Abstract
This was a prospective study that assessed field performance of the INSTI HIV-1/−2 antibody test (INSTI test) in two antenatal clinics in South Africa (SA). INSTI test was evaluated against rapid tests used at these clinics, and pooled nucleic acid amplification testing (NAAT) performed for individuals with negative rapid tests.
386 pregnant women were enrolled; 334 (86.5%) with negative results on the screening rapid test, and 52 (13.5%, 95% confidence interval [CI]: 10.2 – 17.3%) with positive results on screening and confirmatory rapid tests. INSTI test yielded the same results as other rapid tests in all participants, thus showing a 100% sensitivity (95% CI: 93.2 – 100.0%) and specificity (95% CI: 98.9 – 100.0%). Pooled NAAT was performed for 290 participants who had negative rapid tests, and yielded negative results in all pools. These data show excellent field performance of the INSTI test, and highlight that this test can be implemented at SA clinics.
Keywords: INSTI HIV-1/−2 antibody test, Rapid HIV testing, Field performance
Introduction
HIV diagnosis forms a crucial part of the 90–90-90 strategy as this strategy aims to diagnose 90% of all HIV-infected individuals, have 90% of them on antiretroviral therapy (ART), and to have HIV viral load (VL) suppression in 90% of those receiving ART [1]. Serology assays such as rapid tests and enzyme-linked immunoassays (ELISAs) are commonly used for diagnosis of HIV infection in adults. However, molecular assays are used in some instances such as screening for early HIV infections. The use of ELISAs and molecular assays is mostly limited to the laboratories [2, 3]. Rapid HIV tests are commonly used for diagnosis of HIV infection in low resource settings as they are available for use at the point-of-care (POC) facilities, and are cheaper and easier to use compared to laboratory-based assays. Unfortunately, rapid HIV tests have relatively lower sensitivity than ELISAs [4, 5]. As a result, some HIV-infected individuals are misdiagnosed by rapid tests, especially those with early HIV infection [6].
Antibodies are the last diagnostic marker of HIV to appear in the blood following exposure and infection. Even after their appearance they predominantly exist as antigen–antibody complexes owing to higher antigen load during early HIV infection. This makes antigen-bound antibodies difficult to detect until free antibodies appear later as the HIV VL decreases [7, 8]. Unfortunately, the addition of the p24 antigen to some rapid tests has only led to a slight improvement in sensitivity for HIV diagnosis [9].
INSTI HIV-1/−2 Antibody Test (INSTI test), a Food and Drug Administration (FDA)–approved rapid test, is used for detection of HIV-1 and HIV-2 antibodies. It has been shown to have higher sensitivity for detection of HIV-specific antibodies even during early HIV infection compared to other rapid tests [10, 11]. Making an accurate diagnosis of HIV infection is important for management of an infected individual and interruption of further spread of HIV [1]. The aim of this study was to assess the field performance of the INSTI test by comparing this test with other rapid tests that were used at the SA POC facilities.
Materials and Methods
Study population
This was a prospective study conducted in 2016 in two antenatal clinics in the Tshwane district of South Africa (SA), one based in the Pretoria City and the other in Mamelodi Township. Potential participants were pregnant women who were seeking HIV testing during their first antenatal care (ANC) visit. They formed a consecutive series, and were recruited during HIV counseling to be tested on the INSTI test (bioLytical Laboratories Inc., Richmond, Canada) in addition to the rapid tests used in the clinics as part of standard HIV testing service. Exclusion criteria included pregnant women who came for a follow-up ANC visit as they had already been tested for HIV at first visit, male participants, and those who refused HIV testing. The study was approved by the University of Pretoria’s Faculty of Health Sciences Ethics Committee (Protocol number–295/2015) and by Tshwane–Metsweding Region Research Ethics Committee (TMREC 2010/26). All participants provided written informed consent.
HIV testing algorithm in South African POC facilities
In South Africa, a serial testing strategy is employed for HIV testing at POC facilities, where a screening rapid test is performed first and its results are used to determine the need for further testing. If the screening test is negative, then results are reported as HIV-negative, and no further testing is performed. However, if the screening test result is positive, confirmatory testing is performed on a different rapid test. A diagnosis of HIV infection is made if both screening and confirmatory rapid tests are positive. Testing on ELISAs is performed in cases of discrepant results between the screening and confirmatory rapid tests [12]. The rapid tests that were used in the clinics at the time of this evaluation were Advanced Quality HIV test (Intec Products Inc., Xiamen, China) for screening, and First Response HIV test (Premier Medical Corporation Private Limited, Gujarat, India) for confirmatory testing [13], and were both performed on finger-stick whole blood. HIV testing was performed by lay counselors who had received training for counseling and testing. All the counselors also received orientation on the use of the INSTI test.
INSTI test evaluation and procedure
INSTI testing was performed on finger-stick whole blood according to manufacturer’s instructions, while awaiting the results of the screening rapid test. Therefore, it was evaluated as part of the screening algorithm. The INSTI test employs immunofiltration (flow through) principle as opposed to most rapid tests, which use immunochromatographic (lateral flow) principle [14, 15]. With INSTI testing, finger-stick whole blood is added into a vial containing sample diluent, then the mixture is applied to a testing cartridge, followed by addition of colour developer and clarifying solutions, respectively. The results are read 1 minute after addition of the sample diluent-specimen mixture to the testing cartridge [14].
Pooled NAAT among HIV-seronegative participants
Roche COBAS AmpliPrep/COBAS TaqMan HIV-1 VL version 2 assay (Roche Diagnostics, Mannheim, Germany) was employed for pooled NAAT in a mini-pool of 5 samples, using 200 microlitres (μls) from each sample to constitute a 1 millilitre (ml) sample volume required for testing [6]. NAAT was only performed for individuals who had negative rapid HIV tests as the INSTI evaluation was conducted as part of a study that screened for early HIV infections. Those who tested positive on the screening rapid test received further testing on the confirmatory rapid test in accordance to the SA testing protocol mentioned above.
Data analysis
Positive results obtained from both Advanced Quality and First Response tests were considered true positives. Negative results obtained from the Advanced Quality test were considered true negatives. INSTI test results were compared to results obtained from the two rapid tests used at POC facilities and pooled NAAT. MedCalc statistical software (https://www.medcalc.org/calc/diagnostic_test.php) was used for computing 95% confidence intervals (CIs) for point prevalence estimates and assay performance parameters.
Results
There were 386 pregnant women enrolled in this study; 135 (35.0%) from the City-based clinic and 251 (65.0%) from the Township-based clinic. All the participants had testing performed on Advanced Quality and INSTI HIV tests. Three hundred and thirty-four (86.5%) participants tested negative on the Advanced Quality test, and all had negative results on the INSTI test as well. Fifty-two participants were newly diagnosed with HIV infection (13.5%, 95% CI: 10.2 – 17.3%); they tested positive on the Advanced Quality and First Response tests, and were also positive on the INSTI test (Figure 1). All these participants were linked to care. The HIV prevalence distribution in this study was 10.4% (n = 14, 95% CI: 5.8 – 16.8%) and 15.1% (n = 38, 95% CI: 10.9 – 20.2%) in the City-based and Township-based clinics, respectively. There were no discrepancies found among all rapid tests, and no indeterminate results were observed during the evaluation period. The INSTI test demonstrated 100% sensitivity (95% CI: 93.2 – 100.0%) and specificity (95% CI: 98.9 – 100.0%) for detection of HIV antibodies (Table 1).
Figure 1:
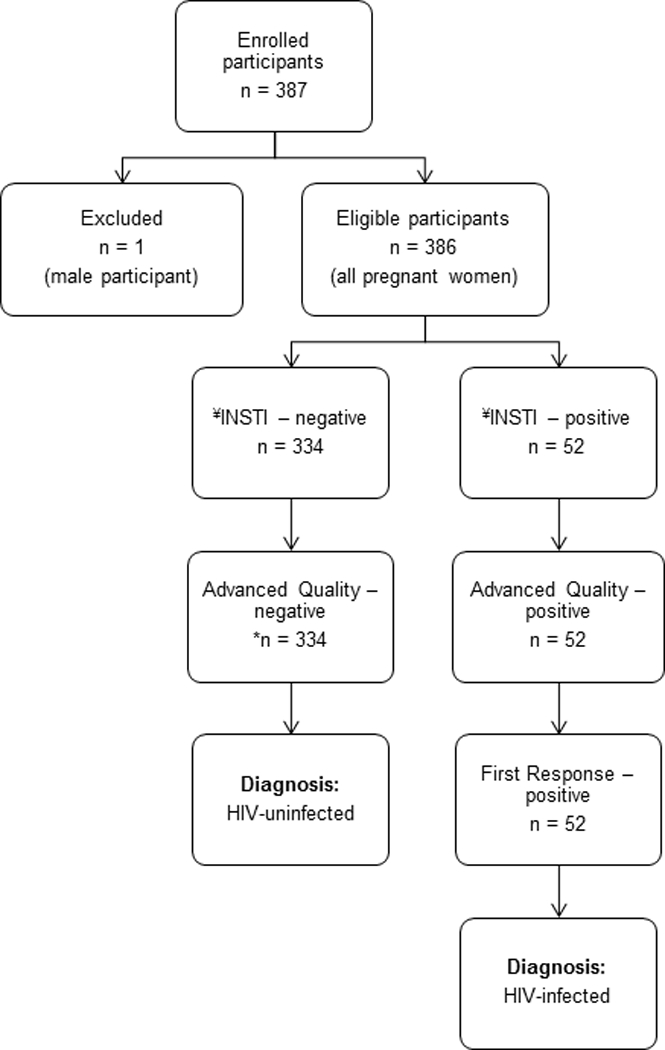
Enrolment of study participants at the two South African antenatal clinics and serial HIV testing algorithm that was used in these clinics [12]. Advanced Quality test was used for screening for HIV infection, and First Response test was used for confirmation of all the positive screening test results. No further testing was done when screening test was negative. There were no indeterminate results observed during the INSTI evaluation period. ¥ = index test, * = 290 participants who had negative rapid test results were available for further testing on pooled nucleic acid amplification test (NAAT).
Table 1.
Field performance of the INSTI rapid HIV test assessed at the two antenatal clinics
| INSTI HIV test results | ||||
|---|---|---|---|---|
| NEG | POS | Sensitivity (95% CI) | Specificity (95% CI) | |
| NEG (n = 334)a | 334 | 0 | -- | 100% (98.9 – 100.0%) |
| POS (n = 52)b | 0 | 52 | 100% (93.2 – 100.0%) | -- |
| Total | 334 | 52 | -- | -- |
All negative (NEG) samples were obtained from the Advanced Quality rapid test at the clinics.
All positive (POS) results were obtained from the Advanced Quality and First Response rapid tests at the clinics. -- = not applicable.
Pooled NAAT was performed for 290 participants who had negative rapid tests, it yielded negative results in all pools. Thus, INSTI and Advanced Quality tests also had 100% correlation with NAAT in these participants. Forty-four participants with negative rapid HIV tests were not available for NAAT as they were not seen for antenatal care on the same day of testing, and were lost to follow-up. The cost of using the INSTI test in 2016 was about R83.60 (5.96 USD) compared to R5.20 (0.37 USD) for the Advanced Quality rapid test, and R6.30 (0.45 USD) for the First Response rapid test. This high cost of the INSTI HIV test was closer to that of pooled NAAT, which was about R92.00 (6.56 USD). However, the costs of the rapid tests used in the clinics are probably based on discounted price negotiated by the government with the suppliers.
Discussion
This study assessed the field performance of the INSTI test in two SA antenatal clinics. There was an overall HIV prevalence of 13.5%, but this varied between the two study clinics. This is not surprising as variation in HIV prevalence has been observed among districts within one SA province [16, 17]. The INSTI test demonstrated excellent field performance in this study as it had 100% correlation with rapid tests used at POC facilities and pooled NAAT. INSTI testing provided parallel testing in this study, and highlights that this strategy is costly [18], as results obtained from the INSTI test were similar to those obtained from the screening rapid test. The excellent field performance of the INSTI test highlights that this test could be easily used by lay counselors at the SA POC facilities, and could be considered for home-based HIV testing. The ease of use of the INSTI HIV test by untrained, non-professional lay users at POC facilities has been reported previously [19]. In November 2018, the INSTI self-test became the first blood-based HIV test to be WHO prequalified [20]. A recent study that assessed the INSTI HIV self-test in Kenya reported an excellent performance of this test, showing that it is a viable option for HIV self-testing [21].
The INSTI test provides results within 1 minute compared to most rapid tests whose results are only read in 15 minutes time interval or longer [14, 15, 22]. Therefore, the use of this test could simplify HIV testing at the POC facilities, and create more time that could be dedicated to other aspects of HIV management or prevention. The INSTI HIV kit comes with a calibrated capillary pipette that enables the exact measurement of blood needed for the test, and thus lessens the chances of using inadequate volume for testing [14].
Studies have shown that the INSTI test has higher sensitivity for detection of early HIV infection than other rapid HIV tests [10, 11], as it is able to detect anti-HIV-1 IgM antibodies during early HIV infection [22]. One study that compared performance of five rapid tests (including INSTI) on finger-stick whole blood and plasma observed that INSTI had higher sensitivity on finger-stick whole blood than other rapid tests [10]. Many SA studies have reported that some individuals with HIV infection are misdiagnosed by the rapid HIV tests at the POC facilities [6, 23, 24]. This highlights a need to consider evaluation and use of highly sensitive rapid tests at the SA POC facilities. High cost was the only disadvantage of the INSTI test. The limitations of this study were that it had small sample size, was only conducted at antenatal clinics, and that early HIV infection could not be excluded in few participants who tested negative on rapid tests but were not available for NAAT.
Conclusions
This study showed an excellent field performance of the INSTI test, highlighting that this rapid test can be considered for use in SA POC facilities. Larger studies are needed for field evaluation of the INSTI test. There is a need to improve the accuracy of HIV diagnosis at POC facilities, particularly in HIV hyper-endemic areas. When this need is met, it could lead to improved assessment of the first 90 of UNAIDS 90–90-90 strategy.
Acknowledgements:
We are grateful to the research assistants for recruiting and enrolling all the study participants.
Sources of funding: This work was supported by South African Medical Research Council – Self Initiated Research (SA MRC-SIR) grant; Discovery Foundation grant; Hamilton Naki Clinical Scholarship; and The Division of Intramural Research, NIAID, NIH.
Footnotes
Conflict of Interest – none declared.
References
- 1.UNAIDS. 90–90-90: An Ambitious treatment target to help end the AIDS epidemic. 2014. Available at http://www.unaids.org/en/resources/documents/2014/90-90-90. Accessed on March 17, 2017.
- 2.Branson BM. The Future of HIV Testing. J Acquir Immune Defic Syndr. 2010;55:S102–S5. [DOI] [PubMed] [Google Scholar]
- 3.Delaney KP, Hanson DL, Masciotra S, Ethridge SF, Wesolowski L, Owen SM. Time Until Emergence of HIV Test Reactivity Following Infection With HIV-1: Implications for Interpreting Test Results and Retesting After Exposure. Clin Infect Dis. 2017;64:53–9. [DOI] [PubMed] [Google Scholar]
- 4.Delaney KP, Branson BM, Uniyal A, Phillips S, Candal D, Owen SM, et al. Evaluation of the Performance Characteristics of 6 Rapid HIV Antibody Tests. Clin Infect Dis. 2011;52:257–63. [DOI] [PubMed] [Google Scholar]
- 5.Galiwango RM, Musoke R, Lubyayi L, Ssekubugu R, Kalibbala S, Ssekweyama V, et al. Evaluation of current rapid HIV test algorithms in Rakai, Uganda. J Virol Methods. 2013;192:25–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayaphi SH, Martin DJ, Quinn TC, Laeyendecker O, Olorunju SAS, Tintinger GR, et al. Detection of Acute and Early HIV-1 Infections in an HIV Hyper-Endemic Area with Limited Resources. PLoS One. 2016;11:e0164943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, et al. Initial B-Cell Responses to Transmitted Human Immunodeficiency Virus Type 1: Virion-Binding Immunoglobulin M (IgM) and IgG Antibodies Followed by Plasma Anti-gp41 Antibodies with Ineffective Control of Initial Viremia. J Virol. 2008;82:12449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. N Engl J Med. 2011;364:1943–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker MM, Bennett SB, Sullivan TJ, Fordan S, Wesolowski LG, Wroblewski K, et al. Performance of the Alere Determine™ HIV-1/2 Ag/Ab Combo Rapid Test with algorithm-defined acute HIV-1 infection specimens. J Clin Virol. 2018;104:89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavie J, Rachline A, Loze B, Niedbalski L, Delaugerre C, Laforgerie E, et al. Sensitivity of Five Rapid HIV Tests on Oral Fluid or Finger-Stick Whole Blood: A RealTime Comparison in a Healthcare Setting. PLoS One. 2010;5:e11581. doi: 10.1371/journal.pone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams S, Luo W, Wesolowski L, Cohen SE, Peters PJ, Owen SM, et al. Performance evaluation of the point-of-care INSTI™ HIV-1/2 antibody test in early and established HIV infections. J Clin Virol. 2017;91:90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SA NDoH. National HIV Testing Services Policy. Department of Health, Republic of South African. 2016. Available at http://www.health.gov.za/index.php/gf-tb-program/332-national-hiv-testing-services. Accessed on September 20, 2017.
- 13.SANAC Secretariat. The National HIV Counselling and Testing Campaign Strategy. South African National AIDS Council. 2010. Available at https://www.westerncape.gov.za/other/2010/6/hct_campaign_strategy_2_3_10_final.pdf. Accessed on November 26, 2010.
- 14.INSTI HIV test. bioLytical Laboratories Inc. 2014. Available from: www.biolytical.com. Accessed on July 27, 2018.
- 15.WHO. HIV assays: laboratory performance and other operational characteristics: rapid diagnostic tests (combined detection of HIV-1/2 antibodies and discriminatory detection of HIV-1 and HIV-2 antibodies): report 18. 2015. Available from: www.who.int/diagnostics_laboratory/en/. Accessed on July 21, 2018.
- 16.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Zungu N, et al. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Cape Town, HSRC Press; 2014. [DOI] [PubMed] [Google Scholar]
- 17.SA NDoH. The 2015 National Antenatal Sentinel HIV & Syphilis Survey, South Africa, National Department of Health 2017. Available from: www.health.gov.za Accessed on August 08, 2018.
- 18.Wilkinson D, Wilkinson N, Lombard C, Martin D, Smith A, Floyd K, et al. On-site HIV testing in resource-poor settings: is one rapid test enough? AIDS. 1997;11:377–81. [DOI] [PubMed] [Google Scholar]
- 19.Galli RA, Green KF, La Marca A, Waldman LF, Powers RE, Daly AC, et al. Evaluation of the accuracy and ease of use of a rapid HIV-1 Antibody Test performed by untrained operators at the point of care. J Clin Virol. 2013;58:e65–e9. [DOI] [PubMed] [Google Scholar]
- 20.WHO. In vitro diagnostics and laboratory technology. 2018. Available from: https://www.who.int/diagnostics_laboratory/evaluations/PQ_list/en/. Accessed on February 22, 2019.
- 21.Bwana P, Ochieng L, Mwau M. Performance and usability evaluation of the INSTI HIV self-test in Kenya for qualitative detection of antibodies to HIV. PLoS One. 2018;13:e0202491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moshgabadi N, Galli RA, Daly AC, Ko SMS, Westgard TE, Bulpitt AF, et al. Sensitivity of a rapid point of care assay for early HIV antibody detection is enhanced by its ability to detect HIV gp41 IgM antibodies. J Clin Virol. 2015;71:67–72. [DOI] [PubMed] [Google Scholar]
- 23.Black V, von Mollendorf CE, Moyes JA, Scott LE, Puren A, Stevens WS. Poor sensitivity of field rapid HIV testing: implications for mother-to-child transmission programme. BJOG. 2009;116:1805–8. [DOI] [PubMed] [Google Scholar]
- 24.Kharsany ABM, Hancock N, Frohlich JA, Humphries HR, Abdool Karim SS, Abdool Karim Q. Screening for ‘window-period’ acute HIV infection among pregnant women in rural South Africa. HIV Med. 2010;11:661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


