Abstract
Objective:
To compare estimates of gastric accommodation (GA) with single photon emission computed tomography (SPECT) to measurements based on intragastric meal distribution immediately post-meal ingestion (IMD0).
Methods:
We evaluated 108 diabetics with upper gastrointestinal (UGI) symptoms who had undergone gastric emptying of solids (GE) by scintigraphy and GA measurements by SPECT. Immediately after ingestion of a 99mTc-labeled egg meal (time 0), we estimated IMD0 as radioactive counts or area of the proximal half of the stomach on 2-dimensional images. Gastric volume (GV) during fasting and after 300mL Ensure® was measured by SPECT to quantify accommodation volume (AV) or postprandial to fasting volume ratio (GVR). From the measured proximal gastric area, we estimated the volume of proximal stomach (4/3* π*r3). We performed regression analyses to assess relationships between IMD0 and GA (AV) and GVR.
Results:
There was a significant correlation between area and radioactivity counts in the proximal stomach (r-0.67, P<0.001); however, there was considerable interpersonal variation [bias 0.20 (95%CI −0.07, 0.47)]. There were no significant correlations between total GV or AV or VR by SPECT and measurements using IMD0: proximal gastric counts, area, and estimated volume as continuous variables of dichotomized patient groups, based on published cut-off values. There were no significant differences in total gastric area or the IMD0 parameters (% area or % radioactive counts) between those with and without UGI symptoms except for fullness and satiety.
Conclusions:
IMD0 is not significantly correlated with GA measurement by SPECT.
Keywords: dyspepsia, gastroparesis
Graphical Abstract

We compared estimates of gastric accommodation (GA) with single photon emission computed tomography (SPECT) to measurements based on intragastric meal distribution immediately post-meal ingestion (IMD0) in 108 diabetics with upper gastrointestinal symptoms who had undergone gastric emptying of solids (GE) by scintigraphy and GA measurements by SPECT. There were no significant differences in total gastric area or the IMD0 parameters (% area or % radioactive counts) between those with and without upper gastrointestinal symptoms except for fullness and satiety. IMD0 is not significantly correlated with GA measurement by SPECT.
INTRODUCTION
Measurement of gastric accommodation (GA) in patients with dyspepsia (1,2) or post-fundoplication (3) has previously been conducted with intragastric balloon under barostatic control; using this method, it was estimated that impaired GA to a meal was found in 40% of the patients with dyspepsia (1,2). Unfortunately, this invasive method has been used predominantly in tertiary centers or in research studies. An alternative intubated method uses high resolution manometry to document the reduced phasic pressure in the proximal stomach denoting accommodation (4).
Impaired GA by noninvasive imaging has been documented in about 50% of 1287 patients and in 39% of individuals with diabetes mellitus type 1 or type 2 (DM1 or DM2) presenting with upper gastrointestinal symptoms (5,6). With novel therapeutic approaches available to treat impaired accommodation, such as buspirone (7,8) and acotiamide (9,10) a recent editorial emphasized the importance of measuring this aspect of gastric motor function and recommended the need to develop and validate a readily accessible method to assess GA (11).
Abnormal intragastric distribution of a meal in patients with dyspepsia and associated symptoms has been identified for over two decades (12,13). Moreover, impaired control of fundic accommodation may lead to overload of a hypersensitive antrum and result in symptoms of functional dyspepsia (14).
At present, there are three imaging approaches to measure GA: single photon emission computed tomography (SPECT), magnetic resonance imaging (MRI), and intragastric meal distribution on scintigraphy (IMD). The SPECT method is the most extensively validated method including comparison with barostat measurements (15), measurements in health and disease including effects of fundoplication, comparison of accommodation in response to equicaloric solid and liquid meals (16), documentation of the effects of pharmacological agents including erythromycin, nitrates, and GLP-1, and definition of performance characteristics (17). The SPECT method has been used clinically at a few centers for over 15 years. MRI measurements have also been described (18), but have not been applied widely. More recently, Orthey et al. have proposed the use of intragastric distribution of the radiolabeled meal (IMD) immediately after ingestion (IMD0) as a method to assess GA based on two-dimensional (2-D) scintigraphy (19,20). Although not validated in relation to a gold standard such as the barostat or SPECT or MRI, it was proposed that IMD0 scintigraphic counts <0.568 of total gastric counts indicate impaired GA (19).
Our aim was to compare the validated SPECT measurement of GA to IMD0 in individuals with DM1 or DM2 with upper gastrointestinal symptoms who had previously undergone gastric emptying (GE) and GA measurements.
METHODS
Study Cohort
In 108 adults with DM1 or DM2 evaluated over a period of 18 years, we evaluated original 2-dimensional images obtained during measurements of GE of solids by scintigraphy and GA by SPECT. The demographics, symptoms, complications of diabetes, medications to treat diabetes, and concomitant medications are described in detail elsewhere (6). The study was approved by Mayo Clinic Institutional Review Board (IRB #16-008776-01).
Patient Symptoms and Clusters
From the medical records review of 108 adults with DM1 or DM2 presenting with upper gastrointestinal symptoms, we summarized symptom clusters: nausea/vomiting, belching/heartburn, bloating/abdominal pain, fullness/satiety, and abdominal discomfort/pain (6).
Measurements of Gastric Emptying and Gastric Accommodation
After a 12-hour fast, the patients ingested a 99mTc-labeled egg meal (296 kcal, 32% fat which included 2 scrambled eggs, one slice of bread, 240mL milk, for an estimated volume of 320mL) and GE was measured over 4 hours. For the purpose of the current study, the scintiscans obtained immediately post-ingestion of the radiolabeled meal (time 0) using 2-D images were analyzed to estimate IMD0. This was calculated from the proportion of radioactive counts in the proximal stomach, drawn as a region of interest corresponding to the upper half of the longitudinal axis of the stomach, as previously described by Orthey et al. (19). The cut-off for abnormal GA, based on counts in the proximal stomach, was reported as 0.568.
All regions of interest were drawn by one investigator (DB) who was blinded to the SPECT data. The analysis was completed on DICOM acquired images using a nuclear medicine GE program from MIM Software Inc., (25800 Science Park Drive, Suite 180, Cleveland, OH). Using the same region of interest, the area of the proximal stomach region of interest was also determined by the same software program. The proximal stomach was defined as the proximal half of the longest axis of the stomach. The length of the long axis was calculated by the same software program.
SPECT transaxial imaging with intravenous injection of 99mTcO4− and rendered images with Analyze™ Software (Biomedical Imaging Resource; Mayo Clinic, Rochester, MN, USA) were used to measure gastric volume (GV) during fasting and after 300mL Ensure® (1kcal/mL; Abbott Laboratories, Chicago, IL, USA). These measurements were used to quantify GA expressed as accommodation volume (AV) or postprandial to fasting volume ratio (GVR). Normal values are >428mL or ratio of postprandial to gastric volume of >2.62, based on 354 healthy volunteers studied in our lab using the same method (17).
Mathematical Estimation of Proximal Gastric Volume Derived from Area of Proximal Stomach
Using the measured proximal gastric area, we estimated its radius (, and, assuming that the shape of the proximal stomach approximated a spheroid, we estimated the volume of the proximal stomach (4/3* π*r3); in this formula, r=0.5*length which is automatically measured by the computer program estimating the area of stomach based on counts at time zero. We also estimated the volume of the proximal stomach assuming it has a shape that approximates a prolate ellipsoid, with equal anteroposterior and lateral transverse diameters. According to this shape, Volume = 4/3 πab2, where a is the length, and b corresponds to transverse diameters. In this formula, it was assumed that a=2b (Volume =4/3 π*a*0.5a*0.5a).
Statistical Analysis
We performed regression analyses to assess relationships between IMD0 measurements or estimated volumes and GA expressed as AV and GVR. In addition, we used 2*2 tables to assess the correlation between SPECT GA normal/abnormal [based on cut-off values of 428mL for AV and 2.62 for GVR (17) and the reported cut-off (0.568) for IMD0 (19)].
We used Student’s t-test to compare stomach parameters between those with and those without the upper gastrointestinal symptom clusters; data are provided as mean ± SD; p<0.05 was considered statistically significant.
RESULTS
Participants
We reviewed data on 108 patients (60.2% females, median age 49.0 years). Overall 71.3% had DM2; one-third of the patients were insulin-requiring and had fairly well controlled diabetes [median HbA1c 6.7% (IQR 6.2; 7.9)]. Manifestations of diabetic triopathy (peripheral neuropathy, nephropathy, and retinopathy) and treatments are documented elsewhere (6). GA was abnormal (<428mL) in 39% patients.
Relationship between Proximal Gastric Radioactivity Counts and Measured Gastric Area on Two-Dimensional Scintigraphy
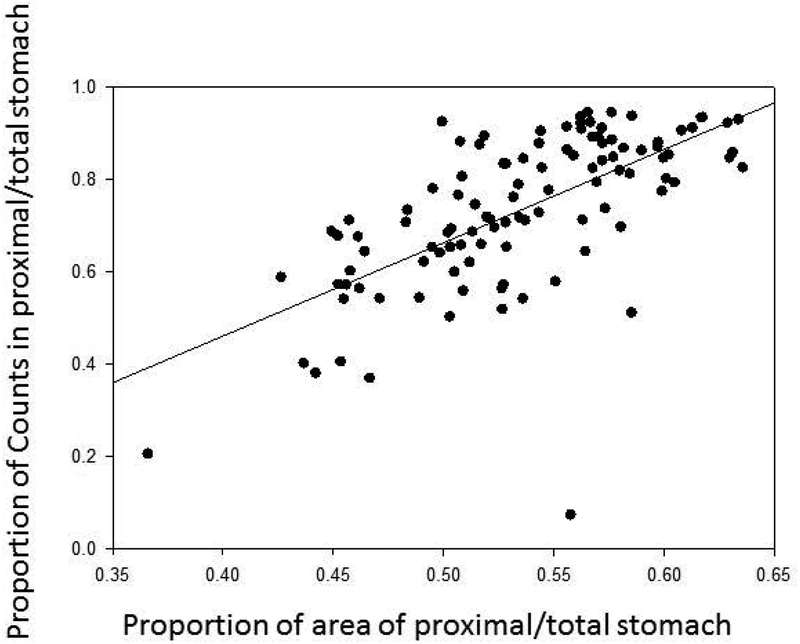
Table 1 shows the measurements of total gastric and proximal stomach regions based on 2-D scintigraphy. Although there was a significant correlation between area of proximal stomach and radioactivity counts in that region, with both being expressed as proportion of total gastric area or counts respectively (r-0.67, p<0.001) (Figure 1), there was considerable variation [bias 0.20 (95%CI −0.07, 0.47)] as illustrated in the Bland-Altman plot (Figure 2).
Table 1.
Data calculated from 2-dimensional scintigraphy immediately after meal ingestion
| Mean ± SD | 10th percentile | 90th percentile | |
|---|---|---|---|
| Total gastric area (cm2) | 108.4 ± 18.9 | 85 | 134.1 |
| Proportion area proximal half | 0.54 ± 0.05 | 0.46 | 0.6 |
| Proportion radioactive proximal half | 0.74 ± 0.16 | 0.54 | 0.92 |
| Estimated proximal half volume (mL) * | 338.4 ± 102.7 | 218.7 | 477.8 |
| Estimated proximal half volume (mL) ^ | 860.9 ± 347.9 | 439.4 | 1399.0 |
assuming proximal stomach is a spheroid (Volume = 4/3 πr3); in this formula, r=0.5*length which is automatically measured by the computer program estimating the area
assuming proximal stomach is a prolate ellipsoid with equal anteroposterior and lateral transverse diameters (Volume = 4/3 πab2), where a is the length, and b corresponds to transverse diameters; in this formula, it was assumed that a=2b (Volume =4/3 π*a*0.5a*0.5a)
Figure 1.
Relationship between counts and area of proximal stomach at IMD0
Figure 2.
Bland-Altman graph comparing percent counts and calculated area of proximal stomach
Measurement of Gastric Volumes and Accommodation using SPECT Compared to IMD0
Abnormal GA by SPECT (<428mL) and IMD0 (<0.568) was detected in 39% and 14.7% respectively. Using continuous variables, there were no significant correlations between SPECT-measured total GV or AV or VR and the following measurements using IMD0: proximal gastric counts, and proximal gastric area. However, there were weak correlations between SPECT-measured AV and total gastric area (r=0.120, p=0.217), and with estimated proximal GV based on either spheroid or ellipsoid shape using the 2-D measurements (R=0.195, p=0.045). These points are shown in Table 2 and Figures 3 and 4.
Table 2.
Correlation coefficients between SPECT estimates of gastric volumes and parameters derived from IMD0
| SPECT measurement of whole stomach by 3D scans | IMD0 parameter of stomach by 2D scans | R | P value |
|---|---|---|---|
| Accommodation volume (mL) | % Area proximal half | −0.08 | 0.43 |
| Accommodation volume ratio | % Area proximal half | 0.05 | 0.58 |
| Accommodation volume (mL) | % Radioactivity proximal half | −0.03 | 0.77 |
| Accommodation volume ratio | % Radioactivity proximal half | 0.01 | 0.93 |
| Accommodation volume (mL) | Total gastric area | 0.12 | 0.22 |
| Total Gastric Volume post-meal | Total gastric area | 0.07 | 0.46 |
| Total Gastric Volume post-meal | Estimated proximal half volume | 0.10 | 0.94 |
| Accommodation volume (mL) | Estimated proximal half volume | 0.06 | 0.59 |
| Abnormal accommodation vol. (≤ 428 mL) | Abnormal % radioactivity proximal half (≤0.568) | −0.02 | 0.84 |
| Abnormal accommodation vol. ratio (≤2.62) | Abnormal % radioactivity proximal half (≤0.568) | 0.01 | 0.91 |
Figure 3. Relationship between total stomach area or proportion of proximal gastric area (relative to whole stomach area) based on 2-dimensional imaging and SPECT-measured gastric accommodation volume.
The correlation between total gastric area and gastric accommodation by SPECT was not statistically significant (r=0.12, p=0.217).
Figure 4. Relationship between estimated proximal gastric volume based on 2-dimensional imaging and SPECT-measured gastric accommodation volume.
Note the weak, but statistically significant correlation based on spheroid or ellipsoid volume assumptions.
Using dichotomized results where SPECT GA was abnormal with AV <428mL and GVR <2.62, and the GA from IMD0 was abnormal if % radioactivity in the proximal half of the stomach was <0.568, there were no correlations between AV or GVR and IMD0 (Table 2).
Relationship between IMD0 or Total Gastric Area and Upper GI Symptom Clusters
Table 3 shows the association of measurements based on IMD0 and upper gastrointestinal symptom clusters. Nausea and vomiting was the most common presenting symptom cluster (81.6%). There were no significant differences in total gastric area or the IMD0 parameters (% area or % radioactive counts) between those with and without upper gastrointestinal symptoms, except in patients with fullness and satiety who had a larger total gastric area (113.8±18.9cm2 vs. 104.4±17.9cm2; p=0.006) and a larger estimated proximal volume (360.9±105.9mL vs. 321.3±97.6mL; p=0.05) compared to those without this cluster. Abdominal pain/discomfort tended to be associated with lower IMD0 parameters.
Table 3.
Association of measurements based on IMD0 and upper gastrointestinal symptom clusters
| Means ± Standard Deviation | Cluster present | Cluster absent | p-value | ||
|---|---|---|---|---|---|
| Nausea and Vomiting, N (%) | 89 (81.6) | 20 (18.3) | |||
| Total Gastric Area (cm2) | 20.22 | 11.51 | 0.40 | ||
| % Area Proximal Half | 0.05 | 0.07 | 0.43 | ||
| % Radioactivity Proximal Half | 0.16 | 0.20 | 0.31 | ||
| Estimated Proximal Half Volume (mL) | 107.24 | 82.10 | 0.91 | ||
| Heartburn and Belching, N (%) | 25 (22.9) | 84 (77.1) | |||
| Total Gastric Area (cm2) | 14.48 | 20.12 | 0.89 | ||
| % Area Proximal Half | 0.05 | 0.05 | 0.48 | ||
| % Radioactivity Proximal Half | 0.16 | 0.17 | 0.61 | ||
| Estimated Proximal Half Volume (mL) | 86.55 | 107.55 | 0.86 | ||
| Bloating and Abdominal Pain, N (%) | 74 (67.9) | 35 (32.1) | |||
| Total Gastric Area (cm2) | 18.92 | 18.99 | 0.41 | ||
| % Area Proximal Half | 0.06 | 0.05 | 0.37 | ||
| % Radioactivity Proximal Half | 0.18 | 0.14 | 0.45 | ||
| Estimated Proximal Half Volume (mL) | 100.80 | 108.22 | 0.87 | ||
| Fullness and Satiety, N (%) | 47 (43.1) | 62 (56.9) | |||
| Total Gastric Area (cm2) | 18.95 | 17.97 | 0.006 | ||
| % Area Proximal Half | 0.06 | 0.05 | 0.68 | ||
| % Radioactivity Proximal Half | 0.17 | 0.17 | 0.75 | ||
| Estimated Proximal Half Volume (mL) | 105.95 | 97.65 | 0.05 | ||
| Abdominal Discomfort/Pain, N (%) | 58 (53.2) | 51 (46.8) | |||
| Total Gastric Area (cm2) | 19.28 | 18.68 | 0.84 | ||
| % Area Proximal Half | 0.06 | 0.05 | 0.08 | ||
| % Radioactivity Proximal Half | 0.19 | 0.13 | 0.06 | ||
| Estimated Proximal Half Volume (mL) | 97.20 | 108.30 | 0.24 | ||
DISCUSSION
Our study has attempted to validate the use of IMD0 using 2-D gastric scintigraphy as a simple measurement of GA. In previous studies, investigators have validated the reproducibility of measurements of radioactivity counts in the proximal stomach (19), but those studies have not actually compared the results obtained with a gold standard such as the intragastric balloon under barostatic control, or with noninvasive imaging methods such as SPECT or MRI. Therefore, it is important to assess the validity of information provided by 2-D scintiscans, which would be widely applicable and simple in clinical practice.
In order to ensure a thorough evaluation of the utility of IMD0 using 2-D gastric scintigraphy, our study included, as originally proposed by Orthey et al. (19), the proportion of radioactive counts in the proximal stomach; in addition, we assessed the automated measurements of area on the proximal stomach, and an estimate of the proximal stomach volume based on the measured length, assuming that the proximal stomach conforms to the shape of a spheroid or a prolate ellipsoid. Irrespective of the measurements obtained from the 2-D imaging, we were unable to identify any significant correlations with measurements of GA, specifically, accommodation volume or gastric volume ratio, except for weak correlations (R2 =0.04) for the correlation between SPECT-measured gastric accommodation and postprandial gastric volume on IMD0.
In addition, we conducted an analysis based on 2*2 tables to assess correlation between SPECT measurements and IMD0-derived GA, dichotomizing the patient groups based on established cut-offs in the published literature. Consistent with the other analyses based on continuous variables, no significant correlation was identified.
The sample size used in our study was almost identical and the patient population similar to that in the prior study by Orthey et al. (19). One limitation of our study is that the cut-off used for abnormal IMD0 was based on the prior publication (19) and has not been confirmed in our laboratory; using the 0.568 cut-off based on radioactivity counts, only 14.7% of the cohort in our study had reduced GA, in contrast to the 39% identified by SPECT imaging. Further studies are, therefore, required to define the cut-off for abnormal IMD0. However, irrespective of the actual cut-off value used to define normality, it is relevant to note that there were no significant differences in total gastric area or the IMD0 parameters between those with and without upper gastrointestinal symptoms, except in those with fullness and satiety who had a larger total gastric area (p=0.006) and a larger estimated proximal volume (p=0.05) compared to those without this cluster. This observation would appear to contradict the previously described association of decreased GA by barostat and postprandial symptoms (1). In contrast, abdominal pain/discomfort tended to be associated with lower IMD0 parameters, which is consistent with the observations of Tack et al. (1).
There are fundamental differences between the two methods that may have also contributed to the lack of correlations observed between the two measurements. SPECT imaging of the stomach assesses the entire content including air, meal ingested and any gastric secretions; none of these can be separated into individual components using this imaging technique, in contrast with MRI which can separate meal from air (21). On the other hand, the labeling of the meal in gastric scintigraphy can only show the labeled contents; one of the desirable qualities of the meal and radioisotope is that the binding of the isotope to the meal needs to be close to 100% in order for the emptying of the isotope to reflect the emptying of the solid phase of the meal, rather than dissociation of the isotope from the solid phase and emptying with the liquid phase of the meal. Therefore, the radiolabeled content of the stomach on scintigraphy does not include any additional secretions which may also layer on top of the meal in the stomach (22) or the air ingested with the meal. In summary, the derived accommodation parameters based on 2-dimensional scintigraphy must rely on several assumptions. In contrast, the gastric volume determined by SPECT directly relates to the accommodation of the stomach to the meal and was validated in comparison to the gold standard, the barostat-controlled balloon (15).
Another limitation of our study is that SPECT estimation of accommodation was based on a liquid nutrient 300kcal meal, whereas IMD0 parameters were calculated on 2-D scintigraphy after ingestion of a 296kcal solid-liquid meal consisting of eggs, bread and skim milk. We have previously shown that equicaloric meals are associated with very reproducible GA responses, at least in healthy volunteers (16). The estimated volumes of the two meals at ingestion were similar: 300mL for the Ensure® meal used in gastric SPECT studies and ~320mL for the mixed meal radiolabeled for the scintigraphic gastric emptying study. However, since the estimate of volume on 2-dimensional scintigraphy is based on imaging the radiolabeled potion of the meal and since the isotope is tightly bound to the egg in the stomach, the volume of the egg is critical to measure surface area or calculate volume of the proximal stomach and is clearly smaller than the volume of Ensure® ingested in the SPECT study.
In conclusion, among referred patients with diabetes with upper gastrointestinal symptoms, IMD0 is not significantly correlated with GA measurements by the current gold standard imaging method, SPECT, and is weakly associated with abdominal pain and discomfort, but not with other upper abdominal symptom clusters. These data suggest that the 2-D measurements based on IMD0 require more validation before they can be applied in clinical practice or research. In particular, simultaneous comparison with a gold standard such as SPECT or MRI and assessment of the same meal of liquid nutrient or solid and liquid nutrient content will provide important insights to validate the IMD0 method.
KEY POINTS.
Gastric accommodation (GA) has been measured by intragastric barostatically-controlled balloon, high resolution manometry, or 3-dimensional imaging with SPECT or MRI; a recent study proposed measurements based on intragastric meal distribution of a radiolabeled solid meal immediately post-meal ingestion (IMD0). Our aim was to compare results from SPECT volume with GA estimated from IMD0 using data from 108 symptomatic diabetic patients.
There were no significant correlations between total GA by SPECT and any measurements using IMD0: proximal gastric counts, area, or estimated volume (using either continuous variables or dichotomized values based on the proposed cut-off in the prior publication.
IMD0 as a measurement of GA requires further validation and comparison with a validated gold-standard GA test before it is applied in clinical research or practice.
Funding support:
Dr. Camilleri is supported by grant R01-DK67071 from National Institutes of Health.
Footnotes
Disclosures: The authors have no relevant disclosures.
REFERENCES
- 1.Tack J, Piessevaux H, Coulie B, et al. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology 1998;115:1346–1352 [DOI] [PubMed] [Google Scholar]
- 2.Thumshirn M, Camilleri M, Saslow SB, Williams DE, Burton DD, Hanson RB. Gastric accommodation in non-ulcer dyspepsia and the roles of Helicobacter pylori infection and vagal function. Gut 1999;44:55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pauwels A, Boecxstaens V, Broers C, Tack JF. Severely impaired gastric accommodation is a hallmark of post-Nissen functional dyspepsia symptoms. Neurogastroenterol Motil 2017;29. doi: 10.1111/nmo.13063. Epub 2017 Mar 20. [DOI] [PubMed] [Google Scholar]
- 4.Janssen P, Verschueren S, Ly HG, Vos R, Van Oudenhove L, Tack J. Intragastric pressure during food intake: a physiological and minimally invasive method to assess gastric accommodation. Neurogastroenterol Motil. 2011. Apr;23(4):316–22, e153–4. [DOI] [PubMed] [Google Scholar]
- 5.Park SY, Acosta A, Camilleri M, Burton D, Harmsen WS, Szarka LA, et al. Gastric motor dysfunction in patients with functional gastroduodenal symptoms. Am J Gastroenterol 2017;112:1689–1699 [DOI] [PubMed] [Google Scholar]
- 6.Chedid V, Brandler J, Vijayvargiya P, Park SY, Szarka LA, Camilleri M. Characterization of upper gastrointestinal symptoms, gastric motor functions, and associations in patients with diabetes at a referral center. Am J Gastroenterol 2018. August 30. doi: 10.1038/s41395-018-0234-1. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7.Chial HJ, Camilleri M, Burton D, Thomforde G, Olden KW, Stephens D. Selective effects of serotonergic psychoactive agents on gastrointestinal functions in health. Am J Physiol Gastrointest Liver Physiol 2003;284:G130–G137 [DOI] [PubMed] [Google Scholar]
- 8.Tack J, Janssen P, Masaoka T, Farré R, Van Oudenhove L. Efficacy of buspirone, a fundus-relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol Hepatol 2012;10:1239–1245 [DOI] [PubMed] [Google Scholar]
- 9.Tack J, Pokrotnieks J, Urbonas G, Banciu C, Yakusevich V, Bunganic I, Tornblom H, Kleban Y, Eavis P, Tsuchikawa M, Miyagawa T. Long-term safety and efficacy of acotiamide in functional dyspepsia (postprandial distress syndrome)-results from the European phase 3 open-label safety trial. Neurogastroenterol Motil 2018;30e13284. [DOI] [PubMed] [Google Scholar]
- 10.Kusunoki H, Haruma K, Manabe N, Imamura H, Kamada T, Shiotani A, Hata J, Sugioka H, Saito Y, Kato H, Tack J. Therapeutic efficacy of acotiamide in patients with functional dyspepsia based on enhanced postprandial gastric accommodation and emptying: randomized controlled study evaluation by real-time ultrasonography. Neurogastroenterol Motil 2012;24:540–545, e250–251 [DOI] [PubMed] [Google Scholar]
- 11.Maurer AH, Parkman HP. Towards a fuller assessment of gastric motility in patients with upper GI dyspepsia: time to accommodate! Am J Gastroenterol. 2018. November 9. doi: 10.1038/s41395-018-0404-1. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12.Troncon LE, Bennett RJ, Ahluwalia NK, Thompson DG. Abnormal intragastric distribution of food during gastric emptying in functional dyspepsia patients. Gut 1994;35:327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piessevaux H, Tack J, Walrand S, Pauwels S, Geubel A. Intragastric distribution of a standardized meal in health and functional dyspepsia: correlation with specific symptoms. Neurogastroenterol Motil 2003;15:447–455 [DOI] [PubMed] [Google Scholar]
- 14.Caldarella MP, Azpiroz F, Malagelada JR. Antro-fundic dysfunctions in functional dyspepsia. Gastroenterology 2003;124:1220–1229 [DOI] [PubMed] [Google Scholar]
- 15.Bouras EP, Delgado-Aros S, Camilleri M, Castillo EJ, Burton DD, Thomforde GM, Chial HJ. SPECT imaging of the stomach: comparison with barostat, and effects of sex, age, body mass index, and fundoplication. Single photon emission computed tomography. Gut 2002;51:781–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Schepper H, Camilleri M, Cremonini F, Foxx-Orenstein A, Burton D. Comparison of gastric volumes in response to isocaloric liquid and mixed meals in humans. Neurogastroenterol Motil 2004;16:567–573 [DOI] [PubMed] [Google Scholar]
- 17.Breen M, Camilleri M, Burton DD, Zinsmeister AR. Performance characteristics of the measurement of gastric volume using single photon emission computed tomography. Neurogastroenterol Motil 2011;23:308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fidler J, Bharucha AE, Camilleri M, Camp J, Burton D, Grimm R, Riederer SJ, Robb RA, Zinsmeister AR. Application of magnetic resonance imaging to measure fasting and postprandial volumes in humans. Neurogastroenterol Motil 2009;21:42–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orthey P, Yu D, Van Natta ML, Ramsey FV, Diaz JR, Bennett PA, Iagaru AH, Fragomeni RS, McCallum RW, Sarosiek I, Hasler WL, Farrugia G, Grover M, Koch KL, Nguyen L, Snape WJ, Abell TL, Pasricha PJ, Tonascia J, Hamilton F, Parkman HP, Maurer AH; NIH Gastroparesis Consortium. Intragastric meal distribution during gastric emptying scintigraphy for assessment of fundic accommodation: correlation with symptoms of gastroparesis. J Nucl Med 2018;59:691–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orthey P, Dadparvar SMD, Parkman HP, Maurer A. Enhanced gastric emptying scintigraphy to assess fundic accommodation using intragastric meal distribution and antral contractility. J Nucl Med Technol 2018. August 23 10.2967/jnmt.118.215566. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21.Fidler J, Bharucha AE, Camilleri M, Camp J, Burton D, Grimm R, Riederer SJ, Robb RA, Zinsmeister AR. Application of magnetic resonance imaging to measure fasting and postprandial volumes in humans. Neurogastroenterol Motil 2009;21:42–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carney BI, Jones KL, Horowitz M, Sun WM, Penagini R, Meyer JH. Gastric emptying of oil and aqueous meal components in pancreatic insufficiency: effects of posture and on appetite. Am J Physiol 1995;268:G925–G932 [DOI] [PubMed] [Google Scholar]