Abstract
Dicer1 functions as a tumor suppressor in mouse models. In humans, somatic mutations are associated with many cancers in adults and DICER1 syndrome patients with DICER1 germline mutations are susceptible to childhood cancers. Dicer is phosphorylated by the ERK-MAP kinase pathway and since this pathway is activated in human cancers, we asked whether phosphorylated Dicer1 contributed to tumor development. In human endometrioid cancers, we discovered that phosphorylated DICER1 is significantly associated with invasive disease. To test a direct involvement of Dicer1 phosphorylation in tumor development, we studied mice with phospho-mimetic alterations at the two conserved Serines phosphorylated by ERK and discovered that a phospho-mimetic Dicer1 drives tumor development and dissemination in two independent murine cancer models (KRas+/LA1 and p53+/−). Our findings demonstrate that Dicer1 phospho-mimetic promotes tumor development and invasion.
Keywords: ERK signaling, endometrioid cancers, p53, KRas, mouse models
Introduction:
Dicer1 is an essential ribo-endonuclease that processes pre-microRNAs into functional microRNAs (1–3). DICER1 acts as a haploinsufficient tumor suppressor: somatic heterozygous mutations are frequently observed in human cancers and heterozygosity promotes tumorigenesis in several murine tumor models (4–8). DICER1 syndrome patients carry germline heterozygous mutations in DICER1 (missense and truncating), and present with increased risk for a variety of cancers including pleuropulmonary blastoma, ovarian sex cord stromal tumors, cystic nephroma, and thyroid cancer (8–10).
Dicer (CDR-1) is phosphorylated by ERK in the KRAS signaling axis in C. elegans (11). In this context, phosphorylated Dicer is nuclear and drives the oocyte to embryo transition, indicating a novel role for Dicer during this key reprogramming event in development (11). The phosphorylation sites (Serines 1712 and 1836 in mice, and Serines 1728 and 1852 in humans) and the nuclear localization of phospho-Dicer1 are well conserved in murine and human cells (11). Using phospho-specific antibodies to cognate residues in human DICER1, we found that DICER1 was phosphorylated in response to fibroblast growth factor activation in cultured human cells (11). We therefore examined a role for Dicer1 phosphorylation in tumor development by assaying human primary endometrioid tumors and by evaluating tumorigenesis in a phospho-mimetic Dicer1 knock-in mouse model wherein Serines 1712 and 1836 were replaced with Aspartic acids. We discovered that 1) phosphorylated DICER1 is present in majority of endometrioid cancers, and is significantly associated with invasive disease and 2) phospho-mimetic Dicer1 drives tumor development and dissemination in two independent murine cancer models (KRas+/LA1 and p53+/−). Our findings implicate phosphorylated Dicer1 in tumor development.
Materials and Methods:
Immunohistochemistry of human tumors
Immunohistochemistry was performed on a tumor microarray (TMA) of 54 primary endometrioid cancer cases. These are untreated tumor samples. Four separate cores were obtained from areas of viable tumor tissue in paraffin blocks of formalin-fixed tumors. The areas for inclusion in the TMA were determined by a gynecologic pathologist. The tumor microarray was then created as previously described (12). Practical methods for tissue microarray construction has been previously described (12). Five micrometer sections of the TMA block were obtained and stained for anti-phospho-DICER1 and anti-phospho-ERK monoclonal antibodies. Anti-phospho-DICER1 monoclonal antibodies were generated in house and tested for specificity as described previously (11). Phospho-ERK staining was performed with anti-phospho-ERK (Thr 202/Tyr 204, #9101 S, rabbit anti-human, Cell Signaling Technology, Inc., Danvers, MA) at 1:100 dilution. Slides were then incubated MACH2 Universal polymer-HRP (#M2U522H, BioCare Medical,LLC., Concord CA). Signals were developed with the 3,3’-diaminobenzidine (DAB) substrate DAB+ Liquid (#K3468, Dako Cytomation, Carpintera, CA ). Slides were read by two blinded independent examiners (BJR). Staining for each antibody was reviewed and recorded as a percentage of cells with nuclear stain and a percentage of cells with cytoplasmic stain. The percentages were averaged between examiners.
Mice:
KRasLA1, p53− and Dicer2SD alleles have been published (13–15). All mice were maintained in >90% C57BL/6 background. All mouse studies were conducted in compliance with an Institutional Animal Care and Use Committee (IACUC) approved protocol.
We crossed Dicer+/2SD and KRas+/LA1 mice to generate a cohort of KRas+/LA1 and KRas+/LA1; Dicer+/2SD mice. The cohort was monitored for 400 days. Moribund animals were euthanized, and their tissues were collected. Animals that were still alive at 400 days were sacrificed and their tissues were collected for pathology. Animals that were euthanized due to non-tumor related issues (dermatitis, rectal prolapse) were not included in the study.
Similarly, we introduced the Dicer2SD allele in the p53+/− driven tumor model. A cohort containing 19 p53+/− mice and 32 p53+/−; Dicer+/2SD mice was generated. The cohort was monitored for 540 days. Moribund animals were euthanized, and their tissues were collected. Animals that were still alive at 540 days were sacrificed and their tissues were collected for pathology. Animals that were euthanized due to non-tumor related issues (dermatitis, rectal prolapse) were not included in the study. Tissues were collected, sectioned and examined for pathology at the same time.
Histopathology:
Tissues harvested from mice were fixed in 10% neutral buffered formalin and paraffin-embedded. Four micrometer sections were stained with hematoxylin and eosin, and examined by light microscopy. Tissue processing, paraffin embedding, sectioning, and H&E staining were performed by the MD Anderson Department of Veterinary Medicine & Surgery Histology Laboratory.
Immunofluorescence staining:
Tissues were harvested from mice, fixed in 10% neutral buffered formalin and paraffin embedded. Tissue processing was performed by the MD Anderson Department of Veterinary Medicine & Surgery Histology Laboratory. Immunofluorescence to detect phosphorylation of Dicer1 was conducted with monoclonal phospho-Dicer1 specific antibodies (clones 1712–26 and 1836–25) (1:200) (11), and DAPI. For each genotype, 50 images at 63X were captured across the full slide. Each image captured 200–500 cells. To avoid bias, staining, image capture, and analyses were performed in a blinded manner.
Statistical Analysis:
Student’s t-tests, Fisher’s exact tests and Kaplan-Meier survival analyses were performed using GraphPad Prism 7 Software. P-values were calculated using Log-rank (Mantel-Cox) test and p<0.05 was considered statistically significant.
Results
DICER1 phosphorylation correlates with endometrioid tumor invasion
Dicer phosphorylation at Serines 1712 and 1836 and nuclear translocation is mediated by Erk downstream of the KRas signaling pathway (11). We therefore examined DICER1 phosphorylation status in 54 endometrioid cancers with increased KRAS or hormonal signaling by immunohistochemistry for phospho-DICER1 and phospho-ERK on a tissue microarray. These 54 endometrioid tumors had an intact DNA mismatch repair (MMR) pathway based on prior microsatellite instability analyses (16). Endometrioid tumors with an intact MMR pathway do not present with DICER1 mutations. POLE mutations also were not identified in these samples (16).
To examine the status of phospho-DICER1 in these tumors, we used two phospho-Dicer1 antibodies mixed together that recognize individual phosphorylated Serines 1728 and 1852. The specificities of these reagents have been validated in C. elegans, murine uterine tissues and human cell lines (11,17). Tumors were also analyzed for phospho-ERK. Each tumor with >10% of cells with phospho-DICER1 or phospho-ERK signal was labeled positive (Figure 1). Phosphorylated DICER1 appears nuclear in all our assays as shown previously (Figure 1) [11, 14]. We observed that 63% (32/51) of endometrioid tumors (three cases were not evaluated due to >2 lost cores of 5 examined for each tumor) were positive for phospho-DICER1 (Figure 1). Overall, 84% of tumors that were positive for phospho-DICER1 were also positive for phospho-ERK (27/32) and 5 were negative for phospho-ERK (either nuclear or cytoplasmic). Of the 19 tumors that were negative for phospho-DICER1, 12 were also negative for phosphor-ERK. The distribution of phospho-DICER1 positive cells did not perfectly overlap with phospho-ERK, which could be an indication of the kinetics of phospho-ERK versus phospho-Dicer turnover due to the presence of different phosphatases. Five tumors that were positive for phospho-Dicer1 were negative for phospho-ERK.
Figure 1: DICER1 phosphorylation is associated with lower BMI and invasion of primary endometrioid cancers.
A. Representative images of endometrioid cancers stained with phospho-specific DICER1 antibodies (top panels) and a phospho ERK antibody (bottom panels). B. Higher magnification of one endometrioid cancer sample displaying nuclear phosphorylated DICER1 staining. C. Based on the number of cells that were positive for phospho-DICER1 (panel A), the tumors were divided into “low to moderate” positivity for phosphorylated DICER1 or “high” positivity for phosphorylated DICER1. Bar graphs display the distribution of tumors with low-to-medium or high phospho-DICER1 with clinicopathologic feature. BMI, body mass index; LVSI, lymphovascular space invasion; Invasion, depth of myometrial invasion. p<0.01 for BMI, p=0.03 for LVSI, and p=0.02 for depth of myometrial invasion.
To dissect the relationship between DICER1 phosphorylation and clinicopathologic features in endometrioid cancers, we classified each tumor with less than 50% of cells with phospho-DICER1 as “low-to-moderate” for DICER1 phosphorylation (n =35), and tumors with over 50% of cells with phospho-DICER1 as “high” for DICER1 phosphorylation (n=16). High phospho-DICER1 was significantly associated with lower body mass index (p<0.01) and deep myometrial invasion (p=0.02) (Figure 1C, Table 1). In addition, there was also a trend wherein presence of lymphovascular space invasion was higher in tumors with high phospho-DICER1 (p=0.03). Deep myometrial invasion and increased lymphovascular space invasion are features of poor outcomes in these cancers. Together, these data indicate that presence of nuclear phosphorylated DICER1 correlates with lower BMI and endometrioid tumor invasion.
Table 1:
Relationship between phospho-DICER1 levels and clinicopathologic features in endometrioid cancer
| Clinicopathologic Factor | Nuclear DICER1 Positivity | |||
|---|---|---|---|---|
| Low | Medium | High | p value* | |
| N (%) | N (%) | N (%) | ||
| Age | ||||
| <60 years | 5 | 10 | 7 | 0.10 |
| ≥60 years | 14 | 6 | 9 | |
| BMI | ||||
| < 25 (normal or underweight) | 2 | 2 | 8 | <0.01 |
| ≥25–30 (overweight) | 16 | 14 | 6 | |
| Grade | ||||
| 1 | 9 | 9 | 9 | 0.80 |
| 2 | 3 | 3 | 1 | |
| 3 | 7 | 4 | 6 | |
| Stage | ||||
| I or II | 12 | 11 | 8 | 0.54 |
| III or IV | 7 | 5 | 8 | |
| LVSI | ||||
| Present | 7 | 2 | 9 | 0.03 |
| Absent | 12 | 14 | 7 | |
| Depth of invasion | ||||
| <50% | 10 | 13 | 5 | 0.02 |
| ≥50% | 9 | 3 | 11 | |
Fisher’s exact test
BMI (body mass index) data missing for 3 cases.
LVSI (lymphovascular space invasion)
Phospho-mimetic Dicer1 cooperates with KRasG12D in transformation
To determine whether phosphorylated Dicer1 could drive tumor progression in vivo, we generated a Dicer1 knock-in mouse model by replacing Serines 1712 and 1836 (corresponding to human Serines 1728 and 1852) with aspartic acid which mimics phosphorylation and renders the protein impervious to dephosphorylation by phosphatases (13). Dicer1 Serines 1712 and 1836 were replaced with Aspartic acids (Dicer2SD) at the endogenous locus. Homozygosity of Dicer1 phospho-mimetic mutations results in highly penetrant post-natal lethality (78%). The few surviving mice are infertile and display accelerated aging phenotypes (13). However, heterozygous mutants are phenotypically normal (13). In light of our observations above that phospho-DICER1 was present in tumors, we tested whether phospho-Dicer1 could modulate tumor progression in two independent cancer models. To assess cancer phenotypes independently of developmental lethality displayed by the homozygous mutants, we used heterozygous Dicer2SD mice in two distinct cancer models carrying KRasLA1 or p53− mutations (14, 15).
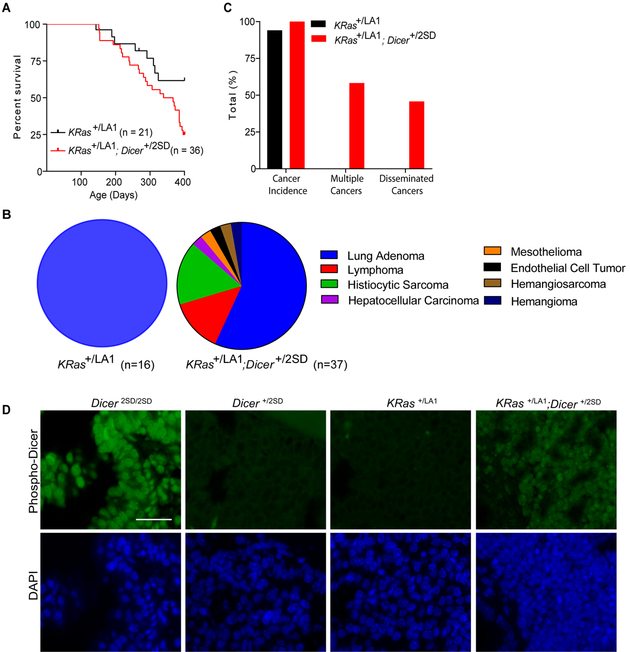
The KRasLA1 allele consists of a duplicated exon1, one of which contains a KRasG12D mutation, that undergoes spontaneous recombination yielding either wild type or mutant KRas alleles (15). This model gives rise to fully penetrant multifocal lung cancer starting at one week of age and low frequency thymic lymphomas that vary by the background of the mouse. We chose this tumor model because heterozygosity of Dicer1 cooperates with KRasG12D to drive lung cancer development in mice. We thus generated a cohort of KRas+/LA1 and KRas+/LA1; Dicer+/2SD mice. KRas+/LA1; Dicer+/2SD mice contain one phospho-mimetic Dicer1 allele that mimics constitutive phosphorylation and one wild type Dicer1 allele that can be potentially regulated by KRas (4). In this background, KRas+/LA1; Dicer+/2SD mice developed a wide spectrum of tumors and had a significantly reduced median survival of 353 days as compared with KRas+/LA1 mice which did not achieve median age at the 400-day end point of the study (p=0.03, Figure 2A). KRas+/LA1 mice developed only lung adenomas/adenocarcinomas (16/17). While KRas+/LA1; Dicer+/2SD mice also developed lung adenocarcinomas (21/24), 58% of mice (14/24) developed additional tumors which included 21% lymphomas (5/24, includes 3 animals without lung adenocarcinoma), 25% histiocytic sarcoma (6/24), and one each of hepatocellular carcinoma, mesothelioma, endothelial cell tumor, hemangiosarcoma and hemangioma (Figures 2B and 2C, Supplementary Figure 1, Table 2). Of note, all lymphomas and histiocytic sarcomas from KRas+/LA1; Dicer+/2SD mice disseminated into multiple organs including lung, liver, heart, kidney, spleen, and thymus (Supplementary Figure 1; Table 2). In one KRas+/LA1; Dicer+/2SD mouse, a lung adenocarcinoma metastasized to the kidney and heart (Supplementary Figure 1).
Figure 2: Phospho-mimetic Dicer1 promotes tumorigenesis in KRas+/LA1 mice.
A. Kaplan-Meier survival curves of KRas+/LA1 mice (n=21) and KRas+/LA1; Dicer+/2SD mice (n=36) followed for 400 days. p=0.03. B. Tumor spectrum of KRas+/LA1 mice (n=16 cancers in 17 mice) and KRas+/LA1; Dicer+/2SD mice (n=37 cancers in 24 mice). C. Frequencies of mice with cancer, multiple cancers, and disseminated cancers in KRas+/LA1 mice (n=17) and KRas+/LA1; Dicer+/2SD mice (n=24). D. Representative immunofluorescence images of lung tissue captured at 63X displaying phosphorylated Dicer1 in green and DAPI in blue, in mice with the indicated genotypes. Scale bar: 50 microns.
Table 2:
Tumors in KRas+/LA1 and KRas+/LA1; Dicer+/2SD mice
| Tumor Type | KRas+/LA1 (n=17) | KRas+/LA1;Dicer+/2SD (n=24) |
|---|---|---|
| Lung Adenocarcinoma | ||
| 16 | 21(1*) | |
| Other Tumors | ||
| Lymphoma | 0 | 5(5*) |
| Histiocytic sarcoma | 0 | 6(6*) |
| Hepatocellular carcinoma | 0 | 1 |
| Mesothelioma | 0 | 1 |
| Endothelial cell tumor | 0 | 1 |
| Hemangiosarcoma | 0 | 1 |
| Hemangioma | 0 | 1 |
| Mice with >1 Tumor Types | 0 | 14 |
| Total mice with tumors | 16 | 24 |
*dissemination/metastasis
To determine whether the phospho-mimetic Dicer1 protein behaved as a constitutively phosphorylated protein, we performed immunofluorescence on lung tissues from each of the genotypes using the phospho-Dicer1 antibodies. All Dicer2SD/2SD lung cells were positive for phospho-Dicer1 and all displayed nuclear localization whereas Dicer+/2SD lung tissues showed little staining likely due to low levels of phosphorylated Dicer1 (Figure 2D). KRas+/LA1 lung tissues showed no staining. In contrast, KRas+/LA1; Dicer+/2SD lung sections showed that >70% of cells were positive for nuclear phospho-Dicer1 staining indicating that increased KRas signaling results in increased phosphorylation and nuclear translocation of wild type Dicer1 in this model (Figure 2D). Combined, these results indicate that phospho-mimetic Dicer1 is nuclear and cooperates with oncogenic KRasG12D resulting in a wide spectrum of tumors, and metastases.
Phospho-mimetic Dicer1 cooperates with p53 heterozygosity in transformation
TP53 loss is associated with DICER1 mutations and an activated KRAS pathway in many human tumors (4,9,18). To test whether the phospho-mimetic Dicer1 is sufficient to promote tumorigenesis in the context of p53 heterozygosity, we next introduced the Dicer2SD allele into p53+/− mice which primarily develop osteosarcomas and lymphomas (14). Similar to the KRas+/LA1; Dicer+/2SD model, p53+/−; Dicer+/2SD mice (n=32) exhibited a reduced median survival of 497 days as compared with p53+/− mice (n=19) which did not achieve median age at the 540-day end point used in this study (p=0.0855, Figure 3A). Additionally, p53+/−; Dicer+/2SD mice exhibited a wide spectrum of tumors and had a significantly reduced tumor-free survival as compared with p53+/− mice (p=0.0273, Figure 3B). 71% (15/21) of the p53+/−; Dicer+/2SD mice developed tumors as compared with 25% (3/12) of the p53+/− mice (Table 3). In contrast to p53+/− mice which only developed osteosarcomas (n=2) and a lymphoma (n=1), the spectrum of tumors in p53+/−; Dicer+/2SD mice included osteosarcomas (n=6), lymphomas (n=6), histiocytic sarcomas (n=2), hemangiosarcoma, lung adenocarcinoma, hepatocellular carcinoma, testicular carcinoma, and two undifferentiated tumors. 24% of p53+/−; Dicer+/2SD mice (5/24) displayed multiple cancers including one mouse which developed an osteosarcoma, a lymphoma and an adenocarcinoma (Figure 3C and3D; Table 3). Interestingly, lymphomas, histiocytic sarcomas, and the testicular carcinoma were disseminated to many organs, a phenotype also observed in KRas+/LA1; Dicer+/2SD mice (Figure 3D; Table 3). None of the p53+/− mice developed multiple cancers.
Figure 3: Phospho-mimetic Dicer1 promotes tumorigenesis in p53+/−mice.
A. Kaplan-Meier survival curve of p53+/− mice (n=19) and p53+/−; Dicer+/2SD mice (n=32) followed for 540 days. p=0.0855. B. Kaplan-Meier survival analysis of tumor-free survival of p53+/− mice (n=12) and p53+/−; Dicer+/2SD mice (n=21) followed for 540 days. p=0.0273. C. Tumor spectrum of p53+/− mice (n=3 cancers in 12 mice) and p53+/−; Dicer+/2SD mice (n=20 cancers in 21 mice). D. Frequencies of mice with cancer, multiple cancers, and disseminated cancers in p53+/−mice (n=12) and p53+/−; Dicer+/2SD mice (n=21). E. Representative immunofluorescence images of lymphomas and osteosarcomas captured at 63X displaying phosphorylated Dicer in green and DAPI in blue, in mice of the indicated genotypes. Scale bar: 50 microns.
Table 3:
Tumors in p53+/− mice and p53+/−; Dicer+/2SD mice
| Tumor Type | p53+/− (n=12) | p53+/−;Dicer+/2SD (n=21) |
|---|---|---|
| Sarcoma | ||
| Ostersarcoma | 2 | 6 |
| Histiocytic sarcoma | 0 | 2(2*) |
| Hemangiosarcoma | 0 | 1 |
| Unspecified | 0 | 1 |
| Lymphoma | ||
| 1(1*) | 6(3*) | |
| Carcinoma | ||
| Lung adenocarcinoma | 0 | 1 |
| Testicular carcinoma | 0 | 1(1*) |
| Hepatocellular carcinoma | 0 | 1 |
| Unspecified | 0 | 1 |
| Mice with >1 Tumor Type | 0 | 5 |
| Total mice with tumors | 3 | 15 |
*dissemination/metastasis
We next performed immunofluorescence to assess the status of Dicer1 phosphorylation in p53+/−; Dicer+/2SD tumors. We could not detect a signal in p53+/− tumors (Figure 3E). In contrast, p53+/−; Dicer+/2SD lymphomas and osteosarcomas revealed over 60% of cells with positive phospho-Dicer1 nuclear signal. In this genotype, we also evidenced some cytoplasmic signal for the antibody which may be an indication of cytoplasmic retention of the phosphorylated protein. These results demonstrate that phospho-mimetic Dicer in nuclear in tumors from p53+/− mice. Combined, these data suggest that phospho-mimetic Dicer1 is a key driver of tumor progression and dissemination.
Discussion
Dicer1 is phosphorylated by ERK upstream of KRas, a well-known and studied oncogene. We show for the first time the phospho-Dicer1 is present in endometrioid cancers, localized to the nucleus and is associated with invasive disease. In this study, the phosphorylation status of Dicer1 and ERK did not completely overlap. Five tumors that were positive for phospho-Dicer1 were negative for phospho-ERK. This observation suggests that either DICER1 may be phosphorylated by additional MAPK’s or that the perdurance of DICER1 phosphorylation is longer than that of phospho-ERK. This observation highlights lack of knowledge regarding the complete regulation of Dicer1 phosphorylation and possible unique functions in the nucleus.
Based on these data, the role of the Dicer1 phospho-mimetic protein in tumorigenesis was examined in this study. We demonstrate, for the first time, that phospho-mimetic Dicer1 cooperates with two distinct oncogenic lesions, KRasLA1 and p53−, to drive tumorigenesis, a diverse tumor spectrum, and dissemination. Additionally, the development of multiple cancers in a high percentage of mice in both models indicates that phospho-mimetic Dicer1 not only plays a role in tumor development and dissemination, but also sensitizes multiple cell types for tumor development. Finally, these observations in mice coupled with the correlation between phospho-DICER1 and invasive endometrioid cancer in humans indicate that Dicer1 phosphorylation promotes tumor invasion and metastasis. Additional data are needed to determine if phosphorylated DICER1 contributes to a broader spectrum of human cancers. Our discovery highlights a need to further investigate DICER1 phosphorylation status as an indicator of tumor progression and dissemination in human cancers.
Supplementary Material
Significance:
This work highlights the relevance of Dicer1 phosphorylation in mammalian tumor development and dissemination.
Acknowledgements:
NIH grants CA82577 to GL and GMR0198200 to SA partially supported this study. This study is also supported in part by P50CA134254 and P50CA16672. SA is an Andrew Sabin Family Foundation Fellow at The University of Texas MD Anderson Cancer Center. NKA is Gigli Family Endowed Scholar at The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences.
Financial support: CA82577 to GL; GMR0198200 to SA; P50CA134254 to The Ohio State University and James Comprehensive Cancer Center and P50CA16672 to The University of Texas MD Anderson Cancer Center.
Footnotes
The authors have no conflict of interests with regard to this study.
References
- 1.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, et al. Dicer is essential for mouse development. Nat Genet 2003;35:215–7 [DOI] [PubMed] [Google Scholar]
- 2.Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem 2005;280:9330–5 [DOI] [PubMed] [Google Scholar]
- 3.Nicholson RH, Nicholson AW. Molecular characterization of a mouse cDNA encoding Dicer, a ribonuclease III ortholog involved in RNA interference. Mamm Genome 2002;13:67–73 [DOI] [PubMed] [Google Scholar]
- 4.Kumar MS, Pester RE, Chen CY, Lane K, Chin C, Lu J, et al. Dicer1 functions as a haploinsufficient tumor suppressor. Genes Dev 2009;23:2700–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshikawa T, Otsuka M, Kishikawa T, Takata A, Ohno M, Shibata C, et al. Unique haploinsufficient role of the microRNA-processing molecule Dicer1 in a murine colitis-associated tumorigenesis model. PLoS One 2013;8:e71969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambertz I, Nittner D, Mestdagh P, Denecker G, Vandesompele J, Dyer MA, et al. Monoallelic but not biallelic loss of Dicer1 promotes tumorigenesis in vivo. Cell Death Differ 2010;17:633–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arrate MP, Vincent T, Odvody J, Kar R, Jones SN, Eischen CM. MicroRNA biogenesis is required for Myc-induced B-cell lymphoma development and survival. Cancer Res 2010;70:6083–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foulkes WD, Priest JR, Duchaine TF. DICER1: mutations, microRNAs and mechanisms. Nat Rev Cancer 2014;14:662–72 [DOI] [PubMed] [Google Scholar]
- 9.Pugh TJ, Yu W, Yang J, Field AL, Ambrogio L, Carter SL, et al. Exome sequencing of pleuropulmonary blastoma reveals frequent biallelic loss of TP53 and two hits in DICER1 resulting in retention of 5p-derived miRNA hairpin loop sequences. Oncogene 2014;33:5295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein S, Lee H, Ghahremani S, Kempert P, Ischander M, Teitell MA, et al. Expanding the phenotype of mutations in DICER1: mosaic missense mutations in the RNase IIIb domain of DICER1 cause GLOW syndrome. J Med Genet 2014;51:294–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drake M, Furuta T, Suen KM, Gonzalez G, Liu B, Kalia A, et al. A requirement for ERK-dependent Dicer phosphorylation in coordinating oocyte-to-embryo transition in C. elegans. Dev Cell 2014;31:614–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fedor HL, De Marzo AM. Practical methods for tissue microarray construction. Methods Mol Med 2005;103:89–101 [DOI] [PubMed] [Google Scholar]
- 13.Aryal NK, Pant V, Wasylishen AR, Parker-Thornburg J, Baseler L, El-Naggar AK, et al. Constitutive Dicer1 phosphorylation accelerates metabolism and aging in vivo. Proc Natl Acad Sci U S A 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol 1994;4:1–7 [DOI] [PubMed] [Google Scholar]
- 15.Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 2001;410:1111–6 [DOI] [PubMed] [Google Scholar]
- 16.Billingsley CC, Cohn DE, Mutch DG, Stephens JA, Suarez AA, Goodfellow PJ. Polymerase varepsilon (POLE) mutations in endometrial cancer: clinical outcomes and implications for Lynch syndrome testing. Cancer 2015;121:386–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burger K, Schlackow M, Potts M, Hester S, Mohammed S, Gullerova M. Nuclear phosphorylated Dicer processes double-stranded RNA in response to DNA damage. J Cell Biol 2017;216:2373–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rakheja D, Chen KS, Liu Y, Shukla AA, Schmid V, Chang TC, et al. Somatic mutations in DROSHA and DICER1 impair microRNA biogenesis through distinct mechanisms in Wilms tumours. Nat Commun 2014;2:4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.