Abstract
Purpose:
Addition of alpelisib to fulvestrant significantly extended progression-free survival in PIK3CA-mutant, hormone receptor-positive (HR+) advanced/metastatic breast cancer in the phase III SOLAR-1 study. The combination of alpelisib and letrozole also had promising activity in phase I studies of HR+ advanced/metastatic breast cancer. NEO-ORB aimed to determine if addition of alpelisib to letrozole could increase response rates in the neoadjuvant setting.
Experimental Design:
Postmenopausal women with HR+, human epidermal growth factor receptor 2-negative, T1c-T3 breast cancer were assigned to the PIK3CA-wild-type or PIK3CA-mutant cohort according to their tumor PIK3CA status, and randomized (1:1) to 2.5 mg/day letrozole with 300 mg/day alpelisib or placebo for 24 weeks. Primary endpoints were objective response rate (ORR) and pathologic complete response (pCR) rate for both PIK3CA cohorts.
Results:
In total, 257 patients were assigned to letrozole plus alpelisib (131 patients) or placebo (126 patients). Grade ≥3 adverse events (≥5% patients) in the alpelisib arm were hyperglycemia (27%), rash (12%), and maculo-papular rash (8%). The primary objective was not met; ORR in the alpelisib vs. placebo arm was 43% vs. 45% and 63% vs. 61% in the PIK3CA-mutant and -wild-type cohorts, respectively. pCR rates were low in all groups. Decreases in Ki-67 were similar across treatment arms and cohorts. In PIK3CA-mutant tumors, alpelisib plus letrozole treatment induced a greater decrease in phosphorylated AKT vs. placebo plus letrozole.
Conclusions:
In contrast to initial results in advanced/metastatic disease, addition of alpelisib to 24-week neoadjuvant letrozole treatment did not improve response in patients with HR+ early breast cancer.
Keywords: alpelisib, PI3K inhibitor, PIK3CA, breast cancer, neoadjuvant treatment
Translational Relevance
Neoadjuvant endocrine therapy is a valuable treatment option for patients with HR+ breast cancer; however, complete pathologic response rates remain low. Genetic alterations of the PI3K pathway, including in PIK3CA, are common in HR+ breast cancer and have been linked to endocrine therapy resistance. Previous studies have suggested that adding the PI3K inhibitor alpelisib to endocrine therapy (letrozole or fulvestrant) leads to improved clinical activity in advanced/metastatic breast cancer, with a trend towards improved benefit in patients with PIK3CA-mutant tumors. Indeed, the phase III SOLAR-1 trial demonstrated a significant and clinically meaningful improvement in progression-free survival with addition of alpelisib to fulvestrant for patients with PIK3CA-mutant metastatic tumors. In this trial, we show that adding alpelisib to 24 weeks of neoadjuvant letrozole treatment does not improve response rate, regardless of PIK3CA mutational status. These data suggest a different role for PI3K pathway alterations in early breast cancer compared with metastatic disease.
Introduction
Treatment options in the neoadjuvant setting for hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) early-stage and locally advanced breast cancer include systemic treatments such as chemotherapy and endocrine therapy (ET) (1). Neoadjuvant ET is an effective therapy used to down-stage tumors and decrease tumor volume, leading to improved surgical outcomes in patients with HR+ early-stage and locally advanced breast cancer (2–5). Similar treatment outcomes, including clinical response and breast conservation surgery rates, are observed for both neoadjuvant ET and chemotherapy (5, 6), and recent analysis in the adjuvant setting suggests that ET alone is just as effective as ET plus chemotherapy in patients with low/intermediate risk HR+ breast cancer (7). As such, given the more favorable side-effect profile of ET compared with chemotherapy (5, 6), neoadjuvant ET has become a clinically acceptable strategy for pre- and postmenopausal patients with HR+ early-stage and locally advanced breast cancer (8, 9). The combination of targeted therapy and ET in metastatic HR+ breast cancer has improved clinical outcomes such as progression-free survival (10, 11); however, the question of whether combining targeted agents with ET can further improve treatment outcomes in the neoadjuvant setting is still under investigation. The utilization of preoperative clinical trials in patients with operable HR+ breast cancer is an attractive strategy to both determine biomarkers of response and mechanisms of resistance and identify cancers that do not require chemotherapy (12).
The phosphoinositide 3-kinase (PI3K) signaling pathway plays a key role in breast cancer development; activation of the PI3K pathway promotes tumor growth and has been associated with resistance to ET (13, 14). Activating mutations in the p110α catalytic subunit of PI3K, PIK3CA, are among the most common genetic alterations in HR+ breast cancer (15). Preclinical studies have demonstrated the potential for combining both estrogen blockade and PI3K inhibition; the addition of PI3K inhibitors to ET potently induces apoptosis of HR+ cells and increases cell death (16, 17). Two such PI3K inhibitors are alpelisib (BYL719; a PI3Kα-specific inhibitor) and buparlisib (BKM120; a pan-PI3K inhibitor) (18–20). Both PI3K inhibitors have demonstrated promising antitumor activity in a number of preclinical models and early phase I studies of advanced breast cancer, including in combination with letrozole (19–22). In the phase III SOLAR-1 study in postmenopausal women with HR+, HER2– advanced/metastatic breast cancer, alpelisib in combination with fulvestrant significantly extended progression-free survival compared with placebo plus fulvestrant in patients with PIK3CA-mutant tumors (hazard ratio 0.65; 95% confidence interval [CI] 0.50–0.85; P = 0.00065; median 11.0 vs. 5.7 months) (23).
Here we present results from the phase II NEO-ORB study investigating the combination of PI3K inhibition with letrozole for the neoadjuvant treatment of postmenopausal patients with HR+, HER2– early breast cancer (NCT01923168).
Materials and Methods
Study design and patients
NEO-ORB is a randomized, double-blind, placebo-controlled study conducted at 87 centers in 17 countries. Postmenopausal women with locally confirmed, HR+, HER2–, T1c-T3 operable breast cancer with known PIK3CA mutation status, who had not previously received treatment with local or systemic therapy and were considered eligible for neoadjuvant ET were included in this study. Postmenopausal status was defined as per the National Comprehensive Cancer Network guidelines according to one or more of the following criteria: prior bilateral oophorectomy; age ≥60; or age <60 with amenorrhea for ≥12 months, and follicle stimulating hormone and estradiol levels considered to be postmenopausal according to the local normal range (1). Patients were required to have measurable disease, defined as any mass that could be reproducibly measured by magnetic resonance imaging (MRI) and/or ultrasound in at least one dimension. A diagnostic biopsy for analysis of PIK3CA mutation status (using the cobas® PIK3CA Mutation Test, F. Hoffmann-La Roche Ltd, Basel, Switzerland; see Supplementary Methods for further details) and Ki-67 level was required for study enrollment. All patients had an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate bone marrow and organ function. Patients with multifocal and/or multicentric disease were eligible; synchronous bilateral breast cancer was permitted provided only one of the tumors in one breast was considered for study purposes. Key exclusion criteria included receipt of prior systemic anticancer therapy, locally recurrent or metastatic disease, inflammatory breast cancer, and fasting plasma glucose >140 mg/dl or HbA1c >6.5%. All patients provided written informed consent. The study was done in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki and was approved by an independent ethics committee. A steering committee supervised the conduct of the study, and a data and safety monitoring committee performed regular safety reviews.
Randomization and study treatment
Prior to randomization, patient tumor samples were prescreened at a central laboratory to determine PIK3CA mutation status and Ki-67 level. Patients were assigned to two similar-sized cohorts according to PIK3CA mutation status; PIK3CA-mutant or PIK3CA-wild-type. Within each cohort, patients were randomly assigned 1:1:1 to receive letrozole with either alpelisib, buparlisib, or placebo using a central patient screening and randomization system. Randomization was stratified according to lymph node status (positive or negative) and centrally assessed Ki-67 level (<14% or ≥14%). Randomization was done with a block size of 12 within each stratum. Interactive response technology (IRT) that included an interactive voice and web response system was used to gather screening information and allocate treatment. Investigators provided identifying information for each patient at enrollment to register them into the IRT system, and each patient was assigned a unique seven-digit patient number, which they retained throughout their participation in the study. Randomization numbers were generated to ensure treatment assignment was unbiased and concealed from patients and investigators: a patient randomization list was produced by the IRT provider using a validated system to automate the random assignment of patient numbers to randomization numbers. Each randomization number was linked to a treatment group and a unique medication number. A separate medication randomization list was produced by Novartis Drug Supply Management with a validated system to automate the random assignment of medication numbers to medication packs containing each study treatment. Randomization numbers were not communicated to investigators. Patients and investigators (including local radiologists) were unaware of the assigned treatments from time of randomization until the study completion. The study was blinded with respect to arm (alpelisib/buparlisib vs. placebo), but due to the differences in the appearance of alpelisib tablets vs. buparlisib capsules it was not blinded with respect to study drug type (alpelisib/alpelisib-matching placebo vs. buparlisib/buparlisib-matching placebo). Premature study drug unblinding was permitted in case of emergency.
Patients were treated with letrozole (2.5 mg, once-daily, continuously) and either alpelisib (300 mg, once-daily, continuously), buparlisib (100 mg, once-daily, continuously; later amended to 5 days on/2 days off on January 29, 2015), or placebo (alpelisib- or buparlisib-matching). Study drugs were self-administered orally for 24 weeks. Definitive breast cancer surgery was performed as soon as possible, but not more than 14 days after the last dose of study drug. Letrozole was continued until the day of surgery. Two levels of dose reductions were permitted for alpelisib/buparlisib/placebo (alpelisib/placebo: 300 mg to 250 mg to 200 mg; buparlisib/placebo: 100 mg to 80 mg to 60 mg). Study discontinuation could occur due to withdrawal of consent, disease progression (radiologically documented according to Response Evaluation Criteria In Solid Tumors [RECIST] version 1.1), unacceptable toxicity, death, protocol violation, loss to follow-up, and/or if treatment was discontinued at the discretion of the investigator or patient. A dose delay of >28 days after the next scheduled dose of alpelisib/buparlisib/placebo necessitated discontinuation from alpelisib/buparlisib/placebo. Patients who discontinued alpelisib/buparlisib/placebo were permitted to remain on study and continue letrozole treatment as per investigator decision. Patients who discontinued all study treatments, but did not withdraw consent, were followed up for pathologic tumor response at the time of surgery and for safety evaluations during the 30 days following the last dose of study treatment.
Enrollment in the buparlisib arm was discontinued on December 22, 2015, due to the non-tolerable toxicity profile associated with buparlisib (24); patients randomized after this date were assigned 1:1 to either alpelisib plus letrozole or placebo plus letrozole. This randomization was also performed centrally by the IRT provider as described above, using a new randomization list and a block size of four within each stratum.
Assessments
Tumor assessments (MRI or ultrasound) were conducted at screening, Cycle 4 Day 1 (12 weeks), and prior to surgery (24 weeks; maximum 7 days before surgery); the same test (MRI or ultrasound) was used for assessment before and after treatment. Surgical breast and axillary node resection specimens were evaluated locally for pathologic tumor response. Pathologic complete response (pCR) was defined as ypT0/Tis ypN0 per the American Joint Committee on Cancer staging system (25). A tissue sample was sent to the central laboratory for assessment of Ki-67 and other biomarkers, including estrogen receptor (ER), progesterone receptor (PgR), and HER2 status, to evaluate the parameters required to calculate the preoperative endocrine prognostic index (PEPI) score. Ki-67 level was determined by the percentage of malignant cells with nuclear Ki-67 immunohistochemical staining. Phospho-AKT scoring was performed using histo-score (H-score) methodology, based on the proportion of positive staining cells (% cells) at each staining intensity graded as 0 (none), 1 (weak), 2 (moderate), and 3 (strong). H-scores were calculated as the fraction of cells with intensity grade 1 + (2 × fraction of cells with intensity grade 2+) + (3 × fraction of cells with intensity grade 3+). Biomarkers were assessed in the tumor sample provided at screening, in a tumor biopsy taken at Cycle 1 Day 15 after 2 weeks of treatment, and in the surgically removed tumor specimen. Next-generation sequencing (NGS) data were generated using the 52-gene Oncomine™ Focus Assay (ThermoFisher Scientific, Waltham, MA, USA; see Supplementary Methods for further details). PIK3CA hot-spot mutations (from exons 9 and 20) were also assessed using conventional polymerase chain reaction (PCR). Safety was monitored throughout the study by physical examination, laboratory evaluations, vital signs, bodyweight, performance status evaluation, electrocardiogram, cardiac imaging, patient self-reported questionnaires, and adverse event (AE) collection. AEs were characterized and graded throughout the study according to the Common Terminology Criteria for AEs (CTCAE) v4.03.
Outcomes
The primary endpoints were locally assessed objective response rate (ORR) and pCR rate in patients who received alpelisib plus letrozole vs. placebo plus letrozole in PIK3CA-mutant and PIK3CA-wild-type tumors based on tumor tissue. ORR was defined as the proportion of patients with a best overall response of complete response or partial response according to RECIST version 1.1. ORR and pCR rate in the buparlisib plus letrozole arm were assessed as an exploratory objective due to the discontinuation of this arm. PEPI score and safety were secondary endpoints. Other secondary endpoints (not reported here) were evaluation of the ORR and pCR rate in PIK3CA-mutant and PIK3CA-wild-type tumors based on circulating tumor DNA, evaluation of the association between changes in Ki-67 level and pCR, rate of breast-conserving surgery, and pharmacokinetics. Phosphoproteomic analysis of AKT, NGS data, and analyses of the Ki-67 proliferation marker from tumor tissue are reported. The data cut-off for the primary analysis was July 8, 2017; this study is complete.
Statistical Analysis
The primary endpoint of ORR as per local investigator review was assessed based on prespecified Bayesian double criteria requiring an estimated difference in ORR between the alpelisib plus letrozole arm and the placebo plus letrozole arm of at least 20% and a posterior probability criterion (difference between groups >0) of more than 0.9. With 60 patients per group in each cohort, there was a 46% chance of meeting the endpoint if the true difference in ORR was 20%, and <5% chance if the true difference in ORR was null. Based on previously published data, the ORR with letrozole alone was expected to be approximately 45% (26, 27). Patients with unknown best overall response were considered as nonresponders for the calculation of ORR. For the primary analysis of pCR rate as per local investigator review, assessment was based on prespecified Bayesian double criteria requiring an estimated difference in pCR rate between the alpelisib plus letrozole arm and the placebo plus letrozole arm of at least 10% and a posterior probability criterion (difference between groups >0) of more than 0.9. With 60 patients per group in each cohort, there was 43% chance of meeting the endpoint if the true difference in pCR rate was 10%, and <5% chance if the true difference in pCR rate was null. The pCR rate with letrozole alone was expected to be 5% or less (28). Patients who experienced progression of disease while undergoing neoadjuvant therapy, or who did not undergo surgery for any reason, or received antineoplastic treatment other than the study drugs before surgery were considered as nonresponders for the calculation of pCR rate. ORR and pCR rate were summarized by cohort and study arm using descriptive statistics including 90% CIs according to the Clopper and Pearson (1934) exact method (29). Summary statistics were also provided for Ki-67 level changes and PEPI score.
Efficacy analyses were performed in the full analysis set including all randomized patients, according to the intention-to-treat principle. All safety analyses were performed in the safety set (patients who received at least one dose of study treatment). For the placebo plus letrozole results, data for the alpelisib-matching and buparlisib-matching placebo plus letrozole arms were combined.
Results
Between April 10, 2014, and December 2, 2016, 257 patients were randomly assigned to receive alpelisib plus letrozole (n = 131) or placebo plus letrozole (n = 126; Fig. 1). Patient characteristics at baseline were generally balanced between treatment arms, with some notable differences (Table 1). Patients with PIK3CA mutations receiving alpelisib plus letrozole had a median age of 65.5 years, compared with 61.0 years for patients receiving placebo plus letrozole. In addition, a higher proportion of patients in the PIK3CA-mutant cohort receiving alpelisib plus letrozole had lymph node involvement (N1 or higher; 35%) and T3-stage tumors (12%) compared with patients who received placebo plus letrozole (27% and none, respectively). During the study period, 83 patients were randomized to receive buparlisib plus letrozole; 37 in the PIK3CA-mutant cohort and 46 in the PIK3CA-wild-type cohort. As the buparlisib arm was discontinued following a program-wide assessment of buparlisib efficacy and safety across different indications, ORR and pCR rate for buparlisib plus letrozole were assessed as exploratory endpoints and the data are presented in Supplementary Tables S1–S4; buparlisib addition to letrozole did not improve ORR or pCR rate in either the PIK3CA-mutant or -wild-type cohorts.
Figure 1.
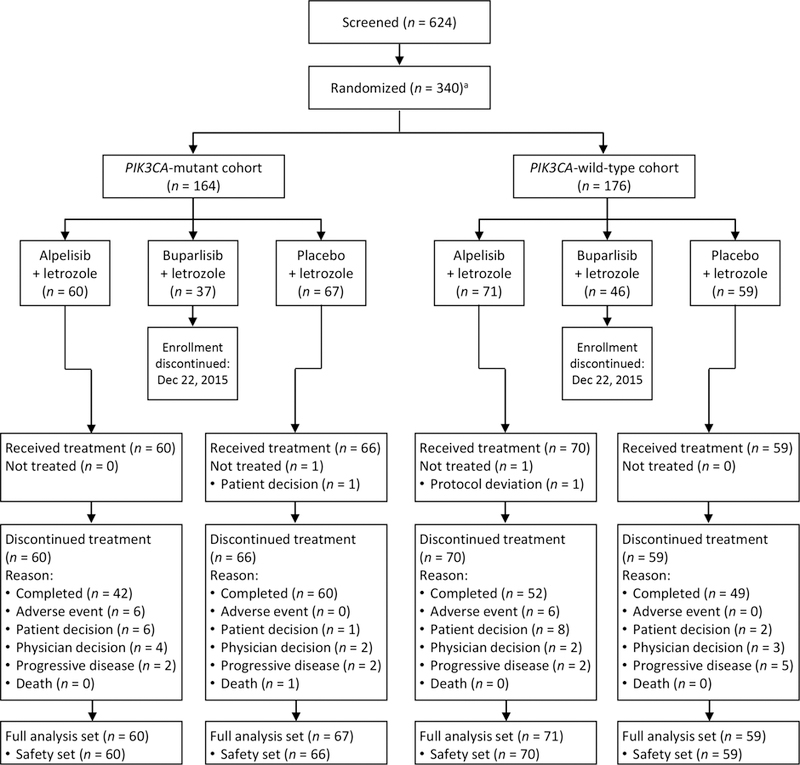
CONSORT diagram
aEnrollment in the buparlisib arm was discontinued on 22 December 2015 and patients randomized after this date were assigned 1:1 to either alpelisib plus letrozole or placebo plus letrozole.
Table 1.
Patient characteristics at baseline
|
PIK3CA-mutant cohort N = 127 |
PIK3CA-wild-type cohort N = 130 | |||
|---|---|---|---|---|
| Characteristic | Alpelisib + letrozole n = 60 | Placebo + letrozole n = 67 |
Alpelisib + letrozole n = 71 | Placebo + letrozole n = 59 |
| Age, median (range), years | 65.5 (47–86) | 61.0 (37–82) | 63.0 (50–84) | 64.0 (49–84) |
| Race, n (%) | ||||
| White | 53 (88.3) | 51 (76.1) | 64 (90.1) | 54 (91.5) |
| Asian | 5 (8.3) | 9 (13.4) | 2 (2.8) | 1 (1.7) |
| Black or African American | 1 (1.7) | 2 (3.0) | 2 (2.8) | 3 (5.1) |
| Unknown | 1 (1.7) | 2 (3.0) | 0 | 1 (1.7) |
| Other | 0 | 3 (4.5) | 3 (4.2) | 0 |
| ECOG performance status, n (%) | ||||
| 0 | 56 (93.3) | 62 (92.5) | 66 (93.0) | 54 (91.5) |
| 1 | 4 (6.7) | 5 (7.5) | 5 (7.0) | 5 (8.5) |
| Hormone receptor status, n (%) | ||||
| Estrogen receptor-positive | 60 (100) | 67 (100) | 70 (98.6) | 59 (100) |
| Progesterone receptor-positive | 48 (80.0) | 60 (89.6) | 65 (91.5) | 48 (81.4) |
| Both positive | 48 (80.0) | 60 (89.6) | 64 (90.1) | 48 (81.4) |
| Details of tumor histology/cytology, n (%) | ||||
| Adenocarcinoma | 1 (1.7) | 2 (3.0) | 1 (1.4) | 2 (3.4) |
| Breast lobular invasive | 14 (23.3) | 13 (19.4) | 19 (26.8) | 8 (13.6) |
| Ductal invasive carcinoma | 41 (68.3) | 49 (73.1) | 49 (69.0) | 45 (76.3) |
| Other | 4 (6.7) | 3 (4.5) | 2 (2.8) | 4 (6.8) |
| Histologic grade, n (%) | ||||
| Well differentiated | 17 (28.3) | 22 (32.8) | 13 (18.3) | 13 (22.0) |
| Moderately differentiated | 30 (50.0) | 30 (44.8) | 37 (52.1) | 33 (55.9) |
| Poorly differentiated | 9 (15.0) | 8 (11.9) | 15 (21.1) | 8 (13.6) |
| Other | 4 (6.7) | 7 (10.4) | 6 (8.5) | 5 (8.5) |
| Primary tumor stage at study entry, n (%) | ||||
| T1b | 0 | 0 | 1 (1.4) | 0 |
| T1c | 19 (31.7) | 16 (23.9) | 18 (25.4) | 15 (25.4) |
| T2 | 34 (56.7) | 51 (76.1) | 39 (54.9) | 37 (62.7) |
| T3 | 7 (11.7) | 0 | 13 (18.3) | 7 (11.9) |
| Lymph node stage at study entry, n (%) | ||||
| N0 | 39 (65.0) | 49 (73.1) | 47 (66.2) | 36 (61.0) |
| N1 | 17 (28.3) | 17 (25.4) | 19 (26.8) | 19 (32.2) |
| N2 | 3 (5.0) | 0 | 4 (5.6) | 2 (3.4) |
| N3 | 1 (1.7) | 1 (1.5) | 1 (1.4) | 2 (3.4) |
| Time since initial diagnosis of primary site, median (range) months | 1.5 (0.6–5.9) | 1.5 (0.8–7.5) | 1.5 (0.7–49.3) | 1.4 (0.8–4.1) |
| Type of lesions at baseline, n (%) | ||||
| Target only | 45 (75.0) | 54 (80.6) | 51 (71.8) | 40 (67.8) |
| Both target and nontarget | 15 (25.0) | 12 (17.9) | 20 (28.2) | 19 (32.2) |
| Unknown | 0 | 1 (1.5) | 0 | 0 |
| Multicentric disease, n (%) | 3 (5.0) | 8 (11.9) | 5 (7.0) | 5 (8.5) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Treatment exposure
The 24-week treatment phase (remaining on alpelisib/placebo and/or letrozole) was completed by 72% and 87% of patients in the alpelisib plus letrozole and placebo plus letrozole arms, respectively (Fig. 1). AEs and patient/physician decision were reported as the primary reasons for discontinuation of study treatment in the alpelisib plus letrozole arm in both the PIK3CA-mutant and -wild-type cohorts. A higher proportion of patients received study treatment for 8 weeks or less in the alpelisib plus letrozole arm (19%) than in the placebo plus letrozole arm (5%; Table 2). Alpelisib dose reductions and interruptions were experienced by 53% and 65%, respectively, of patients in the alpelisib plus letrozole arm.
Table 2.
Exposure and dose modifications
| PIK3CA-mutant cohort N = 127 | PIK3CA-wild-type cohort N = 130 | |||
|---|---|---|---|---|
| Alpelisib + letrozole n = 60 | Placebo + letrozole n = 66 | Alpelisib + letrozole n = 70 | Placebo + letrozole n = 59 | |
| Duration of exposure to study treatment | ||||
| Days, median (Q1–Q3) | 168.0 (102.5–173.0) | 171.0 (168.0–180.0) | 169.0 (165.0–173.0) | 169.0 (167.0–174.0) |
| ≤8 weeks, n (%) | 12 (20.0) | 4 (6.1) | 13 (18.6) | 2 (3.4) |
| >24 weeks, n (%) | 29 (48.3) | 45 (68.2) | 43 (61.4) | 33 (55.9) |
| Duration of exposure to alpelisib/placebo | ||||
| Days, median (Q1–Q3) | 154.0 (30.5–168.0) | 168.0 (167.0–169.0) | 167.0 (43.0–169.0) | 168.0 (166.0–169.0) |
| ≤8 weeks, n (%) | 21 (35.0) | 4 (6.1) | 21 (30.0) | 2 (3.4) |
| >24 weeks, n (%) | 13 (21.7) | 21 (31.8) | 20 (28.6) | 20 (33.9) |
| Dose modifications/discontinuation of alpelisib/placebo, n (%) | ||||
| At least one dose reduction | 34 (56.7) | 3 (4.5) | 35 (50.0) | 2 (3.4) |
| At least one dose interruption | 37 (61.7) | 21 (31.8) | 48 (68.6) | 13 (22.0) |
| Permanent discontinuation reason, n (%) | ||||
| Completed | 28 (46.7) | 60 (90.9) | 40 (57.1) | 47 (79.7) |
| Adverse event | 19 (31.7) | 0 | 18 (25.7) | 2 (3.4) |
| Subject/guardian decision | 7 (11.7) | 1 (1.5) | 7 (10.0) | 2 (3.4) |
| Physician decision | 4 (6.7) | 2 (3.0) | 3 (4.3) | 3 (5.1) |
| Progressive disease | 2 (3.3) | 1 (1.5) | 2 (2.9) | 5 (8.5) |
Abbreviations: Q1, first quartile; Q3, third quartile.
Efficacy
The NEO-ORB study did not meet its primary objectives of improved ORR and pCR rate with the addition of alpelisib to letrozole in either the PIK3CA-mutant or -wild-type cohorts after 24 weeks of neoadjuvant treatment (Table 3). The ORR was similar for the alpelisib plus letrozole vs. placebo plus letrozole arms in both cohorts (PIK3CA-mutant, 43% vs. 45% with a posterior probability that the difference is greater than 0 of 0.435; PIK3CA-wild-type, 63% vs. 61% with a posterior probability that the difference is greater than 0 of 0.611), and the number of patients experiencing pCR was low in all groups. A post-hoc exploratory analysis of ORR was conducted in patients who received treatment for at least 16 weeks and 24 weeks to determine whether the difference in ORR between treatment arms was impacted by treatment duration or discontinuation rate (Supplementary Table S5). Of patients in the alpelisib plus letrozole arm, 60% completed at least 16 weeks of treatment with alpelisib, and only 52% completed 24 weeks of alpelisib treatment. Importantly, in patients who completed 24 weeks of alpelisib treatment, there were no significant differences in ORR between the alpelisib plus letrozole and placebo plus letrozole arms in either the PIK3CA-mutant (n = 88) or PIK3CA-wild-type (n = 87) cohorts; the ORR for alpelisib plus letrozole was 57% in the PIK3CA-mutant group and 78% in the PIK3CA-wild-type cohort, and for placebo plus letrozole the ORR was 50% and 70%, respectively. The ORR for patients who completed at least 16 weeks of alpelisib plus letrozole treatment was 31% in the PIK3CA-mutant cohort (n = 32) and 59% in the PIK3CA-wild-type cohort (n = 46), for patients treated with placebo plus letrozole the ORR was 28% and 45% in the PIK3CA-mutant (n = 60) and PIK3CA-wild-type cohorts (n = 51), respectively. Analysis of ORR in patients with positive PgR status also showed no substantial difference between the alpelisib plus letrozole and placebo plus letrozole arms (Supplementary Table S6). The number of patients with negative PgR status was low (Supplementary Table S6).
Table 3.
ORR, pCR rate, and PEPI score
| PIK3CA-mutant cohort N = 127 | PIK3CA-wild-type cohort N = 130 | |||
|---|---|---|---|---|
| Alpelisib + letrozole n = 60 | Placebo + letrozole n = 67 | Alpelisib + letrozole n = 71 | Placebo + letrozole n = 59 | |
| Primary endpoints: ORR and pCR rate | ||||
| ORR, n (%) | 26 (43.3) | 30 (44.8) | 45 (63.4) | 36 (61.0) |
| Difference (80% crl)a | –1.4% (–12.5, 9.7) | 2.4% (–8.4, 13.2) | ||
| Posterior probabilityb | 0.435 | 0.611 | ||
| pCR rate, n (%) | 1 (1.7) | 2 (3.0) | 2 (2.8) | 1 (1.7) |
| Difference (80% crl)a | –1.3% (–4.5, 1.7) | 1.1% (–1.9, 4.2) | ||
| Posterior probabilityb | 0.282 | 0.697 | ||
| Secondary endpoint: PEPI score | ||||
| Patients with PEPI score at EOT, n (%) | 33 (55.0) | 48 (71.6) | 35 (49.3) | 38 (64.4) |
| PEPI score at EOT, n (%) | ||||
| 0 (low risk) | 1 (3.0) | 0 | 1 (2.9) | 1 (2.6) |
| 1–3 (intermediate risk) | 17 (51.5) | 25 (52.1) | 17 (48.6) | 16 (42.1) |
| ≥4 (high risk) | 15 (45.5) | 23 (47.9) | 17 (48.6) | 21 (55.3) |
Abbreviations: crl, credible interval; EOT, end of treatment; ORR, objective response rate; pCR, pathologic complete response; PEPI, preoperative endocrine prognostic index.
Mean difference between treatment arms.
Posterior probability that the difference between treatment arms is >0.
PEPI scores, that consider pathologic staging variables and biomarker values to define broad groups of patients at risk of relapse, were available for 60% of patients. A similar proportion of patients had low, intermediate, and high PEPI scores in both the PIK3CA-mutant and -wild-type groups and across treatment arms (Table 3).
Safety
The most frequently reported all-grade AEs in the alpelisib plus letrozole arm (≥20% of patients; single preferred term; Table 4) were hyperglycemia (54%), diarrhea (52%), rash (45%), nausea (44%), fatigue (41%), stomatitis (33%), decreased appetite (31%), alopecia (22%), and headache (20%). The most common grade ≥3 AEs in the alpelisib plus letrozole arm (≥5% of patients; single preferred term) were hyperglycemia (27%), rash (12%), and maculo-papular rash (8%). In the alpelisib plus letrozole vs placebo plus letrozole arms there was a higher incidence of treatment-related serious AEs (SAEs; 12% vs 1%) and treatment-related AEs leading to discontinuation of either alpelisib, placebo, or letrozole (27% vs 1%). The most frequent AEs leading to discontinuation of alpelisib or letrozole, regardless of treatment relationship, in the alpelisib plus letrozole arm (≥5% of patients) were hyperglycemia (9%) and rash (5%; Supplementary Table S7). AEs were the cause of discontinuation of both alpelisib and letrozole in 9% of patients in the alpelisib plus letrozole arm; no patients in the placebo plus letrozole arm discontinued both placebo and letrozole due to AEs. No treatment-related deaths occurred.
Table 4.
Most frequent adverse events (≥10% in alpelisib) by single preferred term regardless of treatment relationship in the safety population
| Adverse events by single preferred term, n (%) | Alpelisib + letrozole n = 130 | Placebo + letrozole n = 125 | ||
|---|---|---|---|---|
| All-grade | Grade ≥3 | All-grade | Grade ≥3 | |
| Hyperglycemiaa | 70 (53.8) | 35 (26.9) | 8 (6.4) | 0 |
| Diarrheab | 67 (51.5) | 3 (2.3) | 19 (15.2) | 0 |
| Rashc | 58 (44.6) | 16 (12.3) | 10 (8.0) | 0 |
| Nauseab | 57 (43.8) | 2 (1.5) | 23 (18.4) | 0 |
| Fatigue | 53 (40.8) | 2 (1.5) | 42 (33.6) | 1 (0.8) |
| Stomatitis | 43 (33.1) | 0 | 5 (4.0) | 0 |
| Decreased appetiteb | 40 (30.8) | 0 | 10 (8.0) | 0 |
| Alopecia | 28 (21.5) | 0 | 7 (5.6) | 0 |
| Headache | 26 (20.0) | 0 | 16 (12.8) | 0 |
| Dysgeusia | 24 (18.5) | 0 | 7 (5.6) | 0 |
| Pruritus | 24 (18.5) | 1 (0.8) | 9 (7.2) | 0 |
| Vomitingb | 24 (18.5) | 3 (2.3) | 6 (4.8) | 0 |
| Rash maculo-papularc | 22 (16.9) | 10 (7.7) | 3 (2.4) | 0 |
| Asthenia | 21 (16.2) | 0 | 17 (13.6) | 0 |
| Dry skin | 19 (14.6) | 1 (0.8) | 4 (3.2) | 0 |
| Hypertension | 16 (12.3) | 6 (4.6) | 8 (6.4) | 6 (4.8) |
| Weight decreased | 16 (12.3) | 5 (3.8) | 3 (2.4) | 0 |
| Insomnia | 15 (11.5) | 1 (0.8) | 17 (13.6) | 0 |
| Urinary tract infection | 15 (11.5) | 0 | 7 (5.6) | 0 |
| Alanine aminotransferase increased | 14 (10.8) | 4 (3.1) | 3 (2.4) | 1 (0.8) |
| Dry mouth | 14 (10.8) | 0 | 4 (3.2) | 0 |
| Pyrexia | 14 (10.8) | 0 | 3 (2.4) | 0 |
| Muscle spasms | 13 (10.0) | 0 | 5 (4.0) | 0 |
As a whole, all-grade events of hyperglycemia (including the following preferred terms: diabetes mellitus, hyperglycemia, insulin resistance, metabolic syndrome, and others [see Supplementary Methods for complete list]) were reported in 63.8% of patients in the alpelisib plus letrozole arm (grade ≥3: 30.8%) and 8.0% of patients in the placebo plus letrozole arm (no grade ≥3 events).
All-grade gastrointestinal toxicities (including the following preferred terms: nausea, vomiting, diarrhea, and others [see Supplementary Methods for complete list]) were reported in 70.8% of patients in the alpelisib plus letrozole arm (grade ≥3: 5.4%) and 32.8% of patients in the placebo plus letrozole arm (no grade ≥3 events).
All-grade events of any rash (including the following preferred terms: exfoliative rash, nodular rash, rash follicular, rash generalized, rash maculo-papular, and others [see Supplementary Methods for complete list]) were reported in 68.5% of patients in the alpelisib plus letrozole arm (grade ≥3: 24.6%) and 10.4% of patients in the placebo plus letrozole arm (no grade ≥3 events).
Inhibition of PI3K signaling and tumor cell proliferation
At Cycle 1 Day 15, the combination of alpelisib plus letrozole demonstrated effective inhibition of PI3K signaling in the PIK3CA-mutant cohort as measured by a greater decrease in levels of phosphorylated AKT compared with that observed in the placebo plus letrozole arm (Fig. 2A,B). Interestingly, levels of phosphorylated AKT appeared to increase in the placebo plus letrozole arm of the PIK3CA-mutant cohort at Cycle 1 Day 15 compared with baseline. Levels of phosphorylated AKT were decreased in all patient groups compared with baseline at the end of treatment (Fig. 2C).
Figure 2.
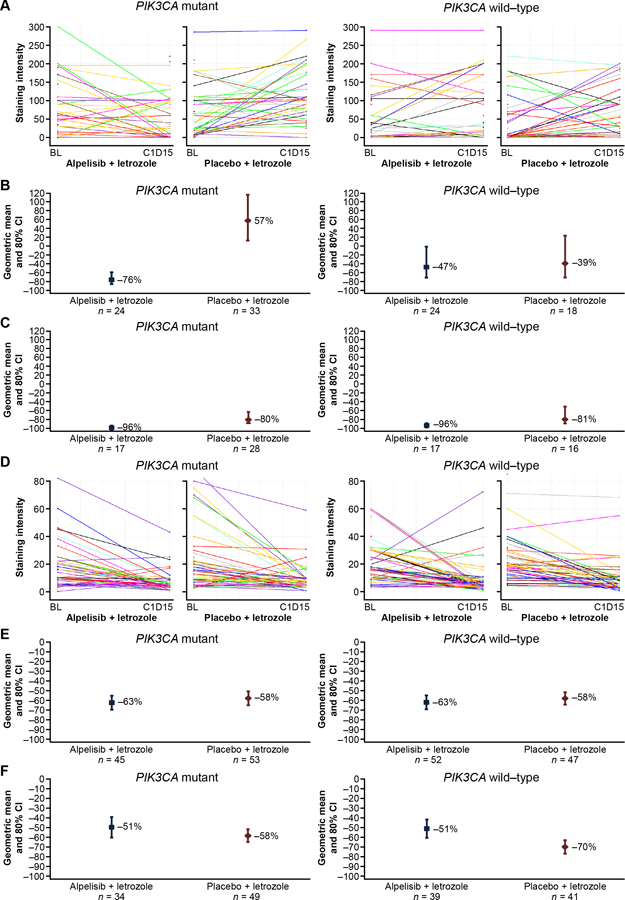
Changes from baseline in phospho-AKT and Ki-67 levels following treatment with alpelisib plus letrozole or placebo plus letrozole. A, phospho-AKT level at baseline and Cycle 1 Day 15 for each patient; B, mean percentage change from baseline in phospho-AKT level at Cycle 1 Day 15; C, mean percentage change from baseline in phospho-AKT level at end of treatment; D, Ki-67 level at baseline and Cycle 1 Day 15 for each patient; E, mean percentage change from baseline in Ki-67 level at Cycle 1 Day 15; F, mean percentage change from baseline in Ki-67 level at end of treatment.
Despite effective inhibition of PI3K pathway activity in situ, assessment of tumor cell proliferation at Cycle 1 Day 15 showed similar inhibition of the Ki-67 proliferation marker following treatment with alpelisib plus letrozole and placebo plus letrozole, independent of PIK3CA mutation status (Fig. 2D,E). At the end of treatment, Ki-67 levels were reduced to a greater extent in the placebo plus letrozole arm vs. the alpelisib plus letrozole arm (Fig. 2F). The termination of alpelisib treatment up to 14 days before surgery per protocol, together with the high rate of premature alpelisib discontinuation, limits interpretation of the Ki-67 data.
Next-generation sequencing
NGS highlighted differences in the genetic landscape of baseline tumor samples between the PIK3CA-mutant and -wild-type cohorts. In tumors with PIK3CA mutations, very few additional mutations were detected across the 52-gene panel. In contrast, PIK3CA-wild-type tumors were enriched for AKT1 hotspot mutations and copy number alterations of CCND1 and FGFR1 (data not shown). At end of treatment, PIK3CA mutations were no longer detected in six patients who received alpelisib plus letrozole and three patients who received placebo plus letrozole in the PIK3CA-mutant cohort. In the PIK3CA-wild-type cohort, a gain of PIK3CA mutations was detected in three patients in the alpelisib plus letrozole arm and one patient in the placebo plus letrozole arm. There was no evidence of mutations associating with a response to treatment. Between NGS and conventional PCR there was high concordance for baseline PIK3CA status; NGS had 97% sensitivity and 97% specificity compared with conventional PCR on the same sample.
Discussion
The NEO-ORB study investigated whether addition of a PI3Kα inhibitor to letrozole could increase ORR and pCR rate in postmenopausal women with early-stage HR+ breast cancer. Alpelisib effectively inhibited PI3K signaling, with a greater decrease in phosphorylated AKT levels observed in the alpelisib plus letrozole arm compared with the placebo plus letrozole arm in the PIK3CA-mutant cohort at Cycle 1 Day 15. However, based on local assessments and protocol criteria, proof of concept was not established for a 24-week neoadjuvant treatment with alpelisib plus letrozole in patients with HR+ operable breast cancer. The addition of alpelisib to letrozole did not improve ORR in either the PIK3CA-mutant or -wild-type cohorts and pCR rates were low in all groups. Preliminary efficacy data for the pan-PI3K inhibitor buparlisib also showed no treatment benefit when added to letrozole. Consistent with the efficacy findings, there were no substantial differences in Ki-67 levels between the alpelisib plus letrozole and placebo plus letrozole treatment arms. However, we recognize that since alpelisib treatment was terminated up to 14 days before surgery per protocol (and was discontinued even earlier in many patients), the Ki-67 value determined by analysis of the surgical specimen may no longer reflect the action of treatment. Exploratory NGS analysis conducted thus far has not revealed any specific biomarkers or pathway mechanisms that could explain the limited efficacy. Analysis of PEPI scores was hindered by the difficult interpretation of the Ki-67 values (due to the discontinuation of alpelisib prior to surgery), which negated the ability to collect complete PEPI data. Interestingly, there appeared to be an increase in phosphorylated AKT levels in the placebo plus letrozole arm at Cycle 1 Day 15 in the PIK3CA-mutant cohort compared with baseline; this could suggest that upon estrogen suppression, PIK3CA-mutant tumors activate PI3K signaling as a compensatory mechanism. The mechanism behind the subsequent decrease in levels of phosphorylated AKT in the placebo plus letrozole arm at end of treatment is unclear, but may also be due to crosstalk between the ER and PI3K signaling pathways (17, 30). The possible full saturation of ER signaling inhibition at end of treatment may have subsequently suppressed the compensatory PI3K signaling. Effects of estrogen suppression with letrozole on PI3K signaling have been observed in other studies. In one study, letrozole reduced PI3K and mTOR expression, but not phosphorylated AKT levels, after 6 months of treatment (31). In another study, after 15 days of treatment, letrozole downregulated expression of PgR and cyclin D1, and to a lesser extent, ribosomal protein S6 (26).
ORR was lower in both arms of the PIK3CA-mutant cohort than in both arms of the PIK3CA-wild-type cohort in this study. A similar finding has been previously observed in HER2-positive early breast cancer, where tumor PIK3CA mutations were associated with a significantly lower rate of pCR, especially in HR+ disease (32). Goel and Krop (2016) speculated that the lower rate of pCR could be due to reduced proliferation rates observed in PIK3CA-mutant vs. PIK3CA-wild-type tumors (33), as some treatments are more effective against highly proliferative tumors. The lower pCR rate in the HER2-positive, PIK3CA-mutant early-stage breast tumors did not appear to impact disease-free survival (32), suggesting that these tumors may have a less virulent biology and lower risk of recurrence than PIK3CA-wild type, HER2-positive cancers even in the absence of pCR following neoadjuvant therapy (33). In a pooled analysis of 19 studies, PIK3CA mutations were significantly associated with better disease-free and overall survival (OS) but had a lesser prognostic effect after adjustment for other prognostic factors (34). Overall, however, the prognostic value of PIK3CA mutations in early breast cancer is unclear (35).
In terms of the predictive value of PIK3CA mutations in early-stage breast cancer, the data presented here indicated no difference between the PIK3CA-mutant and PIK3CA-wild-type cohorts for benefit to PI3K inhibitor treatment (addition of alpelisib to letrozole did not improve response rate in either cohort). Studies with other PI3K inhibitors have also raised questions over the predictive relevance of PIK3CA mutational status for treatment in early-stage breast cancer. In a preoperative window-of-opportunity study of women with operable HR+ breast cancer, the addition of pictilisib (GDC-0941; a pan-PI3K inhibitor) to anastrozole over a 14-day period resulted in a significant decrease in Ki-67 proliferation that was limited to luminal B primary breast cancer subtypes (36). It should be noted, however, that a low proportion of tumors in the control arm (anastrozole alone; 61.5%) exhibited Ki-67 levels of <10% on Day 15 (36). PIK3CA mutations were not predictive of response to pictilisib (36). Similarly, in the LORELEI trial, ORR and pCR rates in postmenopausal women with ER-positive/HER2–, Stage I–III, operable breast cancer who received neoadjuvant treatment with taselisib (PI3Kβ-sparing inhibitor) and letrozole were similar in the overall study population and the PIK3CA-mutant subgroup (37).
There were several limitations to this study. In particular, the extent to which the addition of alpelisib to letrozole improved ORR may have been impacted by the high rate of treatment discontinuations in the alpelisib plus letrozole arm. Only 52% of patients in the alpelisib plus letrozole arm completed 24 weeks of alpelisib treatment. Differences in the baseline patient characteristics between treatment arms, including more patients with a higher tumor stage in the PIK3CA-mutant cohort treated with alpelisib plus letrozole compared with placebo plus letrozole, may have also affected the results. The results of this phase II study were also limited by the lack of consistency in both the timing of response assessments and the method of evaluation (MRI or ultrasound).
Tumor responses have been successfully used as surrogate markers for clinical response in various cancer types (38). For systemic therapies given in the neoadjuvant setting, a pCR endpoint is suggested to predict clinical benefit in high-risk populations (39). Indeed, pCR has been linked to long-term outcomes for neoadjuvant chemotherapy treatment in breast cancer, although the value of pCR for patients with luminal breast cancer, in particular the luminal A subgroup, is questionable (40–42). Data from randomized controlled trials support the use of ORR as a surrogate endpoint for OS in recurrent ovarian cancer with at least two lines of therapy (43). However, although ORR provides evidence of treatment effect, limitations with this surrogate marker include a reliance on imaging technologies, variability in tumor measurements, and the possibility that tumor size may not always correlate with patient OS (38, 39, 44). MRI is considered a valuable imaging method in the neoadjuvant setting, although there is insufficient evidence to support its routine use in assessing responses (41). Ultrasound is also recommended for imaging in neoadjuvant clinical trials when MRI is unavailable or in selected circumstances (41). Modern technologies such as measurement of tumor density or volume and quantitative functional molecular imaging that tracks biochemical processes may hold promise for a better assessment of a treatment effect (38).
Several studies have demonstrated a link between duration of letrozole monotherapy and increased ORR (45–47); both ORR and pCR were greater after 1 year of letrozole treatment compared with 8 and 4 months, respectively, in one study (45), and in another study maximal responses were not observed until after 6–12 months of treatment for a third of patients (47). ORR in patients with early-stage operable breast cancer who received neoadjuvant letrozole or anastrozole ranges (depending on the trial population and method of assessment) from approximately 24% to 70% after 12 weeks of treatment (2, 3, 46) to approximately 55% to 87% for 24–32 weeks of treatment (45, 48–50). Although the optimal duration of neoadjuvant aromatase inhibitor (AI) treatment is unclear (8, 51), durations of 6–12 months show consistently greater benefit compared with shorter durations.
pCR rates following neoadjuvant ET in HR+ breast cancer remain low (5, 6, 26, 45, 47, 50, 52). In particular, pCR rates for neoadjuvant AI treatment have been reported to be less than 2.5% for 16 weeks of treatment (26, 45, 52), 0% for 24 weeks (50), and 5% for 32 weeks (45). The pCR rate results observed for alpelisib plus letrozole in NEO-ORB (1.7% and 2.8% in the PIK3CA-mutant and -wild-type cohorts, respectively) are in line with previous studies of neoadjuvant treatment for HR+ breast cancer, despite the limitations in comparing trials with differing treatment regimens.
In general, combinations of ET and targeted therapy have demonstrated limited efficacy in the neoadjuvant setting. Following 4 weeks of single-agent anastrozole treatment, addition of the AKT inhibitor MK-2206 did not further suppress cell proliferation when evaluated after 2 weeks of combination treatment, with no pCRs observed (53). In the LORELEI study, which compared the β-sparing PI3K inhibitor taselisib plus letrozole with placebo plus letrozole, the observed pCR rate was low and showed no significant difference between treatment arms (37). Patients enrolled in LORELEI received taselisib plus letrozole or placebo plus letrozole for 16 weeks prior to surgery with tumor response assessed centrally by MRI per modified RECIST (37). The ORR based on MRI evaluations after 16 weeks of study treatment was increased in the taselisib plus letrozole arm compared with the placebo plus letrozole arm (50% vs. 39%, odds ratio 1.55, 95% CI 1.00–2.38, P = 0.049) (37); the clinical significance of this difference is unclear. Of note, in contrast to NEO-ORB, the LORELEI study excluded patients with clinical N3, multicentric, or bilateral breast cancers (54).
The AE profile of alpelisib plus letrozole observed in NEO-ORB is consistent with the reported safety profile of alpelisib in combination with ET (21–23). The most frequently reported AEs in the alpelisib plus letrozole arm were hyperglycemia and gastrointestinal events, with hyperglycemia and rash the most common grade 3/4 AEs. AEs were the most common reason for treatment discontinuation in the alpelisib plus letrozole arm for both PIK3CA cohorts; hyperglycemia and rash were the most frequent AEs leading to discontinuation. In the phase Ib study of alpelisib plus letrozole in patients with HR+, HER2– advanced breast cancer, common drug-related AEs were gastrointestinal disorders (including nausea and diarrhea; 73%), hyperglycemia (62%), fatigue (54%), and rash (42%) (22). In contrast to alpelisib, buparlisib displayed an non-tolerable safety profile in NEO-ORB, which included liver toxicity and mood disorders, consistent with that observed in other studies (24, 55). The differences in side effects observed between alpelisib and buparlisib are likely the result of differential target specificity. There are four type 1 PI3K (p110) isoforms, α, β, δ, and γ (56). As a pan-PI3K inhibitor, buparlisib inhibits all four isoforms (18). Conversely, alpelisib specifically inhibits the PI3Kα isoform (19), thus avoiding the cumulative toxicity associated with inhibition of all four PI3K isoforms.
In contrast to this study in early-stage, operable breast cancer, alpelisib has demonstrated clinical activity in PIK3CA-mutant tumors in the metastatic setting (20–23). In the phase III SOLAR-1 trial, addition of alpelisib to fulvestrant resulted in a significant and clinically meaningful improvement in progression-free survival of patients with PIK3CA-mutant, HR+ advanced/metastatic breast cancer (23). It follows that early-stage PIK3CA-mutant breast cancers may be less dependent on PI3K signaling compared with recurrent or metastatic disease. PIK3CA mutations are typically an early event in breast cancer pathogenesis (57). However, PIK3CA mutations do not necessarily correlate with an active pathway at the time of diagnosis (58), and the reliance of the tumor on the PI3K pathway for growth and survival can differ between early- and late-stage disease (59). Studies in early-stage breast cancer indicate that PIK3CA mutations are not an independent predictor of outcome, and PIK3CA mutation status does not strongly correlate with neoadjuvant ET responsiveness in HR+ breast cancer both in previous studies (35, 60, 61) and in the NEO-ORB data presented herein. Based on the results from NEO-ORB, alpelisib does not add clinical benefit in the neoadjuvant setting, but considering the available clinical and experimental data in other indications, the addition of alpelisib to letrozole may still be an effective treatment strategy for HR+ advanced/metastatic breast cancer that harbors somatic alterations in the PI3K pathway.
Supplementary Material
Acknowledgments
The authors would like to thank the patients who participated in NEO-ORB, and their families and caregivers. Medical writing assistance was provided by Cassandra Krone, PhD, and Jenny Winstanley, PhD, of Articulate Science Ltd. and was funded by Novartis Pharmaceuticals Corporation. We also wish to thank the Avon Foundation for Women, Breast Cancer Research Foundation, and Susan G. Komen for their support of the Translational Breast Cancer Research Consortium in the US. I.A. Mayer, A.C. Wolff, E.P. Winer, and C.L. Arteaga are members of the Translational Breast Cancer Research Consortium (TBCRC), with whom this trial was conducted (trial number: TBCRC 025).
Funding
This study was funded by Novartis Pharmaceuticals Corporation.
Clinical Trial Registration: This trial is registered at ClinicalTrials.gov, number NCT01923168.
Financial support: Novartis Pharmaceuticals Corporation.
Footnotes
Disclosure of Potential Conflicts of Interest:
I.A. Mayer has received consultancy/advisory fees from AstraZeneca, Novartis, Genentech, Eli Lilly, Immunomedics, MacroGenics, and GlaxoSmithKline, and received research funding from Novartis, Genentech, and Pfizer; A. Prat has received consultancy/advisory fees and received research funding from Nanostring Technologies, and received lecture fees from Novartis and Pfizer; D. Egle has received consultancy/advisory fees from AstraZeneca, Novartis, Pfizer, and Roche; J. Alejandro Perez Fidalgo has received consulting/advisory fees from Clovis and PharmaMar, and received speakers’ bureaus fees from AstraZeneca, Novartis, and Roche; M. Gnant has received consultancy/advisory fees from Accelsiors, Amgen, AstraZeneca, GlaxoSmithKline, Novartis, OBI Pharma, and Roche, and received research funding from AstraZeneca, Novartis, Pfizer, and Roche; P.A. Fasching has received grants/fees from Amgen, Celgene, Novartis, Pfizer, Puma Biotechnology, Roche, and Teva Pharmaceutical Industries; M. Colleoni has received consultancy/advisory fees from AstraZeneca, Celldex Therapeutics, Novartis, OBI Pharma, Pfizer, Pierre Fabre Laboratories, and Puma Biotechnology; A.C. Wolff has received research funding from Pfizer; E.P. Winer has received consultancy/advisory fees from Genentech, Leap Therapeutics, and Tesaro, and received research funding from Genentech and Merck; C.F. Singer has received consultancy/advisory fees from Amgen, AstraZeneca, Novartis, Pfizer, Roche, and Teva Pharmaceutical Industries, and received research funding from Roche and Novartis; S. Hurvitz has received research funding from Amgen, Bayer, Boehringer Ingelheim, Cascadian Therapeutics, Dignitana, Eli Lilly, Genentech/Roche, Merrimack Pharmaceuticals, Novartis, OBI Pharma, Pfizer, Puma Biotechnology, and Seattle Genetics; P.A. van Dam has received consultancy/advisory fees from Amgen, Novartis, and Roche, and received research funding from Amgen and Roche; S. Kuemmel has received consultancy/advisory fees from Amgen, AstraZeneca, Celgene, Daiichi Sankyo, Genomic Health, Novartis, Pfizer, Roche, and Teva Pharmaceutical Industries, and received research funding from Roche; C. Mundhenke has received consultancy/advisory and speakers’ bureaus fees from Novartis and Pfizer, and received research funding from Novartis; F. Holmes has received consultancy/advisory fees from Myriad Genetics, Novartis, and Puma Biotechnology; N. Babbar, L. Charbonnier, I Diaz-Padilla, and F.D. Vogl are employees of Novartis; D. Sellami is an employee and owns stocks/shares in Novartis; C.L. Arteaga receives or has received research funding from Bayer, Eli Lilly, Pfizer, Radius Health, and Takeda; he serves or has served in advisory roles to Symphogen, Daiichi Sankyo, TAIHO Oncology, Novartis, Merck, PUMA Biotechnology, Eli Lilly, Radius Health, Sanofi, OrigiMed, AbbVie, and H3 Biomedicine; he holds stock options in Provista and Y-TRAP. S. Blau and L. Garcia Estevez declare no conflicts of interest.
References
- 1.National comprehensive cancer network clinical practice guidelines in oncology. Breast cancer. version 1. 2018. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. [DOI] [PubMed]
- 2.Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer JU, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: The immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 2005;23:5108–16. [DOI] [PubMed] [Google Scholar]
- 3.Cataliotti L, Buzdar AU, Noguchi S, Bines J, Takatsuka Y, Petrakova K, et al. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: The pre-operative “arimidex” compared to tamoxifen (PROACT) trial. Cancer 2006;106:2095–103. [DOI] [PubMed] [Google Scholar]
- 4.Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: Clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype - ACOSOG Z1031. J Clin Oncol 2011;29:2342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spring LM, Gupta A, Reynolds KL, Gadd MA, Ellisen LW, Isakoff SJ, et al. Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: A systematic review and meta-analysis. JAMA Oncol 2016;2:1477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semiglazov VF, Semiglazov VV, Dashyan GA, Ziltsova EK, Ivanov VG, Bozhok AA, et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer 2007;110:244–54. [DOI] [PubMed] [Google Scholar]
- 7.Sparano J, Gray R, Makower D, Pritchard K, Albain K, Hayer D, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 2018;379:111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barroso-Soussa R, Silva FRDDA, Alessi JVM, Mano MS. Neoadjuvant endocrine therapy in breast cancer: Current role and future perspectives. Ecancermedicalscience 2016;10:609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agrawal LS, Mayer IA. Optimizing the use of neoadjuvant endocrine therapy. Curr Oncol Rep 2015;17:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016;375:1925–36. [DOI] [PubMed] [Google Scholar]
- 11.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016;375:1738–48. [DOI] [PubMed] [Google Scholar]
- 12.Maugeri-Sacca M, Barba M, Vici P, Pizzuti L, Sergi D, Catenaro T, et al. Presurgical window of opportunity trial design as a platform for testing anticancer drugs: Pros, cons, and a focus on breast cancer. Crit Rev Oncol Hematol 2016;106:132–42. [DOI] [PubMed] [Google Scholar]
- 13.Hosford SR, Miller TW. Clinical potential of novel therapeutic targets in breast cancer: CDK4/6, src, JAK/STAT, PARP, HDAC, and PI3K/AKT/mTOR pathways. Pharmgenomics Pers Med 2014;7:203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer 2015;15:7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chopra N, Turner NC. Targeting PIK3CA-mutant advanced breast cancer in the clinical setting. Lancet Oncol 2017;18:842–3. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez CG, Ma CX, Crowder RJ, Guintoli T, Phommaly C, Gao F, et al. Preclinical modeling of combined phosphatidylinositol-3-kinase inhibition with endocrine therapy for estrogen receptor-positive breast cancer. Breast Cancer Res 2011;13:R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosch A, Li Z, Bergamaschi A, Ellis H, Toska E, Prat A, et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med 2015;7:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maira SM, Pecchi S, Huang A, Burger M, Knapp M, Sterker D, et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther 2012;11:317–28. [DOI] [PubMed] [Google Scholar]
- 19.Fritsch C, Huang A, Chatenay-Rivauday C, Schnell C, Reddy A, Liu M, et al. Characterization of the novel and specific PI3Kalpha inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Mol Cancer Ther 2014;13:1117–29. [DOI] [PubMed] [Google Scholar]
- 20.Juric D, Rodon J, Tabernero J, Janku F, Burris HA, Schellens JHM, et al. Phosphatidylinositol 3-kinase a-selective inhibition with alpelisib (BYL719) in PIK3CA-altered solid tumors: Results from the first-in-human study. J Clin Oncol 2018;36:1291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juric D, Janku F, Rodón J, Burris HA, Mayer IA, Schuler M, et al. Alpelisib Plus Fulvestrant in PIK3CA-Altered and PIK3CA-Wild-Type Estrogen Receptor-Positive Advanced Breast Cancer: A Phase 1b Clinical Trial. JAMA Oncol 2018;December 13:e184475. doi: 10.1001/jamaoncol.2018.4475 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer IA, Abramson VG, Formisano L, Balko JM, Estrada MV, Sanders ME, et al. A phase Ib study of alpelisib (BYL719), a PI3K alpha-specific inhibitor, with letrozole in ER+/HER2- metastatic breast cancer. Clin Cancer Res 2017;23:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.André F, Ciruelos EM, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib (ALP) + fulvestrant (FUL) for advanced breast cancer (ABC): Results of the phase III SOLAR-1 trial. Ann Oncol 2018;29(Suppl 8):Abstract LBA3_PR. [Google Scholar]
- 24.Baselga J, Im SA, Iwata H, Cortes J, De Laurentiis M, Jiang Z, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:904–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hortobagyi GN, Connolly JL, D’Orsi CJ, Edge SB, Mittendorf EA, Rugo H, et al. Part XI: Breast. In: Amin MB et al. , editors. Breast cancer staging system: AJCC Cancer Staging Manual, Eighth Edition. Chicago, Illinois: The American College of Surgeons; 2017. p589–628. [Google Scholar]
- 26.Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, et al. phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol 2009;27:2630–7. [DOI] [PubMed] [Google Scholar]
- 27.Fontein D, Charehbili A, Nortier J, Meershoek-Klein Kranenbarg E, Kroep J, Putter H, et al. Efficacy of six month neoadjuvant endocrine therapy in postmenopausal, hormone receptor-positive breast cancer patients - a phase II trial. Eur J Cancer 2014;50:2190–200. [DOI] [PubMed] [Google Scholar]
- 28.Dixon J, Love C, Bellamy C, Cameron D, Leonard R, Smith H, et al. Letrozole as primary medical therapy for locally advanced and large operable breast cancer. Breast Cancer Res Treat 2001;66:191–9. [DOI] [PubMed] [Google Scholar]
- 29.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26:404–13. [Google Scholar]
- 30.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 2011;62:233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Generali D, Fox SB, Brizzi MP, Allevi G, Bonardi S, Aguggini S, et al. Down-regulation of phosphatidylinositol 3’-kinase/AKT/molecular target of rapamycin metabolic pathway by primary letrozole-based therapy in human breast cancer. Clin Cancer Res 2008;14:2673–80. [DOI] [PubMed] [Google Scholar]
- 32.Loibl S, Majewski I, Guarneri V, Nekljudova V, Holmes E, Bria E, et al. PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: Pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab. Ann Oncol 2016;27:1519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goel S, Krop IE. PIK3CA mutations in HER2-positive breast cancer: An ongoing conundrum. Ann Oncol 2016;27:1368–72. [DOI] [PubMed] [Google Scholar]
- 34.Zardavas D, Te Marvelde L, Milne RL, Fumagalli D, Fountzilas G, Kotoula V, et al. Tumor PIK3CA genotype and prognosis in early-stage breast cancer: A pooled analysis of individual patient data. J Clin Oncol 2018;36:981–90. [DOI] [PubMed] [Google Scholar]
- 35.Yang SX, Polley E, Lipkowitz S. New insights on PI3K/AKT pathway alterations and clinical outcomes in breast cancer. Cancer Treat Rev 2016;45:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmid P, Pinder SE, Wheatley D, Macaskill J, Zammit C, Hu J, et al. Phase II randomized preoperative window-of-opportunity study of the PI3K inhibitor pictilisib plus anastrozole compared with anastrozole alone in patients with estrogen receptor-positive breast cancer. J Clin Oncol 2016;34:1987–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saura C, Azambuja E, Hlauschek D, Oliveira M, Zardavas D, Jallitsch-Halper A, et al. Primary results of LORELEI: A phase II randomized, double-blind study of neoadjuvant letrozole (LET) plus taselisib versus LET plus placebo (PLA) in postmenopausal patients (pts) with ER+/HER2-negative early breast cancer (EBC). Ann Oncol 2017;28(Suppl 5):Abstract LBA10_PR. [Google Scholar]
- 38.The ongoing evolution of endpoints in oncology. Managed Care 2010;19(supplement 1). Available from: https://pdfs.semanticscholar.org/2706/b013de454c37bb217f8f44dbecb5ba3f2046.pdf. [Google Scholar]
- 39.Supplement to Managed Care. Oncology endpoints in a changing landscape. Jan, 2016. Available from: https://www.managedcaremag.com/sites/default/files/graphics/OncoEndpoints_MC.pdf.
- 40.von Minckwitz G, Untch M, Blohmer J, Costa S, Eidtmann H, Fasching P, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012;30:1796–804. [DOI] [PubMed] [Google Scholar]
- 41.Fumagalli D, Bedard PL, Nahleh Z, Michiels S, Sotiriou C, Loi S, et al. A common language in neoadjuvant breast cancer clinical trials: Proposals for standard definitions and endpoints. Lancet Oncol 2012;13:e240–8. [DOI] [PubMed] [Google Scholar]
- 42.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014;384:164–72. [DOI] [PubMed] [Google Scholar]
- 43.Siddiqui MK, Tyczynski J, Pahwa A, Fernandes AW. Objective response rate is a possible surrogate endpoint for survival in patients with advanced, recurrent ovarian cancer. Gynecol Oncol 2017;146:44–51. [DOI] [PubMed] [Google Scholar]
- 44.Kogan AJ, Haren M. Translating cancer trial endpoints into the language of managed care. Biotechnol Healthc 2008;5:22–35. [PMC free article] [PubMed] [Google Scholar]
- 45.Allevi G, Strina C, Andreis D, Zanoni V, Bazzola L, Bonardi S, et al. Increased pathological complete response rate after a long-term neoadjuvant letrozole treatment in postmenopausal oestrogen and/or progesterone receptor-positive breast cancer. Br J Cancer 2013;108:1587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dixon JM, Renshaw L, Macaskill EJ, Young O, Murray J, Cameron D, et al. Increase in response rate by prolonged treatment with neoadjuvant letrozole. Breast Cancer Res Treat 2009;113:145–51. [DOI] [PubMed] [Google Scholar]
- 47.Llombart-Cussac A, Guerrero A, Galan A, Caranana V, Buch E, Rodriguez-Lescure A, et al. phase II trial with letrozole to maximum response as primary systemic therapy in postmenopausal patients with ER/PgR[+] operable breast cancer. Clin Transl Oncol 2012;14:125–31. [DOI] [PubMed] [Google Scholar]
- 48.Olson JA Jr, Budd GT, Carey LA, Harris LA, Esserman LJ, Fleming GF, et al. Improved surgical outcomes for breast cancer patients receiving neoadjuvant aromatase inhibitor therapy: Results from a multicenter phase II trial. J Am Coll Surg 2009;208:906,14; discussion 915–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masuda N, Sagara Y, Kinoshita T, Iwata H, Nakamura S, Yanagita Y, et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): A double-blind, randomised phase 3 trial. Lancet Oncol 2012;13:345–52. [DOI] [PubMed] [Google Scholar]
- 50.Fasching PA, Jud SM, Hauschild M, Kummel S, Schutte M, Warm M, et al. FemZone trial: A randomized phase II trial comparing neoadjuvant letrozole and zoledronic acid with letrozole in primary breast cancer patients. BMC Cancer 2014;14:66,2407–14-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amoroso V, Generali D, Buchholz T, Cristofanilli M, Pedersini R, Curigliano G, et al. International expert consensus on primary systemic therapy in the management of early breast cancer: Highlights of the fifth symposium on primary systemic therapy in the management of operable breast cancer, Cremona, Italy (2013). J Natl Cancer Inst Monogr 2015;2015:90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eiermann W, Paepke S, Appfelstaedt J, Llombart-Cussac A, Eremin J, Vinholes J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann Oncol 2001;12:1527–32. [DOI] [PubMed] [Google Scholar]
- 53.Ma CX, Suman V, Goetz MP, Northfelt D, Burkard ME, Ademuyiwa F, et al. A phase II trial of neoadjuvant MK-2206, an AKT inhibitor, with anastrozole in clinical stage II or III PIK3CA-mutant ER-positive and HER2-negative breast cancer. Clin Cancer Res 2017;23:6823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.A study of neoadjuvant letrozole + taselisib versus letrozole + placebo in post-menopausal women with breast cancer (LORELEI) ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT02273973.
- 55.Di Leo A, Johnston S, Lee KS, Ciruelos E, Lonning PE, Janni W, et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2018;19:87–100. [DOI] [PubMed] [Google Scholar]
- 56.Cintas C, Guillermet-Guibert J. Heterogeneity of phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin activation in cancer: Is PI3K isoform specificity important? Front Oncol 2018;7:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arsenic R, Treue D, Lehmann A, Hummel M, Dietel M, Denkert C, et al. Comparison of targeted next-generation sequencing and sanger sequencing for the detection of PIK3CA mutations in breast cancer. BMC Clin Pathol 2015;15:20, eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swanton C, Soria JC, Bardelli A, Biankin A, Caldas C, Chandarlapaty S, et al. Consensus on precision medicine for metastatic cancers: A report from the MAP conference. Ann Oncol 2016;27:1443–8. [DOI] [PubMed] [Google Scholar]
- 59.Mayer IA, Arteaga CL. PIK3CA activating mutations: A discordant role in early versus advanced hormone-dependent estrogen receptor-positive breast cancer? J Clin Oncol 2014;32:2932–4. [DOI] [PubMed] [Google Scholar]
- 60.Troxell ML. PIK3CA/AKT1 mutations in breast carcinoma: A comprehensive review of experimental and clinical studies. J Clin Exp Pathol 2012:S1:002. [Google Scholar]
- 61.Sabine VS, Crozier C, Brookes CL, Drake C, Piper T, van de Velde CJ, et al. Mutational analysis of PI3K/AKT signaling pathway in tamoxifen exemestane adjuvant multinational pathology study. J Clin Oncol 2014;32:2951–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


