Abstract
Objective:
To compare 1-year all-cause mortality (ACM) and major adverse cardiovascular events (MACE) in cardiac arrest (CA) survivors with and without posttraumatic stress disorder (PTSD) symptomatology at hospital discharge.
Design:
Prospective, observational cohort
Setting:
Intensive care units at a tertiary-care center
Patients:
Adults with return of spontaneous circulation after in-hospital or out-of-hospital CA between 9/2015-9/2017. A consecutive sample of survivors with sufficient mental status to self-report CA and the subsequent hospitalization induced PTSD symptoms (CA-induced PTSS) at hospital discharge were included.
Interventions:
None
Measurements and Main Results:
The combined primary end point was ACM or MACE—hospitalization for nonfatal myocardial infarction (MI), unstable angina (UA), congestive heart failure (CHF), emergency coronary revascularization, or urgent implantable defibrillators/permanent pacemaker (ICD/PPM) placements within 12 months of discharge. An in-person PTSD symptomatology was assessed at hospital discharge via the PTSD Checklist - Specific (PCL-S) scale; a suggested diagnostic cut-off of 36 for specialized medical settings was adopted. Outcomes for patients meeting (vs. not meeting) this cutoff were compared using Cox-hazard regression models. Of 114 included patients, 36 (31.6%) screened positive for CA-induced PTSS at discharge (median 21 days post-CA; interquartile range (IQR) 11-36). During the follow-up period (median = 12.4 months, IQR, 10.2-13.5), 10 (8.8%) died and 29 (25.4%) experienced a recurrent MACE: rehospitalizations due to MI (n=4, 13.8%), UA (n=8, 27.6%), CHF exacerbations (n=4, 13.8%), emergency revascularizations (n=5, 17.2%), and urgent ICD/PPM placements (n=8, 27.6%). CA-induced PTSS was associated with ACM/MACE in univariate (Hazard Ratio [HR] =3.19; 95% confidence interval [CI], 1.7-6.0) and in models adjusted for age, sex, comorbidities, pre-existing psychiatric condition, and nonshockable initial rhythm (HR=3.1; CI, 1.6–6.0).
Conclusions:
PTSD symptomatology is common after CA, and CA-induced PTSS was associated with significantly higher risk of death and cardiovascular events. Further studies are needed to better understand the underlying mechanisms.
Keywords: Cardiac arrest, posttraumatic stress, cardiovascular disease, outcomes, survival
INTRODUCTION:
More than half a million Americans experience cardiac arrest (CA) each year.1 Current survival rates are as high as to 38.6–44.3% in bystander-witnessed cases with shockable rhythms,1 thanks to advances in critical care and effective public health campaigns.
As more patients survive, however, the psychological and secondary cardiovascular risk that arise after CA are becoming clear. Specifically, posttraumatic stress disorder (PTSD) symptoms secondary to the CA are highly prevalent, with rates ranging from 19–38%.2,3 This is considerably higher than in other acute events, including acute coronary syndrome (ACS) and stroke patients (~12% and ~23%, respectively).4,5 Critically, CA and the subsequent hospitalization induced PTSD symptoms (CA-induced PTSS) may confer additional cardiovascular disease (CVD) risk. Acute and potentially life-threatening CVD events can themselves cause PTSD, and in return might be associated with increased risk for subsequent CVD events and mortality.5 In a meta-analysis of 3 studies (total N=600), ACS-induced PTSD was associated with a doubling of risk for subsequent CVD events and mortality in the following 1–3 years.4 No study has assessed the association of CA-induced PTSS with risk for cardiovascular events and mortality.
METHODS:
Participants
This study is a subgroup analysis of an observational, prospective cohort study evaluating the evolution of cognitive, functional, and psychiatric sequelae one-year post-CA. Inclusion criteria were ≥18 years of age, resuscitated following in- or out-of-hospital CA, admitted to Columbia University Medical Center, and survived to hospital discharge between September 2015 and September 2017 (Supplemental Figure 1). CA was defined as a loss of pulse requiring chest compressions, rescue shock, or both. The study was approved by Columbia University’s institutional review board and written consent was obtained.
Details regarding the screening procedure and data collection process have been described previously6 (see also Supplemental Methods). A detailed description of our institutional CA management protocol based on the American Heart Association guidelines is also available.7
Measures
Psychiatric Symptoms
PTSD symptoms were queried with reference to the acute CA event and subsequent hospitalization within 24 hours of hospital discharge via the PTSD Checklist - Specific (PCL-S) scale. PCL-S scores range from 17–85; a cutoff of 36, suggested for specialized medical settings was adopted.8 The median assessment time was 21 days post-CA (interquartile range [IQR] 11–36). Internal consistency was high (Cronbach’s α = .92).
Ascertainment of Medical Outcomes:
The primary end point was either (1) recurrence of a major adverse cardiovascular event (MACE), including hospitalization for nonfatal myocardial infarction (MI), unstable angina (UA), congestive heart failure (CHF) exacerbation, urgent/emergency coronary revascularization procedures (percutaneous coronary intervention, coronary artery bypass grafting, or percutaneous transluminal coronary angioplasty), or urgent implantable cardio-defibrillator (ICD)/pacemaker (PPM) placements, or (2) all-cause mortality (ACM) within 12-months of the hospital discharge interview (reported via telephone or in-person interview). For patient-reported hospitalization with MACE, supporting documentation of the event was confirmed in 25 (86%) patients from hospital electronic medical records. All deaths were verified using the Social Security National Death Index.
Statistical Analysis
The Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables were used to compare those with vs. without CA-induced PTSS on demographic, psychiatric, and medical variables. Cox-hazard regression modeling and Kaplan-Meier survival analysis was used to compare MACE/ACM outcomes between those with and without CA-induced PTSS. Based on research identifying factors that might confound the association between cardiac-event-induced PTSS and MACE/ACM,4 demographic variables (age, sex),1 medical covariates (Charlson Comorbidity Index),1 pre-existing psychiatric disorder,2 and non-shockable rhythm1 were treated as covariates (Supplemental Methods). Analyses with continuous PTSS score are described in the Supplemental Results. Statistical analyses were performed using STATA software (StataCorp, College Station, TX).
RESULTS:
The median PCL-S score in the sample was 29 (IQR 22–39). Nearly one third (n=36; 31.6%) of patients screened positive for CA-induced PTSS. Patients with CA-induced PTSS were younger, more often women, and had higher prevalence of pre-existing psychiatric diagnoses. (Supplemental Table 1).
All-cause Mortality and Major Adverse Cardiac Events
During the median follow-up period of 12.4 months (range 10.2–13.5), 10 (8.8% overall; 6 (5.3%) with PTSS) died and 29 (25.4% overall; 14 (12.3%) with PTSS) experienced a recurrent MACE (Supplemental Table 2).
In an unadjusted model, CA-induced PTSS was associated with MACE/ACM (Hazard Ratio [HR]=3.2; 95% confidence interval [CI], 1.7–6.0) and significant differences in survival times among patients with vs. without CA-induced PTSS (Log-Rank p<0.001) (Figure 1).
Figure 1.
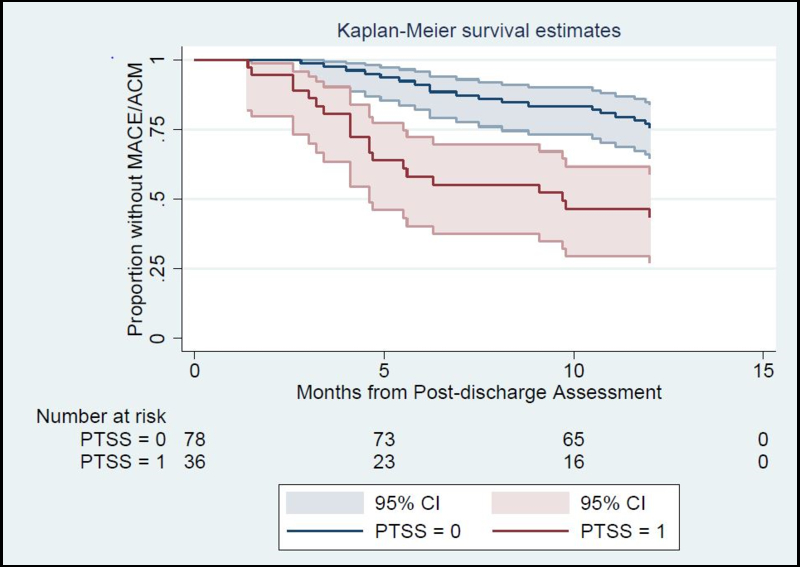
Kaplan-Meier curves for patients with and without CA-induced PTSS symptoms, The number of patients in each group who were at risk for MACE/ACM within first year are presented below the figure.
Abbreviation: CA, cardiac arrest; PTSS, posttraumatic stress symptomatology; MACE/ACM, major adverse cardiovascular event/all-cause mortality.
After adjustment for demographic and medical covariates, CA-induced PTSS remained associated with MACE/ACM (HR=3.2; CI, 1.7–6.1) (Table 1 and Supplemental Figure 2). In sensitivity analyses, CA-induced PTSS was significantly associated with each individual outcome, including different types of MACE and ACM (Supplemental Table 3).
Table 1.
Results of four Cox proportional hazards regression analyses predicting 12-month risk of recurrent major adverse cardiovascular events and all-cause mortality from demographics, clinical characteristics, and PTSDb symptoms after cardiac arrest
| Covariates | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| HRc (95% CId) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| PTSD symptoms | 3.3 (1.7-6.1)a | 3.2 (1.7-6.0)a | 3.2 (1.7-6.1)a | 3.1 (1.6-6.0) a |
| Age | 1.03 (1.0-1.05) | 1.02 (0.9-1.05) | 1.02 (0.99-1.05) | 1.02 (0.99-1.05) |
| Men | 0.9 (0.5-1.8) | 0.9 (0.5-1.8) | 0.92 (0.5-1.8) | 0.9 (0.4-1.8) |
| Charlson Score | …. | 1.04 (0.9-1.2) | 1.08 (0.9-1.3) | 1.1 (0.9-1.3) |
| Non-shockable initial rhythm | …. | …. | 1.5 (0.6-3.9) | 1.6 (0.6-4.3) |
| Pre-existing psychiatric condition | …. | …. | …. | 1.4 (0.6-2.9) |
P < .001
Posttraumatic stress disorder
hazard ratio
confidence interval
DISCUSSION:
CA-induced PTSS was associated with a significantly higher risk of death and poor cardiovascular events within the first year post-CA. These findings supplement those suggesting PTSD due to life-threatening events precipitate worse clinical outcomes, including a meta-analysis that reported ACS-induced PTSD doubled the risk for recurrence or mortality (HR= 2.0).4 Another study found PTSD symptoms increased participants’ odds of hospital readmission within the first month post-ACS by 32%.5 Our study is the first to highlight an independent association of CA-induced PTSS (and not PTSD), with risk of recurrent MACE/ACM. There are several broad considerations that should be addressed in future research to investigate the mechanisms that account for the association of CA-induced PTSS with adverse outcomes. This include autonomic imbalance characterized by an increase in sympathetic activity and a reduction in vagal activity, a pattern that is reflected by decreased high-frequency heart rate variability, baroreflex dysfunction, increased QT intervals, an exaggerated catecholamine response to stressful circumstances, with higher concentrations of circulating catecholamines.9 Both the downstream effects of this imbalance (e.g., increased blood pressure, endothelial dysfunction, excess inflammation, atherosclerosis) and behavioral dysregulation (e.g., medication nonadherence, sedentary behavior, excess tobacco and alcohol use) have been implicated in linking PTSD with risk of recurrent CVD events.5
Furthermore, CA-induced PTSS, in particular, may be distinct from other forms of medical-event induced PTSD. CA survivors rarely remember the arrest, or early post-event period; therefore, distressing and intrusive memories of the event (a hallmark of PTSD) are rare. In that case, traumatizing experiences related to the hospitalization such as delirium, early post-ICU memories of in-ICU frightening psychotic/nightmare experiences, sepsis or administration of high-dose opiates in ICU, as seen after other life-threatening medical events like adult respiratory distress syndrome, 10 could be associated with later PTSD symptoms. An in-depth exploration of the development and unique manifestation of CA-induced PTSS is needed to inform prevention and intervention efforts.
The study has several strengths. This is the first study to assess propsectively, the influence of CA-induced PTSS on prognosis, with low refusal to participation rates along with high retention rates, in a racially and ethnically diverse population. Some limitations warrant consideration. Participants were recruited from a single center, limiting generalizability. The survival and CA-induced PTSS rates are similar to those reported by other prospective cohorts worldwide; however, patients who were unable to participate may have differed from the analytic sample. Despite considering a comprehensive list of pre-, and in-hospital factors underlying PTSS-CVD associations, certain unmeasured covariates and alternative explanations are certainly possible. Our study was also not designed to examine biological or behavioral mechanims underlying associations between CVD events and CA-induced PTSS.
Conclusions:
CA-induced PTSS is associated with substantially elevated mortality and CVD risk. Given its high prevalence, further inquiry is warranted to understand the underlying mechanisms contributing to increased risk, which, in turn, will help define early interventions to offset the adverse impact of CA-induced PTSS.
Supplementary Material
Acknowledgements:
We want to thank Evie Sobczak, and Dr. Nathan Carberry who assisted in gathering follow-up data, and the nurses, physician assistants, nurse practitioners and physicians who take care of these patients. This study was supported by the institutional funds provided to Dr. Agarwal. Dr. Edmondson acknowledges support of the grants HL128310, HL123368, and HL117832 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. Dr. Elknd has no relevant funding to support. Dr. Park acknowledges support of the NIH/NINDS (K01ES026833). The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Copyright form disclosure: Drs. Agarwal and Park received support for article research from the National Institutes of Health. Dr. Agarwal also received support for article research from institutional funds. Dr. Claassen received funding from DANA Foundation (grant in aide), iCE Neurosystems (stock options), and UCB Pharma (one time speaker compensation).
Disclosures: Dr. Claassen reports grants from DANA, grants from McDonell, personal fees from SAGE, grants from SHINE, grants from I-SPOT, from ESETT, grants from SAGE, other from iCE Neurosystems, outside the submitted work.
REFERENCES:
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update: A Report from the American Heart Association. Circulation. 2017. March 7;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaaf KPW, Artman LK, Peberdy MA, et al. Anxiety, depression, and PTSD following cardiac arrest: a systematic review of the literature. Resuscitation. 2013;84(7):873–877. [DOI] [PubMed] [Google Scholar]
- 3.Moulaert VRM, van Heugten CM, Gorgels TPM, et al. Long-term Outcome After Survival of a Cardiac Arrest: A Prospective Longitudinal Cohort Study. Neurorehabil Neural Repair. 2017. June;31(6):530–539. [DOI] [PubMed] [Google Scholar]
- 4.Edmondson D, Richardson S, Falzon L, et al. Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: A meta-analytic review. PLoS ONE. 2012;7:e38915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edmondson D, von Känel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017. April;4(4):320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal S, Presciutti A, Verma J, et al. Women have worse cognitive, functional, and psychiatric outcomes at hospital discharge after cardiac arrest. Resuscitation. 2018. April;125:12–15. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal S, Presciutti A, Roth W, et al. Determinants of Long-Term Neurological Recovery Patterns Relative to Hospital Discharge Among Cardiac Arrest Survivors. RM. Crit Care Med. 2018. February;46(2):e141–e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Center for PTSD. (2012). Using the PTSD Checklist. Retrieved from:https://sph.umd.edu/sites/default/files/files/PTSDChecklistScoring.pdf
- 9.Brudey C, Park J, Wiaderkiewicz J, et al. Autonomic and inflammatory consequences of posttraumatic stress disorder and the link to cardiovascular disease. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2015;309(4):R315–R321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker AM, Sricharoenchai T, Raparla S, et al. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015. May;43(5):1121–9. Review. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


