Abstract
Solitary plasmacytomas are uncommon plasma cell disorders, which may present as a single bone lesion (P-bone) or extramedullary plasmacytoma (P-EM). There is a paucity of large studies analyzing prognostic factors and outcomes of plasmacytomas. While the treatment of choice is radiation therapy (RT), there is a lack of data evaluating optimal RT dose. In this study, we sought to answer these questions by utilizing the National Cancer Data Base plasmacytoma data from 2000–2011. A total of 5,056 patients were included in the study (median age 62 years; range 52–72). To obtain a pure plasmacytoma cohort, potential multiple myeloma patients were excluded from the study (bone marrow involvement, systemic chemotherapy use). P-bone constituted 70% of the patients. The median overall survival (OS) of P-EM was significantly longer than P-bone (132 vs. 85 months); and for soft/connective tissue was worse than remainder of P-EM (82 vs. 148 months). On multivariable analysis, factors associated with worse OS included older age (≥65), presence of P-Bone and treatment with a radiation dose <40 Gy.
Introduction
Plasmacytomas are an uncommon subtype of plasma cell disorders that can present as a solitary bone lesion (P-bone), an extramedullary plasmacytoma (P-EM) or multiple bone and/or extramedullary lesions as in multiple myeloma (MM). The two large studies of plasmacytomas to date consisting of approximately 1,500 patients each from the SEER database demonstrated that P-bone and P-EM constitute approximately 3% and 2% of all plasma cell malignancies, respectively.1, 2 In terms of disease outcomes, the existing studies demonstrate a variable five-year overall survival (OS) rate of 40 to 85%.2–7 The national guidelines recommend treatment of solitary plasmacytomas with radiation therapy (RT) with or without surgery.8 However, the optimal radiation dose has been a subject of debate. In this study, our objective was to describe the patterns of clinical presentation and treatments utilized in a large population in order to then determine their impact on patient survival outcomes using the National Cancer Data Base (NCDB).
Materials and Methods
This is a retrospective study of patients with a plasmacytoma diagnosed from 2000–2011 using the NCDB participant user file. The NCDB is a joint program of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society, which began in 1989. It is a nationwide oncology outcomes database for > 1,500 CoC-accredited cancer programs across the United States and Puerto Rico that report information to the NCDB, and contains ~70% of all new cancer diagnoses. Cases are limited to those diagnosed and/or treated within CoC-approved hospitals. The sites of plasmacytoma presentation were classified according to the International Classification of Diseases for Oncology version 3 (ICD-0–3) codes: 9731 and 9734 (http://codes.iarc.fr/).
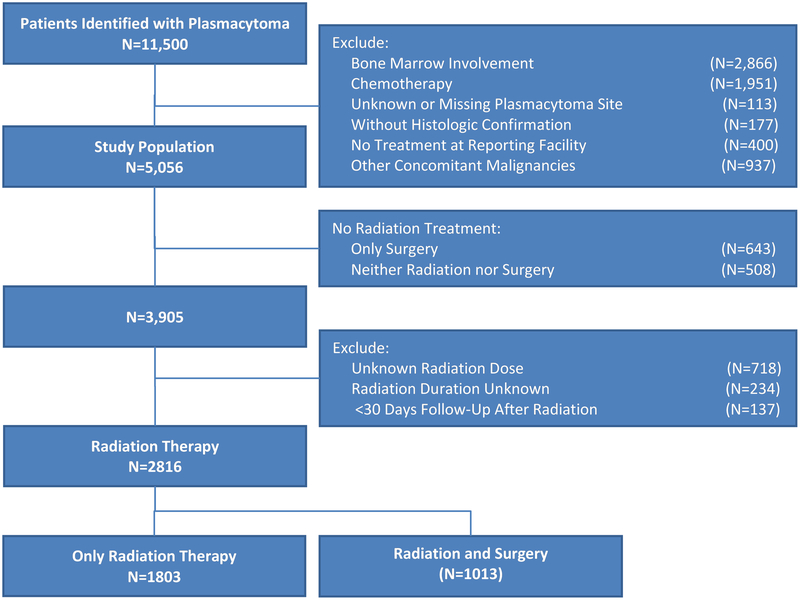
A total of 11,500 patients with plasmacytoma were identified. In order to achieve a pure plasmacytoma cohort, potential MM patients [presence of bone marrow involvement (N=2,866); systemic chemotherapy given, recommended, or chemotherapy status unknown (N=1,951)] were excluded from the analysis. In addition, subjects with unknown or missing plasmacytoma site (N=113), and without histologic confirmation (N=177) were excluded (Figure 1). To ascertain accuracy of follow-up, we excluded patients whose treatment decisions were made at a facility outside of the reporting facility (N=400). In addition, patients who had other concomitant malignancies (N=937) were excluded. For analyses that included dose of radiation as a potential covariate, the following were excluded from OS analysis: subjects who did not receive RT (N=1,151), those with an unknown dose (N=718), and whose RT duration was unknown or those with less than 30 days of follow-up after RT (N=371). The latter were excluded as it was unclear whether they received the intended radiation dose, or if the dose was received due to imminent death.
Figure 1.
CONSORT diagram for cohort selection.
The Kaplan-Meier method was used to estimate median OS and comparisons were made using log-rank test. The OS period was calculated from the diagnosis to the date of last contact or patient death, as reported in the NCDB. A 2-tailed P value of < .05 was considered to indicate statistical significance. Recursive partitioning survival trees were used to assess variable importance. Recursive partitioning is a classification method that can be used in the absence of classic parametric analysis.9 As it is a non-parametric methodology, it is able to perform well in the presence of interactions between predictor variables, especially in complex diseases like plasma cell disorders. Decision trees are created by this method, which attempt to classify the data into sub-populations (partitions) based on the independent variables of interest. The variables of interest for the study cohort were age, sex, site of plasmacytoma, presence of comorbid conditions (Charlson-Deyo score), and dose of RT received. Based on the recursive partitioning results, age, RT dose, and plasmacytoma site were selected for inclusion in the final model. These variables were dichotomized and included in the multivariable survival modeling by Cox regression. Lastly, Cox regression was used to conduct subgroup multivariable survival modeling in each site. For the subgroup(s) in which dose of radiation was found as a significant predictor of survival, a univariate regression tree with dose of radiation being the predictor was analyzed to determine dose cut-point. Statistical analyses were performed using R 3.4.2 (R Foundation, Vienna, Austria).
Results
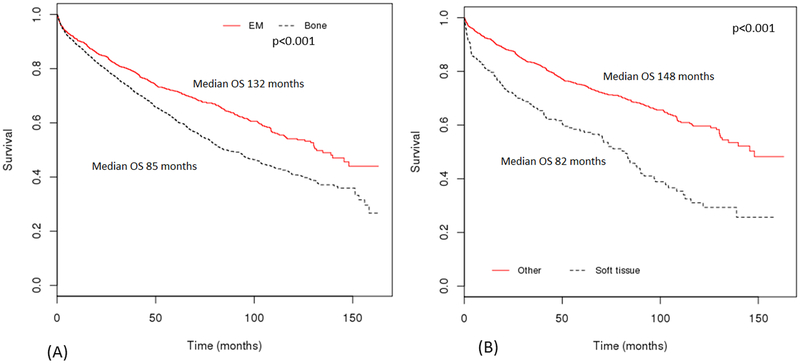
A total of 5,056 patients were included in the study (median age 62 years; Interquartile range 52–72). Of these, 63% were males. P-bone constituted 70% of the patients, with remaining 30% presenting as P-EM. Among P-EM, most common sites of presentation were upper aero-digestive tract (45%) and connective/soft tissue (19%) (Table 1). The median OS of P-EM was significantly longer than P-bone (132 vs. 85 months; Figure 2A). Further analysis among P-EM showed that plasmacytoma of connective/soft tissue had worse OS as compared to other sites (82 vs. 148 months) (Figure 2B). Multivariate analysis of the entire cohort showed age ≥65 years and P-bone location to be associated with worse prognosis (Table 2). In addition, receipt of radiation in combination with surgery was associated with better OS than either monotherapy.
Table 1.
Anatomical distribution and median overall survival (OS) of plasmacytomas.
| Primary site | No. (%) | Median OS (months) | 95% CI |
|---|---|---|---|
| Bone | 3528 (69.8%) | 85 | 8095 |
| Upper aero-digestive tract | 681 (13.5%) | Not reached | 148-not reached |
| Connective/soft tissue | 295 (5.8%) | 82 | 69–92 |
| Digestive system/hepatobiliary | 122 (2.4%) | 129 | 82-not reached |
| Central nervous system | 115 (2.3%) | 96 | 55-not reached |
| Pulmonary | 84 (1.7%) | 135 | 95-not reached |
| Lymph node/spleen | 58 (1.1%) | Not reached | Not reached |
| Endocrine system | 34 (0.7%) | 130 | 90-not reached |
| Genitourinary | 28 (0.6%) | 42 | 16-not reached |
| Eye/Orbit | 30 (0.6%) | Not reached | 110-not reached |
| Cardiac/Mediastinum | 22 (0.4%) | 61 | 25-not reached |
| Breast | 16 (0.3%) | Not reached | Not reached |
| Salivary glands | 17 (0.3%) | 109 | 102-not reached |
| Female reproductive system | 14 (0.3%) | Not reached | Not reached |
| Male reproductive system | 12 (0.2%) | 42 | 23-not reached |
Figure 2.
Overall survival (OS) based on location of plasmacytoma (A) Extramedullary plasmacytoma (P-EM) compared to bone plasmacytoma; (B) Plasmacytoma of soft/connective tissue compared to others (P-EM other than connective/soft tissue or P-bone).
Table 2.
Multivariate analysis of factors affecting survival in plasmacytoma (P- EM: Extramedullary plasmacytoma; P-Bone: Bone plasmacytoma).
| Variable | No. (%) | HR (95% CI) |
|---|---|---|
| Age (years) | ||
| <65 | 2814 (55.7%) | Referent |
| ≥65 | 2242 (44.3%) | 3.46 (3.14–3.81) |
| Location | ||
| P-EM | 1528 (30.2%) | Referent |
| P-Bone | 3528 (69.8%) | 1.48 (1.33–1.65) |
| Treatment | ||
| Neither Surgery nor Radiation | 508 (10.0%) | Referent |
| Both Surgery and Radiation | 1414 (28.0%) | 0.41 (0.35–0.48) |
| Only Radiation | 2491 (49.3%) | 0.53 (0.46–0.60) |
| Only Surgery | 643 (12.7%) | 0.58 (0.48–0.69) |
| Model for Patients With Any Radiation (N=2816) | ||
| Radiation dose (Gy) | ||
| <40 | 703 (25.0%) | Referent |
| ≥40 | 2113 (75.0%) | 0.62 (0.54–0.72) |
| Age (years) | ||
| <65 | 1635 (58.1%) | Referent |
| ≥65 | 1181 (41.9%) | 3.17 (2.74–3.66) |
| Location | ||
| P-EM | 768 (27.3%) | Referent |
| P-Bone | 2048 (72.7%) | 1.47 (1.24–1.73) |
| Treatment | ||
| Only Radiation | 1803 (64.0%) | Referent |
| Both Surgery and Radiation | 1013 (36.0%) | 0.83 (0.71–0.96) |
| Model for P-Bone Primary Site (N=2048) | ||
| Radiation dose (Gy) | ||
| <40 | 572 (27.9%) | Referent |
| ≥40 | 1476 (72.1%) | .66 (0.56–0.77) |
| Age (years) | ||
| <65 | 1183 (57.8%) | Referent |
| ≥65 | 865 (42.2%) | 3.38 (2.87–3.98) |
| Model for P-EM Primary Site (N=768) | ||
| Radiation dose (Gy) | ||
| <40 | 131 (17.1%) | Referent |
| ≥40 | 637 (82.9%) | 0.49 (0.35–0.67) |
| Age (years) | ||
| <65 | 452 (58.9%) | Referent |
| ≥65 | 316 (41.1%) | 2.79 (2.06–3.78) |
A total of 3,905 (77%) patients received some amount of RT, of which 2,816 (56%) patients were included in the analysis of radiation dose due to complete data availability (Figure 1). The median dose of RT received was 45 Gy, with some variations among disease sites (Supplementary Table T1). On multivariable analysis within the RT cohort, factors associated with worse OS included age ≥65 years, P-bone location, and radiation dose <40 Gy (Table 2). Similar to the prior analysis, receipt of surgery followed by adjuvant RT was associated with improved OS as compared to RT alone. When analyzed separately for P-bone and P-EM, age ≥65 years and RT dose <40 Gy maintained their association with worse outcomes. Of note, a higher proportion of patients with P-EM received a radiation dose ≥40 Gy than P-bone (83% vs. 72%, p<0.001). Among the RT cohort, further recursive partitioning survival tree analysis was performed separately for 4 age quartiles, and demonstrated OS differences based on site of plasmacytoma and radiation dose (Supplementary Figure S1–S5).
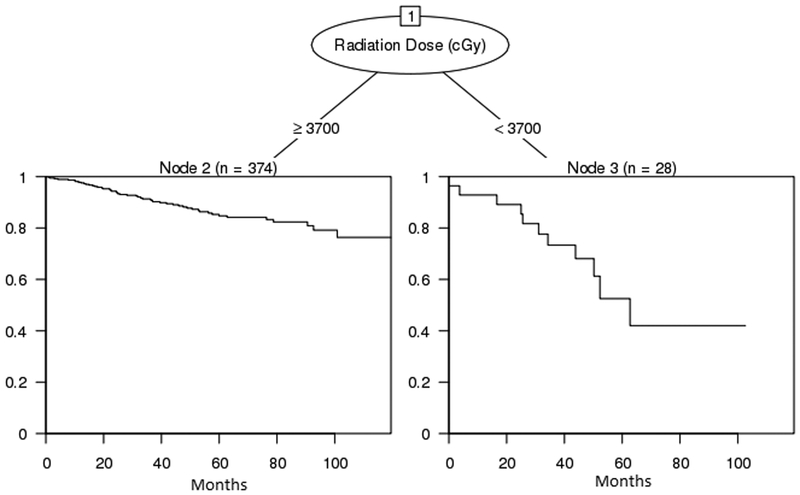
In order to ascertain appropriate radiation dose based on disease location, we pursued a subgroup analysis using regression tree models. However, due to the small sample sizes in each subgroup, we found that only the upper aero-digestive tract site had radiation dose as a significant predictor after accounting for age, sex, and comorbid conditions. The subsequent regression tree (n=402, Figure 3) showed significant difference in survival rates between those receiving 37 Gy or more and those receiving less than 37 Gy. Survival estimates at 63 months (when all in the < 37 Gy group were censored) were 84.2% (79.9 – 88.7%) and 42.0% (22.3, 79.2%), respectively.
Figure 3.
Regression tree for upper aero-digestive tract site subgroup analysis of radiation dose.
As a further exploratory analysis, we sought to analyze other treatment modalities received by plasmacytoma patients in the main cohort of 5,056 patients. We found that surgical therapy was utilized in a proportion of patients, either as monotherapy (13%), or with adjuvant radiation therapy (28%). On multivariate analysis, adjuvant RT after surgery was found to be associated with an improved mortality as compared to either modality as monotherapy (Table 2). As receipt of surgery can be affected by the location of the disease, we evaluated the various treatment modalities based on site of disease. A higher proportion of patients with P-EM underwent surgery alone (21% vs. 9%) and surgery in combination with RT (35% vs. 25%) as compared to P-bone. On the other hand, a higher fraction of patients with P-bone underwent RT alone as compared to P-EM (55% vs. 36%)(Table 3). However, due to the small number of patients in individual disease sites, further subgroup analysis of these treatment modalities was not feasible. In addition, approximately 10% patients did not receive any of these modalities as their planned first course therapy. This group had the worse mortality, but the reasons for non-receipt of treatment were unclear.
Table 3.
Treatment patterns among patients with plasmacytoma (P-bone: Bone plasmacytoma; P-EM:Extramedullary plasmacytoma)
| Site of plasmacytoma | Surgery + Radiation | Neither Surgery nor Radiation | Radiation alone | Surgery alone |
|---|---|---|---|---|
| P-Bone | 886 (25.1%) | 373 (10.6%) | 1948 (55.2%) | 321 (9.1%) |
| P-EM | 528 (34.6%) | 135 (8.8%) | 543 (35.5%) | 322 (21.1%) 95 (2.4%) |
| P-EM subgroups (n=1528) | ||||
| Breast | 6 (37.5%) | 0 (0.0%) | 5 (31.3%) | 5 (31.3%) |
| Cardiac/Mediastinum | 3 (13.6%) | 5 (22.7%) | 13 (59.1%) | 1 (4.5%) |
| Central Nervous System | 64 (55.7%) | 5 (4.3%) | 20 (17.4%) | 26 (22.6%) |
| Digestive System/Hepatobiliary | 14 (11.5%) | 22 (18.0%) | 30 (24.6%) | 56 (45.9%) |
| Endocrine System | 7 (20.6%) | 2 (5.9%) | 13 (38.2%) | 12 (35.3%) |
| Eye/Orbit | 7 (23.3%) | 0 (0.0%) | 14 (46.7%) | 9 (30.0%) |
| Female Reproductive System | 3 (21.4%) | 1 (7.1%) | 3 (21.4%) | 7 (50.0%) |
| Genitourinary | 3 (10.7%) | 4 (14.3%) | 9 (32.1%) | 12 (42.9%) |
| Male Reproductive System | 1 (8.3%) | 0 (0.0%) | 0 (0.0%) | 11 (91.7%) |
| Lymph Node/Spleen | 22 (37.9%) | 10 (17.2%) | 15 (25.9%) | 11 (19.0%) |
| Pulmonary | 1 (1.2%) | 18 (21.4%) | 37 (44.0%) | 28 (33.3%) |
| Salivary Glands | 10 (58.8%) | 0 (0.0%) | 3 (17.6%) | 4 (23.5%) |
| Connective/Soft Tissue | 75 (25.4%) | 44 (14.9%) | 126 (42.7%) | 50 (16.9%) |
| Upper Aero-Digestive Tract | 312 (45.8%) | 24 (3.5%) | 255 (37.4%) | 90 (13.2%) |
Discussion
Our study demonstrates that the outcomes of patients with plasmacytoma vary significantly according to disease location, age, and the dose of RT utilized. We observed a worse OS in patients with P-bone as compared to P-EM. This could be related to higher rates of progression to multiple myeloma from P-bone as compared to P-EM (>75% vs. <30%.).10 Interestingly, presence of soft tissue/connective tissue type of P-EM was associated with outcomes similar to P-bone. The SEER database study showed a 5-year OS of 90% for P-EM arising in skin or lymph nodes, and 48% for eye/brain/central nervous system tumors.1 Our study provides individual survival data in each of 14 sites of presentation of P-EM. This aids in the prognosis of P-EM by sites of presentation so that patients with poor prognosis (connective/soft tissue, cardiac/mediastinal, etc.) can be followed more closely to evaluate the need for systemic therapy. In addition, older age (≥65 years) was associated with worse OS as compared to their younger counterparts. This finding is similar to prior studies, and in our study, is independent of the comorbidities on regression tree analysis.
The optimal RT dose for treatment of plasmacytoma is debated, and most published series use a dose range of 30 to 60 Gy.11–13 One of the first series of P-bone evaluated 46 cases treated with a median radiation dose of 39.75 Gy, and showed no relapses with a radiation dose > 45Gy.11 In contrast to this, in a large retrospective study of 244 RT patients (50% P-bone), 6% of patients treated with ≤30 Gy developed local failure as compared to 13% patients treated with ≥30 Gy.12 Among head and neck plasmacytoma (P-EM), a study of 67 patients treated with a median RT dose of 50 Gy demonstrated no correlation between radiation dose and OS.13 In contrast, another study of 17 P-EM patients with head and neck involvement showed 100% local control with radiation dose >45 Gy, and local control was positively correlated with disease-specific survival.14 The most recent SEER analysis showed improved 5-year OS in P-bone (appendicular skeleton) patients treated with combined surgery and RT as compared to either modality alone. It also showed that upper and lower airway tract disease had better outcomes with surgery alone compared to combination therapy. However, there was no information on dose of RT utilized, which limits drawing of meaningful conclusions. In our study, utilizing data form a large group of patients, we have shown improved outcomes with doses of ≥40 Gy in both P-bone and P-EM, supporting current practice patterns.8 We further attempted to study the ideal radiation dose for specific sites of P-EM based on OS, but were unable to find a specific dose cut off for most sites due to the small sample sizes. The only subgroup that showed a statistically significant cut-off point was upper aerodigestive (head and neck) disease with improved OS at a dose of >37 Gy.
In this study, we found that a proportion of patients underwent surgical treatment, either alone or with adjuvant RT. Hence, we did an exploratory analysis to investigate this in detail. In our analysis, surgery in combination with RT was associated with an improved mortality than either monotherapy. Although this was statistically significant, one has to be cautious in concluding that surgical therapy should be considered in all patients with plasmacytoma as there could be other differences in the disease and patient specific factors that could impact the receipt of surgery. On detailed analysis, we found that a higher proportion of patients with P-EM underwent surgical treatments alone or in combination with RT as compared to patients with P-bone. As the former group includes a large proportion of patients with digestive system and connective tissue disease, it may be possible that surgical excision was performed due to ease of access or local symptoms. Conversely, RT alone group had more patients with P-bone, thus contributing to the worse OS seen in that subgroup.
The strengths of our study lie in the large sample size, making it the largest plasmacytoma study to date. We are able to provide detailed information on the variable sites of presentation and treatment options utilized in this disease. In order to minimize interactions between variables, we utilized a relatively novel statistical method with the regression tree analysis to pick variables of prognostic significance. This study also enabled us to address the important question of the optimum dose of radiation for plasmacytoma, which holds true for both P-bone and P-EM. Although this dose has been recommended by national guidelines, it had not been validated in a large dataset before our study.
The primary limitations of our study are related to its derivation from a database. We did not have access to the individual patient records, which makes it difficult to completely assess for degree of bone marrow involvement, especially in cases of P-bone. We were also unable to ascertain the reason for non-receipt of RT or surgery in 10% of the patients. One of the reasons for this could be that first course therapy was not planned at the reporting facility and the patient decided to get treatment elsewhere later, which would not be captured by NCDB. Although we are able to know the site of the tumor, we do not have accurate tumor volume data, which may affect treatment choice and outcomes. Additionally, there was no information on patients who had disease progression to MM. However, we applied stringent exclusion criteria to aid in obtaining a pure plasmacytoma cohort. Due a lack of information on some of the patient and disease specific factors, our aim was not a direct comparison of surgery to RT, and was performed as an exploratory analysis to prevent bias.
Our study shows that factors that adversely affect prognosis in plasmacytoma are age ≥65 years and bone involvement of plasmacytoma. In addition, radiation doses ≥40 Gy were associated with significantly improved survival as compared to lower RT doses in the entire cohort. In the subgroup of upper-aerodigestive tract disease, a dose ≥37 Gy was found to be associated with the best OS. Hence, this study provides important information on prognostic factors in P-bone and P-EM in a large cohort of patients.
Supplementary Material
Acknowledgments
Portions of this study were presented at the 2016 American Society of Hematology and 2017 American Society of Clinical Oncology meetings.
Funding: This research was funded by the Mayo Clinic Division of Hematology funds for the Hematology and Oncology Outcomes Research (HONOR) Group. This publication was also supported by the National Cancer Institute of the National Institutes of Health under Award Number K23CA218742.
Conflict of Interest Disclosures
S.K. has research support for clinical trials from Abbott Laboratories, Amgen Inc.; Celgene Corporation; Janssen Pharmaceutica Products, LP; Novartis Pharmaceuticals Corporation; sanofi-aventis U.S.; and Takeda Pharmaceuticals North America, Inc. He serves as a scientific advisor for Amgen Inc., Bristol-Myers Squibb Company, Celgene Corporation, GlycoMimetics, Janssen Pharmaceutica Products, LP, sanofi-aventis U.S., and Takeda Pharmaceuticals North America, Inc Supplementary information is available at Leukemia’s website.
References
- 1.Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992–2004. Br J Haematol 2009. January; 144(1): 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thumallapally N, Meshref A, Mousa M, Terjanian T. Solitary plasmacytoma: population-based analysis of survival trends and effect of various treatment modalities in the USA. BMC Cancer 2017. January 05; 17(1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holland J, Trenkner DA, Wasserman TH, Fineberg B. Plasmacytoma. Treatment results and conversion to myeloma. Cancer 1992. March 15; 69(6): 1513–1517. [DOI] [PubMed] [Google Scholar]
- 4.Knobel D, Zouhair A, Tsang RW, Poortmans P, Belkacemi Y, Bolla M, et al. Prognostic factors in solitary plasmacytoma of the bone: a multicenter Rare Cancer Network study. BMC Cancer 2006; 6: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knowling MA, Harwood AR, Bergsagel DE. Comparison of extramedullary plasmacytomas with solitary and multiple plasma cell tumors of bone. J Clin Oncol 1983. April; 1(4): 255–262. [DOI] [PubMed] [Google Scholar]
- 6.Warsame R, Gertz MA, Lacy MQ, Kyle RA, Buadi F, Dingli D, et al. Trends and outcomes of modern staging of solitary plasmacytoma of bone. Am J Hematol 2012. July; 87(7): 647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weberpals J, Pulte D, Jansen L, Luttmann S, Holleczek B, Nennecke A, et al. Survival of patients with lymphoplasmacytic lymphoma and solitary plasmacytoma in Germany and the United States of America in the early 21st century. Haematologica 2017. June; 102(6): e229–e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. Multiple Myeloma (Version 3.2017). Accessed July 24th, 2017. [DOI] [PubMed]
- 9.Strobl C, Malley J, Tutz G. An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol Methods 2009. December; 14(4): 323–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soutar R, Lucraft H, Jackson G, Reece A, Bird J, Low E, et al. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Br J Haematol 2004. March; 124(6): 717–726. [DOI] [PubMed] [Google Scholar]
- 11.Frassica DA, Frassica FJ, Schray MF, Sim FH, Kyle RA. Solitary plasmacytoma of bone: Mayo Clinic experience. Int J Radiat Oncol Biol Phys 1989. January; 16(1): 43–48. [DOI] [PubMed] [Google Scholar]
- 12.Ozsahin M, Tsang RW, Poortmans P, Belkacemi Y, Bolla M, Dincbas FO, et al. Outcomes and patterns of failure in solitary plasmacytoma: a multicenter Rare Cancer Network study of 258 patients. Int J Radiat Oncol Biol Phys 2006. January 01; 64(1): 210–217. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki R, Yasuda K, Abe E, Uchida N, Kawashima M, Uno T, et al. Multi-institutional analysis of solitary extramedullary plasmacytoma of the head and neck treated with curative radiotherapy. Int J Radiat Oncol Biol Phys 2012. February 01; 82(2): 626–634. [DOI] [PubMed] [Google Scholar]
- 14.Tournier-Rangeard L, Lapeyre M, Graff-Caillaud P, Mege A, Dolivet G, Toussaint B, et al. Radiotherapy for solitary extramedullary plasmacytoma in the head-and-neck region: A dose greater than 45 Gy to the target volume improves the local control. Int J Radiat Oncol Biol Phys 2006. March 15; 64(4): 1013–1017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.