Abstract
Previous studies have shown that cisplatin requires metabolic activation to become nephrotoxic. The activation is proposed to be via the metabolism of a glutathione-platinum conjugate to a cysteinyl-glycine-platinum conjugate, which is further processed to a cysteine conjugate. Preincubating cisplatin with glutathione (GSH), cysteinyl-glycine, or N-acetylcysteine (NAC) results in a transient increase in the toxicity of cisplatin toward renal proximal tubular cells. In this study, the preincubation solutions were analyzed by high pressure liquid chromatography (HPLC), atomic absorption spectrometry, and mass spectrometry (MS) to characterize the formation and structure of the platinum conjugates. HPLC analysis of the cisplatin-GSH, cisplatin-cysteinyl-glycine, and cisplatin-NAC preincubation solutions revealed two new platinum-containing peaks in each of the solutions. MS-MS analysis of the peaks revealed a diplatinum- and a monoplatinum conjugate in each of the solutions. Analysis of the composition and toxicity of the solutions with time showed that the transient increase in toxicity correlated with the formation of the monoplatinum conjugate whereas prolonged preincubation decreased toxicity and correlated with the formation of the diplatinum conjugate. The monoplatinum-monoglutathione conjugate is a substrate for γ-glutamyl transpeptidase, an enzyme that is essential for the nephrotoxicity of cisplatin. The monoplatinum-mono-NAC conjugate can be deacetylated to a cysteine conjugate, which is a substrate for pyroxidol phosphate (PLP)-dependent cysteine S-conjugate β-lyase. This PLP-dependent enzyme is proposed to catalyze the final step in the metabolic activation of cisplatin. Identification of the structure and toxicity of these conjugates further elucidates the metabolism of cisplatin to a nephrotoxin.
The use of cisplatin in the treatment of ovarian, germ-cell, head and neck, bladder, and other tumors is limited by its nephrotoxicity. Cisplatin kills dividing cells by forming platinum-DNA cross-links that prevent DNA synthesis and result in cell death (O’Dwyer et al., 1999). In the kidney, cisplatin is toxic to the proximal tubular cells, which are not replicating, suggesting an alternative mechanism of toxicity. Daley-Yates and McBrien showed the first evidence that indicated biotransformation products of cisplatin were the nephrotoxic compounds (Daley-Yates and McBrien, 1984). These investigators reported that seven platinum containing species were present in plasma that could be separated via HPLC2 following a single dose of cisplatin. The mixture of platinum-containing species was injected into rats and was more nephrotoxic than cisplatin. However, the anti-cancer activity of the mixture of platinum-containing species was less effective than cisplatin in a mouse leukemia model. The mechanism of cisplatin-induced nephrotoxicity was not identified until studies in our laboratory demonstrated that cisplatin-induced renal toxicity is due to the metabolism of cisplatin to a nephrotoxin via γ-glutamyl transpeptidase (GGT) and a cysteine S-conjugate β-lyase (Hanigan et al., 1994, 2001; Townsend and Hanigan, 2002).
GGT is a cell surface enzyme that cleaves γ-glutamyl bonds found in glutathione (GSH) and glutathione conjugates (Hanigan and Pitot, 1985). GGT has been shown to metabolize the glutathione conjugates of the nephrotoxic halogenated alkenes, such as trichloroethylene and hexachlorobutadiene, as part of the pathway that bioactivates these compounds (Lash et al., 2000) (Anders and Dekant, 1998). The bioactivation of the nephrotoxic halogenated alkenes requires the cleavage of the GSH S-conjugate to a cysteinyl-glycine conjugate by GGT, followed by the cleavage of the cysteinyl-glycine conjugate to a cysteine conjugate by diaminopeptidase and finally metabolism of the cysteine conjugate to a reactive thiol. Several pyroxidol phosphate (PLP)-dependent enzymes present in the cytosol and mitochondria of rat kidneys have been shown to metabolize cysteine conjugates to reactive thiols (Cooper, 1998). The cysteine S-conjugate β-lyase enzymes can catalyze a β-elimination reaction to produce a reactive thiol. In addition, in transamination reaction. The transamination reaction produces a keto acid that undergoes rearrangement to a reactive thiol (Cooper, 1998). Aminooxyacetic acid, a general inhibitor of PLP-dependent enzymes, blocks the toxicity of trichloroethylene and hexachlorobutadiene in vitro and in vivo (Jaffe et al., 1983; Stevens et al., 1986). We propose that cisplatin is bioactivated by the same pathway that activates the halogenated alkenes. Inhibition of GGT blocks the nephrotoxicity of cisplatin (Hanigan et al., 1994, 2001). Aminooxyacetic acid blocks the nephrotoxicity of cisplatin (Townsend and Hanigan, 2002; Townsend et al., 2003).
We propose that the initial step in the activation pathway is the formation of a GSH-platinum conjugate. GSH conjugates of cisplatin have been shown to form spontaneously in solution and have been isolated from the kidneys of rats treated with cisplatin (Mistry et al., 1989; Ishikawa and Ali-Osman, 1993; Bernareggi et al., 1995). However, these GSH-platinum conjugates have been proposed to be inactive forms of the drug and have not been implicated in the nephrotoxicity. Although cisplatin has not been identified as a substrate of glutathione S-transferases (GSTs), pretreating rats with inhibitors of GSTs reduced the nephrotoxicity of cisplatin (Sadzuka et al., 1994). Buthionine-sulfoximine, a glutathione-depleting agent, diminished the nephrotoxic effects of cisplatin in rats when injected 2 h prior to treatment (Mayer et al., 1987; Mayer et al., 1989). These data suggest that decreased levels of GST activity reduce the formation of the GSH-platinum conjugate. In the renal proximal tubules, the GSH-platinum conjugate can be hydrolyzed by GGT to a cysteinyl-glycine conjugate, then further metabolized by diaminopeptidases to a cysteine S-conjugate. Both GGT and diaminopeptidase are on the cell surface (Hanigan, 1998). The first two steps in the metabolism of the GSH conjugate would occur extracellularly. The resulting cysteine S-conjugate would enter the proximal tubular cells, where it could be metabolized by cysteine S-conjugate β-lyase into a toxic thiol. The structure of the nephrotoxic metabolites has not been elucidated.
Studies in our laboratory have shown that incubating cisplatin with GSH, cysteinyl-glycine (Cys-Gly), and N-acetylcysteine increases the toxicity of cisplatin toward LLC-PK1 cells, a proximal tubular cell line (Townsend et al., 2003). Confluent monolayers of LLC-PK1 cells express all the enzymes involved in the metabolic activation of the halogenated alkenes, and they have been used to study the metabolism of S-(1,2-dichlorovinyl)glutathione (Stevens et al., 1986). In this study, we analyzed the composition of the cisplatin-glutathione, cisplatin-cysteinyl-glycine, and cisplatin-NAC solutions. We identified the structure of the new compounds that formed within the solutions by HPLC and electrospray ionization mass spectrometry.
Materials and Methods
Cell Culture.
LLC-PK1 cells (ATCC CRL 1392), a proximal tubular cell line isolated from pig kidney, was purchased from American Type Culture Collection at passage 196 (Manassas, VA). The cells were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA), 5% fetal bovine serum (Hyclone Laboratories, Logan, UT), 50 units penicillin G and 50 µg of streptomycin/ml (Invitrogen). Subconfluent cultures were passaged every 3 to 4 days. For toxicity experiments, LLC-PK1 cells were seeded in 96-well plates at 104 cells/well. On the third day after plating, confluent monolayers formed and the media were replaced with fresh media. Cells were used for experiments on day 7.
Toxicity Assays.
Cisplatin (3.33 mM) (0.9% NaCl; Bristol-Myers Squibb Company, Princeton, NJ) was combined 1:1 with 3.33 mM reduced glutathione (GSH; Sigma-Aldrich, St. Louis, MO), Cys-Gly (Bachem Biosciences, King of Prussia, PA), or N-acetylcysteine (NAC; Sigma-Aldrich) in Hanks’ balanced salt solution (HBSS; Invitrogen) with 5 mM N-[2-hydroxyethyl]piperazine-N-[2-ethane sulfonic acid] (HEPES, pH 7.2). The solutions were preincubated in a 37°C water bath to allow for the spontaneous formation of cisplatin conjugates. At the end of the preincubation, the solutions were diluted to 100 µM with HBSS/HEPES. The media was removed from the cells and 150 µl of the preincubation mixture was added to each well. The cells were incubated in the solutions at 37°C in an air incubator. The mixture was removed after a 3-h exposure and replaced with Dulbecco’s modified Eagle’s medium, 5% fetal bovine serum, 50 units penicillin G and 50 µg of streptomycin/ml. The cells were incubated for an additional 69 h at 37°C in 5% CO2. The number of viable cells was determined 72 h after the start of the experiment by the MTT assay (Mosmann, 1983). A standard curve was developed relating cell number to MTT results.
High Pressure Liquid Chromatography (HPLC).
Cisplatin (3.33 mM in 0.9% NaCl) was combined 1:1 with 3.33 mM GSH, Cys-Gly, or NAC in HBSS with 5 mM HEPES (pH 7.2). The solutions were incubated in a 37°C water bath for 30 min to allow for the spontaneous formation of cisplatin conjugates. At the end of the preincubation the mixtures were analyzed by HPLC. A Waters 600 HPLC (Houston, TX) with a model 486 UV Detector with the detector focused at 230 nm and a Lichrosorb column RP18 (25 cm × 4.6 mm i.d.), 10-µm particle size (Merck, Darmstadt, Germany) was used. The mobile phase was 15 mM formic acid, pH 2.2, filtered through a 0.2-µm membrane (Millipore Corporation, Bedford, MA) and degassed in an ultrasonic bath. The flow rate was 1 ml/min. Aliquots of 20 µl were analyzed. The peaks in the HPLC eluates were collected on dry ice, lyophilized, and stored (—80°C) for electrospray ionization mass spectrometry analysis.
Atomic Absorption Analysis.
Aliquots from each of the peaks identified by HPLC were collected for platinum analysis. The aliquots were frozen and stored (—80°C). Platinum was detected in the samples with a Varian Analytical Instruments (Walnut Creek, CA) SPECTRAA-220Z graphite furnace double beam atomic absorption spectrophotometer with Zeeman background correction.
MS Parameters on the LCQ Ion Trap Mass Spectrometer.
The lyophilized products were dissolved in 15 mM formic acid with 20% methanol and infused (5 µl/min) directly into a Finnigan LCQ ion trap mass spectrometer (ThermoQuest, San Jose, CA). The LCQ ion trap mass spectrometer was operated in positive ion mode. The initial MS scan recorded mass to charge (m/z) ratios of ions over the range of 200 to 1500. Full-scan mass spectra (300 Š m/z Š5000) were collected at approximately one scan per second, with typically 5,000 to 10,000 mass resolving power. The ions of interest in each of the lyophilized products were manually selected for MS-MS, subsequent collision-activated dissociation to identify the fragmentation pattern.
Data Analysis.
Statistically significant differences in toxicity with preincubation time were detected with a one-way analysis of variance. A Tukey test was used for pairwise comparisons to determine which time points differed significantly from the To value.
Results
Toxicity of the Cisplatin Solutions.
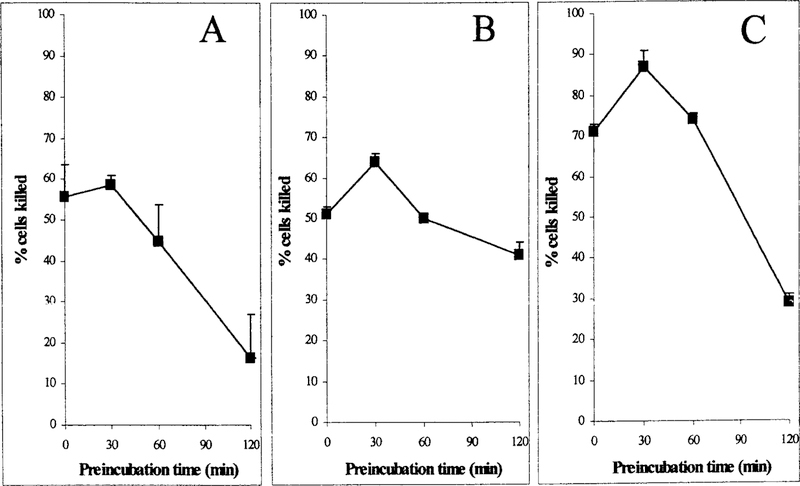
Prior studies in our laboratory have shown that short-term preincubation of cisplatin with GSH, Cys-Gly, or NAC potentiates the toxicity toward isolated proximal tubules (Townsend et al., 2003). However, the toxicity of the mixtures is transient. Preincubating cisplatin with GSH, Cys-Gly, or NAC longer than 30 min at 37°C prior to treating the cells resulted in the inactivation of the mixture toward LLC-PK1 cells (Fig. 1). Cisplatin was preincubated with equimolar GSH from 0 min to 2 h. LLC-PK1 cells were exposed to the GSH-cisplatin preincubation mixture (100 µM) for 3 h. Cell viability was measured at 72 h. A solution of GSH and cisplatin that was added to the cells without preincubation (T0) killed 55% ± 8 of the cells (Fig. 1A). After a 30-min preincubation, the cisplatin solution killed 58% ± 2. With increased time of preincubation, the mixture became less toxic. After 2 h of preincubation at 37°C, the toxicity of the GSH-cisplatin solution had decreased significantly, killing 16% ± 10 ( p < 0.05).
FIG. 1. Preincubation of cisplatin with GSH, Cys-Gly, or NAC; effect of preincubation on the toxicity of cisplatin toward LLC-PK1 cells.
Cisplatin was preincubated with equimolar GSH, Cys-Gly, or N-acetylcysteine in HBSS with 5 mM HEPES (pH 7.2) at 37°C for the times indicated to allow for the spontaneous formation of cisplatin conjugates. The toxicity of the mixture was assayed by treating LLC-PK1 cells for 3 h with 100 µM cisplatin preincubated with GSH (A), 100 µM cisplatin preincubated with Cys-Gly (B), or 100 µM cisplatin preincubated with NAC (C). The number of viable cells was determined at 72 h. Bars, values are the mean of three measurements ± S.D.
Cisplatin was preincubated with equimolar Cys-Gly from 0 min to 2 h. With no prior preincubation (T0) the 100 µM Cys-Gly-cisplatin mixture killed 51% ± 2 of the cells (Fig. 1B). After a 30-minpreincubation, Cys-Gly-cisplatin solution was significantly more toxic, killing 64% ± 2 ( p < 0.05). With increased time of preincubation the mixture became less toxic. After 2 h, the Cys-Gly-cisplatin solution was significantly less toxic; 41% ± 3 of the cells were killed ( p < 0.02). Preincubation of cisplatin alone for 3 h at 37°C had no effect on its toxicity; 51% ± 2 of the cells were killed.
Cisplatin was incubated with equimolar NAC. Without prior preincubation (T0) the solution containing 100 µM NAC and cisplatin killed 71% ± 2 of the cells (Fig. 1C). After a 30-min preincubation, the NAC-cisplatin solution was significantly more toxic, killing 87% ± 4 ( p < 0.05). With increased time of preincubation, the mixture became less toxic. After 2 h, the preincubation mixture was significantly less toxic, with only 29% ± 2 of the cells killed ( p < 0.05).
Chromatograph of the GSH-Cisplatin Solution.
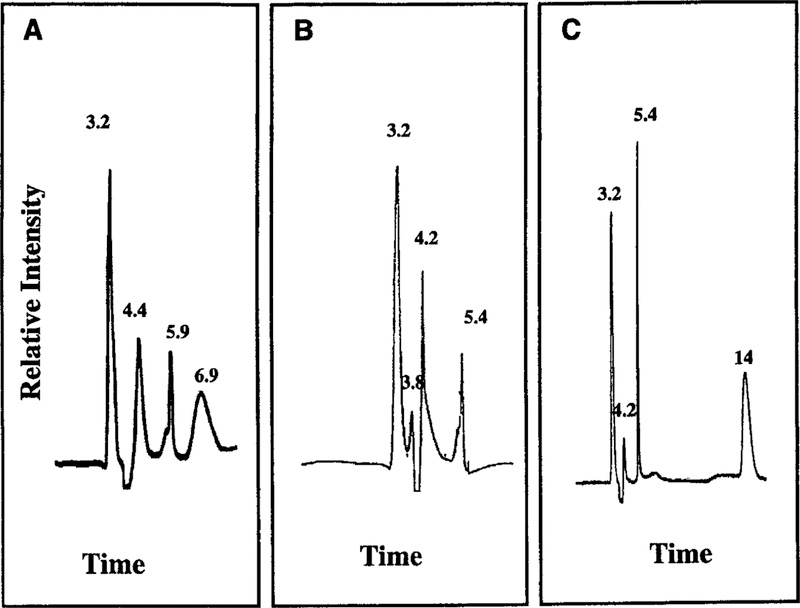
The preincubation solutions containing cisplatin with equimolar GSH, Cys-Gly, and NAC in HBSS with 5 mM HEPES (pH 7.2) were evaluated to determine the components of the solutions that gave rise to a difference in toxicity over time. The solutions analyzed by HPLC and MS were identical to those used to treat the cells. Analysis by HPLC showed that cisplatin and GSH alone had retention times (tR) of 3.2 and 6.9 min, respectively. A single peak was present following preincubation of cisplatin alone at 37°C for 30 min, indicating that neither hydrolysis nor degradation of cisplatin occurred during the preincubation or the HPLC analysis (data not shown). The GSH-cisplatin preincubation mixture was analyzed by HPLC after 0, 30,and 120 min. Two new peaks were present at 30 and 120 min, GSH-1and Cys-Gly-2 (t 5.4 min; Fig. 2B). Atomic absorption analysis(tR 4.4 min) and GSH-2 (tR 5.9 min; Fig. 2A). Platinum was detected by atomic absorption analysis in both of these two new peaks.
FIG. 2. HPLC elution profiles of cisplatin incubated for 30 min with GSH, Cys-Gly, or NAC.
Cisplatin was preincubated with equimolar GSH, Cys-Gly, or NAC in HBSS with 5 mM HEPES (pH 7.2) at 37°C for 30 min to allow for the spontaneous formation of cisplatin conjugates. Elution profiles of solutions containing cisplatin preincubated with GSH (A), Cys-Gly (B), or NAC (C) are shown. The elution time (min) of each of the peaks is noted.
The area under the curve (AUC) for cisplatin and the GSH-1 and GSH-2 were calculated and averaged for three separate HPLC profiles (Table 1). At T0, all of the platinum was in the cisplatin peak. By 30 min the unreacted cisplatin (tR 3.2 min) decreased to 44% of the total platinum-containing peaks and further decreased by 120 min to 30%. GSH-1 (tR 4.4 min) increased to 35% of the total platinum-containing peaks by 30 min and 56% by 120 min. GSH-2 (tR 5.9 min) comprised 21% at 30 min and 14% at 120 min. The toxicity of the preincubation mixture toward LLC-PK1 cells decreased as the relative abundance of GSH-2 decreased and GSH-1 increased (Fig. 1 and Table 1). These data suggest that GSH-2 is the nephrotoxic species whereas GSH-1 is a nontoxic cisplatin-GSH conjugate.
TABLE 1.
The HPLC profile of the cisplatin solutions with equimolar GSH, Cys-Gly, and NAC
| Percentage of Area Under the Curve |
||
|---|---|---|
| 30 min | 120 min | |
| % | ||
| GSH-platinum mixture | ||
| Cisplatin | 44 | 30 |
| GSH-1 | 35 | 56 |
| GSH-2 | 21 | 14 |
| Cys-Gly-platinum mixture | ||
| Cisplatin | 69 | 59 |
| Cys-Gly-1 | 25 | 20 |
| Cys-Gly-2 | 6 | 21 |
| NAC-platinum mixture | ||
| Cisplatin | 54 | 23 |
| NAC-1 | 17 | 21 |
| NAC-2 | 29 | 56 |
Chromatograph of the Cys-Gly-Cisplatin Solution.
Cys-Gly had a retention time (tR) of 4.2 min under our HPLC conditions. Preincubation of Cys-Gly in HBSS with 5 mM HEPES (pH 7.2) at 37°C did not result in the formation of new peaks. The equimolar solution of Cys-Gly and cisplatin in HBSS with 5 mM HEPES (pH 7.2) was analyzed by HPLC at T0, and after 30 and 120 min preincubation at 37°C. Two new peaks were present at 30 min, Cys-Gly-1 (tR 3.8 min) confirmed the new peaks contained platinum.
AUC for cisplatin, Cys-Gly-1, and Cys-Gly-2 were calculated and averaged for three separate HPLC profiles (Table 1). At T0, unreacted cisplatin was 100%. Unreacted cisplatin (tR 3.2 min) decreased to 69% of the total platinum-containing peaks by 30 min and further decreased to 59% by 120 min. Cys-Gly-1 (tR 3.8 min) comprised 25% of the total platinum-containing peaks by 30 min and 20% by 120 min. The average of three HPLC runs showed Cys-Gly-2 (tR 5.4 min) was 6% at 30 min and increased to 21% by 120 min. The toxicity of the preincubation mixture toward LLC-PK1 cells decreased as the relative abundance of Cys-Gly-1 decreased and as Cys-Gly-2 increased (Fig. 1 and Table 1). These data suggest that Cys-Gly-1 is the nephrotoxic species whereas Cys-Gly-2 is a nontoxic cisplatin-Cys-Gly conjugate.
Chromatograph of the NAC-Cisplatin Solution.
NAC had a retention time (tR) of 14 min. Preincubation of NAC HBSS with 5 mM HEPES (pH 7.2) at 37°C did not result in the formation of new peaks. The equimolar solution of NAC and cisplatin in HBSS with 5 mM HEPES (pH 7.2) was analyzed by HPLC at T0, and after 30- and 120-min preincubation at 37°C. Two new peaks were present at 30 and 120 min, NAC-1 (tR 4.2 min) and NAC-2 (tR 5.4 min; Fig. 2C). Atomic absorption analysis indicated the new peaks contained platinum.
AUC for cisplatin and the NAC-1 and NAC-2 are shown in Table 1. Unreacted cisplatin (tR 3.2min) decreased to 54% of the total platinum-containing peaks by 30 min and further decreased to 23% by 120 min. NAC-1 (tR 4.2 min) comprised 17% of the total platinum-containing peaks by 30 min and 21% at 120 min. NAC-2 (tR 5.4 min) constituted 29% at 30 min and 56% by 120 min. The toxicity of the preincubation mixture toward LLC-PK1 cells decreased as the relative abundance of NAC-2 increased (Fig. 1 and Table 1). These data suggest that NAC-1 is the nephrotoxic species whereas NAC-2 is a nontoxic cisplatin-NAC conjugate.
MS Analysis of GSH-1 and GSH-2.
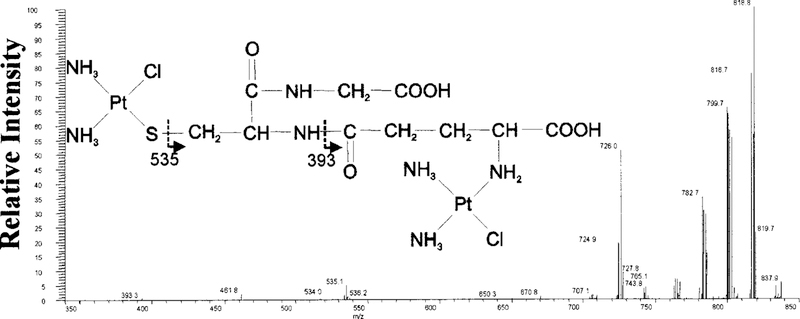
MS analysis of the parental compounds, cisplatin and GSH, showed an abundant ion cluster at m/z 300 and 308, respectively (data not shown). Platinum, a neutral heavy metal, ionizes poorly. Hence, the lyophilized products from each peak were evaluated by MS, with analysis of the individual ions of interest by MS-MS. The MS spectra of all platinum and chlorine containing species are in isotopic clusters that relate to the isotopic distribution of platinum and chlorine. GSH-1 (tR 4.4 min) was collected from HPLC and analyzed by MS. The mass spectrum of GSH-1 showed an abundant isotopic cluster ion [M + H]+ at m/z 835. MS-MS analysis of m/z 835 showed an ion with a fragmentation pattern that is consistent with the structure of the diplatinum-monoglutathione conjugate shown in Fig. 3. The larger fragments are consistent with the loss of amine and chloride groups: m/z 819 (—NH3), 801 (—Cl), 783 (—NH3, —Cl), 765 (—2NH3, —Cl), 726 (—NH3, —2Cl). The smaller fragments, 535 and 393, are consistent with the fragments indicated in Fig. 3.
FIG. 3. MS-MS analysis of the GSH-1.
MS-MS spectrum of the m/z 835 precursor ion, the dominant ion in GSH-1. A diplatinum-monoglutathione structure is proposed for the precursor ion based on the fragmentation pattern.
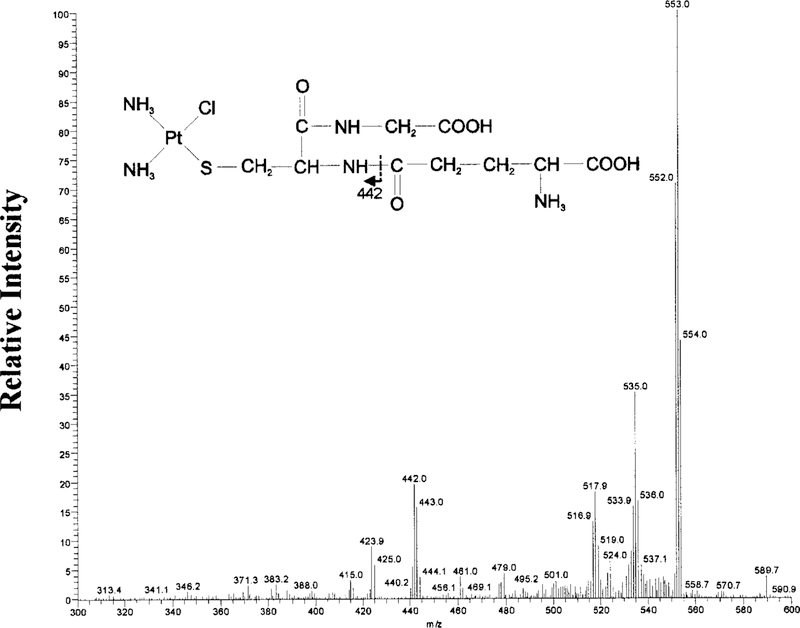
GSH-2 (tR 5.9 min) was collected from HPLC and analyzed by MS. The mass spectrum of GSH-2 showed an isotopic cluster ion [M + H]+ at m/z 570. MS-MS analysis of m/z 570, showed a fragmentation pattern consistent with the structure of a monoplatinum-monoglutathione conjugate shown in Fig. 4. The larger fragments are consistent with the loss of amine and chloride groups: m/z 553 (—NH3), 535 (—Cl), 517 (—NH3, —Cl). The fragment at 442 is consistent with the fragment indicated in Fig. 4. The ion at m/z 424 represents the m/z 442 ion minus one amine group.
FIG. 4. MS-MS Analysis of the GSH-2.
MS-MS spectrum of the m/z 570 precursor ion, the dominant ion in GSH-2. A monoplatinum-monoglutathione structure is proposed for the precursor ion based on the fragmentation pattern.
MS Analysis of Cys-Gly-1 and Cys-Gly-2.
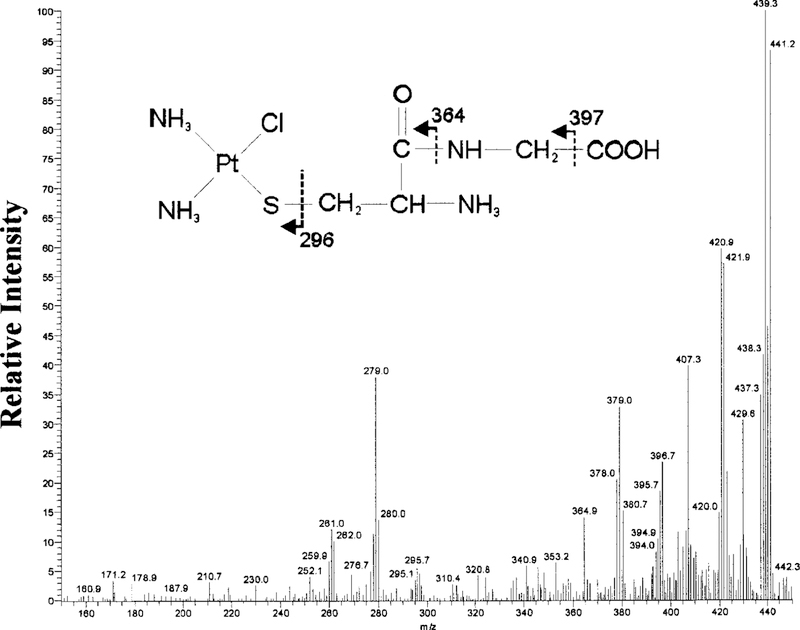
MS analysis of the parent compounds, cisplatin and Cys-Gly, showed the formation of an abundant ion at m/z 300 and 178, respectively (data not shown). Cys-Gly-1 (tR 3.8 min) was collected and analyzed by MS. Cys-Gly-1 showed an isotopic cluster ion [M + H]+ at m/z 439. MS-MS analysis of m/z 439 showed a fragmentation pattern consistent with the structure of a monoplatinum-monocysteinyl-glycine conjugate shown in Fig. 5. The larger ions are consistent with the loss of amine and chloride groups: m/z 421 (—NH3), 407 (—Cl). The structure of the ions at m/z 397, 364, and 296 are shown in Fig. 5 as indicated by arrows. The ion at m/z 379 represents the loss of an amine group from the ion at m/z 397. The ion at m/z 279 represents the loss of an amine group from the ion at m/z 296.
FIG. 5. MS-MS analysis of the Cys-Gly-1.
MS-MS spectrum of the m/z 439 precursor ion, the dominant ion in Cys-Gly-1. A monoplatinum-mono-Cys-Gly structure is proposed for the precursor ion based on the fragmentation pattern.
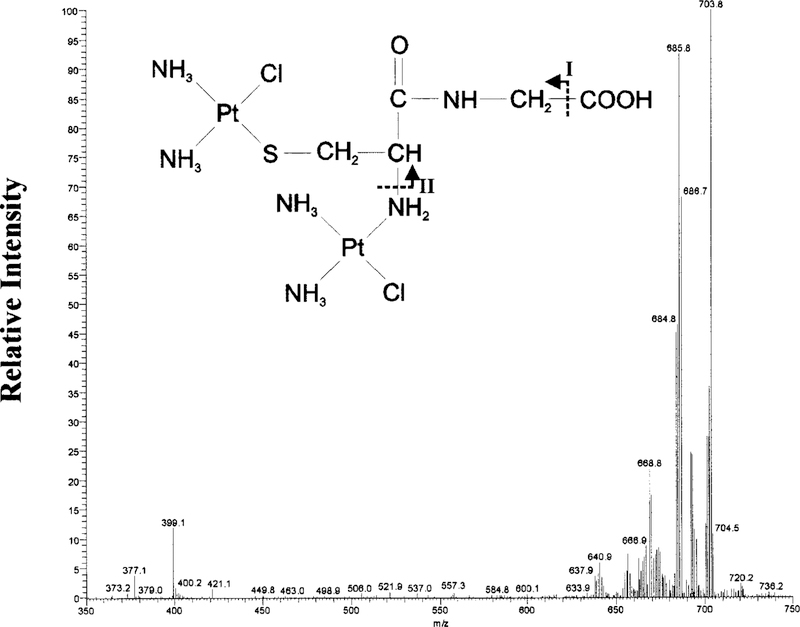
Cys-Gly-2 showed an isotopic cluster ion [M + H]+ at m/z 702. MS-MS analysis of m/z 702 showed a fragmentation pattern consistent the structure of a diplatinum-monocysteinyl-glycine conjugate shown in Fig. 6. The larger ions are consistent with the loss of the following amine and chloride groups: m/z 686 (—NH3), 669 (—2NH3). The ion at m/z 640 is consistent with fragment I minus an amine group, and m/z 399 is consistent with fragment II minus an amine group.
FIG. 6. MS-MS analysis of the Cys-Gly-2.
MS-MS spectrum of the m/z 702 precursor ion, the dominant ion in Cys-Gly-2. A diplatinum-mono-Cys-Gly structure is proposed for the precursor ion based on the fragmentation pattern.
MS-Analysis of NAC-1 and NAC-2.
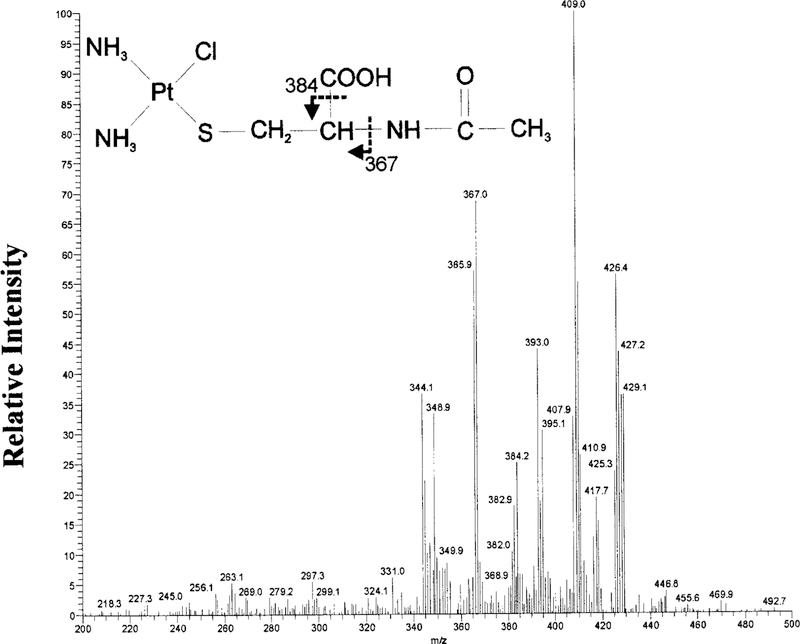
MS analysis of the parent compounds, cisplatin and NAC, showed an abundant ion at m/z 300 and 163, respectively (data not shown). NAC-1 was collected, lyophilized, and the product was analyzed via MS. NAC-1 showed an isotopic cluster ion [M + H]+ at m/z 427. MS-MS analysis of m/z 427 showed a fragmentation pattern consistent with the structure of a monoplatinum-mono-NAC conjugate shown in Fig. 7. The larger ions are consistent with the loss of the following amine and chloride groups: m/z 409 (—NH3), 393 (—Cl). The structure of the ions at m/z 384 and 367 are shown in Fig. 7 as indicated by arrows. Loss of an amine group from m/z 367 is represented in m/z 349.
FIG. 7. MS-MS analysis of NAC-1.
MS-MS spectrum of the m/z 427 precursor ion, the dominant ion in NAC-1. A monoplatinum-mono-NAC structure is proposed for the precursor ion based on the fragmentation pattern.
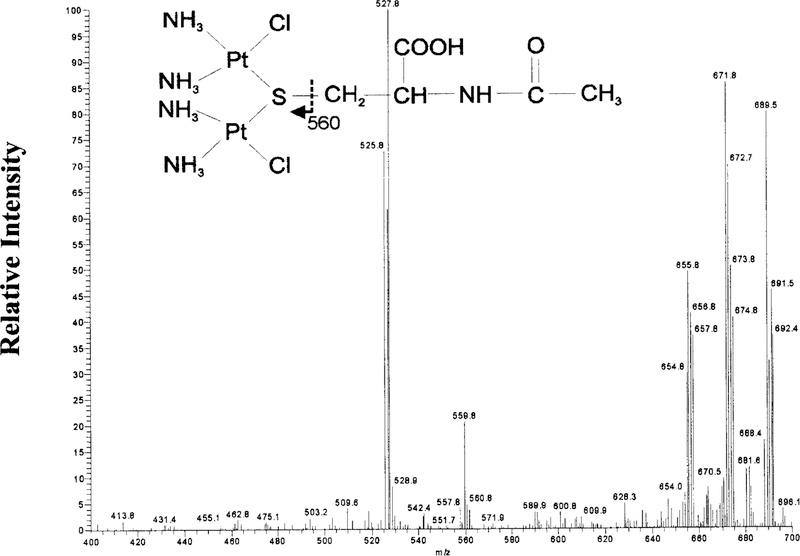
NAC-2 showed an isotopic cluster ion [M + H]+ at m/z 690. MS-MS analysis of m/z 690 showed a fragmentation pattern consistent with the structure of the diplatinum-mono-NAC conjugate shown in Fig. 8. The larger ions are consistent with the loss of the following amine and chloride groups: m/z 672 (—NH3), 656 (—Cl). The structure of m/z 560 ion is indicated by the arrows in Fig. 8. The ion at m/z 526 represents the loss of an amine group from m/z 560.
FIG. 8. MS-MS analysis of NAC–2.
MS-MS spectrum of the m/z 690 precursor ion, the dominant ion in NAC-2. A diplatinum-mono-NAC structure is proposed for the precursor ion based on the fragmentation pattern.
Discussion
Preincubation of cisplatin with GSH, Cys-Gly, or NAC results in a transient increase in the toxicity of the mixture toward LLC-PK1 cells (Townsend et al., 2003). In this study, we have shown that, initially, monoplatinum conjugates form spontaneously when GSH, Cys-Gly, or NAC is preincubated in solution with cisplatin. The presence of these conjugates correlates with increased nephrotoxicity. MS analysis revealed that the structures of the GSH-monoplatinum conjugate, GSH-2, the Cys-Gly-monoplatinum conjugate, Cys-Gly-1, and the NAC-monoplatinum conjugate, NAC-1, are similar. In each of the monoplatinum conjugates, the sulfur moiety of the GSH, Cys-Gly, or NAC is bound to the platinum with the loss of a chloride from the platinum. These compounds are substrates for the enzymes that we have found to be essential for the nephrotoxicity of cisplatin (Hanigan et al., 1996, 2001; Townsend and Hanigan, 2002). GSH-2 has a free γ-glutamyl group and therefore is a substrate for GGT (Tate and Meister, 1978). We propose that GSH-2 is the GSH-platinum conjugate that is metabolized to a nephrotoxin. The monoplatinum-NAC conjugate, NAC-1 would be deacetylated to a platinum-cysteine conjugate by the cell. Following deacetylation, this conjugate would be a substrate for PLP-dependent cysteine S-conjugate β-lyase (Cooper, 1998). Prolonged preincubation caused an inactivation of the toxicity. The loss of nephrotoxicity of the preincubation mixtures correlates with the formation of diplatinum conjugates in each of the mixtures. The diplatinum-GSH, GSH-1, and diplatinum-Cys-Gly, Cys-Gly-2, conjugates are both composed of the corresponding monoplatinum adduct with a second cisplatin bound to the nitrogen of the free amine of the amino acid with the loss of a chloride from the platinum. GSH-1 is not a substrate for GGT due to the lack of a free μ-glutamyl group (Tate and Meister, 1978). Diplatinum-NAC, NAC-2, consists of the monoplatinum-NAC with the second cisplatin also bound to the sulfur with the loss of a chloride from the platinum. In NAC the amine group of the amino acid is acetylated, therefore, there is no free nitrogen to bind the platinum. The NAC-2 conjugate is unlikely to be a substrate for the PLP-dependent cysteine S-conjugate β-lyase due to the two platinums bound to the sulfur, which would not favor the β-elimination reaction necessary to form the toxic thiol (Cooper et al., 2002).
The glutathione conjugate of hexachlorobutadiene, one of the nephrotoxic halogenated alkenes, is metabolized by GGT, and the cysteine conjugate is metabolized by cysteine S-conjugate β-lyase to a toxic thiol (Jaffe et al., 1983; Jones et al., 1985). Both mono- and bis-GSH conjugates of hexachlorobutadiene have been identified by HPLC analysis (Jones et al., 1985). However, only the mono-GSH conjugates of hexachlorobutadiene and other halogenated alkenes are substrates of GGT (Stevens et al., 1986; Finkelstein et al., 1992; Dekant et al., 1995).
Formation of platinum-GSH conjugates and platinum-cysteine conjugates has been demonstrated in several laboratories (Ishikawa and Ali-Osman, 1993; Bernareggi et al., 1995). Bernareggi and coworkers identified a monoplatinum-GSH conjugate that formed spontaneously in solution under incubation conditions that differed slightly from those used in this study (Bernareggi et al., 1995). The MS fragmentation profile and structure proposed by Bernareggi are the same as those of GSH-2 identified in this study. Bernareggi and coworkers also identified a diplatinum-GSH conjugate, however, the fragmentation pattern and proposed structure of the m/z 835 precursor ion obtained by Bernareggi differ from GSH-1 identified in this study. Ishikawa and Ali-Osman reported a monoplatinum-diglutathione complex that was not toxic in tumor cells (Ishikawa and Ali-Osman, 1993). In a separate series of experiments we found that increasing the ratio of GSH to cisplatin from 1:1 to 2:1 or 4:1 resulted in a more rapid inactivation of the solution (data not shown). The increased concentration of GSH would favor the formation of the inactive monoplatinum-diglutathione complex identified by Ishikawa and Ali-Osman. Bose and coworkers reported a kinetic analysis of the reaction of cisplatin with cysteine (Bose et al., 1997). Cysteine conjugates of cisplatin have been identified in the kidney (Maines, 1986).
Studies in vivo have indicated that a GSH conjugate of cisplatin is metabolized by GGT and the cysteine conjugate is metabolized by a PLP-dependent enzyme within the renal proximal tubules to a nephrotoxic species(Hanigan et al., 1996, 2001; Townsend and Hanigan, 2002; Townsend et al., 2003). In vitro studies have shown that preincubation of cisplatin with GSH, cysteinyl-glycine, or NAC potentiate the nephrotoxicity of cisplatin. In this study, we have identified the toxic platinum-GSH, platinum-Cys-Gly, and platinum-NAC conjugates that form spontaneously in solution. We have also shown that the toxicity of the solution is transient and decreases as diplatinum conjugates form. The toxic monoplatinum conjugates are substrates for the enzymes that metabolize cisplatin to a nephrotoxin. There are several renal enzymes that have cysteine S-conjugate β-lyase activity (Cooper et al., 2002). We are currently investigating which of these enzymes metabolizes the cysteine conjugate of cisplatin to a reactive thiol.
Acknowledgments.
We are grateful to Christine Diekhaus (Department of Chemistry, The University of Virginia) for her assistance in the HPLC analysis and to Amy West (Department of Cell Biology, University of Oklahoma Health Sciences Center) for the platinum analysis.
This work was supported by Grant R01CA57530 to M.H.H. from the National Cancer Institute. M.D. was supported by a grant from the Martha Jefferson Hospital.
2 Abbreviations used are:
- HPLC
high pressure liquid chromatography
- GGT γ
glutamyl transpeptidase
- GSH
glutathione
- PLP
pyroxidol phosphate
- AOAA
aminooxyacetic acid
- GSTs
glutathione S-transferases
- Cys-Gly
cysteinyl-glycine
- NAC
N-acetyl cysteine
- HBSS
Hanks’ balanced salt solution
- MS-MS
tandem mass spectrometry
- tR
retention times
- AUC
area under the curve
Contributor Information
DANYELLE M. TOWNSEND, Department of Cell Biology, University of Virginia, Charlottesville, Virginia.
JARROD A. MARTO, Department of Chemistry, University of Virginia, Charlottesville, Virginia
MEI DENG, Department of Cell Biology, University of Virginia, Charlottesville, Virginia.
TIMOTHY J. MACDONALD, Department of Chemistry, University of Virginia, Charlottesville, Virginia
MARIE H. HANIGAN, Department of Cell Biology, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma
References
- Anders MW and Dekant W (1998) Glutathione-dependent bioactivation of haloalkenes. Annu Rev Pharmacol Toxicol 38:501–537. [DOI] [PubMed] [Google Scholar]
- Bernareggi A, Torti L, Facino RM, Carini M, Depta G, Casetta B, Farrell N, Spadacini S, Ceserani R, and Tognella S (1995) Characterization of cisplatin-glutathione adducts by liquid chromatography-mass spectrometry: evidence for their formation in vitro but not in vivo after concomitant administration of cisplatin and glutathione to rats and cancer patients. J Chromatogr 669:247–263. [DOI] [PubMed] [Google Scholar]
- Bose RN, Ghosh SK, and Moghaddas S (1997) Kinetic analysis of the cis-diamminedichloroplatinum (II)– cysteine reaction: implications to the extent of platinum–DNA binding. J Inorg Biochem 65:199–205. [DOI] [PubMed] [Google Scholar]
- Cooper AJ (1998) Mechanisms of cysteine S-conjugate β-lyases. Adv Enzymol Relat Areas Mol Biol 72:199–238. [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Bruschi SA, and Anders MW (2002) Toxic, halogenated cysteine S-conjugates and targeting of mitochondrial enzymes of energy metabolism. Biochem Pharmacol 64:553–564. [DOI] [PubMed] [Google Scholar]
- Daley-Yates PT and McBrien DCH (1984) Cisplatin metabolites in plasma, a study of their pharmacokinetics and importance in the nephrotoxic and antitumour activity of cisplatin. Biochem Pharmacol 33:3063–3070. [DOI] [PubMed] [Google Scholar]
- Dekant W, Vamvakas S, and Anders MW (1995) Formation and fate of nephrotoxic and cytotoxic glutathione S-conjugates: cysteine conjugate β-lyase pathway. Adv Pharmacol 27:115–162. [DOI] [PubMed] [Google Scholar]
- Finkelstein MB, Baggs RB, and Anders MW (1992) Nephrotoxicity of the glutathione and cysteine conjugates of 2-bromo-2-chloro-1,1-difluoroethene. J Pharmacol Exp Ther 261: 1248–1252. [PubMed] [Google Scholar]
- Hanigan MH (1998) Gamma-glutamyl transpeptidase, a glutathionease: its expression and function in carcinogenesis. Chem Biol Interact 111–112:333–342. [DOI] [PubMed] [Google Scholar]
- Hanigan MH, Gallagher BC, and Taylor PT Jr (1996) Cisplatin nephrotoxicity: inhibition of gamma-glutamyl transpeptidase blocks the nephrotoxicity of cisplatin without reducing platinum concentrations in the kidney. Am J Obstet Gynecol 175:270–274. [DOI] [PubMed] [Google Scholar]
- Hanigan MH, Gallagher BC, Taylor PT Jr, and Large MK (1994) Inhibition of gamma-glutamyl transpeptidase activity by acivicin in vivo protects the kidney from cisplatin-induced toxicity. Cancer Res 54:5925–5929. [PubMed] [Google Scholar]
- Hanigan MH, Lykissa ED, Townsend DM, Ou C, Barrios R, and Lieberman MW (2001) Gamma-glutamyl transpeptidase-deficient mice are resistant to the nephrotoxicity of cisplatin. Am J Pathol 159:1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanigan MH and Pitot HC (1985) Gamma-glutamyl transpeptidase—its role in hepatocarcino-genesis. Carcinogenesis 6:165–172. [DOI] [PubMed] [Google Scholar]
- Ishikawa T and Ali-Osman F (1993) Glutathione-associated cis-diamminedichloroplatinum (II) metabolism and ATP-dependent efflux from leukemia cells. J Biol Chem 268:20116–20125. [PubMed] [Google Scholar]
- Jaffe DR, Hassall CD, Brendel K, and Gandolfi AJ (1983) In vivo and in vitro nephrotoxicity of the cysteine conjugate of hexachlorobutadiene. J Toxicol Environ Health 11:857–867. [DOI] [PubMed] [Google Scholar]
- Jones TW, Gerdes RG, Ormstad K, and Orrenius S (1985) The formation of both a mono-and bis-substituted glutathione conjugate of hexachlorobutadiene by isolated hepatocytes and following in vivo administration to the rat. Chem Biol Interact 56:251–267. [DOI] [PubMed] [Google Scholar]
- Lash LH, Fisher JW, Lipscomb JC, and Parker JC (2000) Metabolism of trichloroethylene. Environ Health Perspect 108 (Suppl 2):177–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines MD (1986) Differential effect of cis-platinum (cis-diamminedichloroplatinum) on regulation of liver and kidney haem and haemoprotein metabolism. Biochem J 237:713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer RD, Lee K, and Cockett ATK (1987) Inhibition of cisplatin-induced nephrotoxicity in rats by buthionine sulfoximine, a glutathione synthesis inhibitor. Cancer Chemother Pharmacol 20:207–210. [DOI] [PubMed] [Google Scholar]
- Mayer RD, Lee KE, and Cockett AT (1989) Improved use of buthionine sulfoximine to prevent cisplatin nephrotoxicity in rats. J Cancer Res Clin Oncol 115:418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry P, Lee C, and McBrien DC (1989) Intracellular metabolites of cisplatin in the rat kidney. Cancer Chemother Pharmacol 24:73–79. [DOI] [PubMed] [Google Scholar]
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival:Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. [DOI] [PubMed] [Google Scholar]
- O’Dwyer PJ, Stevenson JP, and Johnson SW (1999) Clinical status of cisplatin, carboplatin and other platinum-based antitumor drugs, in Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug (Lippert B ed) pp 29–70. Wiley-VCH, Zurich. [Google Scholar]
- Sadzuka Y, Shimizu Y, Takino Y, and Hirota S (1994) Protection against cisplatin-induced nephrotoxicity in the rat by inducers and an inhibitor of glutathione S-transferase. Biochem Pharmacol 48:453–459. [DOI] [PubMed] [Google Scholar]
- Stevens J, Hayden P, and Taylor G (1986) The role of glutathione conjugate metabolism and cysteine conjugate β-lyase in the mechanism of S-cysteine conjugate toxicity in LLC-PK1 cells. J Biol Chem 261:3325–3332. [PubMed] [Google Scholar]
- Tate SS and Meister A (1978) Serine-borate complex as a transition-state inhibitor of gamma-glutamyl transpeptidase. Proc Natl Acad Sci USA 75:4806–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DM, Deng M, Zhang L, Lapus MG, and Hanigan MH (2003) Metabolism of cisplatin to a nephrotoxin in proximal tubule cells. J Am Soc Nephrol 14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DM and Hanigan MH (2002) Inhibition of gamma-glutamyl transpeptidase or cysteine S-conjugate β-lyase activity blocks the nephrotoxicity of cisplatin in mice. J Pharmacol Exp Ther 300:142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]