Abstract
Carbon nanotubes (CNTs) have emerged as promising drug delivery systems particularly for cancer therapy, due to their abilities to overcome some of the challenges faced by cancer treatment, namely non-specificity, poor permeability into tumour tissues, and poor stability of anticancer drugs. Encapsulation of anticancer agents inside CNTs provides protection from external deactivating agents. However, the open ends of the CNTs leave the encapsulated drugs exposed to the environment and eventually their uncontrolled release before reaching the desired target. In this study, we report the successful encapsulation of cisplatin, a FDA-approved chemotherapeutic drug, into multi-walled carbon nanotubes and the capping at the ends with functionalised gold nanoparticles to achieve a “carbon nanotube bottle” structure. In this proof-of-concept study, these caps did not prevent the encapsulation of drug in the inner space of CNTs; on the contrary, we achieved higher drug loading inside the nanotubes in comparison with data reported in literature. In addition, we demonstrated that encapsulated cisplatin could be delivered in living cells under physiological conditions to exert its pharmacological action.
1. Introduction
Carbon nanotubes (CNTs) are currently explored in many fields of nanomedicine owing to their unique physicochemical properties such as high aspect ratio, high tensile strength, ultra light weight, high thermal conductivity, and electronic properties ranging from metallic to semiconducting [1], [2], [3]. These properties guarantee efficient cellular uptake of CNTs, high stability of the nanotubes, and enhancement of mechanical properties of composite materials, therefore raising interesting prospects in electronics [4], [5], material science [6] and even tissue repair [7]. There is also growing interest in exploring the possibilities of using CNTs as drug delivery systems [8]. In principle CNTs can be used as carriers for all kind of drugs, although current effort focuses more on their potential application in cancer therapy, mainly due to poor distribution and penetration of anti-cancer drugs into tumour tissues and the limited stability of several bioactive agents, rendering their administration a challenge.
CNTs may be able to overcome some of these problems. First, CNTs have high aspect ratio, which enhances their cell penetration capability. It has been postulated that the high internalisation capacity of CNTs by cells is due to their ability to pierce through the cell membrane like needles [9]. Second, drugs can be encapsulated inside CNTs and hence be protected from deactivation before reaching the target sites. In addition, the properties of encapsulated drugs can be indirectly altered by functionalisation of the external walls [1], [2], [3], [10], [11]. Encapsulating drugs inside the tubes seems to be more beneficial than attaching drugs on the external walls. Attaching a molecule on external walls of CNTs typically involves covalent or non-covalent conjugations which may not be ideal since any structural changes may change the pharmacological activities of the drugs. In addition, the interior of CNTs has a more favourable binding energy towards adsorption of molecules, making it possible for encapsulated drug molecules to interact with CNTs by simple adhesive forces. This eliminates any need of forming chemical bonds between drug molecules and CNTs [10]. Another benefit of encapsulating drugs inside CNTs would be to shield them from external deactivating agents or unfavourable environmental conditions which may affect their stability. We have previously demonstrated that drug molecules can be further protected by capping the ends of the tubes with different molecules as the basis of the so-called “carbon nanotube bottle” concept [12].
In this manuscript, we study the use of multi-walled carbon nanotubes (MWCNTs), rather than single-walled tubes (SWCNTs), as drug delivery systems because the multiple wall layers minimise the possibility of leakage of encapsulated drugs from the side walls, which may affect our measurements of drug release from the tubes. In addition, the larger diameter of MWCNTs facilitates easier entry of materials and thus more substances could be encapsulated in a shorter period of time. Cisplatin (cis-diamminedichloroplatinum(II, CDDP) was chosen as the carrier drug because it is a highly potent and widely used anticancer agent, particularly in testicular, ovarian, breast and bladder cancer, but its use is limited by high systemic toxicities arising from non-specific binding away from its biological target, DNA [13]. Caps were made of gold nanoparticles (GNPs) functionalised with alkanethiols in order to tune their size and surface properties. It has been demonstrated that alkanethiols readily assemble as a monolayer on the surface of gold [14], [15], [16], [17], [18], [19], [20], [21], [22], which is facilitated by strong bonds between gold and sulphur, and readily formed under ambient conditions. In addition, GNPs can be detected quite easily under the Transmission Electron Microscope (TEM). This combination of MWCNTs, CDDP, and thiol-functionalised GNPs (f-GNPs) constituted the carbon nanotube bottle, which was further investigated in this study.
2. Materials and methods
Spherical GNPs (4 nm diameter) were purchased from Nanopartz™. Cisplatin (CDDP) and 1-octadecanethiol (ODT) were purchased from Sigma Aldrich. Organic solvents were obtained from Fischer Scientific. TEM was carried out using JEM 2010F HRTEM (JEOL) and TEM-EDX analysis (Energy Dispersive X-ray) using JEM 3010 HRTEM & EDX 300KV LaB6 (JEOL). IR spectra were recorded on a Spectrum100 FT-IR (PerkinElmer) and UV–vis spectra on a BioSpec-nano (Shimadzu). Thermogravimetric analysis (TGA) and determination of Pt levels using ICP-OES Optima 5300 DV (PerkinElmer) were performed by CMMAC (NUS).
2.1. Synthesis of ODT-functionalised GNPs as caps
Coating of bare GNPs by ODT was exhaustively optimised using different stoichiometric ratios of reagents; only optimised coating conditions are reported. Briefly, 1.044 mg of bare GNPs were pre-concentrated to a volume of 3.2 mL using Vivaspin 20 PES 3 K MWCO (Stedim Biotech), and 1.044 mg (1044 μL of 1 mg/mL of ODT in EtOH) of ODT were added. The mixture was agitated for 24 h and subjected to four cycles of washing to remove unbound ODT, comprising centrifugation at 10,000g for 20 min, removal of supernatant and addition of fresh EtOH for one washing cycle. After the last washing cycle, EtOH was removed and ODT-f-GNPs were re-dispersed in suitable solvents for subsequent characterisation steps by TEM, IR and UV–vis analysis.
The ODT-f-GNPs were dispersed in various organic solvents, namely dichloromethane, EtOH, ethyl acetate (EA), hexane, dimethyl sulfoxide, and toluene, to determine a suitable medium for nano-extraction. Thus ODT-f-GNPs, equivalent to 0.2 mg of bare GNPs, were dispersed in the mentioned organic solvents and their stability and suspendibility were qualitatively evaluated after 24 h.
2.2. Encapsulation of CDDP into MWCNTs
Ultrapure MWCNTs were prepared as previously reported [23], [24]. Briefly, MWCNTs were synthesised by chemical vapour decomposition of acetylene over rare earth-based AB2 alloy hydride catalysts. The crude MWCNTs were purified by air oxidation and heating at 400 °C for 3 h to remove amorphous carbon, followed by acid treatment to remove catalytic impurities by refluxing in concentrated HNO3 for 24 h. After repeated washings with de-ionised water, the product was air-dried at 100 °C for 30 min. TEM characterisation of the MWCNTs (images not shown) indicated that the outer diameter of MWCNTs was 30–40 nm with average internal diameter of about 10 nm, while their length ranged from a few hundred nanometres to 1 μm.
Encapsulation of CDDP into MWCNTs was based on the principles of nano-extraction [22], for which EA was selected as the encapsulation medium. MWCNTs (5 mg) and CDDP (14 mg) were mixed in EA (4.5 mL) and ultrasonicated for 30 min. After stirring for 40 h in the dark, EA was evaporated and the residue re-dispersed in a solvent mixture comprising EA (2.6 mL), EtOH (1 mL) and water (1 mL). The mixture was then filtered through 0.22 μM PTFE membrane and washed with a solvent mixture EA:EtOH:water (2.6:1:1 v/v) to remove unencapsulated CDDP molecules. Dry MWCNTs containing encapsulated CDDP (MWCNT-CDDP) were recovered from the membrane and analysed using TEM and TEM-EDX. Pt encapsulation levels were evaluated using TGA and ICP-OES. The sample was heated in air at a rate of 10 °C/min until a final temperature 1000 °C was reached. The residue was dissolved in aqua regia, diluted and analysed by ICP-OES to determine the amount of elemental Pt.
2.3. Capping of MWCNT-CDDP with ODT-f-GNPs
Based on the stability of ODT-f-GNPs in various organic solvents, EA was chosen for nano-extraction of ODT-f-GNPs into MWCNT-CDDP. MWCNT-CDDP (1 mg) and ODT-f-GNPs (equivalent to 1 mg of bare GNPs) were mixed in EA (1 mL). The mixture was ultrasonicated for 30 min and stirred in the dark for 63 h. EA was evaporated to leave a mixture of free ODT-f-GNPs and capped MWCNT-CDDP. EtOH was used to separate the two components since capped MWCNT-CDDP was dispersible in EtOH while free ODT-f-GNPs remained on the reaction flask. The capped MWCNT-CDDP was characterised using TEM, which indicated the presence of ODT-f-GNPs inside the MWCNTs.
2.4. In vitro release of CDDP
In vitro release of CDDP from both capped and uncapped MWCNTs was evaluated using a dialysis bag diffusion method. Spectra/Por Dialysis Membrane 12–14 K MWCO was used, which allowed the free exchange of CDDP and buffer as the release media. Therefore, phosphate buffer at pH 5.5 was selected to simulate the slightly acidic condition in tumour cells [25], and phosphate-buffered saline (PBS) at pH 7.4 mimicked the physiological conditions. Uncapped MWCNT-CDDP (containing 1 mg CDDP) was dispersed in 2 mL of medium in a dialysis bag and in turn immersed in a tank of release media (298 mL). An aliquot of 1 mL was withdrawn periodically and Pt levels quantified using ICP-OES. After each withdrawal, equivalent volumes of release media were replaced. Based on the comparable results of the drug release in both pH conditions, subsequent release of CDDP from the capped formulation was only performed at physiological conditions.
2.5. Cell viability assays of MWCNTs, CDDP, uncapped MWCNT-CDDP, and capped MWCNT-CDDP on MCF-7 cells
2.5.1. Cell culture
Human mammary gland adenocarcinoma epithelial cell (MCF-7) was obtained courtesy of Prof. Victor Yu (NUS).
Breast cancer cells (MCF-7) were chosen as a common prototype of cancer cells, since CDDP is a FDA approved drug for the treatment of solid malignancies. In particular, it has shown an important role as a front-line therapy for metastatic breast cancer [26].
MCF-7 cells were cultured in DMEM medium (Invitrogen) supplemented with 10% FBS (HyClone) and 1% Penicillin–Streptomycin (Invitrogen).
2.5.2. MTT cell viability assay
In order to compare the efficacy of encapsulated CDDP against the free drug, nine concentrations (between 0.1 and 80 μM) of free CDDP were initially chosen for the MTT assay [27], [28] involving MCF-7 cells. The corresponding concentrations of uncapped MWCNT-CDDP, capped MWCNT-CDDP and MWCNTs alone were calculated based on our results that CDDP content of MWCNT-CDDP were 62.1% and 44.44% for uncapped MWCNT-CDDP and capped MWCNT-CDDP, respectively. The negative control consisting of pristine MWCNTs was included to demonstrate that the cytotoxic effects of both MWCNT-CDDP samples were specifically stemmed from the CDDP encapsulated. Thus, the assay were carried out with pristine MWCNTs that were at same amount as capped MWCNT-CDDP, as the amount of MWCNTs present was greater than uncapped MWCNT-CDDP due to difference in CDDP content. Table 1 summarises the concentrations of MWCNTs, CDDPs, and MWCNT-CDDP (capped and uncapped) used for the purpose.
Table 1.
Concentrations of CDDP, MWCNT-CDDP, and MWCNTs used for the cell treatment. Note: the concentrations of CDDP and MWCNTs were comparable to the corresponding concentration of CDDP and MWCNTs in MWCNT-CDDP. The molar concentration is the molar concentration of CDDP.
| Molar concentration (μM) | Cisplatin (μg/mL) | MWNT-CDDP (μg/mL) | MWNT-CDDP with cap (μg/mL) | MWNT pristine (μg/mL) |
|---|---|---|---|---|
| 0.1 | 0.03 | 0.055 | 0.075 | 0.075 |
| 1 | 0.3 | 0.55 | 0.75 | 0.75 |
| 2 | 0.6 | 1.1 | 1.5 | 1.5 |
| 5 | 1.5 | 2.8 | 3.78 | 3.78 |
| 10 | 3 | 5.6 | 7.58 | 7.58 |
| 15 | 4.5 | 8.3 | 11.36 | 11.36 |
| 20 | 6 | 11.2 | 15.16 | 15.16 |
| 50 | 15 | 27.8 | 37.88 | 37.88 |
| 80 | 24 | 44.8 | 60.64 | 60.64 |
MWCNTs, CDDP, uncapped MWCNT-CDDP, and capped MWCNT-CDDP solutions were prepared by dissolving the samples in plain DMEM and the solutions were sonicated briefly to ensure an even suspension.
For the MTT assay, the cultured cells were plated on 96-well plates at a density of 5000 cells per well and the plates were incubated at 37 °C and 5% CO2 for 24 h prior to drug treatment. Before treating the cells, the medium was removed from each well. MWCNTs, CDDP, uncapped MWCNT-CDDP, and capped MWCNT-CDDP solutions were added into each designated well at 100 μL while wells without any treatment were used as negative controls. The cells were then incubated for further 6 h. After 6 h of drug treatment, the drug solution were replaced with FBS supplemented DMEM and the cells were allowed to grow further for 66 h.
Assessment of cell viability was done using 0.5 mg/mL solution of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Alfa Aesar) prepared by dissolving MTT in sterile PBS and filtering through 0.2 μM PES filter. After incubation for 72 h, the solution in each well was aspirated and MTT solution (100 μL) was added and the plates were incubated for another 4 h. Subsequently, MTT solution was thoroughly aspirated out and DMSO was added to solubilise the insoluble formazan dye. The plates were shaken for 3 min to ensure homogeneity and aid proper solubilisation of the dye prior to measure absorbance. The absorbance at 595 nm was recorded using Microplate Reader Infinite Series M200 (Tecan). The readings were converted into survival (%) and the concentrations were transformed logarithmically. The data were analysed by non-linear regression and plotted via GraphPad Prism.
3. Results and discussion
3.1. Synthesis of capped MWCNTs containing encapsulated CDDP
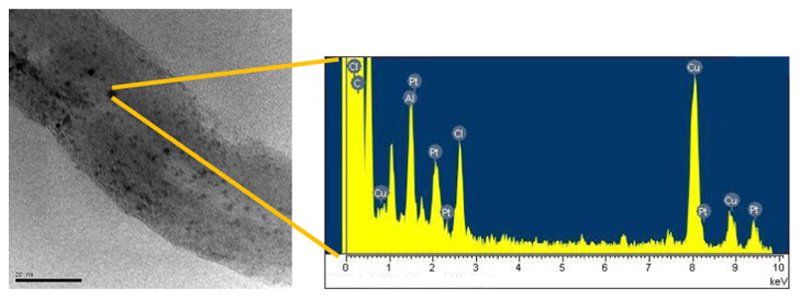
Carbon nanotubes can easily undergo chemical modifications in order to introduce suitable functional groups at their surface. However, this procedure is associated with loss of the aromaticity at the benzene rings and concomitant decrease of the characteristic high aspect-ratio. On the contrary, the inner encapsulation of molecules inside the hollow space of nanotubes is based on different strategies, which rely on favourable binding energies towards adsorption of molecules and do not affect the integrity of the tubes. Therefore, in our case pristine, non-functionalised CNTs were either used directly as control or subjected to nano-extraction [22] to incorporate CDDP and further seal the extremities with surface-modified gold nanoparticles (ODT-f-GNPs). The successful preparation of the samples was confirmed using UV–vis and FT-IR spectroscopy [29]. UV–vis spectra showed a red shift once 1-octadecanethiol (ODT) was incorporated on GNPs. Indeed, ODT-f-GNPs showed a slight increase in SPR λmax for ODT-f-GNPs (Fig. S1). Moreover, the absence of the peaks at about 2400 cm−1, attributable to S–H stretching in the IR spectrum of ODT-f-GNPs, provided further evidence that ODT were attached to the GNP surface via the formation of Au–S bond (Fig. S2). To confirm that CDDP was successfully encapsulated inside MWCNTs, MWCNT-CDDP sample was observed under TEM. CDDP, which appeared as black dots, was found inside the nanotubes (Fig. 1a). The identity of black dots was verified using TEM-EDX analysis. The presence of chlorine and platinum peaks in the TEM-EDX spectrum (Fig. 1b) confirmed that the black dots inside the MWCNTs were indeed CDDP.
Fig. 1.
(a) TEM image showing encapsulated CDDP inside a nanotube. (b) EDX spectrum of MWCNT-CDDP. Al and Cu peaks could be attributed to the TEM supporting grid, which was made of aluminium and copper.
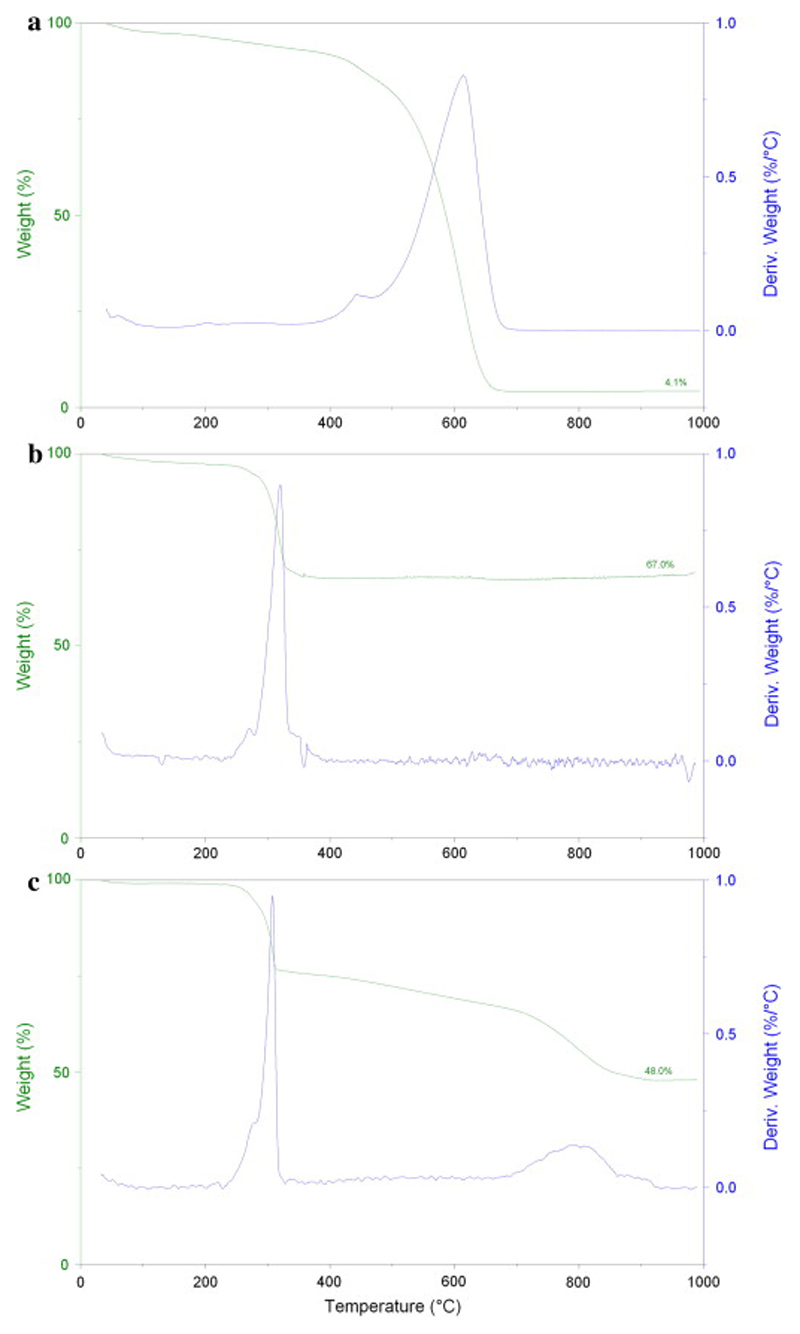
The loading of CDDP in MWCNTs was evaluated with TGA and ICP-OES. First, MWCNT-CDDP was combusted by TGA, and the remaining residue was dissolved and analysed by ICP-OES to determine the amount of elemental platinum. Using 10 mg of MWCNT-CDDP for analysis, 4.04 mg of elemental platinum (MW = 195) was quantitated on ICP-OES which amounted to approximately 621 μg CDDP (MW = 300) within 1 mg MWCNT-CDDP.
In the analysis of MWCNTs alone, the residue collected after heating up to 1000 °C was 4.1% of initial weight (Fig. 2a). At 1000 °C, the carbon atoms would be completely combusted and thus the residue was most likely due to metal catalyst impurities present in the MWCNTs during their production. This was in agreement with the residue found in other samples used by our group [7]. Notably, no Pt was present in the starting material, which has been proven by ICP-OES measurement, as shown previously [30].
Fig. 2.
TGA of (a) MWCNTs alone, (b) CDDP alone and (c) MWCNT-CDDP. TGA are labelled with the wt.% of metal residue after ramping the sample to 1000 °C at a rate of 10 °C/min in air.
In TGA analysis of pure CDDP, degradation occurred at about 320 °C (Fig. 2b). The amine and chloride groups were combusted leaving behind platinum residue which was 67% of initial weight. This value is comparable to the theoretical platinum content of CDDP. Based on the atomic weight of platinum (195.08) and the molecular weight of CDDP (300.05), the theoretical platinum content of CDDP is 65%. In contrast, the residue obtained for the MWCNT-CDDP was 48% of its initial weight. The first weight loss at about 320 °C corresponded to CDDP degradation while the second weight loss corresponded to MWCNT degradation (Fig. 2c). Taking into account that our MWCNTs contained about 4.1% of metal catalyst impurities (Fig. 2a), the platinum content in our formulation and as obtained from ICP-OES measurements was 43.9%, thus corresponding to 0.621 mg of CDDP for each 1 mg of MWCNT-CDDP sample (i.e. 62.1%). In another report, Tripisciano et al. encapsulated CDDP into MWCNTs with a main diameter of about 13 nm, via nano-extraction of CDDP from DMF for 3 days. However, their final product only contained 21% of CDDP [31]. In comparison, our method successfully encapsulated a significantly greater amount of CDDP (62.1%) in a shorter period of time (40 h).
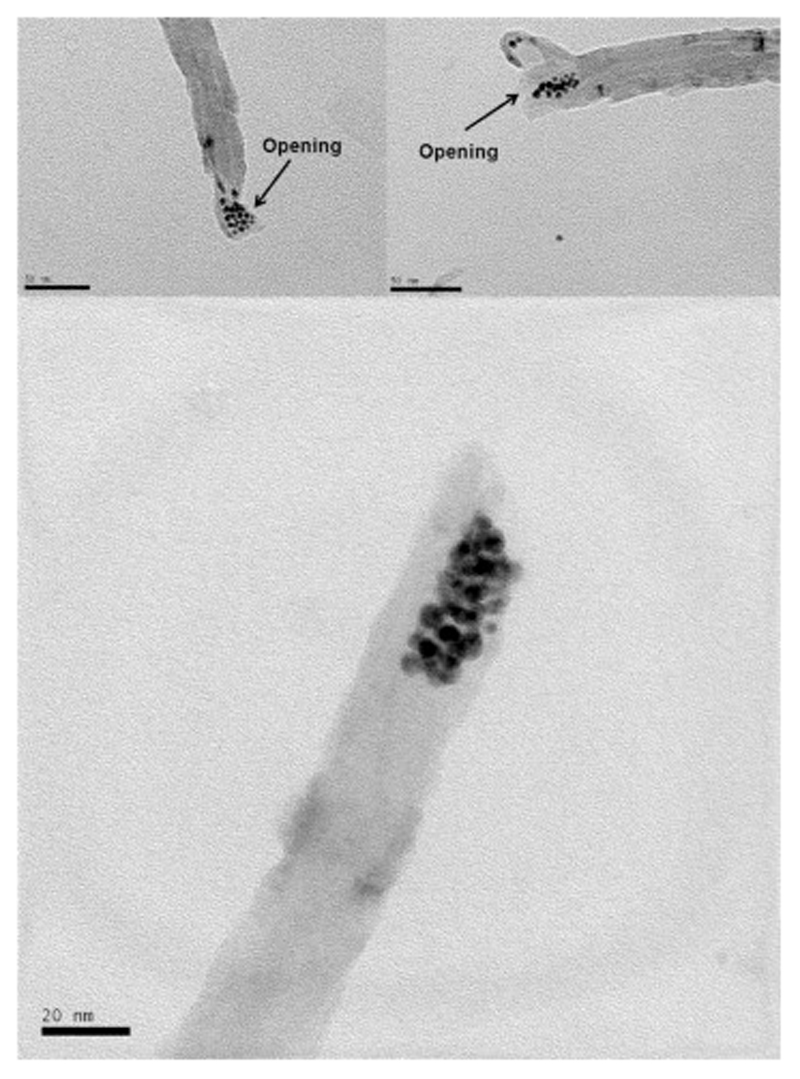
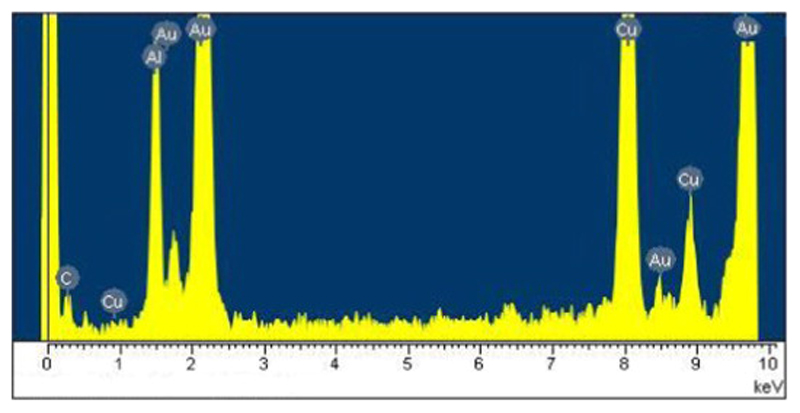
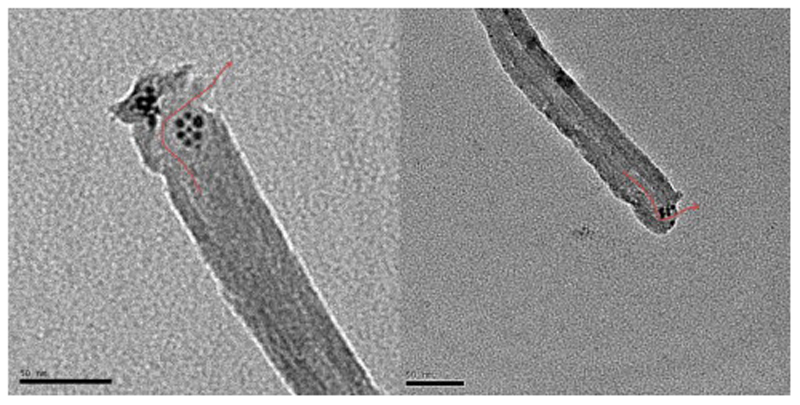
The ODT-f-GNPs-filled MWCNTs were observed under TEM, and the loading of CDDP in this capped-sample was also evaluated by TGA and ICP-OES. Unlike CDDP, which was distributed along the inner cavities of MWCNTs, ODT-f-GNPs were mostly found at the tips (Fig. 3). TEM-EDX analysis confirmed the identity of these black circles to be ODT-f-GNPs (Fig. 4). We postulated that the migration of ODT-f-GNPs into the middle part of MWCNTs was not favourable due to their larger particle size. In addition, the hydrophobic C18 ODT chain which surrounded the GNPs made the surface of the GNPs sticky. Consequently, the ODT-f-GNPs readily adsorbed on the internal walls, preventing adsorption of other ODT-f-GNPs. Based on qualitative evaluation of the TEM images, we estimated that at least 30% of the tubes were capped with ODT-f-GNPs. Moreover, in the case of capped MWCNT-CDDP, the co-localisation of ODT-f-GNPs at the tips of the tubes decreased the levels of CDDP that could be encapsulated inside the tubes. In comparison with uncapped tubes, the procedure required an additional washing step after the co-encapsulation of ODT-f-GNPs, further decreasing CDDP loading inside the tubes while increasing the total weight of the nano-bottle (capped-sample). Nevertheless, CDDP levels quantitated within the capped MWCNT-CDDP corresponded to 44.4% of the overall weight, which was still much higher than previously reported values [31].
Fig. 3.
TEM images of ODT-f-GNPs at the tips of MWCNTs without CDDP.
Fig. 4.
EDX spectrum of MWCNTs containing ODT-f-GNPs without CDDP. Al and Cu peaks could be attributed to the TEM supporting grid, which was made of aluminium and copper.
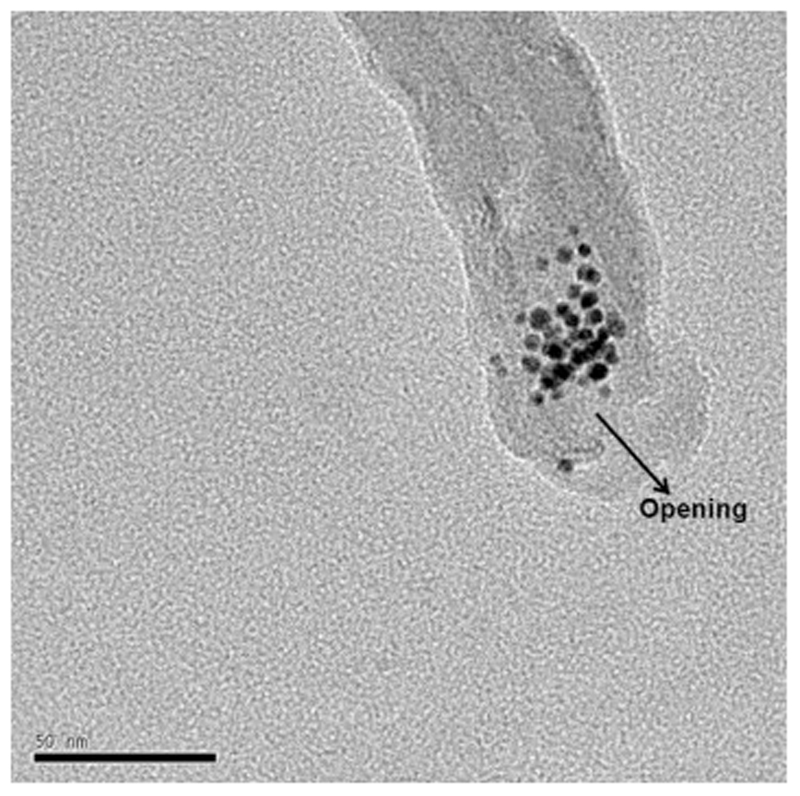
We also observed that ODT-f-GNPs did not completely block the MWCNT openings (Fig. 5, Fig. 6, Fig. 7). Considering that CDDP is less than 1 nm in diameter, we expected that it could still pass through the gaps formed among the ODT-f-GNPs to exit the MWCNTs. Their rate of exit, however, would be slower in comparison to uncapped MWCNTs since the exits were partially blocked. In other words, ODT-f-GNPs were expected to narrow the exit openings, resulting in a reduced rate of release.
Fig. 5.
TEM images of ODT-f-GNPs in MWCNT-CDDP. Red arrows denote the possible passage for the exit of encapsulated CDDP. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
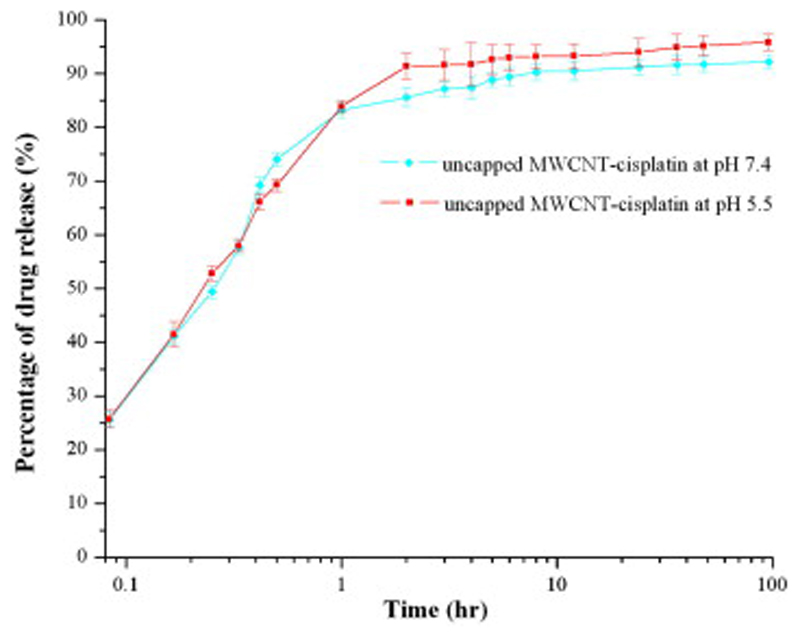
Fig. 6.
Plot showing release patterns of CDDP from uncapped MWCNTs at different pH.
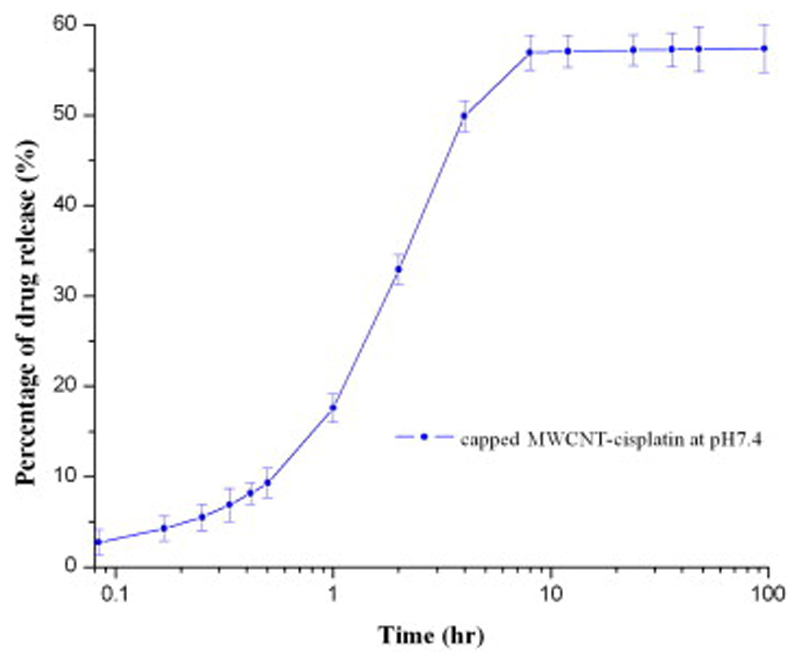
Fig. 7.
Plot showing release patterns of CDDP from capped MWCNTs at pH = 7.4.
3.2. In vitro release of CDDP from MWCNTs
Cancer needs an acidic and oxygen-poor environment to survive and flourish within. Therefore, the tumour environment has a remarkably lower pH in comparison to physiological conditions: for example, the lumen of recycling endosomes and trans-Golgi network in MCF-7 human breast cancer cell line has a pH of 6.0–6.3 and that of the lysosomes is as low as 4.9 [25]. Therefore, the rate of CDDP released from uncapped MWCNT-CDDP was quantified in pH environments mimicking tumoural (pH = 5.5) and physiological (pH = 7.4) conditions, and observed to be comparable (Fig. 6). Rapid release of encapsulated drugs occurred mainly within the first hour. Approximately 89% and 93% of encapsulated CDDP were released at pH 7.4 and 5.5 during 6 h, respectively. This was in agreement with Tripisciano et al., who reported that 95% of encapsulated CDDP was recovered from their formulation, although their drug release mainly occurred after 48 h [31]. The fast and almost complete release of CDDP from MWCNTs suggested that there was no significant interaction between encapsulated CDDP and the walls of MWCNTs. As the formulation was immersed in aqueous environment, water could enter and exit the MWCNTs freely. CDDP, being soluble in water and having higher affinity to water as compared to MWCNTs, could be displaced from MWCNTs as it would prefer to exist in an aqueous environment as opposed to being enclosed inside hydrophobic MWCNTs.
Since variations in pH did not affect drug release significantly, we only tested our capped MWCNT-CDDP in a medium that was similar to physiological conditions (Fig. 7). The presence of caps decreased the rate of CDDP release from MWCNTs sealed by ODT-f-GNPs especially in the first 1 h, although about 40% of CDDP still remained inside capped MWCNT-CDDP.
This result suggested that ODT-f-GNP caps reduced the release rate of CDDP. It is possible that the passage of exit was partially blocked and thus the encapsulated CDDP could not pass through this narrow exit opening freely, which also resulted in incomplete release extent. This is in agreement with the ODT-f-GNPs-filled MWCNTs observed under TEM. To that end, caps of different dimensions and material will be investigated. For example, pH-sensitive polymers that shrink only in case of acidic pH could represent a valuable alternative to our structurally-rigid GNPs.
In addition, due to C18-alkyl chains on the surface of the GNPs, these caps were hydrophobic in nature and insoluble in water. Therefore, we did not expect them to be displaced from the MWCNTs by water. To validate this hypothesis, we characterised the MWCNTs by TEM after the 72 h drug release experiment. Indeed, the ODT-f-GNPs remained at the tips of the MWCNTs, suggesting that indeed they were not removed (Fig. 8).
Fig. 8.
A TEM image showing ODT-f-GNPs caps remained at the tip of the nanotube after the in vitro drug release experiment. The grey layer surrounding the tube was a trace from the release medium.
3.3. Cell viability assay of MCF-7 cells incubated with capped and uncapped MWCNT-CDDP samples
Nine concentrations of free CDDP were initially chosen for the MTT assay against MCF-7 cells [27], [28]. The corresponding concentrations of uncapped MWCNT-CDDP and capped MWCNT-CDDP, and MWCNTs were calculated based on our results that CDDP content of MWCNT-CDDP were 62.1% and 44.44% for uncapped MWCNT-CDDP and capped MWCNT-CDDP, respectively.
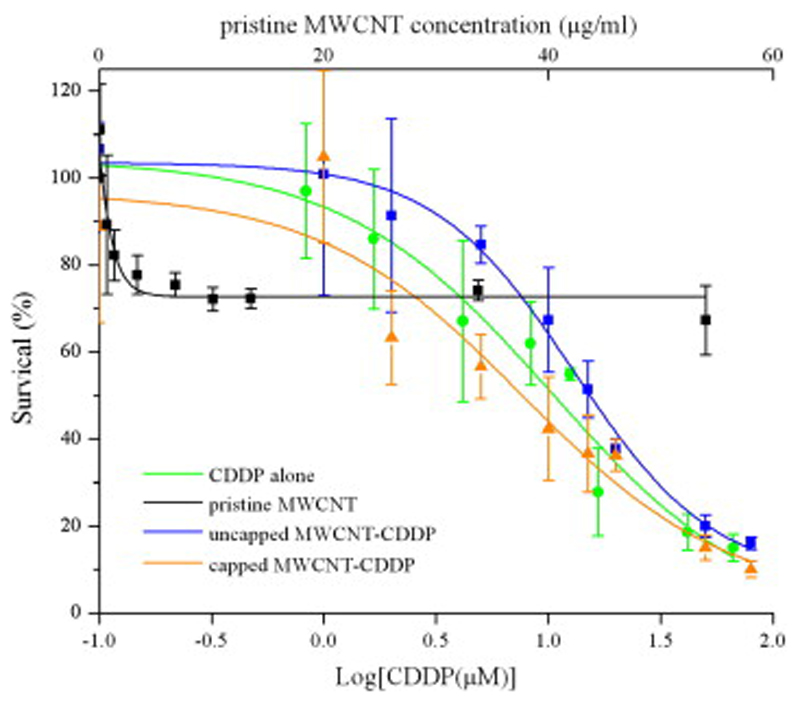
The results on cell viability are illustrated in Fig. 9. At lower concentrations, (0.1–1 μM) cell viability was maintained across the samples, suggesting that cells could tolerate these low doses of CDDP. We found in our previous study that several influencing factors are responsible for MWCNTs potential cytotoxicity, such as nanotubes length, surface functionalisation, purity and dispersibility [30]. Although ultrapure MWCNTs were used for entrapment of cisplatin and subsequent drug delivery, the lack of functionalisation still led to 30% reduction in cell viability for higher concentrations of ultrapure pristine MWCNTs (5–80 μM). This effect, most probably due to lower dispersibility of the nanotubes in aqueous media, remained quite constant across several concentrations of MWCNTs. On the basis of our cytotoxicity study, in future studies it will be possible to minimise such a decrease in cell viability by functionalising the surface of MWCNTs (e.g. through pegylation), which can also increase MWCNTs dispersibility.
Fig. 9.
Graph showing percentage of cell viability after 6 h treatment with nine different molar concentrations of CDDP and corresponding w/v concentration of MWCNTs alone and MWCNT-CDDP (capped and uncapped) on MCF7 cells as determined by MTT assay.
On the other hand, we observed a remarkable reduction in cell viability as the cells were exposed to higher concentrations of CDDP, uncapped MWCNT-CDDP, and capped MWCNT-CDDP. The IC50 of CDDP alone, uncapped MWCNT-CDDP, and capped MWCNT-CDDP were 11.74 μM, 12.92 μM, and 7.74 μM, respectively. Uncapped MWCNT-CDDP did not exhibit significant difference compared to free CDDP in terms of their pharmacological profile. This could be attributed to the findings of in vitro release experiment that around 90% of CDDP was leaked out from inner space of MWCNT by 2 h. These leaked CDDP would become the same as free CDDP in the medium and therefore no significant difference was observed in their IC50.
Importantly, as for the capped MWCNT-CDDP treatment, there was a slight left shift in the curve, indicating the pharmacological actions of CDDP had been augmented. In addition, considering the in vitro release findings of capped MWCNT-CDDP, only 50% of the encapsulated CDDP would be released within 6 h of drug treatment. Therefore, this infers that at 50% of survival level, only 50% of 7.74 μM of CDDP was available and thus the actual IC50 of capped MWCNT-CDDP would be less than 7.74 μM. Additionally, the enhanced pharmacological characteristic of encapsulated CDDP could stem from the role of capped MWCNTs as an effective drug depot. When the drug treatment solution was replaced with fresh growth medium at 6 h, it might be possible to postulate that if one cell internalises few capped MWCNT-CDDP and one of them was brought close to nucleus via intracellular trafficking, the drug depot would continue releasing CDDP beyond 6 h and this would enhance the cellular bioavailability of CDDP for its therapeutic action. Moreover, as capped MWCNT-CDDP slowly release their cargo, the freshly released CDDP would be able to exert its pharmacological effects while the un-released CDDP would be protected inside MWCNT against possible inactivations. Furthermore, the lack of a surge in the initial release in the capped MWCNT-CDDP might be advantageous as there would be less excessive reactive form of CDDP at the initial stage to attack other proteins and enzymes non-specifically and this might confer a decrease in unwanted side effects. Such enhanced pharmacological properties and the ability of decreasing or delaying tumour cell growth both in vitro and in vivo of CDDP in several nano-carriers has also been shown [32], [33], [34].
Overall, these findings supported the concept of employing MWCNTs as a drug delivery system. First, capped MWCNTs could act as a “depot” to store the drug and shielding it from the environment to enhance stability. Second, the encapsulated drug could be released at physiological pH with retention of its pharmacological activity. The results obtained at a dose as low as <7.74 μM suggest that it is possible to use less amount of drug, with concomitant lower side-effects. Also, current direct administration of high amount of drugs into cancer tissues is not possible as the drugs may not be able to penetrate the cancer cells fast enough. As a result, any unabsorbed drugs would be distributed to other tissues, causing toxicity. With MWCNTs, direct administration into cancer tissues may become feasible as MWCNTs have shown to penetrate cells [9]. Coupled with the possibility of adding targeting molecules on the external walls of MWCNTs, we can increase the specificity towards cancer cells. Another possible application is to anchor MWCNTs outside the cells, rather than letting them enter the cells. With “caps”, we can regulate the amount of drug released per unit time to be similar to the rate of drug taken up by cancer cells. Thus, we could prevent any excessive build-up of drug, which will result in the distribution of any unabsorbed drugs into surrounding tissues.
4. Conclusion
In this study, we have achieved a few important goals such as successful synthesis of “carbon nanotube bottle”, higher amount of drug loading inside the nanotubes in comparison to published literature, showing that “carbon nanotube bottle” does not prevent the release of encapsulated drug while improving its pharmacological effects. On the basis of these results, we could conclude that “carbon nanotube bottle” remains a promising alternative drug delivery system. Future investigations would focus on the use of caps that could be removed, rather than permanently blocking the tips of MWCNTs, under specific temperature or pH conditions [35], [36]. This would increase the specificity of anticancer therapy as encapsulated cancer drugs will only be released in acidic tumour tissues, but not in normal tissues.
Supplementary Material
Acknowledgements
This research has been supported by the National University of Singapore, Department of Pharmacy ((AcRF) Tier 1-FRC Grant R-148-000-129-112) and by MOE of Singapore (Grant MOE2009-T2-2-011, R-398-000-068-112). A.T. was funded in part by National Institute for Health and Research (Department of Health) NIHR L100. The authors G.P. and W.H. A. acknowledge support also by A*STAR SERC TSRP-Integrated Nano-Photo-Bio Interface grant (Project Number: 102 152 0016).
References
- 1].Bianco A, Sainz R, Li S, Dumortier H, Lacerda L, Kostarelos K, et al. Medicinal chemistry and pharmacological potential of fullerenes and carbon nanotubes series: carbon materials: chemistry and physics. Vol. 1. Springer Science + Business Media B.V.; 2008. Biomedical applications of functionalised carbon nanotubes; pp. 23–50. [Google Scholar]
- [2].Pastorin G. Carbon nanotubes: from bench chemistry to promising biomedical applications. 1st ed. Singapore: Pan Stanford Publishing Pte Ltd.; 2010. [Google Scholar]
- [3].Vashist SK, Zheng D, Pastorin G, Al-Rubeaan K, Luong JHT, Sheu F-S. Delivery of drugs and biomolecules using carbon nanotubes. Carbon. 2011;49(13):4077–97. [Google Scholar]
- [4].Avouris P. Nanotube electronics-electronics with carbon nanotubes. Phys World. 2007;20(3):40–5. [Google Scholar]
- [5].Avouris P, Chen Z, Perebeinos V. Carbon-based electronics. Nat Nanotechnol. 2007;2(10):605–15. doi: 10.1038/nnano.2007.300. [DOI] [PubMed] [Google Scholar]
- [6].Sinnott SB, Andrews R. Carbon nanotubes: synthesis, properties, and applications. Crit Rev Solid State. 2001;26(3):145–249. [Google Scholar]
- [7].Li J, Phua LC, Ho HK, Ren Y, Pastorin G. Thin films of functionalized multiwalled carbon nanotubes as suitable scaffold materials for stem cells proliferation and bone formation. ACS Nano. 2010;4(12):7717–25. doi: 10.1021/nn102738c. [DOI] [PubMed] [Google Scholar]
- [8].Pastorin G. Crucial functionalizations of carbon nanotubes for improved drug delivery: a valuable option? Pharm Res. 2009;26(4):746–69. doi: 10.1007/s11095-008-9811-0. [DOI] [PubMed] [Google Scholar]
- [9].Serag MF, Kaji N, Gaillard C, Okamoto Y, Terasaka K, Jabasini M, et al. Trafficking and subcellular localization of multiwalled carbon nanotubes in plant cells. ACS Nano. 2011;5(1):493–9. doi: 10.1021/nn102344t. [DOI] [PubMed] [Google Scholar]
- [10].Ajima K, Yudasaka M, Maigné A, Miyawaki J, Iijima S. Effect of functional groups at hole edges on cisplatin release from inside single-wall carbon nanohorns. J Phys Chem B. 2006;110(11):5773–8. doi: 10.1021/jp056813x. [DOI] [PubMed] [Google Scholar]
- [11].Foldvari M, Bagonluri M. Carbon nanotubes as functional excipients for nanomedicines: II drug delivery and biocompatibility issues. Nanomed Nanotech Biol Med. 2008;4(3):183–200. doi: 10.1016/j.nano.2008.04.003. [DOI] [PubMed] [Google Scholar]
- [12].Ren Y, Pastorin G. Incorporation of hexamethylmelamine inside capped carbon nanotubes. Adv Mater. 2008;20(11):2031–6. [Google Scholar]
- [13].O’Dwyer PJ, Stevenson JP, Johnson SW. Clinical status of cisplatin, carboplatin, and other platinum-based anti- tumour drugs. In: Lippert B, editor. Cisplatin: chemistry and biochemistry of a leading anticancer drug. Zurich (Switzerland), Weinheim (Germany): Verlag Helvetica Chimica Acta (VHCA), Wiley-VCH; 1999. pp. 29–69. [Google Scholar]
- [14].Daniel M-C, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104(1):293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- [15].Shon Y-S. Dekker encyclopedia of nanoscience and nanotechnology. Vol. 6 New York: Marcel Dekker, Inc.; 2004. Metal nanoparticles protected with monolayers: synthetic methods. [Google Scholar]
- [16].Thanh NTK, Green LAW. Functionalisation of nanoparticles for biomedical applications. Nano Today. 2010;5(3):213–30. [Google Scholar]
- [17].Vericat C, Vela ME, Benitez GA, Gago JAM, Torrelles X, Salvarezza RC. Surface characterization of sulfur and alkanethiol self-assembled monolayers on Au(1 1 1) J Phys: Condens Matter. 2006;18(48):R867–900. [Google Scholar]
- [18].Yeh J-M, Huang K-Y, Lin S-Y, Wu Y-Y, Huang C-C, Liou S-J. Noncovalent interaction between gold nanoparticles and multiwalled carbon nanotubes via an intermediatory. J Nanotech. 2009:1–7. [Google Scholar]
- [19].Yee CK, Jordan R, Ulman A, White H, King A, Rafailovich M, et al. Novel one-phase synthesis of thiol-fuctionalized gold, palladium, and iridium nanoparticles using superhydride. Langmuir. 1999;15(10):3486–91. [Google Scholar]
- [20].Cao C, Sim SJ. Preparation of highly stable oligo(ethylene glycol) derivatives-functionalized gold nanoparticles and their application in LSPR-based detection of PSA/ACT complex. J Nanosci Nanotechnol. 2007;7(11):3754–7. [PubMed] [Google Scholar]
- [21].Tielens F, Santos E. AuS and SH bond formation/breaking during the formation of alkanethiol SAMs on Au(1 1 1): a theoretical study. J Phys Chem C. 2010;114(20):9444–52. [Google Scholar]
- [22].Yudasaka M, Ajima K, Suenaga K, Ichihashi T, Hashimoto A, Iijima S. Nano-extraction and nano-condensation for C60 incorporation into single-wall carbon nanotubes in liquid phases. Chem Phys Lett. 2003;380(1–2):42–6. [Google Scholar]
- [23].Rakhi RB, Sethupathi K, Ramaprabhu S. Synthesis and hydrogen storage properties of carbon nanotubes. Int J Hydrogen Energ. 2008;33(1):381–6. [Google Scholar]
- [24].Chiang IW, Brinson BE, Smalley RE, Margrave JL, Hauge RH. Purification and characterization of single-wall carbon nanotubes. J Phys Chem B. 2001;105(6):1157–61. [Google Scholar]
- [25].Simon SM. Role of organelle pH in tumor cell biology and drug resistance. Drug Discov Today. 1999;4(1):32–8. doi: 10.1016/s1359-6446(98)01276-8. [DOI] [PubMed] [Google Scholar]
- [26].Sledge GW, Loehrer PJ, Roth BJ, Einhorn LH. Cisplatin as first- line therapy for metastatic breast-cancer. JCO. 1988;6(12):1811–4. doi: 10.1200/JCO.1988.6.12.1811. [DOI] [PubMed] [Google Scholar]
- [27].Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- [28].Plumb JA. Cell sensitivity assays: the MTT assay. Meth Mol Med. 1999;28:25–30. doi: 10.1385/1-59259-687-8:25. [DOI] [PubMed] [Google Scholar]
- [29].Tosa N, Olenic L, Bratu I, Turdeanu R, Turcu I. Infrared and UV–vis spectroscopic study of 3,7,10-substituted- phenothiazine derivatives adsorbed on gold nanoparticles. J Phys: Conf Ser. 2009;182 012019. [Google Scholar]
- [30].Nayak TR, Leow PC, Ee P-LR, Arockiadoss T, Ramaprabhu S, Pastorin G. Crucial parameters responsible for carbon nanotubes toxicity. Curr Nanosci. 2010;6(2):141–54. [Google Scholar]
- [31].Tripisciano C, Kraemer K, Taylor A, Borowiak-Palen E. Single- wall carbon nanotubes based anticancer drug delivery system. Chem Phys Lett. 2009;478(4–6):200–5. [Google Scholar]
- [32].Wada R, Hyon S-H, Nakamura T, Ikada Y. In vitro evaluation of sustained drug release from biodegradable elastomer. Pharm Res. 1991;8(10):1292–6. doi: 10.1023/a:1015860030772. [DOI] [PubMed] [Google Scholar]
- [33].Basma H, El-Refaey H, Sgagias MK, Cowan KH, Luo X, Cheng P-W. BCL-2 antisense and cisplatin combination treatment of MCF-7 breast cancer cells with or without functional p53. J Biomed Sci. 2005;12(6):999–1011. doi: 10.1007/s11373-005-9025-y. [DOI] [PubMed] [Google Scholar]
- [34].Xiao C, Qi X, Maitani Y, Nagai T. Sustained release of cisplatin from multivesicular liposomes: potentiation of antitumor efficacy against S180 murine carcinoma. J Pharm Sci. 2004;93(7):1718–24. doi: 10.1002/jps.20086. [DOI] [PubMed] [Google Scholar]
- [35].Sethuraman VA, Lee MC, Bae YH. A biodegradable pH- sensitive micelle system for targeting acidic solid tumors. Pharm Res. 2008;25(3):657–66. doi: 10.1007/s11095-007-9480-4. [DOI] [PubMed] [Google Scholar]
- [36].Ewesuedo RB, Ratain MJ. Systemically administered drug. In: Brown DM, editor. Drug delivery systems in cancer therapy. New Jersey: Humana Press Inc.; 2004. pp. 3–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.