Abstract
Objective:
Acute kidney injury (AKI) is a common complication of major surgery. However, AKI occurring within the first 48h after surgery (early AKI) and therefore likely related to the surgery itself is possibly different from AKI occurring after 48h (late AKI). The aim of this study was to describe the epidemiology and identify differences in risk factors and outcomes between early and late AKI following major surgery.
Design:
Retrospective cohort study.
Setting:
Academic Medical Center.
Patients:
Patients admitted to ICU following non-cardiac major surgery.
Interventions:
None.
Measurements and Main Results:
We analyzed data from 3499 patients and defined AKI according to full KDIGO criteria and classified as early (48h or less) or late (>48h to 7 days) based on time from surgery. Separate multivariable logistic regression models were fit to identify risk factors of early AKI compared with no AKI and risk factors of late AKI compared with no AKI. Overall 41.7% (1459/3499) developed early AKI vs 14.4% (504/3499) late AKI. Most AKI occurred within 48h following surgery and 12h was the peak interval. Risk factors for early AKI included increased age, BMI, decreased eGFR and anemia, while late AKI cases were closely associated with postoperative factors, like sepsis, mechanical ventilation, positive fluid balance, blood transfusions and exposure to diuretics, vasopressors, and nonsteroidal anti-inflammatory drugs. After adjusting for age, BMI, eGFR, comorbidities, surgery type, both early AKI (OR [95%CI]: 1.84 [1.50, 2.27]) and late AKI (OR [95%CI]: 1.42 [1.09, 1.85]) were associated with higher one-year mortality compared to patients without AKI. We found similar results in a validation cohort of 10723 patients admitted between 2008 and 2014.
Conclusions:
Most surgery-related AKI occurred within 48h of surgery. AKI occurring within the first 48h was associated with underlying health, while AKI occurring after 48h was related to postoperative complications or drugs. Design of clinical and experimental interventions for AKI in this population should consider these differences.
Keywords: non-cardiac major surgery, early AKI, late AKI, risk factor, prognosis
Introduction
Acute kidney injury (AKI) is a severe complication of major surgery and is associated with high risk of morbidity, mortality, prolonged hospitalization, and high economic costs (1-4). Several studies have examined AKI following cardiac surgery and this procedure carries a high risk for AKI (5). However, a meta-analysis showed that pooled estimated incidence of AKI, after major abdominal surgery, was 13% [10.9, 16.4] (6). Moreover, the incidence of surgery-associated AKI, among patients going to the ICU, was reported to be 32% (7). Even though most of the AKI following cardiac surgery is mild to moderate, it is still strongly associated with progression to CKD and/or mortality (2, 7, 8). Currently, there is no effective therapy for AKI. Early identification of high risk patients, enhanced clinical monitoring, optimal fluid administration, and avoiding nephrotoxic exposure are the most effective preventive interventions for AKI. Studies focusing on surgery-associated AKI have defined AKI within variable timeframes such as 48h, 72h or 7day post-surgery (2, 9-12). Increases in serum creatinine may be delayed by as much as 24-48 hours after an insult and urine output may not be measured. However, causes and clinical outcomes for post-operative AKI diagnosed within 48 hours post surgery (early AKI) are likely to be different from those diagnosed after 48 hours (late AKI). The aim of our study was to identify differences (including risk factors, prognosis) for AKI in different time intervals following non-cardiac major surgery in a multi-ICU, single-center cohort of patients, and determine potentially modifiable risk factors that could be targeted in future studies.
Materials and Methods
Patients:
We formed derivation cohort from the HIgh DENsity Intensive Care (HiDenIC-08) database which includes 54,810 patients admitted to 8 ICUs at the University of Pittsburgh Medical Center from 2000 to 2008. We limited the current analysis to ICU patients undergoing a major surgery defined as opening a major body cavity (cranium, chest, abdomen, or pelvis). We excluded patients undergoing cardiac surgery, emergent surgery or transplantation, as well as patients with RRT or ESRD before surgery. For those who underwent multiple surgeries during the time interval, we only selected the first surgery. Finally, there were 3499 patients in our cohort. We used ICD-9 codes to identify surgical procedures (CM implementation) and we extracted data on demographics, health characteristics, postoperative treatments and complications.
In order to validate the epidemiology of AKI post non-cardiac major surgery has not changed during the past decade, we used a validation cohort from a dataset including 122,000 patients admitted into an ICU at the University of Pittsburgh Medical Center from 2008-2014. We included and excluded patients according to the same criteria as we did in derivation cohort. In total, 10,723 patients comprised the validation cohort. This database only included age, baseline sCr / eGFR, ESRD, RRT (starting and ending time), ICU admission and discharge time, procedure name, surgery starting and ending time, sCr values and Urine Output (UO) post- surgery, and date of death.
The study was approved by the institutional review board of the University of Pittsburgh.
Outcomes:
In derivation cohort, the primary outcome was AKI (all stages). We classified AKI first manifesting within 48 hours of surgery (i.e. the time at which AKI criteria were first met) as -early AKI and AKI manifesting after 48 hours (up to 7 days) as -late AKI. We defined AKI according to KDIGO criteria (13) using both urine output and creatinine. Baseline serum creatinine (sCr) was defined as follows: 1). If >2 sCr values were available in the 6 months prior to surgery (including the day of admission), then we used the median of these values. 2). If only two values were present in the prior 6 months, then we used the mean. 3). If <2 values were present in the prior 6 months, we expanded the window to 12 months taking the median if there were 3 or more values or the most recent if fewer than 3 values were available. 4). If no prior sCr values were present prior to admission then we used the admission value as the baseline. The historical baseline sCr was chosen over the immediate pre-operative sCr in order to be more sensitive to changing renal function that began before surgery. Secondary outcomes included mortality and “death or dialysis” at 90-day and one-year, length of hospital and ICU stay. Dialysis information was pulled from the US Renal Data System (USRDS)(14). Death data came from the National Death Index (NDI). We defined recovery as the absence of any AKI stage at hospital discharge (15). In validation cohort, the primary outcome was AKI (all stages), and the secondary outcome was 1-year mortality post surgery.
Statistical analysis:
Baseline characteristics among patients who did not develop, developed early, and developed late AKI were compared using chi-squared or Fisher’s exact test for categorical variables and using ANOVA or Kruskal-Wallis test for continuous variables. Bonferroni adjustments were used for multiple comparisons. Outcomes of interest were further compared among patients with 3 different post-operative AKI statuses. These outcomes included length of stay (LOS) in ICU, LOS in hospital, hospital mortality, 90-day mortality, 365-day mortality, 90-day dialysis or death, and 365-day dialysis or death. Among those who developed early AKI, we compared baseline characteristics and outcomes of interest among patients with different 48h AKI stage. Same comparison was repeated among those who developed late AKI. Separate multivariable logistic regression models were fit to identify risk factors for early AKI and late AKI respectively (compared with those who did not develop AKI). Missing data were imputed by using the Multivariate Imputation by Chained Equations (MICE) method(16). Among patients who did not develop AKI and who developed early AKI, we fit separate multivariable logistic regression models to estimate the effect of early AKI on 90-day and 365-day mortality. Two sets of adjusting covariates were used. Regression models were redeveloped among patient who did not develop AKI and who developed late AKI. Variable selection in these regression models were based on forward stepwise selection using Akaike Information Criterion (AIC). Model goodness of fit was evaluated via the Hosmer-Lemeshow test. For sensitivity analysis, we redid the analyses by excluding patients with unstable sCr. Statistical analyses were performed using R software Version 3.4.1, with statistical significance level at p-value<0.05.
Results
Overall 41.7% (1459/3499) of patients developed AKI within 48h after surgery (early AKI) (Table 1); and the severity distribution for early AKI was 43% stage 1, 49% stage 2 and 7.8% stage 3 (Supplementary Table 1). Another 14.4% (504/3499) patients developed AKI during 48h-7day following surgery (late AKI) (Table 1); and the severity distribution for late AKI was similar at 51% stage 1, 45% stage 2 and 3.8% stage 3 (Supplementary Table 2). Baseline characteristics by post-operative AKI status are presented in Table 1. Both early AKI and late AKI patients had similar baseline sCr. However, among 3313 patients (95%) who had a hospital admission sCr value prior to surgery, 439 (13%) had an unstable baseline sCr—hospital admission sCr was >0.3mg/dl higher compared to the defined baseline sCr as described in methods. Compared to patients with late AKI, those with early AKI were older (62.96 (16.06) vs 57.04 (18.17), p <0.001) and had higher BMI (29.94 (9.96) vs 26.8 (7.12), p <0.001) (Table 1). Early AKI patients also had higher APACHE-III scores (69.01 (28.19) vs. 60.58 (24.97), p <0.001) and more positive fluid balance (5% vs. 3%, p <0.001) compared to late AKI patients. In order to further compare baseline characteristics between early and late AKI patients, we built a multivariable logistic regression with late AKI patients as the reference of outcome. After adjustment, age (OR [95%CI]: 1.02[1.01, 1.02], p <0.001) and BMI (OR [95%CI]: 1.06[1.04, 1.08], p <0.001) were significantly associated with early AKI.
Table 1.
Baseline characteristics of patients undergoing non-cardiac major surgery stratified by post-operative AKI.
| Variable (n=3499) | No AKI (n=1536) | Early AKI(n=1459) | Late AKI (n=504) | p-value* | p-value** |
|---|---|---|---|---|---|
| Age (n=3498) | 54.72(18.28) | 62.96(16.06) | 57.04(18.17) | <0.001 | <0.001 |
| BMI (n=2251) | 25.29(5.74) | 29.94(9.96) | 26.80(7.12) | <0.001 | <0.001 |
| Male | 829(54.0%) | 843(57.8%) | 308(61.1%) | 0.009 | 0.21 |
| Race (n=3300) | 0.12 | - | |||
| White | 1352(92.0%) | 1247(92.0%) | 420(88.6%) | ||
| Black/Others | 87(5.9%)/31(2.1%) | 81(6.0)/28(2.1%) | 36(7.6%)/18(3.8%) | ||
| Baseline sCr | 0.81(0.22) | 0.88(0.23) | 0.86(0.25) | <0.001 | 0.06 |
| sCr within 24h hospital admission (n=3313) | 0.86(0.26) | 1.13(0.65) | 0.93(0.32) | <0.001 | <0.001 |
| eGFR | <0.001 | <0.001 | |||
| < 60 | 61(4.0%) | 97(6.6%) | 24(4.8%) | ||
| 60 - 90 | 599(39.0%) | 869(59.6%) | 233(46.2%) | ||
| > 90 | 876(57.0%) | 493(33.8%) | 247(49.0%) | ||
| 48h Blood transfusion# | 0.21(0.40) | 0.37(0.48) | 0.34(0.47) | <0.001 | 0.33 |
| 7-day Blood transfusion# | 0.91(2.75) | 2.39(5.13) | 1.99(3.47) | <0.001 | 0.1 |
| Anemia | 461(30.0%) | 567(38.9%) | 187(37.1%) | <0.001 | 0.52 |
| Diabetes | 127(8.3%) | 196(13.4%) | 52(10.3%) | <0.001 | 0.08 |
| Cardiac disease | 50(3.3%) | 72(4.9%) | 27(5.4%) | 0.03 | 0.80 |
| Hypertension | 308(20.1%) | 455(31.2%) | 141(28.0%) | <0.001 | 0.20 |
| Liver transplantation | 12(0.8%) | 19(1.3%) | 3(0.6%) | 0.26 | - |
| Myocardiac Infarction | 50(3.3%) | 72(4.9%) | 27(5.4%) | 0.03 | 0.80 |
| COPD | 78(5.1%) | 109(7.5%) | 36(7.1%) | 0.02 | 0.89 |
| Vascular disease | 86(5.6%) | 130(8.9%) | 37(7.3%) | 0.002 | 0.32 |
| APACHE III (n=3492) | 47.15(22.12) | 69.01(28.19) | 60.58(24.97) | <0.001 | <0.001 |
| Normalized fluid (n=2671) | 0.01(0.04) | 0.03(0.05) | 0.02(0.04) | <0.001 | <0.001 |
| Normalized fluid within 48h (n =2351) | 0.02(0.04) | 0.05(0.06) | 0.03(0.05) | <0.001 | <0.001 |
| Mechanical ventilation | 617(40.2%) | 1043(71.5%) | 343(68.1%) | <0.001 | 0.16 |
| Vasopressors& | 138(9.0%) | 347(23.8%) | 110(21.8%) | <0.001 | 0.40 |
| Suspected Sepsis## | 203(13.2%) | 432(29.6%) | 191(37.9%) | <0.001 | <0.001 |
| Aminoglycoside& | 8(0.5%) | 12(0.8%) | 5(1.0%) | 0.44 | |
| Amphotericin& | 1(0.1%) | 1(0.1%) | 0(0%) | 1.00 | - |
| ACEInhibitor/ARB & | 41(2.7%) | 37(2.5%) | 16(3.2%) | 0.75 | - |
| NonSteroidal& | 127(8.3%) | 162(11.1%) | 58(11.5%) | 0.01 | 0.87 |
| Diuretic& | 129(8.4%) | 220(15.1%) | 86(17.1%) | <0.001 | 0.32 |
| Surgery | <0.001 | <0.001 | |||
| Vascular | 100(6.5%) | 203(13.9%) | 53(10.5%) | ||
| General | 795(51.8%) | 911(62.4%) | 284(56.3%) | ||
| Neuro | 416(27.1%) | 130(8.9%) | 88(17.5%) | ||
| Ortho | 102(6.6%) | 48(3.3%) | 32(6.3%) | ||
| Thoracic | 123(8.0%) | 167(11.4%) | 47(9.3%) |
sCr: serum creatinine. Age, BMI, baseline sCr, sCr within 24h hospital admission, 48h blood transfusion, 7-day blood transfusion, APACHE III score, normalized fluid, normalized fluid within 48h were expressed in mean ±SD. The remaining variables were expressed in n(%).
amount of blood transfusion within first 48h/ 7 days from ICU admission.
Information for various drugs was collected within first 24h from ICU admission. They were taken before late AKI occurred.
Suspected sepsis was defined by the first time an order of antibiotics and blood cultures was taken within 24hrs of each other. Most of them occurred before late AKI. For continuous variables, ANOVA was used; for categorical variables, Chi-square test or Fisher’s exact test was used.
overall p-value is for comparison among no AKI, early AKI (AKI occurred within 48h following surgery) and late AKI (AKI occurred during 48h-7day post surgery).
p-value is for comparison between early AKI and late AKI, less than 0.0167 indicates significance due to multiple comparison adjustment; only shown if overall p-value is significant.
We analyzed peri-operative risk factors for postoperative AKI in two different multivariable logistic regressions, which set early AKI or late AKI as primary outcome, respectively. In the early AKI model, only preoperative variables were included. We included different types of surgery treating “general” as the reference (Table 2). Risk factors for early AKI included age (OR: 1.02, 95%CI: [1.02, 1.03], p <0.001), BMI (OR: 1.09, 95%CI: [1.07, 1.10], p <0.001), baseline eGFR (60~90 vs. >90, OR: 1.51, 95%CI: [1.26, 1.81], p <0.001), anemia (OR: 1.35, 95%CI: [1.15, 1.58], p =0.0002). Among them, baseline eGFR was the risk factor with the largest effect, followed by anemia. Neurosurgery was the lowest risk procedure with OR of 0.33 (95%CI: [0.26, 0.41], p <0.001) (Table 2). The late AKI model included both preoperative and postoperative variables. Risk factors mainly came from postoperative variables, which included sepsis (OR: 3.45, 95%CI: [2.67, 4.47], p <0.001), mechanical ventilation (OR: 2.35, 95%CI: [1.83, 3.01], p <0.001), normalized fluid balance within 48h (OR: 1.04, 95%CI: [1.01, 1.07], p =0.02), blood transfusion (OR: 1.05, 95%CI: [1.01, 1.09], p =0.02), use of diuretic (OR: 2.10, 95%CI: [1.50, 2.96], p <0.001), vasopressors ( OR: 1.41, 95%CI: [1.01, 1.95], p =0.04), use of nonsteroidal (OR: 1.46, 95%CI: [1.00, 2.11], p =0.048) (Table 2). Among all the preoperative variables, only age (OR: 1.01, 95%CI: [1.00, 1.01], p =0.047), male patients (OR: 1.37, 95%CI: [1.09, 1.73], p =0.007), BMI (OR: 1.05, 95%CI: [1.02, 1.07], p <0.001) and history of hypertension (OR: 1.52, 95%CI: [1.16, 1.98], p =0.002) were risk factors for late AKI. Again, neurosurgery (OR: 0.55, 95%CI: [0.41, 0.74], p <0.001) was the only procedure with relatively lower risk for late AKI compared to general surgery (Table 2). The use of laparoscopic technique did not have an effect on either early or late AKI (data not shown).
Table 2.
Risk factors for postoperative AKI following surgery in multivariable logistic models.
| Early AKI vs. No AKI (n=3499) | Late AKI vs. No AKI (excluded early AKI, n=2040) |
|||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Age, year | 1.02 | 1.02 - 1.03 | <0.001 | 1.01 | 1.00 - 1.01 | 0.047 |
| Male* | 1.16 | 0.99 - 1.35 | 0.06 | 1.37 | 1.09 - 1.73 | 0.007 |
| BMI | 1.09 | 1.07 - 1.10 | <0.001 | 1.05 | 1.02 - 1.07 | <0.001 |
| eGFR# | <0.001 | |||||
| <60 | 1.32 | 0.93 - 1.88 | 0.12 | |||
| 60-90 | 1.51 | 1.26 - 1.81 | <0.001 | - | - | |
| Anemia | 1.35 | 1.15 - 1.58 | 0.0002 | - | - | |
| Liver transplant | 1.97 | 0.95 - 4.09 | 0.07 | - | - | |
| History hypertension | - | - | - | 1.52 | 1.16 - 1.98 | 0.002 |
| Nonsteroidal | - | - | - | 1.46 | 1.00 - 2.11 | 0.048 |
| Diuretic | - | - | - | 2.10 | 1.50 - 2.96 | <0.001 |
| Blood transfusions | - | - | - | 1.05 | 1.01 - 1.09 | 0.02 |
| Vasopressors | - | - | - | 1.41 | 1.01 - 1.95 | 0.04 |
| Normalized fluid balance 48h (1%) | - | - | - | 1.04 | 1.01 - 1.07 | 0.02 |
| Mechanical ventilation | - | - | - | 2.35 | 1.83 - 3.01 | <0.001 |
| Sepsis | - | - | - | 3.45 | 2.67 - 4.47 | <0.001 |
| Surgery** | <0.001 | 0.002 | ||||
| Vascular | 1.26 | 0.99 - 1.61 | 0.06 | 1.01 | 0.67 - 1.53 | 0.97 |
| Neuro | 0.33 | 0.26 - 0.41 | <0.001 | 0.55 | 0.41 - 0.74 | <0.001 |
| Orhto | 0.41 | 0.28 - 0.60 | <0.001 | 0.70 | 0.44 - 1.11 | 0.13 |
| Thoracic | 1.09 | 0.85 - 1.39 | 0.50 | 0.91 | 0.60 - 1.37 | 0.64 |
female, eGFR (>90) and general surgery were reference levels, respectively. Two multivariable logistic models were established using stepwise model selection with AIC criteria. Early AKI or late AKI was set as outcome, respectively. For early AKI model, only preoperative variables were included for selection. While both preoperative variables and postoperative variables were included in late AKI model. Hosmer-Lemeshow p-values for early AKI model and late AKI model were 0.88 and 1.00, respectively.
When compared with late AKI patients, patients with early AKI had higher mortality at one year (26.5% vs 21.4%, p = 0.03), and higher incidence of “death or dialysis” at 90 days (16.0% vs 12.1%, p = 0.04) as well as at one year (26.8% vs 21.6%, p = 0.03) (Table 3). Outcomes stratified by early AKI stage or late AKI stage are shown in Supplementary Table 3 and 4, respectively. Patients without AKI were set as reference and 90-day or one-year mortality post surgery were the outcomes respectively. After adjusting by age, BMI, eGFR, comorbidities and surgery type, both early AKI (OR: 2.24, 95%CI: [1.72, 2.92], p <0.001 and OR: 1.84, 95%CI: [1.50, 2.27], p <0.001, respectively) and late AKI (OR: 1.68, 95%CI: [1.19, 2.36], p =0.003 and OR: 1.42, 95%CI: [1.09, 1.85], p =0.01, respectively) were significantly associated with 90-day and one-year mortality, respectively (Table 4).
Table 3.
Outcomes of patients undergoing non-cardiac major surgery by post-operative AKI.
| Variable (n=3499) |
No AKI (n=1536) |
Early AKI (n=1459) |
Late AKI (n=504) |
p-value* | p-value** |
|---|---|---|---|---|---|
| LOS in ICU, median(IQR) | 3.00(2.00) | 4.00(6.00) | 6.00(9.00) | <0.001 | <0.001 |
| LOS in hospital, median(IQR) | 10.00(10.00) | 14.0(14.00) | 17.50(18.25) | <0.001 | <0.001 |
| Hospital mortality, n(%) | 67(4.4%) | 155(10.6%) | 38(7.5%) | <0.001 | 0.06 |
| 90-day mortality, n(%) | 123(8.0%) | 229(15.7%) | 61(12.1%) | <0.001 | 0.06 |
| 365-day mortality, n(%) | 260(16.9%) | 387(26.5%) | 108(21.4%) | <0.001 | 0.03 |
| Death or dialysis at day 90, n(%) | 123(8.0%) | 233(16.0%) | 61(12.1%) | <0.001 | 0.04 |
| Death or dialysis at day 365, n(%) | 260(16.9%) | 391(26.8%) | 109(21.6%) | <0.001 | 0.03 |
LOS: length of stay. For continuous variables, ANOVA or Kruskal-Wallis test was used; for categorical variables, Chi-square test was used.
overall p-value is for comparison among no AKI, early AKI and late AKI.
p-value is for comparison between early AKI and late AKI, less than 0.0167 indicates significance due to multiple comparison adjustment; only shown if overall p-value is significant.
Table 4.
Multivariable logistic models for comparing mortality among AKI diagnosed at different time windows.
| Model | Outcomes | Variable | Adjusted Odds Ratio |
95% CI of Odds Ratio |
p-value |
|---|---|---|---|---|---|
| 1 | 90-day mortality# | AKI | <0.001 | ||
| Early AKI vs no AKI | 2.24 | 1.72-2.92 | <0.001 | ||
| Late AKI vs no AKI | 1.68 | 1.19-2.36 | 0.003 | ||
| 2 | 365-day mortality## | AKI | <0.001 | ||
| Early AKI vs no AKI | 1.84 | 1.50-2.27 | <0.001 | ||
| Late AKI vs no AKI | 1.42 | 1.09-1.85 | 0.01 | ||
| 3 | 90-day mortality* | AKI | 0.12 | ||
| Early AKI vs no AKI | 1.32 | 0.99-1.77 | 0.06 | ||
| Late AKI vs no AKI | 1.04 | 0.72-1.50 | 0.83 | ||
| 4 | 365-day mortality** | AKI | 0.01 | ||
| Early AKI vs no AKI | 1.39 | 1.12-1.73 | 0.003 | ||
| Late AKI vs no AKI | 1.09 | 0.82-1.43 | 0.56 |
Models(#,##) were adjusted by age, BMI, eGFR, comorbidities, surgery type. Models(*,**) were adjusted by age, BMI, eGFR, comorbidities, surgery type and postoperative complications.
Sensitivity analysis
After excluding patients with changes in sCr from baseline to admission (n=439), we repeated the analysis. Compared to patients with late AKI, early AKI patients were older (1.02 [1.01, 1.02], p< 0.001) and had higher BMI (1.06 [1.04, 1.08], p<0.001). Risk factors for early AKI and late AKI can be found in supplementary table 5. Effects of early AKI versus late AKI on outcomes are provided in supplementary table 6. Results were similar to our primary analysis.
Validation analysis
Selecting non-cardiac major surgery patients admitted to multiple ICUs at the University of Pittsburgh Medical Center from 2008 to 2014 by the same inclusive and exclusive criteria, we identified 10,723 patients. Analysis of this cohort yielded similar results to the primary analysis with 58.6% (6287/10723) being classified as early (within 48h) and 8.4% (907/10723) late. Most AKI following major non-cardiac surgical disease occurred within the first 48h after surgery, and 12h post surgery was the peak interval (Fig 1). One-year mortality for patients with early and late AKI were 41.74% and 36.03%, respectively (Supplementary Table 7).
Figure 1.
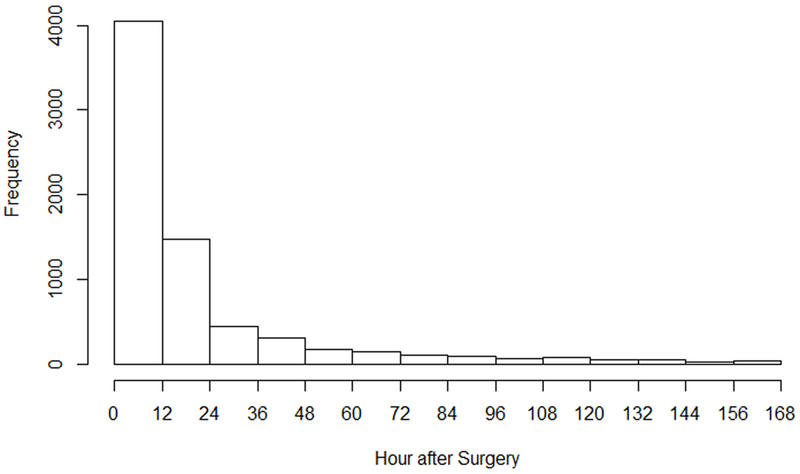
Distribution of AKI frequency following surgery.
Discussion
In this study we demonstrated that AKI occurring before and after 48hrs post-surgery are quite different in terms of risk factors and outcomes. Our data strongly support the need to treat these conditions separately when designing trials and conducting quality improvement. While it may seem obvious that interventions during surgery are unlikely to affect AKI rates several days later, many trials have been conducted exactly this way.
Overall rates of AKI post-surgery observed in our study were higher compared to many other studies (6, 7, 10, 17) but quite consistent with studies in the ICU which have used full KDIGO criteria (18, 19). A special point on baseline sCr also deserves our attention. We found that 13% of patients already had evidence of AKI by sCr at hospital admission (i.e. before surgery). Thus, it may not be reasonable to set hospital admission sCr as baseline for all surgical patients. In addition, most AKI manifest within 48h after surgery, and 12h after surgery was the peak interval. This suggests that surgical patients in the ICU require close renal function monitoring for the first 48h post-surgery—perhaps longer than the current standard of care.
Even though the concepts of early AKI or late AKI have been mentioned previously in acute myocardial infarction (AMI) (20, 21), burn (22) and ICU (23, 24) patients, definitions and time boundaries vary among them. Moriyama et al. included 760 patients with AMI, and AKI was defined according to AKIN criteria. The time boundary for early AKI and late AKI was 48h after hospital admission (20). In another AMI study of 971 patients, early AKI was defined as AKI occurred within 24h post percutaneous coronary intervention (PCI) in AKIN criteria (21). In a study of 221 burn patients, early AKI was defined as AKI occurred within 24h post hospital admission according to RIFLE criteria (22). While another two studies focusing on critically ill patients defined early AKI as AKI diagnosed within 24h of ICU admission (23, 24). We chose to define early AKI as within 48h of surgery as this represents a plausible delay in reaching serum creatinine criteria and because the 48h window is codified in the KDIGO definition of AKI (13).
Our results reveal that patients manifesting AKI early (within 48 hours) were different from late AKI patients in terms of baseline characteristics and prognosis. Importantly, the differences in risk factors for the early and late AKI permit us to speculate that these are in fact distinct phenotypes, and interventions that are developed for one may not be effective for the other. Furthermore, pooling of patients developing AKI at these different times into a single outcome is likely to be wrong for interventions that specifically target a mechanism that only apply to one group (e.g. improving intraoperative hemodynamics).
Apart from age and sex, BMI is the only risk factor associated with both early and late AKI in our study. A relationship between BMI and postoperative AKI has been shown in previous studies and surgery types included orthotopic liver transplantation (25), non-cardiac surgery and cardiac surgery (12, 26-28). By contrast, preoperative anemia was associated only with early AKI, while blood transfusion after surgery was associated with late AKI. The relationship between anemia, blood transfusion and postoperative AKI has been demonstrated before (29, 30). In cardiac surgery, preoperative anemia itself increased AKI rates from 1.4% to 1.9%, while blood transfusion amplified the difference—AKI was more than two times higher in patients receiving transfusion (6% vs. 2.8%)(29). Blood transfusion exacerbated renal damage based on anemia (30). These may be explained in several ways. First, anemia itself may make the kidney more vulnerable to hypoperfusion. Indeed, we found that anemia was a risk factor for early AKI but not late AKI, suggesting that intraoperative hypoperfusion might be the main mechanism. Second, blood transfusion-related direct renal injury also cannot be ignored (31). The adverse effects of storage on red blood cells may be a critical factor. Storage of red blood cells alters cell deformability and shortens cell lifespan, possibly resulting in hemolysis and iron-mediated oxidative kidney injury (32, 33).
We observed similar outcomes for patients with AKI as reported in previous studies (2, 10, 11). One-year mortality rates of 20-25% are often reported. The incidence of “death or dialysis” at 90 days of about 15% is also consistent with prior studies (34).
There are several important strengths of our study to highlight. First, we studied a relatively large population with complete data to calculate AKI using full KDIGO criteria with precise information on timing. Second, we were able to link our data with national databases (USRDS and NDI) to ascertain long-term outcomes. Finally, we had reasonably comprehensive data on pre- and post-operative variables that could reasonably be expected to impact AKI rates. However, there are also several limitations. First, as an observational study, we cannot make causal inferences between risk factors and AKI. Second, our cohort came from a single center and is now more than a decade old. However, we validated the incidence of AKI and 1-year mortality in a newer dataset, which includes patients from 2008-2014. Third, because of our specific patient population which were admitted into the ICU following surgery, our conclusions may not be generalizable to other populations.
Conclusions
In conclusion, most surgery-related AKI occurred within the first 48h after surgery, and 12h after surgery was the peak interval. Early AKI (with 48h) was significantly different from late AKI in baseline characteristics, risk factors and long-term outcomes.
Supplementary Material
Acknowledgments
Financial Disclosure and Conflicts of Interest: This work was funded by institutional grant R01 DK083961-121860 from the National Institutes of Health to John Kellum. The remaining authors have disclosed that they do not have any conflicts of interest. The content is solely the responsibility of the authors.
Copyright form disclosure: Drs. Li, Wang, and Kellum received support for article research from the National Institutes of Health (NIH). Drs. Wang and Kellum’s institutions received funding from NIH R01 DK083961-121860. Dr. Priyanka disclosed work for hire.
Footnotes
Reprints: No reprints will be ordered.
USRDS Disclaimer: The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government.
References
- 1.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 2008;3(3):844–861. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor ME, Hewson RW, Kirwan CJ, et al. Acute kidney injury and mortality 1 year after major non-cardiac surgery. Br J Surg 2017. [DOI] [PubMed] [Google Scholar]
- 3.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. . Nat Rev Nephrol 2014;10(4):193–207. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui NF, Coca SG, Devereaux PJ, et al. Secular trends in acute dialysis after elective major surgery-1995 to 2009. CMAJ 2012;184(11):1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson T Quan S, Cheema K, Zarnke K, Quinn R, de Koning L, Dixon E, Pannu N, James MT. Risk prediction models for acute kidney injury following major noncardiac surgery: systematic review. Nephrol Dial Transplant 2016:31(32):231–240. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor ME, Kirwan CJ, Pearse RM, et al. Incidence and associations of acute kidney injury after major abdominal surgery. . Intensive Care Med 2016;42(4):521–530. [DOI] [PubMed] [Google Scholar]
- 7.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. . Ann Surg 2009;249(5):851–858. [DOI] [PubMed] [Google Scholar]
- 8.Korenkevych D, Ozrazgat-Baslanti T, Thottakkara P, et al. The Pattern of Longitudinal Change in Serum Creatinine and 90-Day Mortality After Major Surgery. . Ann Surg 2016;263(6):1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long TE, Helgason D, Helgadottir S, et al. Acute Kidney Injury After Abdominal Surgery: Incidence, Risk Factors, and Outcome. Anesth Analg 2016;122(6):1912–1920. [DOI] [PubMed] [Google Scholar]
- 10.Grams ME, Sang Y, Coresh J, et al. Acute Kidney Injury After Major Surgery: A Retrospective Analysis of Veterans Health Administration Data. Am J Kidney Dis 2016;67(6):872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilmi IA, Damian D, Al-Khafaji A, et al. Acute kidney injury following orthotopic liver transplantation: incidence, risk factors, and effects on patient and graft outcomes. Br J Anaesth 2015;114(6):919–926. [DOI] [PubMed] [Google Scholar]
- 12.O’Sullivan KE, Byrne JS, Hudson A, et al. The effect of obesity on acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg 2015;150(6):1622–1628. [DOI] [PubMed] [Google Scholar]
- 13.Workgroup: KDIGO AKI. Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for acute kidney injury. . Kidney Int 2012(Suppl 2):1–138. [Google Scholar]
- 14.System U.S. Renal Data. 2016 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2016. [Google Scholar]
- 15.Kellum JA, Sileanu FE, Bihorac A, et al. Recovery after Acute Kidney Injury. Am J Respir Crit Care Med 2017;195(6):784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buuren Stef van, Groothuis-Oudshoorn Karin. MICE: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software 2011;45(3):51554. [Google Scholar]
- 17.Gameiro J, Neves JB, Rodrigues N, et al. Acute kidney injury, long-term renal function and mortality in patients undergoing major abdominal surgery: a cohort analysis. Clin Kidney J 2016;9(2):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015;41(8):1411–1423. [DOI] [PubMed] [Google Scholar]
- 19.Kellum JA, Sileanu FE, Murugan R, et al. Classifying AKI by Urine Output versus Serum Creatinine Level. . J Am Soc Nephrol 2015;26(9):2231–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moriyama N, Ishihara M, Noguchi T, et al. Early development of acute kidney injury is an independent predictor of in-hospital mortality in patients with acute myocardial infarction. J Cardiol 2017;69(1):79–83. [DOI] [PubMed] [Google Scholar]
- 21.Kim JH, Lee JH, Jang SY, et al. Prognostic value of early acute kidney injury after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Am J Cardiol 2014;114(8):1174–1178. [DOI] [PubMed] [Google Scholar]
- 22.Mosier MJ, Pham TN, Klein MB, et al. Early acute kidney injury predicts progressive renal dysfunction and higher mortality in severely burned adults. J Burn Care Res 2010;31(1):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poukkanen M, Vaara ST, Reinikainen M, et al. Predicting one-year mortality of critically ill patients with early acute kidney injury: data from the prospective multicenter FINNAKI study. Crit Care 2015;19:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagshaw SM, George C, Bellomo R, et al. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care 2007;11(3):R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iglesias Jose I, DePalma John A, Levine Jerrold S. Risk factors for acute kidney injury following orthotopic liver transplantation: the impact of changes in renal function while patients await transplantation. BMC Nephrol 2010(11):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007;107(6):892–902. [DOI] [PubMed] [Google Scholar]
- 27.Billings Frederic T., Pretorius Mias, Schildcrout Jonathan S., et al. Obesity and Oxidative Stress Predict AKI after Cardiac Surgery. J Am Soc Nephrol 2012;23(7):1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedersen AB, Gammelager H, Kahlert J, et al. Impact of body mass index on risk of acute kidney injury and mortality in elderly patients undergoing hip fracture surgery. Osteoporos Int 2017;28(3):1087–1097. [DOI] [PubMed] [Google Scholar]
- 29.Karkouti K, Wijeysundera DN, Yau TM, et al. Influence of erythrocyte transfusion on the risk of acute kidney injury after cardiac surgery differs in anemic and nonanemic patients. Anesthesiology 2011;115(3):523–530. [DOI] [PubMed] [Google Scholar]
- 30.Karkouti K, Grocott HP, Hall R, et al. Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anaesth 2015;62(4):377–384. [DOI] [PubMed] [Google Scholar]
- 31.Karrowni W, Vora AN, Dai D, et al. Blood Transfusion and the Risk of Acute Kidney Injury Among Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Circ Cardiovasc Interv 2016;9(9). [DOI] [PubMed] [Google Scholar]
- 32.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood 2010;115:4284–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozment CP, Turi JL. Iron overload following red blood cell transfusion and its impact on disease severity. . Biochim Biophys Acta 2009;1790:694–701. [DOI] [PubMed] [Google Scholar]
- 34.Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med 2017;43(11):1551–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


