Abstract
Objective:
Intracranial pressure (ICP) over 20 mmHg is associated with poor neurologic prognosis, but measuring ICP directly requires an invasive procedure. Dilation of the optic nerve sheath on axial ultrasound of the eye has been correlated with elevated ICP, but optimal cutoffs have been inconsistent possibly related to the measurement technique. A coronal technique has been studied on healthy volunteers but not on patients with high ICP. We compared two measurement techniques (axial and coronal) in patients with suspected high ICP due to trauma, bleeding, tumor, or infection.
Design:
Prospective blinded observational study.
Setting:
Two tertiary referral center intensive care units.
Patients:
20 adults admitted to the ICU at risk for increased ICP expected to receive invasive intracranial monitoring.
Interventions:
Ultrasound measurements of the optic nerve sheath in axial and coronal views either averaged between eyes or the highest in either eye.
Measurements and Main Results:
Coronal measurements showed less variability between each eye than axial measurements (mean difference 0.5mm vs. 1mm, p=0.03) and were associated with high ICP at first measurement and over 24 hours (AUROC range 0.7 to 0.8). Mean and highest axial measurements showed improved association with first (AUROC 0.87, 0.94) and highest ICP measurement (AUROC 0.89, 0.96) within 24 hours. A cutoff of highest axial measurement in either eye greater than 6.2mm or mean axial measurement between eyes of 5.6mm had a sensitivity of 100% in predicting high ICP over the following 24 hours.
Conclusions:
The highest axial measurement of optic nerve sheath diameter in either eye is the most predictive of patients with high ICP in our population. This comparison of measurement techniques has not previously been described and should be further explored to set test cutoffs for ultrasound of the optic nerve sheath diameter.
Keywords: Optic Nerve Sheath Diameter, Intracranial Pressure, Optic Ultrasound, ultrasonography, point of care ultrasound
Introduction
Elevated intracranial pressure (ICP), defined as sustained pressure > 20 mmHg, is associated with poor clinical outcomes in patients with neurologic injury. Currently, obtaining this pressure requires placement of an invasive monitor by a neurosurgeon, which may not be available in all hospitals. With the development of more sophisticated ultrasound technology, there has been significant research focus on whether optic nerve sheath diameter (ONSD) can be used to identify patients with increased ICP, and whether the ONSD can be followed along with the neurologic exam. These non-invasive measurements could be helpful in triage decisions at community hospitals, in the emergency department, or in the intensive care unit (ICU).
Previous studies show that patients with fluctuating ICPs due to CSF absorption disorders or due to tracheal stimulation while intubated show similarly reliable increases in ONSD and return to baseline after stimulation or saline instillation ceases.(1, 2) Observational studies in healthy patients confirm that normal ONSD ranges from about 2.2–5mm.(3–5) In multiple neurologic populations including traumatic brain injury (TBI), subarachnoid hemorrhage (SAH), and intracranial hemorrhage (ICH), increased ICP has been associated with increased ONSD.(6–18) However, despite reliably high sensitivity and specificity, a consistent ONSD cutoff to predict ICP>20mmHg has been elusive, with various studies determining an optimal cutoff anywhere from 5–6.2 mm.(19–22)
One explanation for this variability may be the technique used to sonographically measure the optic nerve. MRI and cadaveric studies show the optic nerve is thickest about 3mm behind the retina, and then narrows as it makes a curved path posterior and medially.(23–25) However, the most common method of ultrasound measurement, which involves transverse orientation through the upper eyelid to obtain a longitudinal cut of the optic nerve, shows a parallel set of lines or a pyramidal structure that widens as it travels posteriorly.(26, 27) Given the anatomic evidence that the optic nerve narrows posteriorly, this set of parallel lines or expanding pyramidal structure is thought to be artifact, either shadowing of the lamina cribrosa or shadowing of the optic nerve sheath.(28–30) This artifact may increase as the optic nerve swells, explaining the consistent correlation between ICP and ONSD but also the variability in exact numeric cutoffs between studies. This artifact may also explain why studies examining normal ultrasound ONSD have some healthy individuals above 5mm, while cadaveric and MRI studies consistently show lower upper limits of normal optic nerves.(4)
Ophthalmologic ultrasonographers often use a different technique for measuring ONSD, placing a probe in a vertical orientation on the temporal surface of the eye or the eyelid, aiming nasally and posterior.(19, 31, 32) This technique, which creates a coronal plane through the patient, identifies a cross-section of the optic nerve as a circular shape with less artifact compared to the transverse or axial orientation. Studies that have compared these two techniques in healthy patients find that the coronal imaging technique provides more accurate measurements consistent with known normal optic nerve measurements, whereas measurements made through a transverse approach inflate the size of the optic nerve sheath.(33–35) However, though it is critical to establish the optimal ultrasound technique in patients who are at risk for elevated ICP, no study has yet compared these two measurement strategies in these patients.
Materials and Methods
Study Design
This study is a prospective blinded observational study in two academic tertiary referral center intensive care units (ICU).
Study Population
We enrolled adult patients admitted to the ICU from the emergency department or transferred from outside hospitals with moderate-severe traumatic brain injury (TBI) with a GCS of 3–12, subarachnoid hemorrhage (SAH), intracerebral hemorrhage with or without associated intraventricular hemorrhage (IVH), or suspected increased intracranial pressure due to infection or tumor who received an invasive intracranial pressure monitor (parenchymal monitor or external ventricular drain [EVD]). Patients were excluded if they had an EVD in place prior to transfer from an outside hospital, received a decompressive procedure (ie. surgical hemicraniectomy) prior to admission to the intensive care unit, or had an ophthalmologic condition that would preclude ultrasound (globe rupture, synthetic eye). Though not an exclusion criteria, we did not enroll any patients who had direct optic nerve compression from a focal lesion.
Consent
This study was approved by our hospital’s institutional review board with an initial waiver of consent to obtain the time-sensitive minimal-risk ultrasound on admission to the ICU. Consent was subsequently obtained from the patient’s designated surrogate. Consent was reobtained from the patient if they regained ability to consent during their ICU stay.
Optic Nerve Sheath Measurements
Optic nerve sheath diameter (ONSD) measurements were made with a Sonosite X-porte ultrasound by two different methods with the patient in a supine position with the head raised 30 degrees. In the first method (axial method), a Sonosite L25×p 13–6MHz linear probe was placed on the upper eyelid with sterile ultrasound gel in a horizontal orientation creating an axial line through the eyelid of the patient until the optic nerve could be seen longitudinally behind the orbit at its widest diameter. The diameter was measured 3mm behind the retina. In the second method (coronal method), the same ultrasound probe was placed in a vertical (cephalad-caudal) orientation at the lateral canthus and aimed nasally and posterior until a circular optic nerve was seen at its largest diameter. Measurements were taken in a superior-inferior direction to minimize artifact if the image was taken at a slightly oblique angle (Figure 1). Each measurement was then repeated on the other eye. All measurements were taken prior to EVD placement and the provider was blinded to ICP measurements until after images were processed and measured. All individuals performing the ultrasound were clinical fellows who had undergone a 1 hour hands-on group training in optic nerve sheath ultrasound as well as one-on-one training with the first author or senior author. All measurements were made by the primary author who was blinded to the outcome variables until after measurement.
Figure 1:
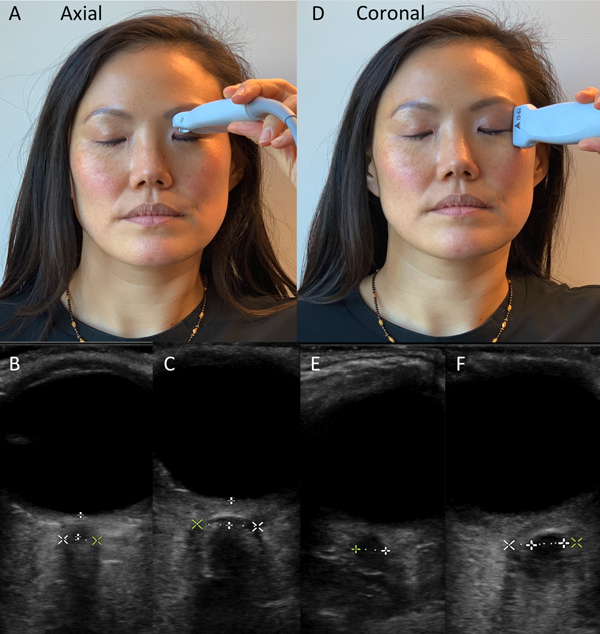
The axial approach involves probe placement as in A. The view obtained through the globe is a pyramidal structure which fans outward posteriorly in the eye. Measurements are made 3mm behind the retina (B). If edema is seen, measurements are made at the outer edge of edema, as presumably that is the optic nerve sheath. The coronal approach (D) involves placing the probe cranial-caudal. The view obtained is a sharply demarcated circle, measured at its widest diameter (E). If edema is seen the measurement is taken at the outer edge (F, outer calipers).
Data Storage
Study data was collected and managed using REDCap electronic data capture tools hosted at University of California, San Francisco. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies.(36)
Statistical Analysis
Research data was analyzed with STATA/IC 15.1 (StataCorp 2017). Coronal measurements and axial measurements were compared with a paired T-test of mean difference between measurements in each eye. Four predictor variables were predetermined prior to analysis: Highest axial measurement of optic nerve sheath diameter between right and left eye, mean axial measurement between eyes, highest coronal measurement, and mean coronal measurement. The first intracranial pressure was measured after the placement of an EVD or parenchymal monitor. However, because the process of placing an EVD can often result in the loss of CSF, we suspected that initial ICP measurement may be inaccurate. Thus, our predetermined secondary outcome included the highest ICP recorded over the first 24 hours. Outcome variables were analyzed as both continuous variables and as dichotomized variables with a cutpoint of ICP = 20 cm H20 since this is generally accepted as clinically significant intracranial hypertension. ICPs were measured and recorded at least hourly in our ICU, or more frequently if clinical changes warranted. Each predictor was compared to the outcome with a nonparametric Spearman’s rank correlation coefficient (continuous outcome) and nonparametric Wilcoxon Rank-Sum test (dichotomized outcomes). Area Under the Receiver Operating Characteristic (AUROC) was calculated for each test and outcome. Optimum test cutoffs were chosen to maximize sensitivity in identifying patients with high ICP. In order to test the fragility of our significant results, we performed a bootstrap sample with 50 repetitions from our cohort.
Results
Baseline characteristics from 20 patients are summarized in Table 1. The average age was 53 years (range 21–85 years), and most patients had either subarachnoid hemorrhage (35%) or intracerebral hemorrhage (25%). 45% of our patients received mannitol or other ICP lowering therapies, and 10% required surgical decompressive hemicraniectomy. ICU mortality among our cohort was 20%. The mean time from ONSD measurement to first ICP measurement after EVD placement was 82 minutes (range 0–196 minutes). Median first ICP measurement was 9.5 cm H2O (IQR: 5.5–17.5) with median highest ICP within 24 hours of 18 cm H2O (IQR: 13–26.5).
Table 1:
Baseline Characteristics
| N = 20 Patients | |
|---|---|
| Variables | Number of patients (%) |
| Age | 53.2 years (range 21–85) |
| Male Gender | 5 (25%) |
| Admission Diagnosis | |
| Subarachnoid Hemorrhage | 7 (35%) |
| Intracerebral Hemorrhage | 5 (25%) |
| Infection-related Hydrocephalus | 4 (20%) |
| Tumor-related Hydrocephalus | 3 (15%) |
| Traumatic Brain Injury | 1 (5%) |
| Midline shift on Imaging | 6 (30%) |
| Mannitol or Hypertonic Saline | 9 (45%) |
| Decompressive Hemicraniectomy | 2 (10%) |
| Intensive Care Unit Mortality | 4 (20%) |
Among all patients, the mean axial measurement between eyes was 5.6mm (range 3.7–8.1mm) and mean coronal measurement was 5.7mm (range 3.1–7.6mm). Coronal measurements showed smaller differences between each eye than axial measurements (mean difference 0.5mm vs. 1mm, p=0.03). Both mean and highest axial diameters were higher in patients who had high first measured ICP or high ICP within the first 24 hours (p=0.006–0.03). However, coronal measurements did not meet statistical significance (Table 2). Both mean and highest axial measurements had strong correlation with ICP within the first 24 hours (Rho = 0.7, 0.79 respectively, p<0.0005 for each, Figure 2) and moderate correlation with first measured ICP (Rho = 0.4, 0.5 respectively, p=0.07 and p=0.02). Mean and highest coronal measurements showed moderate correlation at best with initial ICP and highest ICP within 24 hours (Rho 0.4–0.5, p=0.02–0.1).
Table 2:
Summary Statistics (in mm)
| Mean (SD) | ||||
|---|---|---|---|---|
| Measurement Technique | First ICP measurement >20mmHg | ICP measurement >20 mmHg within 24 hours | ||
| Yes (N=4) | No (N=16) | Yes (N=8) | No (N=12) | |
| Highest Axial Measurement | 7.5* (1) | 5.7* (1) | 7.1* (0.9) | 5.4* (0.7) |
| Highest Coronal Measurement | 6.9 (1) | 5.7 (1.4) | 6.5 (1.2) | 5.5 (1.4) |
| Average Axial Measurement | 6.6* (1.1) | 5.3* (0.9) | 6.4* (0.9) | 5.1* (0.8) |
| Average Coronal Measurement | 6.8 (0.9) | 5.4 (1.4) | 6.4 (1.2) | 5.2 (1.4) |
ICP = Intracranial Pressure, SD = Standard Deviation
Statistically significant p<0.05
Figure 2:
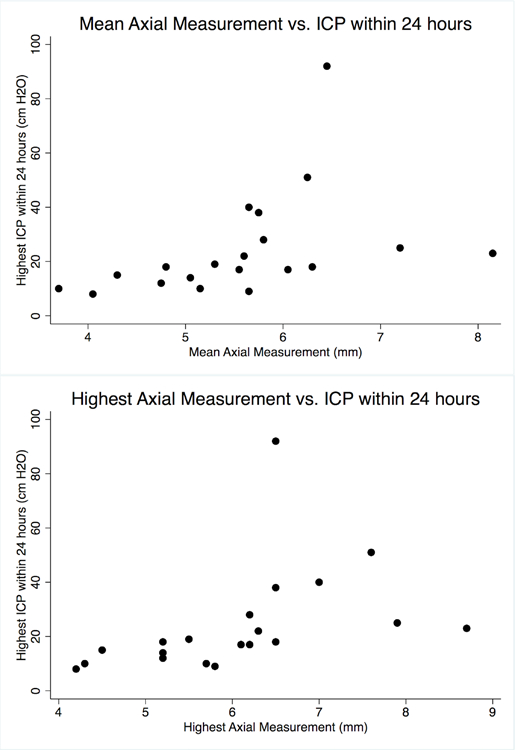
Both mean (top graph) and highest (bottom graph) axial measurements had strong correlation with ICP within the first 24 hours (Rho = 0.7, 0.79 respectively, p<0.0005 for each).
Both mean and highest coronal measurements were correlated with first ICP measurement (AUROC 0.8 and 0.73, respectively) and highest ICP measurement (AUROC 0.74, 0.7) within 24 hours (Figure 3). Mean and highest axial measurements were also correlated with first (AUROC 0.87, 0.94) and highest ICP measurement (AUROC 0.89, 0.96) within 24 hours. Highest axial measurement had better prediction of high ICP within 24 hours than highest coronal measurement (AUROC 0.96 vs. 0.7, p=0.04), while other comparisons did not meet statistical significance.
Figure 3:
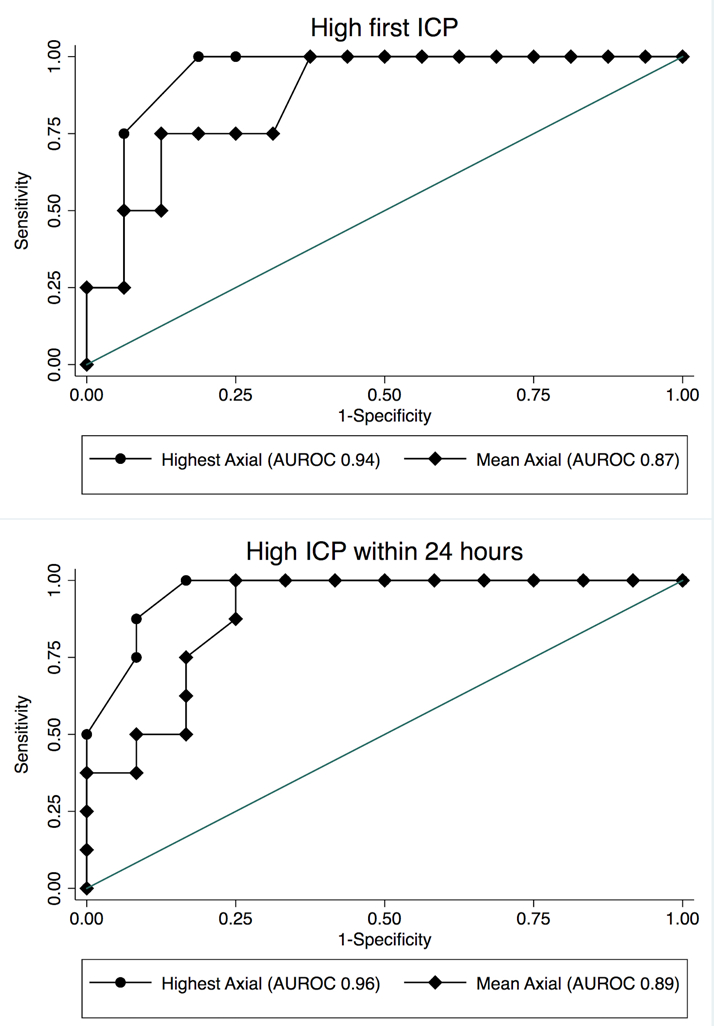
Both highest axial measurement and mean axial measurement of the optic nerve sheath diameter predict high ICP (>20 mmHg) on first measurement (Top graph) and over the first 24 hours (Bottom graph).
A cutoff of highest axial measurement in either eye greater than 6.2mm had a sensitivity of 100% and specificity of 83% in predicting high ICP over the following 24 hours and a sensitivity of 100% and specificity of 63% at predicting high ICP first measured after EVD placement (positive predictive value [PPV] 80% and 57% respectively, negative predictive values [NPV] both 100%). A mean axial measurement between eyes of 5.6mm also had a sensitivity of 100% and specificity of 75% in predicting high ICP over the following 24 hours and a sensitivity of 100% and specificity of 63% at predicting high ICP first measured after EVD placement (PPV 73% and 40% respectively, NPVs both 100%).
One patient in our population required surgical hemicraniectomy due to worsening neurologic decompensation and active herniation on CT scan, despite no recorded high ICP events. If this patient was reclassified as having high ICP, then the highest axial measurement of >6.2mm was 100% sensitive and specific in identifying patients who developed high ICP within 24 hours.
In order to test the fragility of our analyses, we performed a bootstrap sample of our main results, which did not change their significance.
Discussion
Despite a large body of literature examining ultrasound of the optic nerve sheath diameter in a variety of healthy and sick patient populations, descriptions of the procedural technique and the image measurement have been poor. To our knowledge, this is the first study to compare two different measurement techniques (axial and coronal) and two different interpretation techniques (highest measurement vs. average measurement) in a diverse patient population at risk for increased intracranial pressure.
Our study confirms that the coronal measurement showed less variability between eyes than the axial view. This is consistent with studies in healthy human volunteers that show the coronal measurement has less artifact and likely more accurate measurement of the optic nerve sheath.(33, 34) However, our study shows that despite the narrower range of variability with the coronal method, the axial method is a better estimate of true increased intracranial pressure. As all prior studies on the coronal method have been done on healthy volunteers, this is a novel finding and may imply that the artifact of the axial method may be important in its clinical utility as a predictor of increased intracranial pressure.
Furthermore, though previous studies have either averaged measurements between eyes or chosen to evaluate the highest measurement between eyes, we directly compared the test characteristics of both of these interpretation methods. We found that the largest axial diameter in either eye was the most predictive of patients who were diagnosed with high ICP after placement of their intracranial monitor or within 24 hours afterwards. Our cutoff of 6.2mm for highest diameter and 5.6mm for mean diameter for 100% sensitivity is consistent with the range seen in prior studies. Similarly, our excellent AUROC is comparable to the higher range of previously published studies.
Our study has a few unique strengths compared to other literature. First, the direct comparison of axial vs. coronal and highest vs. mean measurements has not previously been reported. Second, our study was performed with a diverse patient population including a range of potential risk factors for increased intracranial pressure. As a result, our findings are likely to have broader generalizability to other patients at risk for high ICP.
Our decision to make our ONSD measurements prior to placement of invasive intracranial monitoring deserves further discussion. Because there was a delay of 82 minutes on average between our measurements and the first recorded ICP, it is possible that ICP may have changed in the interim due to medical management (hypertonic therapy) or CSF decompression while placing the intracranial monitor. For this reason, we prespecified another outcome, increased ICP within the first 24 hours, presuming that patients who presented with high ICP who were temporized would be likely to have repeated events of intracranial hypertension. However, it is not clear that making our ONSD measurement simultaneously with invasive ICP measurement would have eliminated this problem. Though there are some studies that show transient increases in ICP are translated to the optic nerve sheath immediately, others show that after prolonged intracranial hypertension the optic nerve can remain dilated and take time to return to normal even after increased ICP has been relieved.(2, 37) Thus, invasive pressure measurements made immediately after EVD placement may show “normal” ICP after CSF loss but still enlarged optic nerve sheath by ultrasound and be subject to the same error. By making our ONSD measurements prior to placement of an intracranial monitor, we preserve two strengths. First, we preserve blinding for the clinician performing the scan and reduce the introduction of bias. Second, this timing mimics the real-world utility of the technique, where it would be used prior to invasive monitor placement to identify patients at risk of decompensation at that time or over the next day and perhaps arrange for transfer if at a facility that lacks advanced neurosurgical care.
There are two main limitations with the interpretation of our study. First, given the four predictors and the two outcomes, there is the potential for false positives due to multiple comparisons. We reduced this risk by prespecifying our predictors and outcomes and following a prespecified statistical analysis plan, and our strongest findings survive even an overly conservative 8-fold Bonferroni correction. We are also encouraged that our findings replicate test characteristics in past studies, so we believe that this risk is low. Second, though we had an adequate number of patients to answer our primary question, our overall number of patients studied was low. This can increase the risk of variability in our data, and we did not have power to explore subgroups such as the effect of mannitol or hypertonic saline on our measurements or to analyze outcomes such as mortality. Lastly, because our ONSD measurement was not performed at the exact same time as ICP measurement, we relied on surrogate outcome measures, such as first ICP measured or highest ICP measured in the first 24 hours.
Conclusions
We performed a blinded observational study in two academic tertiary referral centers to compare multiple potential measurement techniques of the optic nerve sheath diameter in a diverse population of patients at risk for increased intracranial pressure. Our results suggest that the highest measured diameter in either eye using the axial technique is the most likely to be predictive of high first-measured ICP as well as high ICP over the next 24 hours. In a group of 20 patients, this measurement showed promising test characteristics and a clinically-relevant cutoff of 6.2mm that had 100% sensitivity for identifying patients at risk of decompensation. Future studies should prospectively validate this cutoff with larger patient cohorts.
Acknowledgements
We would like to thank Jenny Wilson MD and Nathan Teismann MD for their advice and support with this project as well as Isabel Allen PhD for statistical advice.
This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through UCSF-CTSI Grant Number UL1 TR001872. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Fujifilm Sonosite provided an ultrasound probe for the duration of this study. They did not provide any direct financial contribution to the project.
Footnotes
Potential conflicts of interest: Sonosite provided a loaner ultrasound probe for the duration of this project. There were no direct financial contributions to the project nor to any authors on this manuscript.
Copyright form disclosure: Dr. Agrawal received support for article research from the National Institutes of Health; he disclosed that Sonosite provided a loaner ultrasound probe for the study which was returned at study termination; and he disclosed that the authors are employees of the University of California, which is a state government agency. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Hansen HC, Helmke K: Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J Neurosurg 1997; 87:34–40 [DOI] [PubMed] [Google Scholar]
- 2.Maissan IM, Dirven PJAC, Haitsma IK, et al. : Ultrasonographic measured optic nerve sheath diameter as an accurate and quick monitor for changes in intracranial pressure. J Neurosurg 2015; 123:743–747 [DOI] [PubMed] [Google Scholar]
- 3.Ballantyne SA, O’Neill G, Hamilton R, et al. : Observer variation in the sonographic measurement of optic nerve sheath diameter in normal adults. Eur J Ultrasound 2002; 15:145–149 [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Ding G-S, Zhao Y-C, et al. : Ultrasound measurement of optic nerve diameter and optic nerve sheath diameter in healthy Chinese adults. BMC Neurol 2015; 15:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goeres P, Zeiler FA, Unger B, et al. : Ultrasound assessment of optic nerve sheath diameter in healthy volunteers. J Crit Care 2016; 31:168–171 [DOI] [PubMed] [Google Scholar]
- 6.Helmke K, Hansen HC: Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension II. Patient study. Pediatr Radiol 1996; 26:706–710 [DOI] [PubMed] [Google Scholar]
- 7.Newman WD, Hollman AS, Dutton GN, et al. : Measurement of optic nerve sheath diameter by ultrasound: a means of detecting acute raised intracranial pressure in hydrocephalus. Br J Ophthalmol 2002; 86:1109–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaivas M, Theodoro D, Sierzenski PR: Elevated intracranial pressure detected by bedside emergency ultrasonography of the optic nerve sheath. Acad Emerg Med 2003; 10:376–381 [DOI] [PubMed] [Google Scholar]
- 9.Tayal VS, Neulander M, Norton HJ, et al. : Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med 2007; 49:508–514 [DOI] [PubMed] [Google Scholar]
- 10.Geeraerts T, Launey Y, Martin L, et al. : Ultrasonography of the optic nerve sheath may be useful for detecting raised intracranial pressure after severe brain injury. Intensive Care Med 2007; 33:1704–1711 [DOI] [PubMed] [Google Scholar]
- 11.Kimberly HH, Shah S, Marill K, et al. : Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med 2008; 15:201–204 [DOI] [PubMed] [Google Scholar]
- 12.Goel RS, Goyal NK, Dharap SB, et al. : Utility of optic nerve ultrasonography in head injury. Injury 2008; 39:519–524 [DOI] [PubMed] [Google Scholar]
- 13.Moretti R, Pizzi B: Optic nerve ultrasound for detection of intracranial hypertension in intracranial hemorrhage patients: confirmation of previous findings in a different patient population. J Neurosurg Anesthesiol 2009; 21:16–20 [DOI] [PubMed] [Google Scholar]
- 14.Skoloudík D, Herzig R, Fadrná T, et al. : Distal enlargement of the optic nerve sheath in the hyperacute stage of intracerebral haemorrhage. Br J Ophthalmol 2011; 95:217–221 [DOI] [PubMed] [Google Scholar]
- 15.Rajajee V, Vanaman M, Fletcher JJ, et al. : Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care 2011; 15:506–515 [DOI] [PubMed] [Google Scholar]
- 16.Amini A, Kariman H, Arhami Dolatabadi A, et al. : Use of the sonographic diameter of optic nerve sheath to estimate intracranial pressure. Am J Emerg Med 2013; 31:236–239 [DOI] [PubMed] [Google Scholar]
- 17.Frumin E, Schlang J, Wiechmann W, et al. : Prospective analysis of single operator sonographic optic nerve sheath diameter measurement for diagnosis of elevated intracranial pressure. West J Emerg Med 2014; 15:217–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robba C, Cardim D, Tajsic T, et al. : Ultrasound non-invasive measurement of intracranial pressure in neurointensive care: A prospective observational study. PLoS Med 2017; 14:e1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soldatos T, Chatzimichail K, Papathanasiou M, et al. : Optic nerve sonography: a new window for the non-invasive evaluation of intracranial pressure in brain injury. Emerg Med J 2009; 26:630–634 [DOI] [PubMed] [Google Scholar]
- 20.Dubourg J, Javouhey E, Geeraerts T, et al. : Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med 2011; 37:1059–1068 [DOI] [PubMed] [Google Scholar]
- 21.Dubourg J, Messerer M, Karakitsos D, et al. : Individual patient data systematic review and meta-analysis of optic nerve sheath diameter ultrasonography for detecting raised intracranial pressure: protocol of the ONSD research group. Syst Rev 2013; 2:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohle R, McIsaac SM, Woo MY, et al. : Sonography of the Optic Nerve Sheath Diameter for Detection of Raised Intracranial Pressure Compared to Computed Tomography: A Systematic Review and Meta-analysis. J Ultrasound Med 2015; 34:1285–1294 [DOI] [PubMed] [Google Scholar]
- 23.Karim S, Clark RA, Poukens V, et al. : Demonstration of systematic variation in human intraorbital optic nerve size by quantitative magnetic resonance imaging and histology. Invest Ophthalmol Vis Sci 2004; 45:1047–1051 [DOI] [PubMed] [Google Scholar]
- 24.Lagrèze WA, Lazzaro A, Weigel M, et al. : Morphometry of the retrobulbar human optic nerve: comparison between conventional sonography and ultrafast magnetic resonance sequences. Invest Ophthalmol Vis Sci 2007; 48:1913–1917 [DOI] [PubMed] [Google Scholar]
- 25.Bäuerle J, Schuchardt F, Schroeder L, et al. : Reproducibility and accuracy of optic nerve sheath diameter assessment using ultrasound compared to magnetic resonance imaging. BMC Neurol 2013; 13:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strumwasser A, Kwan RO, Yeung L, et al. : Sonographic optic nerve sheath diameter as an estimate of intracranial pressure in adult trauma. J Surg Res 2011; 170:265–271 [DOI] [PubMed] [Google Scholar]
- 27.Dubost C, Geeraerts T: Possible pitfalls when measuring the optic nerve sheath with sonography. J Surg Res 2012; 173:e43–4; author reply e44–5 [DOI] [PubMed] [Google Scholar]
- 28.Copetti R, Cattarossi L: Optic nerve ultrasound: artifacts and real images. Intensive Care Med 2009; 35:1488–9; author reply 1490–1 [DOI] [PubMed] [Google Scholar]
- 29.Geeraerts T, Bergès O, Merceron S, et al. : Reply to Copetti and Cattarossi. Intensive Care Med 2009; 35:1490–1491 [Google Scholar]
- 30.Teismann NA, Lenaghan P, Stein J, et al. : Will the real optic nerve sheath please stand up? J Ultrasound Med 2012; 31:130–131 [DOI] [PubMed] [Google Scholar]
- 31.Gans MS, Byrne SF, Glaser JS: Standardized A-scan echography in optic nerve disease. Arch Ophthalmol 1987; 105:1232–1236 [DOI] [PubMed] [Google Scholar]
- 32.Beatty S, Good PA, McLaughlin J, et al. : Echographic measurements of the retrobulbar optic nerve in normal and glaucomatous eyes. Br J Ophthalmol 1998; 82:43–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blehar DJ, Gaspari RJ, Montoya A, et al. : Correlation of visual axis and coronal axis measurements of the optic nerve sheath diameter. J Ultrasound Med 2008; 27:407–411 [DOI] [PubMed] [Google Scholar]
- 34.Amini R, Stolz LA, Patanwala AE, et al. : Coronal Axis Measurement of the Optic Nerve Sheath Diameter Using a Linear Transducer. J Ultrasound Med 2015; 34:1607–1612 [DOI] [PubMed] [Google Scholar]
- 35.Teismann N: What is the Correct Approach for Measuring Optic Nerve Sheath Diameter Using Point-Of-Care Ultrasound? A Comparison of Two Techniques
- 36.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajajee V, Fletcher JJ, Rochlen LR, et al. : Comparison of accuracy of optic nerve ultrasound for the detection of intracranial hypertension in the setting of acutely fluctuating vs stable intracranial pressure: post-hoc analysis of data from a prospective, blinded single center study. Crit Care 2012; 16:R79. [DOI] [PMC free article] [PubMed] [Google Scholar]


