Abstract
Introduction:
Prior studies showed that intranasally administered ST266, a novel biological secretome of Amnion-derived Multipotent Progenitor cells containing multiple growth factors and anti-inflammatory cytokines, attenuated visual dysfunction and prevented retinal ganglion cell (RGC) loss in experimental optic neuritis. Long-term effects and dose escalation studies examined here have not been reported previously.
Methods:
Optic neuritis was induced in the multiple sclerosis model experimental autoimmune encephalomyelitis (EAE). EAE and control mice were treated once or twice daily with intranasal placebo/vehicle or ST266 beginning after onset of optic neuritis for either 15 days or continuously until sacrifice. Visual function was assessed by optokinetic responses (OKR). RGC survival and optic nerve inflammation and demyelination were measured.
Results:
Both once and twice daily continuous intranasal ST266 treatment from disease onset to 56 days post-EAE induction significantly increased OKR scores, decreased RGC loss, and reduced optic nerve inflammation and demyelination compared with placebo (saline, non-specific protein solution, or cell culture media)-treated EAE mice. ST266 treatment given for just 15 days after disease onset, then discontinued, only delayed OKR decreases, and had limited effects on RGC survival and optic nerve inflammation 56 days after disease induction.
Conclusions:
ST266 is a potential neuroprotective therapy to prevent RGC damage, and intranasal delivery warrants further study as a novel mechanism to deliver protein therapies for optic neuropathies. Results suggest that once daily ST266 treatment is sufficient to sustain maximal benefits, and demonstrate that neuroprotective effects promoted by ST266 are specific to the combination of factors present in this complex biologic therapy.
Keywords: Neuroprotection, optic neuritis, multiple sclerosis, amnion cell-derived therapy, intranasal drug delivery, ST266
Optic neuritis (ON) is an inflammatory demyelinating disease often associated with the central nervous system autoimmune disease multiple sclerosis (MS) (1). Neuronal and axonal damage also are key features of the pathophysiology of MS and ON, and result in long-term vision loss and neurologic disability in patients (2-4). Inflammation-mediated apoptotic retinal ganglion cell (RGC) loss occurs in eyes of MS patients with or without a history of ON (5), and significant RGC loss also occurs in the experimental autoimmune encephalomyelitis (EAE) model of MS (6,7). Intravenous corticosteroids frequently are used to hasten visual recovery in acute ON, but have no effect in preventing RGC loss and associated permanent visual deficits (8,9). No current therapy improves final visual outcomes in ON. Therefore, improved and safe potential neuroprotective therapies that prevent RGC loss from ON are needed.
Studies of experimental ON in EAE animals have identified potential immunomodulatory and neuroprotective compounds effective in attenuating RGC loss during ON (10-12), and demonstrate the usefulness of this model. Our prior studies, for example, have demonstrated that pharmaceutical approaches that promote mitochondrial biogenesis and function using SIRT1 activating compounds or mitochondrial uncoupling drugs, are beneficial in ON with improved cellular function, increased biomarkers and reduced oxidative stress resulting in improved RGC preservation and visual outcomes (13-15). Recent advances in stem cell-based therapies offer a new, potentially multifactorial approach in the treatment of retinal and optic nerve diseases (16). Transplanted stem cells have shown significant neuroprotective effects in preclinical models of glaucoma (17), transient ischemia and ocular hypertension models (18,19). Continuous intraocular secretion of several growth factors by stem cells, including BDNF (20), CNTF (21), and PDGF (22) provide immunomodulatory and neuroprotective effects in several retinal or optic neuropathies. Although stem cell therapies have shown promise in numerous studies, there are potential risks associated with transplantation, including immune rejection and unwanted cell growth. Further research has focused on stem cell secretion-based therapies that can be used in place of cells (23,24). Secretions from stem cells provide some key advantages, including the potential to be purified, stored and dosed precisely compared with stem cells (25,26).
ST266, (formerly Amnion-derived Cellular Cytokine Solution, (ACCS)), is the proprietary biologic secretome of Amnion-derived Multipotent Progenitor (AMP) cells. ST266 modulates inflammatory responses by decreasing vascular permeability, immune cell infiltration, and edema (27,28); accelerates wound healing (27,29,30); and attenuates the effects of a penetrating ballistic brain injury by inducing persistent motor improvement and ameliorating neuro-inflammation (31,32). We have shown that intranasally-delivered ST266 accumulates rapidly in rodent eyes and optic nerves 30 minutes after administration, not via systemic absorption but rather likely through direct CNS entry (33). A single 6 μL intranasal drop of ST266 given once daily beginning after the onset of EAE ON attenuated visual dysfunction, prevented RGC loss, and reduced both inflammation and demyelination six weeks after induction of EAE, as compared to saline (PBS)-treated EAE mice (33). Thus, ST266 is a potential new neuroprotective therapy for ON that may be delivered by a novel, noninvasive route of administration. However, the optimal dosage and frequency of administration of ST266 needed to achieve maximal therapeutic effect, and its ability to confer longer-term neuroprotective effects, remain to be determined. Therefore, in the current studies, we examined the ability of different ST266 treatment regimens to prevent neuronal damage during EAE-induced experimental optic neuritis. In addition, we compared effects of ST266 with additional controls containing cell culture medium nutrients and high protein levels to confirm that ST266-mediated effects are due to its specific protein secretome composition.
Methods
Animals
Six-week-old female C57BL6/J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and housed at an approved animal facility at the University of Pennsylvania. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania and adhered to all applicable international, national, and institutional guidelines for the care and use of animals.
Induction and Scoring of EAE
EAE induction was carried out as in our prior studies (7). Eight-week-old mice were anesthetized using isoflurane and were injected subcutaneously at two sites on the back with myelin oligodendrocyte glycoprotein (200 μg, MOG35–55 peptide; Genscript, Piscataway, NJ, USA) emulsified in Complete Freund’s Adjuvant (Difco, Detroit, MI, USA) containing 2.5 mg/ml mycobacterium tuberculosis (Difco). An equal volume of PBS in Complete Freund’s Adjuvant was injected into control mice. In addition, each animal received 200 ng pertussis toxin (List Biological, Campbell, CA, USA) in 0.1 ml PBS by intraperitoneal (i.p.) injection at 0 h and 48 h post-immunization. As in prior studies, severity of EAE spinal cord disease was monitored to confirm successful disease induction (data not shown) (13,14,34,35).
ST266 Treatment
ST266 (Noveome Biotherapeutics, Inc., Pittsburgh, PA) was aliquoted and stored at −20 °C. A fresh aliquot was thawed and warmed to room temperature each day and administered to the mice as a single 6 μL drop placed in the nose (33, 36). Mice were grouped and treated once or twice with one drop of ST266, PBS, STM100 (the cell culture medium used to culture AMP cells and collect ST266 protein secretions), or 0.5% human serum albumin (HSA) in PBS intranasally beginning after the onset of optic neuritis (day 15 post-immunization). Treatment was continued through day 30, 42 or 56 as indicated in each experiment. Mice receiving ST266 for only days 15 to 30 were treated with PBS during the interval when they were not receiving ST266 (days 31 to 56) so that all control and treated mice received the same total number of days of intranasal fluid administration. In the 42-day treatment study, 5 mice were included in each treatment group (PBS-treated control non-EAE mice; PBS-treated EAE mice; STM100-treated EAE mice; ST266-treated EAE mice). In the 56-day treatment study, the control non-EAE mouse group contained 4 mice, and each of the six experimental EAE groups listed in Table 1 contained 6 mice. No mice died or were otherwise removed from any of the studies; thus all eyes of all mice in each experimental group were used in analyses of visual function and RGC survival. In the 56-day treatment study, the right optic nerve from 3 randomly selected mice in each treatment group was removed for protein isolation and Western blot analysis of inflammatory and apoptotic markers (data not shown due to undetectable levels). The left optic nerves of these 3 mice/group, and both optic nerves from all other mice, were used for histopathologic analyses of optic nerve inflammation and demyelination.
Table 1.
ST266 Treatment Groups
| Group | Treatment Cohort | ST266 treatment | Placebo treatment |
|---|---|---|---|
| 1 | Control (non-EAE) mice | - | PBS daily, days 15-56 |
| 2 | EAE mice | - | PBS daily, days 15-56 |
| 3 | EAE mice | - | 0.5% HSA daily, days 15-56 |
| 4 | EAE mice | Once/day, days 15-30 | PBS daily, days 31-56 |
| 5 | EAE mice | Twice/day, days 15-30 | PBS twice daily, days 31-56 |
| 6 | EAE mice | Once/day, days 15-56 | - |
| 7 | EAE mice | Twice/day, days 15-56 | - |
Measurement of optokinetic responses (OKR)
The methods for OKR recording to assess visual function were similar to prior studies (7). OKR experiments were performed using OptoMotry software and apparatus (Cerebral Mechanics Inc., Medicine Hat, AB, Canada). OKR function was determined by the highest spatial frequency at which mice track a 100% contrast grating projected at varying spatial frequencies, and data are reported as cycles/degree.
Quantification of RGC survival
RGCs were immunolabeled with Brn3a (RGC marker) antibody and counted as described previously (33). Briefly, mice were sacrificed at day 42 or 56 as indicated, and retinas were isolated, prepared as flattened whole mounts, washed, and permeabilized in 0.5% Triton X 100 in PBS by freezing them for 15 min at −70 °C. The specimens were incubated overnight at 4 °C with goat-anti-Brn3a antibody (Santa Cruz) diluted 1:100 in blocking buffer (PBS, 2% bovine serum albumin, 2% Triton X 100). The retinas were then washed three times in PBS and incubated for 2 hours at room temperature with anti-goat secondary antibody diluted 1:500 in blocking buffer. Sections were washed in PBS and mounted vitreous side up on slides in anti-fading solution. Brn3a-labelled RGCs were photographed at 40X magnification in 12 standard fields: 1/6, 3/6, and 5/6 of the retinal radius from the center of the retina in each quadrant and counted by a masked investigator using image analysis software (Image-Pro Plus 5.0; Media Cybernetics, Silver Spring, MD).
Evaluation of Optic Nerve Inflammation and Demyelination
Inflammation and demyelination in the optic nerves were evaluated as described previously (10,33). Briefly, optic nerves were isolated, fixed in 4% paraformaldehyde for 15 min, embedded in paraffin, and then cut into 5 μm longitudinal sections. Hematoxylin and eosin (H&E) staining was used to evaluate inflammation in the optic nerves. For histological analysis of inflammatory cell infiltration, the entire length of each optic nerve section was examined with light microscopy by a blinded investigator and scored according to a 0–4 point scale: 0 = no infiltration; 1 = mild cellular infiltration of the optic nerve or optic nerve sheath; 2 = moderate infiltration; 3 = severe infiltration; and 4 = massive infiltration. Luxol fast blue (LFB) staining was done to evaluate demyelination in the optic nerve. The entire length of each optic nerve section was examined by light microscopy and scored on relative scale by a blinded investigator according to a 0–3 point scale: 0 = no demyelination; 1 = scattered foci of demyelination; 2 = prominent foci of demyelination; and 3 = large (confluent) areas of demyelination.
Experimental Design and Statistics
Mice were randomly numbered and assigned to treatment groups. All outcome measures including vision testing and tissue analysis were quantified in a masked fashion. Only female mice were used because they are more susceptible to the EAE disease, similar to human MS. Statistical comparisons were made using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA). Because optic neuritis occurs as an independent event that can affect one or both eyes, each eye was analyzed similar to prior studies (10,15,33). OKR responses measured across time were compared by ANOVA of repeated measures. RGC numbers and the level of optic nerve inflammation and demyelination were measured at a single time point, and differences were compared between mice in different treatment groups using one-way ANOVA followed by Tukey’s Multiple Comparison. Data are expressed as means ± SEM. Differences were considered statistically significant at p < 0.05.
Results
Ability of ST266 to attenuate experimental optic neuritis was mediated by factors secreted by AMP cells.
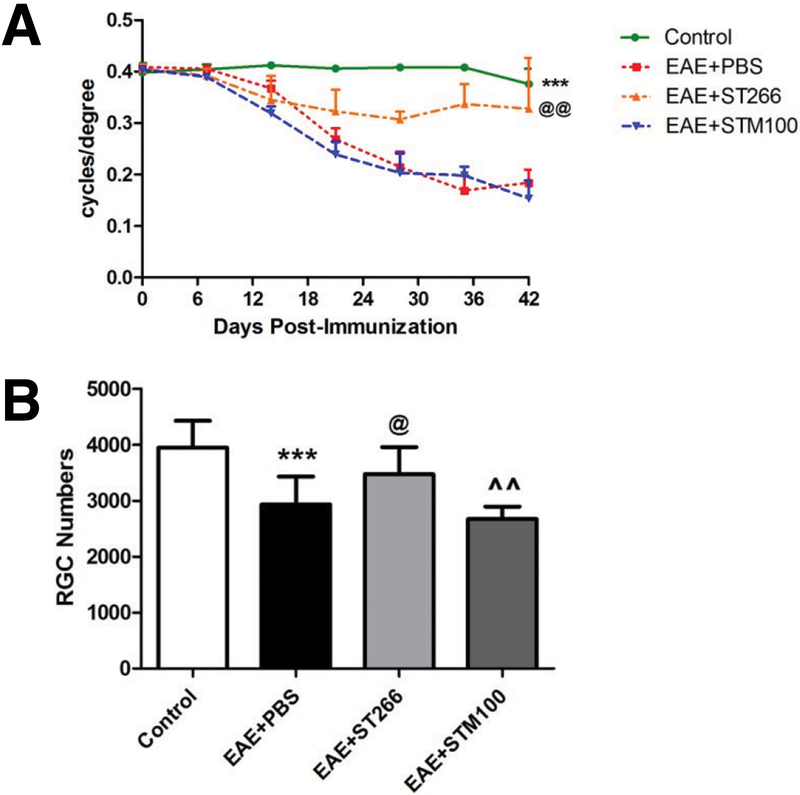
OKR responses significantly decreased in the eyes of PBS- and STM100-treated EAE mice starting from day 14 and progressed through day 42, and ST266 treatment attenuated this decrease (Fig 1A). Following sacrifice at day 42, RGC numbers were significantly decreased in the eyes of PBS- or STM100-treated EAE mice compared to control, non-EAE mice; and EAE mice that received daily ST266 showed a significant reduction in RGC loss (Fig 1B).
Figure 1. ST266, not the media it is collected in, suppresses loss of vision and RGCs in experimental optic neuritis.
(A) OKR responses significantly (***p<0.001) decreased in the eyes of PBS- (N=10) or STM100-treated EAE mice (N=10) compared with control (non-EAE) mice (N=10) starting from day 14 post-immunization and progressing through day 42. EAE mice that received intranasal ST266 once daily (N=10) from days 15 to 42 show significantly (@@p<0.01 vs EAE treated with PBS, ^^^p<0.001 vs EAE treated with STM100) higher OKR scores. (B) RGCs were labeled and counted in retinas isolated 42 days post-immunization. Data show a significant decrease in the number of RGCs in eyes of PBS- (***p<0.001, N=10) or STM100 (^^p<0.01, N=10)-treated EAE mice compared with control mice. EAE mice that received ST266 daily from day 15 to 42 (N=10) show significant (@p<0.05 vs EAE treated with PBS, @p<0.05 vs EAE treated with STM100) attenuation of RGC loss.
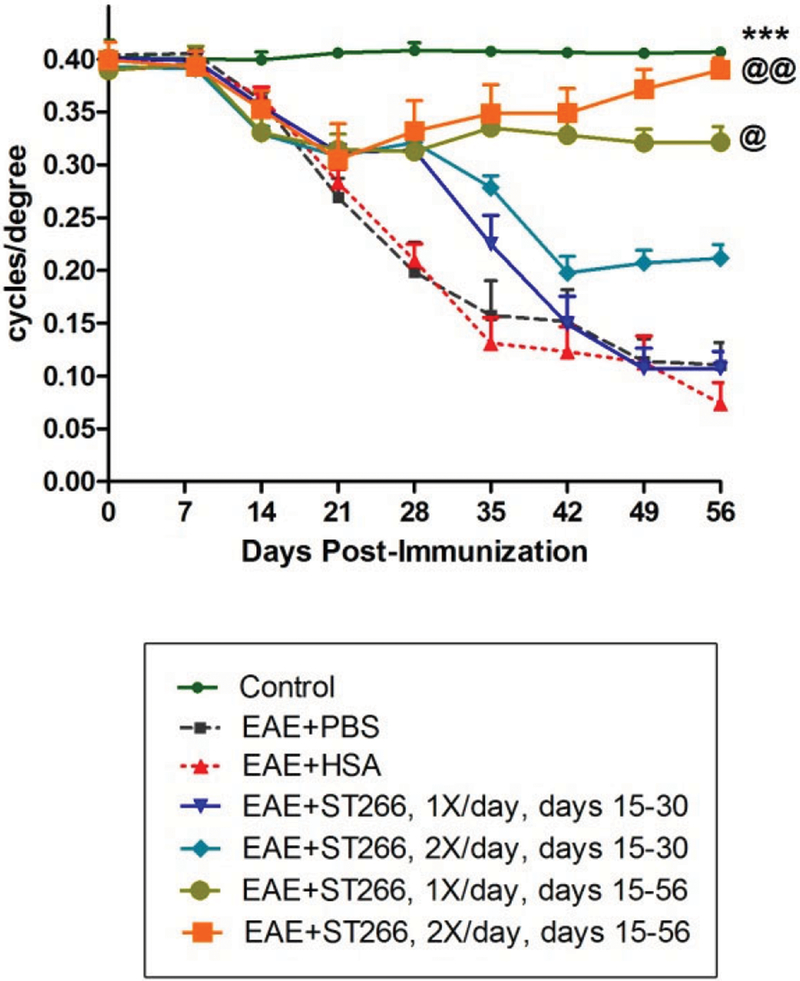
Continuous ST266 treatment promoted longer-term preservation of visual function.
Table 1 indicates the specific treatment received by each experimental group. OKR responses progressively decreased in the eyes of PBS-treated EAE mice from day 14 through day 56 post-induction (Fig 2), with similar OKR decreases in EAE mice treated with 0.5% HSA (HSA is the most abundant protein in ST266 and is present at this concentration), which served as a control for potential non-specific effects of high protein levels in solution. EAE mice that received either once or twice daily intranasal drops of ST266 starting from day 15 and repeated continuously through day 56 showed a significant preservation of OKR scores compared with PBS- or HSA-treated EAE mice (Fig 2). In contrast, EAE mice that received once or twice daily ST266 drops only from days 15 to 30, showed a delayed loss of OKR responses at days 35-42 that was not maintained from days 42-56, although twice daily treatment over this abbreviated period did show a trend toward improved OKR scores (Fig 2).
Figure 2. Continuous ST266 treatment preserves OKR for 8 weeks in EAE optic neuritis.
OKR responses significantly (***p<0.001) decreased in the eyes of PBS- (N=12) or HSA-treated EAE mice (N=12) compared with control mice (N=8), starting from day 14 and progressing over time until sacrifice at day 56 post-immunization. EAE mice that received either once or twice daily doses of intranasal ST266 from days 15 to 30 (N=12), showed a delay in OKR decreases but no significant preservation of OKR by day 56. EAE mice that received either once (@p<0.05) or twice (@@p<0.01) daily doses of intranasal ST266 continuously from day 15 to 56 (N=12) showed a significant preservation of OKR compared with both PBS- and HSA-treated EAE mice.
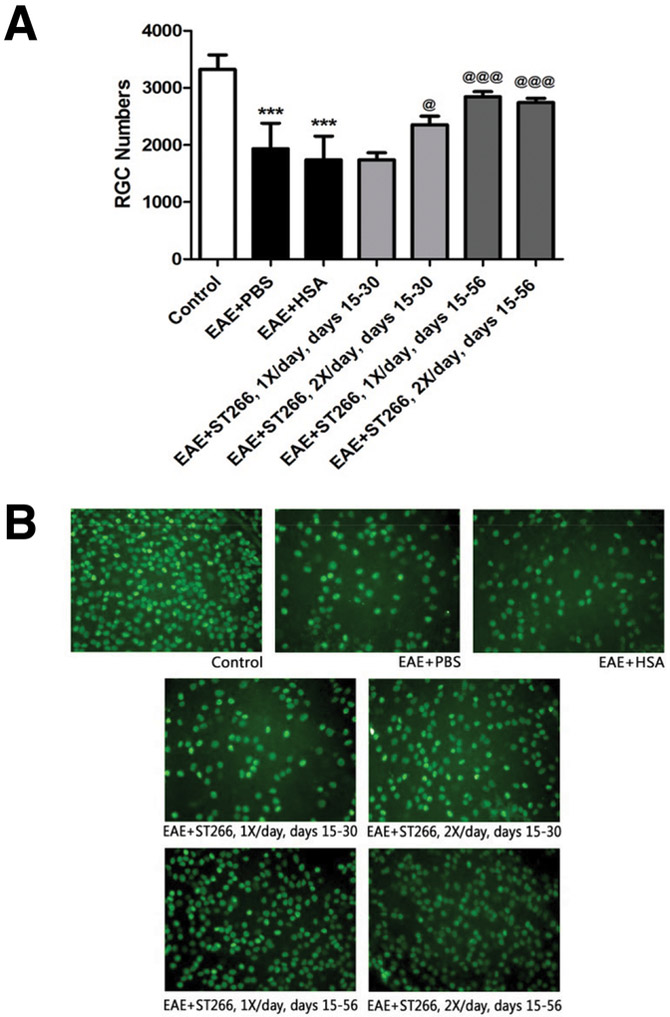
ST266 treatment reduced RGC loss in EAE optic neuritis.
EAE mice (treated as detailed in Table 1) were sacrificed on day 56. RGC numbers were significantly decreased in the eyes of PBS- or HSA-treated EAE mice compared with control mice (Fig 3). EAE mice that received either once or twice daily intranasal drops of ST266 starting from day 15 and repeated continuously through day 56 showed a significant preservation of RGCs compared with PBS- or HSA-treated EAE mice (Fig 3). EAE mice that received twice daily ST266 drops only on days 15-30, also showed a small but significant increase in RGC numbers compared to placebo-treated EAE mice, whereas once daily ST266 treatment limited to days 15-30 failed to prevent RGC loss measured at day 56 (Fig 3).
Figure 3. ST266 treatment reduces RGC loss in EAE optic neuritis.
(A) RGC counts 56 days post-immunization were significantly (***p<0.001) decreased in the eyes of PBS- (N=12) or HSA-treated EAE mice (N=12) compared with control mice (N=8). EAE mice that received once daily doses of intranasal ST266 from day 15 to 30 (N=12), showed no significant preservation of RGCs whereas twice daily ST266 given only from day 15-30 (N=12) did promote a significant increase (@p<0.05) in RGC survival. EAE mice that received either once (N=12) or twice daily (N=12) intranasal doses of ST266 continuously from day 15 to 56 showed a significant (@@@p<0.001) preservation of RGC numbers by day 56 compared with PBS- or HSA-treated EAE mice. (B) Representative images show RGCs (green) in one retina from each group (original magnification X40).
ST266 treatment reduced inflammation in the optic nerve during EAE optic neuritis.
Optic nerves from control mice showed normal histology whereas optic nerves from PBS- and HSA-treated EAE mice showed significant inflammatory cell infiltration (Fig 4). EAE mice that received either once or twice daily intranasal drops of ST266 starting from day 15 and repeated continuously through day 56 showed a significant reduction in optic nerve inflammation compared with PBS- or HSA-treated EAE mice, whereas EAE mice that received once or twice daily ST266 drops only from days 15 to 30 showed only a non-significant trend toward decreased inflammation (Fig 4).
Figure 4. ST266 treatment reduces optic nerve inflammation during EAE optic neuritis.
(A) Optic nerves from PBS- (N=9) and HSA-treated EAE mice (N=9) showed significant (*p<0.05) levels of inflammation compared with controls (N=5). EAE mice that received either once (N=9) or twice (N=9) daily doses of intranasal ST266 from days 15 to 30, showed a trend toward, but no significant, attenuation of inflammation. EAE mice that received either once (N=9) or twice (N=9) daily intranasal doses of ST266 continuously from day 15 to 56 showed a significant reduction of inflammation scores compared with PBS- or HSA-treated EAE mice (@p<0.05). (B) Representative images of H&E stained optic nerves from each group show the relative cell density (original magnification X20).
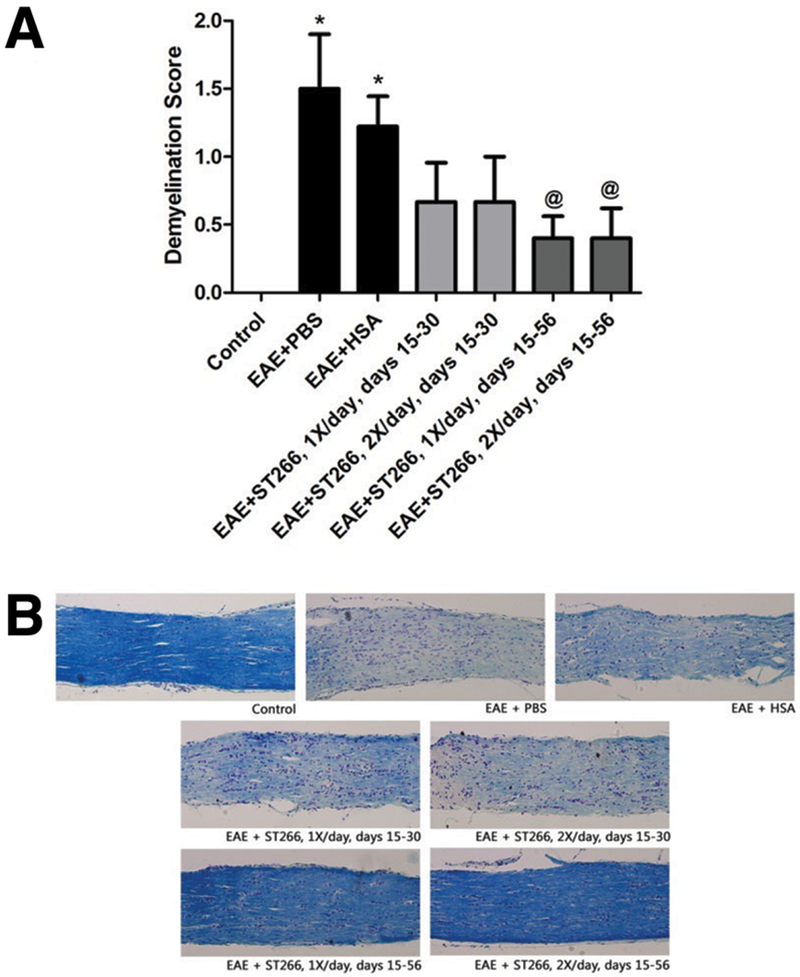
ST266 treatment reduced demyelination in the optic nerve during EAE optic neuritis.
Optic nerves from control mice showed normal myelin staining whereas optic nerves from PBS- and HSA-treated EAE mice showed significant areas of myelin loss (Fig 5). EAE mice that received either once or twice daily intranasal drops of ST266 starting from day 15 and repeated continuously through day 56 showed a significant reduction in optic nerve demyelination compared to PBS- or HSA-treated EAE mice, whereas EAE mice that received once or twice daily ST266 drops only from days 15 to 30 showed only a non-significant trend toward decreased demyelination (Fig 5).
Figure 5. ST266 treatment reduces demyelination in the optic nerve during EAE optic neuritis.
(A) Optic nerves from PBS- (N=9) and HSA-treated EAE (N=9) mice showed significant (*p<0.05) levels of demyelination compared with controls (N=5). EAE mice that received either once (N=9) or twice (N=9) daily doses of intranasal ST266 from days 15 to 30, showed a trend toward, but no significant, attenuation of demyelination. EAE mice that received either once (N=9) or twice (N=9) daily intranasal doses of ST266 continuously from day 15 to 56 showed a significant reduction of demyelination scores compared to PBS- or HSA-treated EAE mice (@p<0.05). (B) Representative images of LFB stained optic nerves images from each group show the relative level of myelin staining (original magnification X20).
Discussion
The current studies demonstrated that intranasal delivery of the novel biologic therapy ST266 promoted beneficial effects on multiple features of experimental optic neuritis, including attenuating vision loss, promoting RGC survival, and reducing the degree of optic nerve inflammation and demyelination. Results are consistent with prior studies of optic neuritis (33), as well as neuroprotective effects mediated by ST266 in a model of traumatic optic neuropathy (36). Importantly, the current results suggest that once daily ST266 dosing is sufficient to promote maximal neuroprotective effects, as twice daily treatment led to equivalent outcomes. That is, continuous treatment with either once or twice daily ST266 for 6 weeks beginning after onset of optic neuritis (days 15-56) significantly improved OKR responses and reduced RGC loss compared with placebo-treated EAE mice, whereas there was no significant difference in OKR responses or RGC numbers between once daily ST266-treated and twice daily ST266-treated mice.
In a prior study, ST266 was able to promote continued attenuation of optic neuritis 6 weeks after EAE induction even when treatment was given for only 15 days after disease onset (days 15-30 post-immunization) and then was discontinued (33). Here, using the same mouse model, results suggest that the protective effects of ST266 are diminished by 8 weeks after EAE induction when treatment is stopped at day 30, but benefits are maintained with continuous treatment through day 56. The MOG-induced EAE model in C57BL6/J mice used is known to induce an acute onset of optic neuritis with peak inflammation by day 15 post-immunization, but also exhibits continued low level inflammation for several weeks (7). This ongoing inflammation may explain why continuous ST266 treatment is needed to maintain effects in this model. In the human disease, optic neuritis is typically felt to be a self-limited inflammatory condition, thus it is likely that ST266 therapy may be able to promote improved long-term outcomes if it is administered only during the period of active inflammation. Interestingly, anti-MOG antibodies increasingly have been recognized in a subset of human optic neuritis patients and some studies have suggested these patients may have distinct phenotypes from typical MS-related optic neuritis (37). Although the MOG-induced EAE disease in C57BL/6J mice recapitulates many clinical features of human MS and optic neuritis, it is unlikely that this model specifically relates to human anti-MOG antibody positive optic neuritis because this EAE model is known to be mediated by MOG-reactive T cells, as opposed to B cells/antibodies (38).
Although specific mechanisms mediating transport of proteins from the nose to the eye and optic nerve are not known, previous studies showed that radiolabeled ST266 proteins administered intranasally accumulated at high levels in the optic nerve and vitreous within 30 minutes of treatment (33). ST266 contains numerous factors, including cytokines and growth factors, secreted by AMP cells, such as angiogenin, platelet derived growth factor BB, vascular endothelial growth factor, transforming growth factor–beta 2, amphiregulin, decorin, secreted protein, acidic and rich in cysteine, and hyaluronic acid (39). ST266 also contains anti-inflammatory factors, including macrophage inhibitory cytokine-1, macrophage migration inhibitory factor, the protease dipeptidyl peptidase-IV, and the lipid resolvin D1, as well as anti-apoptotic factors such as soluble tumor necrosis factor receptor 1, soluble tumor necrosis factor-related apoptosis-inducing ligand receptor-3, Axl, and tissue inhibitors of metalloproteinases (39). Individual proteins in ST266 are found at concentrations in the pg/mL to ng/mL range, with the total concentration of secretome proteins approximating 100 μg/mL (27). The consistent reproducibility of specific constituents of ST266 previously have been demonstrated, including similar levels of PDGF, VEGF, Angiogenin, TGF-β2, TIMP-1, and TIMP-2 across multiple lots of ST266 (27), and reproducible function in MMP-9 inhibition and Schwann cell proliferation assays (data not shown). ST266 also has shown no serious adverse effects in 8 human clinical trials using topical, oral, intravenous and ocular applications, including topical skin application to reduce UV light-induced damage (40) and ophthalmic drops for allergic conjunctivitis (Unpublished data: NCT02978183, Evaluation of the Effectiveness of ST266 Ophthalmic Drops Compared to Placebo to Treat Allergic Conjunctivitis, available at: https://clinicaltrials.gov/ct2/show?term=Noveome&rank=6).
Like other cell culture media, the STM100 growth medium that is used to grow the AMP cells contains HSA, amino acids, vitamins, salts and buffers. Therefore, to rule out the possibility that observed neuroprotective effects are due to some components in the STM100 medium other than the cell secretions in ST266, effects of intranasal delivery of STM100 alone were compared with ST266 in EAE optic neuritis. HSA, a major component of STM100 and ST266 which is not expected to affect neuronal survival, was used in a separate experiment to rule out the possibility that high levels of non-specific protein may induce any beneficial effects. Results revealed that neither HSA nor STM100 exerted any effects on inflammation, demyelination and RGC loss mediated visual loss in EAE optic neuritis mice, as predicted. These controls further support the idea that it is a combination of the multiple growth factors and anti-inflammatory cytokines present in ST266 that promote its anti-inflammatory and neuroprotective effects. While it is likely that multiple factors are needed to exert combined effects, it will be useful to identify and assess the most prominent components of ST266 as potential mediators of RGC neuroprotection in future studies.
The potential role of intranasal delivery of proteins to the cribriform plate as a novel route to target the eye and optic nerve is supported by the current studies, although specific mechanisms mediating delivery to these tissues remain to be determined. The rapid protein accumulation in rodent eyes within 30 minutes following intranansal administration reported previously (33) suggests proteins spread by local diffusion after gaining direct access to the CNS. Published studies have demonstrated that intranasal delivery of proteins to the CNS primarily occurs through absorption by the olfactory nerves adjacent to the cribriform plate, and the trigeminal nerve (41-43), with deposition of radiolabeled interferon found at lower levels throughout the brain and in the optic nerve 60 minutes after intranasal administration (42). The mechanism of absorption of intranasally applied macromolecules appears to bypass the blood–brain barrier. Instead, the macromolecules rapidly enter the primate CNS along olfactory- and trigeminal-associated extracellular pathways. In similar intranasal delivery studies in cynomolgus monkeys using Evans Blue Dye bound to ST266 proteins, the dye deposited along the nasal turbinates to the cribriform plate, the olfactory bulb and olfactory nerve, and in the orbits surrounding the eyes (unpublished data). It appears that the general proximity of the olfactory nerves within the brain to the optic nerves allows for extracellular transport by diffusion, but additional studies are needed to better understand these mechanisms.
Development of potential neuroprotective therapies for optic neuropathies depends not only on identifying drugs capable of preventing RGC damage, but also on the ability to deliver such drugs to the RGC cell bodies in the retina or axons in the optic nerve in a timely manner. A major issue with targeting therapeutic agents to these tissues is the presence of the blood-brain and blood-retinal barriers, which impede large molecular therapeutics from gaining access to the injured retina and optic nerve. Intravitreal injections are now a common delivery method for retinal therapy, currently used to treat millions of patients with degenerative retinal diseases including macular degeneration and diabetic retinopathy (44). However, repeat injections are required, and each injection increases the potential for complications including endophthalmitis, retinal detachment, ocular hypertension, inflammation or systemic toxicity (45-48). For optic neuropathies possibly requiring neuroprotective therapies on a daily basis, intravitreal delivery is not a viable method. The intranasal delivery method allows for large molecular therapeutics to gain access to the eye and CNS in a rapid noninvasive manner in rodents, making it a potential drug delivery system for targeting neuroprotective compounds to the retina and optic nerve that warrants further investigation in humans.
Overall, results confirm significant neuroprotection mediated by intranasal delivery of ST266 in experimental optic neuritis, and demonstrate that effects are mediated specifically by ST266 as opposed to growth factors present in STM100 cell culture media or any non-specific effects of any protein such as HSA. Results further demonstrate that once daily treatment is sufficient to sustain maximal benefits of this novel therapy.
Acknowledgments of funding
The authors thank Noveome Biotherapeutics, Inc. for providing ST266 for all studies reported in this manuscript. This work was supported by NIH grant EY015014, Research to Prevent Blindness, and the F. M. Kirby Foundation. Portions of this work were subcontracted from grants to Noveome Biotherapeutics, Inc. through US Navy Contract # N62645-13-C-4014, Cell-Based Wound Therapeutics for Combat Casualties and The State of Pennsylvania, 2014 Bio-Technology Grant SAP#4100068500.
Footnotes
Disclosure of potential competing financial interests
HW and LB are full time employees of Noveome Biotherapeutics, Inc., which provided the ST266 product used in these studies. KSS received research funding and has received consulting fees for discussions of clinical needs for optic neuritis from Noveome.
References
- 1).Beck RW, Cleary PA, Anderson MM Jr, Keltner JL, Shults WT, Kaufman DI, Buckley EG, Corbett JJ, Kupersmith MJ, Miller NR. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med. 1992; 326: 581–588 [DOI] [PubMed] [Google Scholar]
- 2).Trip SA,Schlottmann PG,Jones SJ,Altmann DR,Garway-Heath DF,Thompson AJ,Plant GT,Miller DH. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005; 58: 383–391. [DOI] [PubMed] [Google Scholar]
- 3).Costello F,Coupland S,Hodge W,Lorello GR,Koroluk J,Pan YI,Freedman MS,Zackon DH,Kardon RH. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006; 59: 963–969. [DOI] [PubMed] [Google Scholar]
- 4).Dutta R, Trapp BD. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol. 2011; 93: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Graham EC,You Y,Yiannikas C,Garrick R,Parratt J,Barnett MH,Klistorner A Progressive loss of retinal ganglion cells and axons in non-optic neuritis eyes in multiple sclerosis: Invest Ophthalmol Vis Sci. 2016; 57: 2311–2317. [DOI] [PubMed] [Google Scholar]
- 6).Shindler KS,Ventura E,Dutt M,Rostami A. Inflammatory demyelination induces axonal injury and retinal ganglion cell apoptosis in experimental optic neuritis. Exp Eye Res.2008; 87:208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Quinn TA,Dutt M, Shindler KS. Optic neuritis and retinal ganglion cell loss in a chronic murine model of multiple sclerosis. Front Neurol. 2011; 2:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Beck RW, Gal RL. Treatment of acute optic neuritis: a summary of findings from the optic neuritis treatment trial. Arch Ophthalmol. 2008;126:994–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol. 2008; 65: 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Khan RS, Dine K, Luna E, Ahlem C, Shindler KS. HE3286 reduces axonal loss and preserves retinal ganglion cell function in experimental optic neuritis. Invest Ophthalmol Vis Sci. 2014; 55: 5744–5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Liu Q, Li H, Yang J, Niu X, Zhao C, Zhao L,Wang Z. Valproic acid attenuates inflammation of optic nerve and apoptosis of retinal ganglion cells in a rat model of optic neuritis. Biomed Pharmacother. 2017; 96:1363–1370. [DOI] [PubMed] [Google Scholar]
- 12).Yang H, Liu C, Jiang J, Wang Y, Zhang X. Celastrol attenuates multiple sclerosis and optic neuritis in an experimental autoimmune encephalomyelitis model. Front Pharmacol. 2017; 8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Fonseca-Kelly Z,Nassrallah M,Uribe J,Khan RS,Dine K,Dutt M,Shindler KS. Resveratrol neuroprotection in a chronic mouse model of multiple sclerosis. Front Neurol. 2012; 3:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Shindler KS, Ventura E, Dutt M, Elliott P, Fitzgerald DC, Rostami A. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J Neuroophthalmol. 2010; 30:328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Khan RS, Dine K, Geisler JG, Shindler KS. Mitochondrial Uncoupler Prodrug of 2,4-Dinitrophenol, MP201, prevents neuronal damage and preserves vision in experimental optic neuritis. Oxid Med Cell Longev. 2017; 2017:7180632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Dahlmann-Noor A,Vijay S,Jayaram H,Limb A,Khaw PT. Current approaches and future prospects for stem cell rescue and regeneration of the retina and optic nerve. Can J Ophthalmol. 2010; 45:333–341. [DOI] [PubMed] [Google Scholar]
- 17).Yu S,Tanabe T,Dezawa M,Ishikawa H,Yoshimura N. Effects of bone marrow stromal cell injection in an experimental glaucoma model. Biochem Biophys Res Commun. 2006; 344:1071–1079. [DOI] [PubMed] [Google Scholar]
- 18).Li N,Li XR,Yuan JQ. Effects of bone- marrow mesenchymal stem cells transplanted arrow into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch Clin Exp Ophthalmol. 2009; 247:503–514. [DOI] [PubMed] [Google Scholar]
- 19).Harper MM,Grozdanic SD,Blits B,Kuehn MH,Zamzow D,Buss JE,Kardon RH,Sakaguchi DS. Transplantation of BDNF-secreting mesenchymal stem cells provides neuroprotection in chronically hypertensive rat eyes. Invest Ophthalmol Vis Sci. 2011; 52:4506–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Wilkins A, Kemp K, Ginty M,Hares K, Mallam E,Scolding N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009; 3: 63–70. [DOI] [PubMed] [Google Scholar]
- 21).Jung G,Sun J,Petrowitz B,Riecken K,Kruszewski K,Jankowiak W,Kunst F,Skevas C,Richard G,Fehse B,Bartsch U. Genetically modified neural stem cells for a local and sustained delivery of neuroprotective factors to the dystrophic mouse retina. Stem Cells Transl Med. 2013; 2:1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Osborne A,Sanderson J,Martin KR. Neuroprotective effects of human mesenchymal stem cells and platelet-derived growth factor on human retinal ganglion cells. Stem Cells. 2018; 36:65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Cantinieaux D,Quertainmont R,Blacher S,Rossi L,Wanet T,Noël A,Brook G,Schoenen J,Franzen R. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: an original strategy to avoid cell transplantation. PLoS One. 2013; 8:e69515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Mead B,Logan A,Berry M,Leadbeater W,Scheven BA. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One. 2014;9:e109305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int. 2014; 2014:965849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Vizoso FJ,Eiro N,Cid S,Schneider J,Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017; 18: E1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Steed DL,Trumpower C,Duffy D,Smith C,Marshall V,Rupp R,Robson M. Amnion-derived cellular cytokine solution: a physiological combination of cytokines for wound healing. Eplasty. 2008; 8:e18. [PMC free article] [PubMed] [Google Scholar]
- 28).Franz MG,Payne WG,Xing L,Naidu DK,Salas RE,Marshall VS,Trumpower CJ,Smith CA,Steed DL,Robson MC. The use of amnion derived cellular cytokine solution to improve healing in acute and chronic wound models. Eplasty. 2008; 8:e21. [PMC free article] [PubMed] [Google Scholar]
- 29).Payne WG,Wachtel TL,Smith CA,Uberti MG,Ko F,Robson MC. Effect of amnion-derived cellular cytokine solution on healing of experimental partial-thickness burns. World J Surg. 2010; 34:1663–1668. [DOI] [PubMed] [Google Scholar]
- 30).Uberti MG,Pierpont YN,Ko F,Wright TE,Smith CA,Cruse CW,Robson MC,Payne WG. Amnion-derived cellular cytokine solution (ACCS) promotes migration of keratinocytes and fibroblasts. Ann Plast Surg. 2010; 64:632–635. [DOI] [PubMed] [Google Scholar]
- 31).Deng-Bryant Y,Readnower RD,Leung LY,Cunningham TL,Shear DA,Tortella FC. Treatment with amnion-derived cellular cytokine solution (ACCS) induces persistent motor improvement and ameliorates neuroinflammation in a rat model of penetrating ballistic-like brain injury. Restor Neurol Neurosci. 2015; 33:189–203. [DOI] [PubMed] [Google Scholar]
- 32).Deng-Bryant Y,Chen Z,van der Merwe C,Liao Z,Dave JR,Rupp R,Shear DA,Tortella FC. Long-term administration of amnion-derived cellular cytokine suspension promotes functional recovery in a model of penetrating ballistic-like brain injury. J Trauma Acute Care Surg. 2012; 73:S156–164. [DOI] [PubMed] [Google Scholar]
- 33).Khan RS,Dine K,Bauman B,Lorentsen M,Lin L,Brown H,Hanson LR,Svitak AL,Wessel H,Brown L,Shindler KS. Intranasal delivery of a novel amnion cell secretome prevents neuronal damage and preserves function in a mouse multiple sclerosis model. Sci Rep. 2017; 7:41768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Shindler KS,Ventura E,Rex TS,Elliott P,Rostami A. SIRT1 activation confers neuroprotection in experimental optic neuritis. Invest Ophthalmol Vis Sci. 2007; 48:3602–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Shindler KS,Revere K,Dutt M,Ying GS,Chung DC. In vivo detection of experimental optic neuritis by pupillometry. Exp Eye Res. 2012; 100:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Grinblat GA,Khan RS,Dine K,Wessel H,Brown L,Shindler KS. RGC Neuroprotection following optic nerve trauma mediated by intranasal delivery of amnion cell secretome. Invest Ophthalmol Vis Sci. 2018; 59:2470–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Chen JJ, Flanagan EP, Jitprapaikulsan J, López-Chiriboga ASS, Fryer JP, Leavitt JA, Weinshenker BG, McKeon A, Tillema JM, Lennon VA, Tobin WO, Keegan BM, Lucchinetti CF, Kantarci OH, McClelland CM, Lee MS, Bennett JL, Pelak VS, Chen Y, VanStavern G, Adesina OO, Eggenberger ER, Acierno MD, Wingerchuk DM, Brazis PW, Sagen J, Pittock SJ. Myelin oligodendrocyte glycoprotein antibody (MOG-IgG)-positive optic neuritis: clinical characteristics, radiologic clues and outcome. Am J Ophthalmol. 2018; 195:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006; 1:1810–1819. [DOI] [PubMed] [Google Scholar]
- 39).Du Y, Banas RA, McCart EA, George J, Oakley K , Han Y , Landauer MR, Day RM. Effect of human amnion-derived multipotent progenitor cells on hematopoietic recovery after total body irradiation in C57BL/6 mice. Int J Rad Res. 2018; 16:155–168. [Google Scholar]
- 40).Guan L, Suggs A, Galan E, Lam M, Baron ED. Topical application of ST266 reduces UV-induced skin damage. Clin Cosmet Investig Dermatol. 2017;10:459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Thorne RG, Pronk GJ, Padmanabhan V, Frey WH. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496. [DOI] [PubMed] [Google Scholar]
- 42).Thorne RG, Hanson LR, Ross TM, Tung D, Frey WH. Delivery of interferon-beta to the monkey nervous system following intranasal administration. Neuroscience. 2008;152:785–797. [DOI] [PubMed] [Google Scholar]
- 43).Alcalá-Barraza SR, Lee MS, Hanson LR, McDonald AA, Frey WH, McLoon LK. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J Drug Target. 2010:18:179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Kopić A,Biuk D,Barać J,Vinković M,Benašić T,Kopić V. Retinal nerve fiber layer thickness in glaucoma patients treated with multiple intravitreal anti-vegf (Bevacizumab) Injections. Acta Clin Croat. 2017; 56:406–414. [DOI] [PubMed] [Google Scholar]
- 45).Xu K,Chin EK,Bennett SR,Williams DF,Ryan EH,Dev S,Mittra RA,Quiram PA,Davies JB,Parke DW 3rd,Johnson JB,Cantrill HL,Almeida DRP. Endophthalmitis after intravitreal injection of vascular endothelial growth factor inhibitors: management and visual outcomes. Ophthalmology. 2018; 125:1279–1286. [DOI] [PubMed] [Google Scholar]
- 46).Gupta A, Sun JK, Silva PS. Complications of intravitreous injections in patients with diabetes. Semin Ophthalmol. 2018; 33:42–50. [DOI] [PubMed] [Google Scholar]
- 47).Ercalik NY,Imamoglu S,Turkseven Kumral E,Yenerel NM,Bardak H,Bardak Y. Influence of serous retinal detachment on the outcome of ranibizumab treatment in diabetic macular oedema. Cutan Ocul Toxicol. 2018:1–4. [DOI] [PubMed] [Google Scholar]
- 48).Moraru A,Pînzaru G,Moţoc A,Costin D,Brănişteanu D. Incidence of ocular hypertension after intravitreal injection of anti-VEGF agents in the treatment of neovascular AMD. Rom J Ophthalmol. 2017;61:207–211 [DOI] [PMC free article] [PubMed] [Google Scholar]