vitro kinase assay was carried out using purified enzyme. As shown in Fig. 6, preincubation of DNA-PK with TLK286 effectively inhibited the kinase activity in a dose-dependent fashion with an IC50 value of ~1 µm; conversely, preincubation with cisplatin did not significantly alter the kinase activity.
Fig. 6.
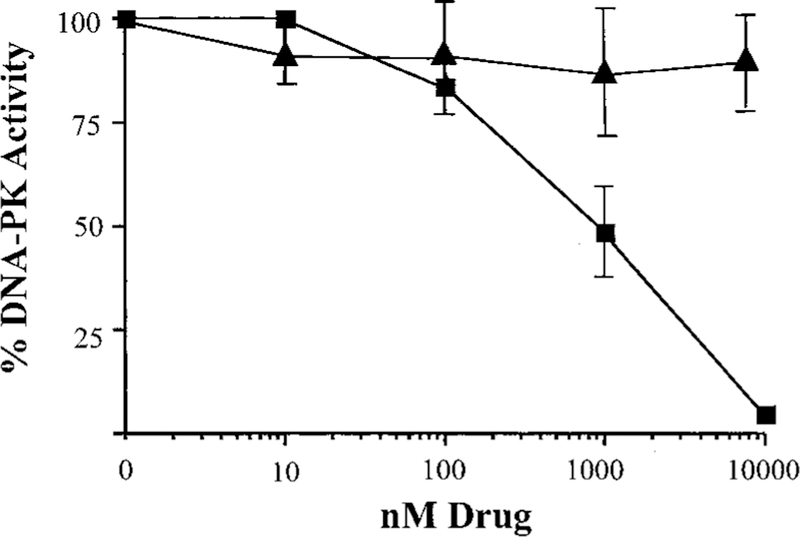
Inhibition of the kinase assay of DNA-PK by TLK286 (■) and cisplatin (▲) under in vitro conditions. All data points are calculated by subtracting background values (assays without DNA). The data are the mean of three experiments; bars, ±SD.
TLK286 was preincubated with purified GSTπ to determine whether metabolism/activation of the drug alters the inhibition of DNA-PK. HPLC analysis was carried out to confirm activation of TLK286 via GSTπ in vitro. The HPLC profile of TLK286 alone showed a single peak with a TR of 6.6 min, indicating the parent compound. However, preincubation of TLK286 with purified GSTπ for 1 h showed the formation of a second peak (TR = 4.3 min). These peaks are consistent with TLK286 (TR = 6.6 min) and the vinyl sulfone derivative (TR = 4.3 min).3 Fig. 7 represents the formation of the vinyl sulfone derivative over time and confirms the activation of TLK286 in vitro. Preincubation of TLK286 with purified GSTπ for 1 h did not alter the pattern of inhibition of DNA-PK activity (data not shown), suggesting that metabolism/activation of the drug is not a prerequisite for this effect.
Fig. 7.
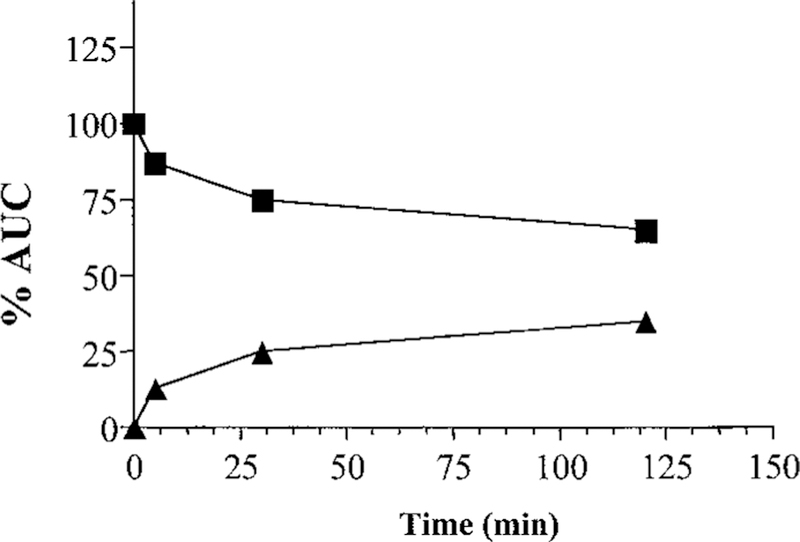
In vitro activation of TLK286 via purified GSTπ. After a 0–2 h incubation, formation of the vinyl sulfone derivative (▲) compared with unchanged TLK286 (■) is represented by the percentage area under the curve (AUC).
Immunoprecipitation of Ku70 was carried out to determine whether the inhibitory mechanism of TLK286 could be attributed to a drug-induced dissociation of the DNA-PKcs from the Ku heterodimeric complex. Purified DNA-PK containing the catalytic subunit and the Ku heterodimer was immunoprecipitated using a monoclonal antibody to Ku70 (Fig. 8A). In the absence of drug, the catalytic subunit coimmunoprecipitated with the Ku70 and Ku80 subunits (Fig. 8A, Lanes 1–2). DNA-PK was preincubated with 150 nm TLK286 for 0.5 and 1 h (Fig. 8A, Lanes 3– 4). After treatment for 1 h, the catalytic subunit did not coprecipitate with the Ku heterodimer. A similar disruption of the complex was seen after incubation for1h with1 mm GSSG (Fig. 8A, Lane 5). Fig. 8B confirms that 150 µm cisplatin preincubated with purified DNA-PK does not alter coprecipitation of the catalytic subunit with the heterodimer (Fig. 8B, Lane 2). To test the effects of TLK286 in vivo, this experimental approach was repeated with lysates from C70 cells treated with 150 µm TLK286 for 1 h (Fig. 8C). The data were consistent with those using purified protein. Fig. 8C, Lane 1, confirms the coimmunoprecipitation of the trimeric complex by the Ku70 antibody. Pretreatment of cells with TLK286 (150 µm) for 1 h resulted in a disassociation of the catalytic subunit (Fig. 8C, Lane 2). Dissociation was also apparent after treatment with 1 mm GSSG for 1 h (Fig. 8C, Lane 3). Fig. 8C, Lane 4, provided negative control conditions in the absence of protein. These data indicate that TLK286 destabilizes the protein-protein interactions of the catalytic subunit with the heterodimer in vivo and in vitro.
Fig. 8.
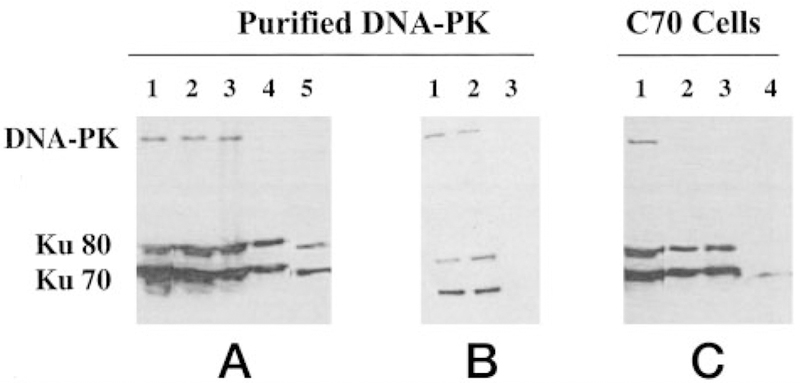
Immunoprecipitation analysis showed that TLK286 treatment destabilizes the protein-protein interaction of the DNA-PK holoenzyme. Protein or cell lysate was first immunoprecipitated with anti-Ku70. The immunoprecipitate was separated on gel and transferred to a nitrocellulose membrane. The blots were used for Western analysis for DNA-PKcs and Ku70/Ku80. Purified DNA-PK (A): purified DNA-PK (Lane 1); immunoprecipitation of untreated DNA-PK (Lane 2); preincubation with 150 nm TLK286 for 30 min (Lane 3) and 1 h (Lane 4); preincubation with 1 mm GSSG for 1 h (Lane 5). Purified DNA-PK (B): untreated DNA-PK (Lane 1); preincubation with 150 µm cisplatin for 1 h (Lane 2); negative control for anti-Ku70 in the absence of purified DNA-PK (Lane 3). Immunoprecipitation of lysates from C70 cells treated with TLK286 (C): untreated cell lysate (Lane 1); pretreatment with 150 µm TLK286 for 1 h (Lane 2); pretreatment with 1 mm GSSG for 1 h (Lane 3); negative control for anti-Ku70 in the absence of cell lysate (Lane 4).
Discussion
Although partial success has been achieved in the chemotherapeutic treatment of ovarian cancer, many patients eventually develop resistance in platinum-based chemotherapy. Identifying the cellular adaptations leading to resistance allows a more rational approach to the design of novel drug therapies. Our earlier data showed that overexpression of DNA-PK confers resistance to Adriamycin (3). The present results support a similar role for DNA-PK in cisplatin resistance. The overexpression of the DNA-PK complex (DNA-PKcs, Ku70, and Ku80) is consistent with the fact that cisplatin induces DNA damage. The cisplatin-resistant cells have been selected through chronic, long-term exposure, and a reasonable conclusion would be that at least one adaptation to drug exposure was to enhance DNA repair capacities. This result extends the link between DNA-PK and therapeutic resistance beyond the published reports for γ-irradiation, Adriamycin, nitrogen mustards, and bleomycin (3, 5, 6).
The overexpression of GSTπ in drug-resistant cell lines has been reported for a broad range of drugs used in cancer treatment. Whereas conclusions derived have assumed frequently that detoxification of cytotoxic drug species is causal to the resistant phenotype, in fact, many of the selecting drugs are neither subject to conjugation with glutathione nor substrates for GSTπ (10). More recently, our studies have provided an alternative insight into the reason for high GSTπ expression in solid tumors and drug-resistant cells, where a role for this protein in the regulation of cell proliferation has been described (19, 20). Whereas the mechanism by which GSTπ influences regulation primarily depends on its ligand-binding properties with critical cellular kinases (e.g., JNK1), the protein-protein interactions are distal to the catalytic domains of GSTπ with the consequence that catalysis remains intact (21). Because activation of TLK286 requires GSTπ catalyzed cleavage (9), high intratumoral levels of GSTπ provide an environment for enhanced therapeutic index. Metabolic activation results in two molecular species, one containing alkylating aziridinium ions and another a glutathione-conjugate mimetic moiety (Fig. 1). Increased expression of DNA-PK correlated with an increased DNA repair capacity in Adriamycin-resistant cells (3) We propose that the absence of collateral resistance to TLK286 in cisplatin and Adriamycin-resistant cell lines may be because of the ability of TLK286 to inhibit DNA-PK. In this regard, elevated expression of the DNA-PK complex in cisplatin-resistant cells does not lead to collateral resistance to TLK286. In addition, HL60 cells made resistant to TLK286 (22) do not overexpress DNA-PK. This implies either that the type and/or extent of DNA damage caused by TLK286 is distinct from these other drugs. It is also conceivable that the concentrations of TLK286 are saturating for DNA-PK activity in both wild-type and resistant cells, diminishing any differences in response. We have reported previously that certain glutathione-conjugates and glutathione mimetics can inhibit DNA-PK activity by interfering with the formation of, or causing a disassociation of, the holoenzyme complex (18). Because preincubation of TLK286 with GSTπ caused no apparent change in the ability of the parent drug to inhibit DNA-PK, the presence of the derivatized reduced glutathione tripeptide backbone seems to be a critical determinant to the inhibitory properties. This observation is consistent with the effects of either GSSG or APA-SG (18), which caused a similar pattern of inhibition, albeit at concentrations much higher than those for TLK286.
By using a photoaffinity-labeled APA-SG, we have shown previously direct binding of the glutathione mimetic to the catalytic subunit of DNA-PK. The specificity of this binding was confirmed by competition assays, either directly with unlabeled APA-SG or with other glutathione conjugates (18) Earlier studies by Henkels and Turchi (23) showed that cisplatin decreased the kinase activity of DNA-PK by binding to and altering the DNA template, a cofactor required for the activity of the enzyme. In the present study, preincubation of cisplatin with the DNA-PK assay components did produce a slight decrease in kinase activity, although the drug concentrations required were high, and no clear dose dependency was apparent (Fig. 6). Cisplatin treatment did not alter protein-protein interactions of the holoenzyme (Fig. 8). Thus, our data suggest a mechanism of inhibition for TLK286 that is distinct from cisplatin. Specifically, the lack of coimmunoprecipitation after TLK286 results from the disruption of the protein-protein interactions of DNA-PKcs with the Ku heterodimer, with a concomitant decrease in kinase activity. By analogy with cisplatin, it could be argued that the alkylating species of TLK286 inhibit DNA-PK by causing direct DNA damage. However, using either COMET assays on cells treated with TLK286 or an in vitro plasmid strand breakage technique, significant levels of TLK286-induced DNA strand breaks were not detected (data not shown). Thus, both qualitative and quantitative differences in drug-induced DNA damage are apparent. This, together with the dose response of DNA-PK inhibition by TLK286 at biologically meaningful drug concentrations would support a mechanism of inhibition through drug-protein interactions causing interference with the ligand interactions of DNA-PKcs with the Ku heterodimer.
In the recently completed Phase I study of TLK286, the drug was well tolerated, with fatigue and nausea being primary drug-associated toxicities. Myelotoxicity was not reported, and evidence of partial response and disease stabilization was apparent (11). The absence of bone marrow toxicity indicates that TLK286 does not share the dose-limiting tissue characteristics of other clinically used nitrogen mustards and gives credence to the low DNA damaging properties of the tetrakis chloroethylating moiety. As such, the importance of DNA-PK inhibition to the overall therapeutic properties of the drug seems reasonable.
Presently, the most effective treatment for advanced stage ovarian cancer after cytoreductive surgery is combination chemotherapy with cisplatin-containing regimens (2). Unfortunately, the majority of patients eventually relapse and become refractory to additional treatment (24). The efficacy, lack of collateral resistance, and identification of DNA-PK as a plausible drug target provide a rational preclinical platform for implementation of Phase II trials incorporating TLK286.
Acknowledgments
We thank Telik Inc., San Francisco, CA, for the provision of TLK286.
Supported in part by NIH Grants CA06927, CA83638 (to Robert F. Ozols), and CA75266 and CA85660 to (K. D. T.), and by an appropriation from the Commonwealth of Pennsylvania. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
The abbreviations used are:
- DNA-PK
DNA-dependent protein kinase
- TLK286
γ-glutamyl-α-amino-β [2-ethyl-N,N,N′,N′-tetrakis (2-chloroethyl) phosphorodiamidate]-sulfonyl-propionyl-(R)-(—) phenylglycine
- GST
glutathione S-transferase
- MTS
3-(4,5-dimethylthiazol-2,3-carboxy-methoxylphenyl)-2-(4-sulfohenyl)-2H-tetrazolium inner salt
- HPLC
high pressure liquid chromatography
- TR
retention time
- GSSG
oxidized glutathione
- APA-SG
azidophenacyl glutathione
Footnotes
Telik, Inc., unpublished data.
References
- 1.Eastman A Separation and characterization of products resulting from the reaction of cis-diamminedichloroplatinum (II) with deoxyribonucleosides. Biochemistry, 21: 6732–6736, 1982. [DOI] [PubMed] [Google Scholar]
- 2.Ozols RF Ovarian cancer. Part II: Treatment. Curr. Probl. Cancer, 16: 61–126, 1992. [PubMed] [Google Scholar]
- 3.Shen H, Schultz M, Kruh GD, and Tew KD Increased expression of DNA-dependent protein kinase confers resistance to adriamycin. Biochim. Biophys. Acta, 1381: 131–138, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Frit P, Bergmann E, and Egly JM Transcription factor IIH: a key player in the cellular response to DNA damage. Biochimie, 81: 27–38, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Muller C, Christodoulopoulos G, Salles B, and Panasci L DNA-Dependent protein kinase activity correlates with clinical and in vitro sensitivity of chronic lymphocytic leukemia lymphocytes to nitrogen mustards. Blood, 92: 2213–2219, 1998. [PubMed] [Google Scholar]
- 6.Kim SH, Kim D, Han JS, Jeong CS, Chung BS, Kang CD, and Li GC Ku autoantigen affects the susceptibility to anticancer drugs. Cancer Res, 59: 4012–4017, 1999. [PubMed] [Google Scholar]
- 7.Jackson SP, and Jeggo PA DNA double-strand break repair and V(D)J recombination: involvement of DNA-PK. Trends Biochem. Sci, 20: 412–415, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Weaver DT What to do at an end: DNA double-strand-break repair. Trends Genet, 11: 388–392, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Lyttle MH, Satyam A, Hocker MD, Bauer KE, Caldwell CG, Hui HC, Morgan AS, Mergia A, and Kauvar LM Glutathione-S-transferase activates novel alkylating agents. J. Med. Chem, 37: 1501–1507, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Tew KD Glutathione-associated enzymes in anticancer drug resistance. Cancer Res, 54: 4313–4320, 1994. [PubMed] [Google Scholar]
- 11.Rosen L, Kadib L, Laxa B, Brown J, Kilfoil G, Gomez R, Wick M, C., V., and Brown B TLK286: Phase I dose-escalation trial in patients with advanced malignancies. In: 11th NCI EORTC AACR Symposium on New Drugs in Cancer Therapy, Amsterdam, 2000. [Google Scholar]
- 12.Schisselbauer JC, Hogan WM, Buetow KH, and Tew KD Heterogeneity of glutathione S-transferase enzyme and gene expression in ovarian carcinoma. Pharmacogenetics, 2: 63–72, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Lewis AD, Hayes JD, and Wolf CR Glutathione and glutathione-dependent enzymes in ovarian adenocarcinoma cell lines derived from a patient before and after the onset of drug resistance: intrinsic differences and cell cycle effects. Carcinogenesis (Lond.), 9: 1283–1287, 1988. [DOI] [PubMed] [Google Scholar]
- 14.Johnson SW, Swiggard PA, Handel LM, Brennan JM, Godwin AK, Ozols RF, and Hamilton TC Relationship between platinum-DNA adduct formation and removal and cisplatin cytotoxicity in cisplatin-sensitive and -resistant human ovarian cancer cells. Cancer Res, 54: 5911–5916, 1994. [PubMed] [Google Scholar]
- 15.McGrath T, and Center MS Mechanisms of multidrug resistance in HL60 cells: evidence that a surface membrane protein distinct from P-glycoprotein contributes to reduced cellular accumulation of drug. Cancer Res, 48: 3959–3963, 1988. [PubMed] [Google Scholar]
- 16.Chomczynski P, and Sacchi N Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem 162: 156–159, 1987. [DOI] [PubMed] [Google Scholar]
- 17.Hartley KO, Gell D, Smith GC, Zhang H, Divecha N, Connelly MA, Admon A, Lees-Miller SP, Anderson CW, and Jackson SP DNA-dependent protein kinase catalytic subunit: a relative of phosphatidylinositol 3-kinase and the ataxia telangiectasia gene product. Cell 82: 849–856, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Shen H, Schultz MP, and Tew KD Glutathione conjugate interactions with DNA-dependent protein kinase. J. Pharmacol. Exp. Ther, 290: 1101–1106, 1999. [PubMed] [Google Scholar]
- 19.Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR, Davis RJ, and Ronai Z Regulation of JNK signaling by GSTp. EMBO J, 18: 1321–1334, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruscoe JE, Rosario LA, Wang T, Gate L, Arifoglu P, Wolf CR, Henderson CJ, Ronai Z, and Tew KD Pharmacologic or genetic manipulation of glutathione S-transferase π (GSTpi) influences cell proliferation pathways. J. Pharmacol. Exp Ther, 298: 339–345, 2001. [PubMed] [Google Scholar]
- 21.Wang T, Arifoglu P, Ronai Z, and Tew KD Glutathione S-transferase π (GSTπ) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J. Biol. Chem, 276: 20999–21003, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Rosario LA, O’Brien ML, Henderson CJ, Wolf CR, and Tew KD Cellular response to a glutathione S-transferase π activated prodrug. Mol. Pharmacol, 58: 167–174, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Henkels KM, and Turchi JJ Induction of apoptosis in cisplatin-sensitive and -resistant human ovarian cancer cell lines. Cancer Res 57: 4488–4492, 1997. [PubMed] [Google Scholar]
- 24.Perez RP, Hamilton TC, Ozols RF, and Young RC Mechanisms and modulation of resistance to chemotherapy in ovarian cancer. Cancer (Phila.), 71: 1571–1580, 1993. [DOI] [PubMed] [Google Scholar]


