Abstract
Objective
Persistent renal damage (RD) three months after exposure to contrast media is associated with contrast-induced acute kidney injury (CI-AKI) and poor clinical outcomes. Little is known about the role of preprocedural hydration on persistent RD in patients with chronic kidney disease [CKD; estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2] undergoing percutaneous coronary intervention (PCI). We therefore examined the use of preprocedural hydration to decrease the incidence of persistent RD.
Methods
Between 2012 and 2015, 1,230 consecutive patients undergoing PCI, except for patients with an eGFR ≥60 mL/min/1.73 m2, on dialysis, having acute myocardial infarction, or recently having started renin-angiotensin inhibitors, were screened (n=333). Before their index PCI, the 12-h saline group (n=103) received 1 mL/kg/h 0.9% sodium chloride for 12 hours, and the 1-h bicarbonate group (n=63) received 3 mL/kg 154 mEq/L sodium bicarbonate for 1 hour. The control group (n=167) received no pre-procedural hydration. The study outcome of kidney function decline was investigated using the percent-change (%-change) of the calculated creatinine clearance between the baseline value and the lowest value recorded three to six months after index PCI.
Results
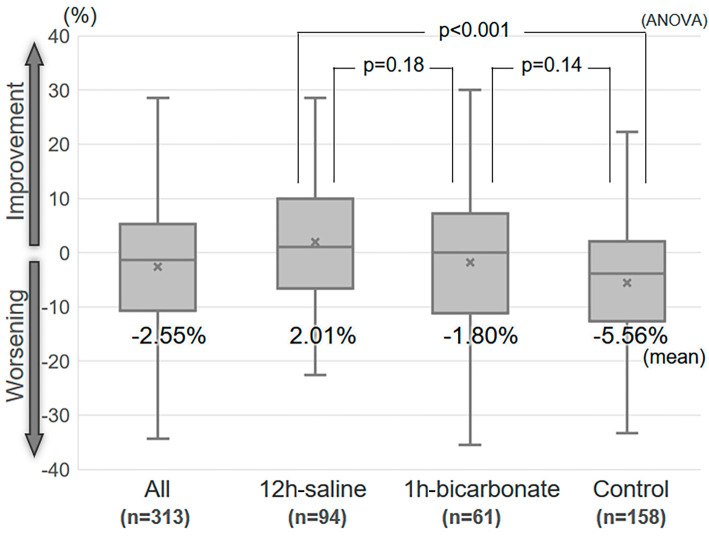
There was less renal function deterioration in the saline group than in the control group, and the bicarbonate group showed deterioration similar to the other groups (%-change; 12-h saline 2.0±11.3% vs. control −5.6±12.6%, p<0.001; vs. 1-h bicarbonate −1.8±14.1%, p=0.18; 1-h bicarbonate vs. control, p=0.14 ANOVA). A multiple regression analysis adjusted for risk factors for persistent RD showed that saline hydration correlated independently with a higher %-change (r=0.262, p<0.001).
Conclusion
Preprocedural 12-h saline may be better than no preprocedural hydration in preventing mid-term renal insufficiency in CKD patients undergoing PCI.
Keywords: contrast-induced nephropathy, acute kidney injury, persistent renal damage, contrast media, percutaneous coronary intervention, chronic kidney disease
Introduction
Contrast-induced acute kidney injury (CI-AKI) three days following contrast media exposure and persistent renal damage (RD) three months after exposure correlate with poor clinical outcomes in patients undergoing percutaneous coronary intervention (PCI) (1, 2). Transient RD is reported in 81% of CI-AKI patients, but the baseline renal function is restored by 3 months after PCI. However, 19% of CI-AKI patients and 1.0% of non-CI-AKI patients develop persistent RD (2). Although persistent RD is less common in non-CI-AKI patients than in CI-AKI, patients with a history of PCI are susceptible to repeat diagnostic and therapeutic procedures that require contrast media (29-44%), and a lower baseline kidney function has been reported as a risk factor for adverse outcomes (3-5). Therefore, preventing renal function deterioration is important for patients undergoing PCI.
Isotonic (0.9%) saline preprocedural hydration, sodium bicarbonate (154 mEq/L) preprocedural hydration, and furosemide diuresis with matched hydration have been reported to prevent CI-AKI. However, preprocedural hydration is still controversial owing to differences in patient background characteristics as well as differences in emergent versus elective procedures, therapeutic versus diagnostic procedures, and intra-arterial versus venous procedures (6-11).
PCI is considered a high-risk cause of CI-AKI (1). Isotonic saline preprocedural hydration is recommended by the current American College of Cardiology/American Heart Association (ACC/AHA, 2011) and European Society of Cardiology (ESC, 2014) PCI guidelines (12, 13), and both isotonic saline and sodium bicarbonate preprocedural hydration are recommended in the local 2012 Japanese guidelines (14). Unfortunately, few data support the use of hydration to prevent persistent RD in patients with chronic kidney disease (CKD).
The objective of the present study was to compare the efficacy of preprocedural hydration with isotonic saline or sodium bicarbonate versus no hydration (control) for prophylaxis of persistent RD in CKD patients undergoing PCI.
Materials and Methods
Population
This study was a retrospective, single-center, observational study. Analyses were done per-protocol (Fig. 1). From January 2012 to April 2015, 1,230 consecutive patients underwent PCI for ischemic heart disease at a single cardiovascular center (Fukuyama Cardiovascular Hospital); 698 of the 1,230 patients (56.7%) were identified as having CKD [defined for Japanese as an estimated glomerular filtration rate (15) (eGFR) <60 mL/min/1.73 m2] and were selected for the study. Patients with end-stage renal failure requiring dialysis (n=45), patients with acute myocardial infarction (n=233), and patients newly started on renin-angiotensin inhibitors (angiotensin-converting enzyme inhibitors, angiotensin-II receptor blockers, or mineralocorticoid receptor antagonists) (n=87) were excluded (16-18). From May 2013 to June 2014, CKD patients routinely received 0.9% sodium chloride at a rate of 1 mL/kg/h for 12 hours before and after index PCI (6). During the study period, 31 patients received no hydration because of either clinical emergencies or missing hydration and were excluded from the analysis, leaving 103 patients in the 12-h saline group. From July 2014 to April 2015, CKD patients instead received sodium bicarbonate (154 mEq/L in dextrose and water) at a rate of 3 mL/kg for 1 hour before PCI, and at 1 mL/kg/h for 6 hours after PCI (8). Six patients actually received no hydration and were excluded (1-h bicarbonate group, n=63). From January 2012 to April 2013, 167 CKD patients received postprocedural 0.9% sodium chloride hydration at a rate of 1 mL/kg/h for 12 hours after index PCI, with no patient receiving preprocedural hydration. The 12-h saline and 1-h bicarbonate groups were compared with the control group using an on-treatment analysis. Twenty patients with missing creatinine values between 3-6 months were excluded from the analyses (313/333, follow-up rate 94.0%).
Figure 1.
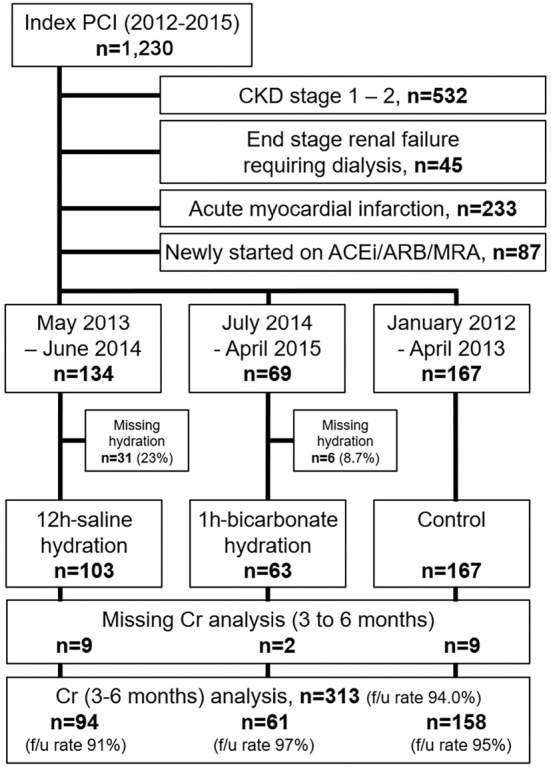
Study flow. PCI: percutaneous coronary intervention, CKD: chronic kidney disease, defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2, ACEi: angiotensin-converting enzyme inhibitor, ARB: angiotensin-II receptor blocker, MRA: mineralocorticoid receptor antagonist, 12h-saline: pre-procedural saline hydration group at a rate of 1 mL/kg/h for 12 hours before PCI, 1h-bicarbonate: pre-procedural sodium bicarbonate hydration group at a rate of 3 mL/kg/h for 1 hour before PCI, Cr: serum creatinine, f/u: follow-up
In all cases, iohexol (350 mg・I/mL, Omnipaque; Daiichi-Sankyo Pharmaceutical, Tokyo, Japan), a nonionic, low-osmolar contrast medium, was used during PCI.
Age, body mass index, systolic blood pressure, diastolic blood pressure, and left ventricular ejection fraction (LVEF) by echocardiographic evaluations were obtained on the day of admission. Laboratory test findings, including serum creatinine (Cr), hematocrit, random blood glucose, hemoglobin A1c (HbA1c), total cholesterol, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), triglycerides, uric acid, and proteinuria on a dipstick urinalysis, were assessed the day before angiography (pre-hydration). Cr was measured again at three to six months from the time of follow-up after index PCI. All tests were carried out in our hospital laboratory with consistent methodology.
Clinical and procedural data were recorded prospectively for all patients in a dedicated database. The study protocol was approved by the regional ethics committee. All patients gave their informed consent.
Endpoint and clinical definitions
The primary endpoint was the severity of persistent RD. In this study, the severity of persistent RD was assessed by the percent-change (%-change) in the calculated creatinine clearance (CC) based on the baseline creatinine value and the highest value during the three to six months after index PCI. A negative value for the %-change was considered to indicate renal functional deterioration. The CC was assessed by the Cockcroft-Gault formula: (19) (140-age in years) × body weight in kg/(72×Creatinine in mg/dL) adjusted by sex (×0.85 in female gender). The baseline CC was calculated from the baseline Cr. The lowest value of the CC was calculated from the highest creatinine value during the three to six months after index PCI.
The eGFR was estimated using the equation for Japanese patients (15), and classified into the following CKD stage ranges: ≥60 mL/min/1.73 m2 (stage 1 or 2), 45-59 mL/min/1.73 m2 (stage 3A), 30-44 mL/min/1.73 m2 (stage 3B), 15-29 mL/min/1.73 m2 (stage 4), and <15 mL/min/1.73 m2 (stage 5), according to the Kidney Disease Outcomes Quality Initiative. Anemia was defined as a baseline hematocrit <39% for men and <36% for women. Proteinuria was defined as ≥1+ protein on a baseline dipstick urinalysis. Periprocedural hypotension was defined as a systolic blood pressure <80 mmHg for at least 1 hour requiring an inotropic agent or intra-aortic balloon pump (IABP) within 24 hours periprocedurally. Advanced congestive heart failure was defined as a New York Heart Association (NYHA) functional class III or IV. The contrast nephropathy risk score was calculated as specified by Mehran et al. (1) Angiotensin-converting enzyme inhibitors (ACEis) and/or angiotensin-II receptor blockers (ARBs) and loop diuretics were noted if they had been started at least three months before index PCI and continued during follow-up. Unstable angina was defined as either new-onset exertional angina within the preceding two months (Canadian Cardiovascular Society Class III), or angina at rest with no evidence of myonecrosis or ST elevation. Multivessel disease was defined as ≥2 diseased epicardial coronary arteries with a diameter stenosis of ≥50% by visual estimation.
Statistical analyses
Data are presented as the mean±standard deviation for continuous variables and as counts and percentages for categorical variables. Multiple comparisons were made among three or more groups using a one-way analysis of variance (ANOVA) with the Scheffe post-hoc test for continuous variables and the chi-square test for categorical variables. Linear regression models require only two subjects per variable (20), univariate and multivariate regression analyses were performed to identify the variables that predicted a %-change in creatinine clearance. Glucose, triglyceride, contrast volume, and contrast volume-to-creatinine clearance ratio were log-converted to fit the regression analyses. The multivariate regression analysis included known and persistent risk factors for RD (LVEF and the nephropathy risk score) (2) and hydration method (12-h saline or 1-h bicarbonate). The statistical analyses were performed using the Statview software program, version 5.0 (SAS Institute, Cary, USA) and JMP version 8 (SAS Institute). P values less than 0.05 were considered significant.
Results
Clinical characteristics
Baseline clinical, laboratory, and procedural characteristics of the study patients are shown in Table 1. The number of patients with unstable angina in the 12-h saline group was lower than that in the other groups [12-h saline vs. 1-h bicarbonate vs. control, 2/94 (2.1%) vs. 12/61 (20%) vs. 20/158 (13%), respectively, p=0.002]. IABPs were used more often in the 1-h bicarbonate group than in the other groups [12-h saline vs. 1-h bicarbonate vs. control, 0/94 (0%) vs. 3/61 (4.9%) vs. 1/158 (0.6%), respectively, p=0.017], but IABP use occurred in settings other than periprocedural hypotension (e.g., unprotected left main disease). Periprocedural hypotension was observed in 1/94 (1.1%) in the 12-h saline group, 0/61 (0%) in the 1-h bicarbonate group, and 3/158 (1.9%) in the control group. The systolic blood pressure in the control group was higher than in the 1-h bicarbonate group (1-h bicarbonate vs. control, 130±20 vs. 143±25 mmHg, respectively, p=0.001). Other variables did not significantly differ between groups. No patients developed congestive heart failure in either the 12-h saline or 1-h bicarbonate groups.
Table 1.
Baseline Clinical, Laboratory, and Procedural Characteristics.
| All (n=313) |
12-h saline (n=94) |
1-h bicarbonate (n=61) |
Control (n=158) |
p value | |||
|---|---|---|---|---|---|---|---|
| 12-h saline vs. control |
1-h bicarbonate vs. control |
12-h saline vs. 1-h bicarbonate |
|||||
| Age, (yr) | 72.9±8.2 | 73.8±7.4 | 73.5±9.1 | 72.2±8.3 | 0.32 | 0.58 | 0.97 |
| Female, n (%) | 88 (28%) | 24 (26%) | 17 (28%) | 47 (30%) | 0.89 | ||
| Body mass index, (kg/m2) | 25.0±3.7 | 25.3±3.6 | 25.0±4.1 | 24.8±3.6 | 0.65 | 0.94 | 0.93 |
| Smoking, n (%) | 150 (48%) | 45 (48%) | 29 (48%) | 76 (48%) | 1.00 | ||
| LVEF, (%) | 59.6±11.0 | 59.3±11.2 | 61.0±10.9 | 59.2±11.0 | 1.00 | 0.54 | 0.64 |
| Unstable angina, n (%) | 34 (11%) | 2 (2.1%) | 12 (20%) | 20 (13%) | 0.002# | ||
| Multi-vessel disease, n (%) | 136 (43%) | 39 (41%) | 27 (44%) | 70 (44%) | 0.90 | ||
| NYHA functional class III-IV, n (%) | 9 (2.9%) | 2 (2.1%) | 2 (3.3%) | 5 (3.2%) | 0.87 | ||
| Periprocedural hypotension, n (%) | 4 (1.3%) | 1 (1.1%) | 0 (0%) | 3 (1.9%) | 0.52 | ||
| Intra-aortic balloon pump, n (%) | 4 (1.3%) | 0 (0%) | 3 (4.9%) | 1 (0.6%) | 0.017# | ||
| ACEi/ARB, n (%) | 220 (70%) | 67 (71%) | 43 (70%) | 110 (70%) | 0.96 | ||
| Loop diuretics, n (%) | 81 (26%) | 21 (22%) | 12 (20%) | 48 (30%) | 0.17 | ||
| Hematocrit, (%) | 38.1±5.1 | 39.0±4.8 | 37.9±4.5 | 37.6±5.5 | 0.11 | 0.92 | 0.44 |
| Anemia, n (%) | 158 (50%) | 44 (47%) | 33 (54%) | 81 (51%) | 0.65 | ||
| Systolic blood pressure, (mmHg) | 139±23 | 139±21 | 130±20§ | 143±25§ | 0.43 | 0.001§ | 0.05 |
| Diastolic blood pressure, (mmHg) | 71±13 | 69±11 | 73±14 | 72±13 | 0.15 | 0.84 | 0.11 |
| Glucose, (mg/dL) | 140±58 | 141±57 | 141±63 | 139±58 | 0.98 | 0.98 | 1.00 |
| HbA1c, (%) | 6.35±0.94 | 6.34±0.81 | 6.36±0.92 | 6.36±1.03 | 0.99 | 1.00 | 0.99 |
| Total cholesterol, (mg/dL) | 171±37 | 169±35 | 170±32 | 173±41 | 0.81 | 0.92 | 0.99 |
| HDL-cholesterol, (mg/dL) | 45.3±11.3 | 45.3±12.2 | 47.4±11.0 | 44.5±10.8 | 0.87 | 0.24 | 0.52 |
| LDL-cholesterol, (mg/dL) | 100±32 | 99±30 | 99±26 | 100±35 | 0.98 | 0.97 | 1.00 |
| Triglyceride, (mg/dL) | 143±77 | 146±78 | 141±74 | 143±78 | 0.97 | 0.98 | 0.93 |
| Uric acid, (mg/dL) | 6.0±1.4 | 6.0±1.4 | 6.0±1.2 | 6.1±1.4 | 1.00 | 0.98 | 0.99 |
| Creatinine, (mg/dL) | 1.14±0.35 | 1.14±0.35 | 1.11±0.32 | 1.15±0.37 | 0.98 | 0.73 | 0.85 |
| eGFR, (mL/min/1.73 m2) | 48.2±10.2 | 47.9±9.0 | 49.3±9.9 | 48.0±11.0 | 1.00 | 0.70 | 0.71 |
| CKD stage | 0.55 | ||||||
| stage 3A (60>eGFR≥45), n (%) | 226 (72%) | 72 (77%) | 47 (77%) | 107 (68%) | |||
| stage 3B (45>eGFR≥30), n (%) | 64 (20%) | 17 (18%) | 10 (16%) | 37 (23%) | |||
| stage 4 (30>eGFR≥15), n (%) | 21 (6.7%) | 5 (5.3%) | 3 (4.9%) | 13 (8.2%) | |||
| stage 5 (15>eGFR), n (%) | 2 (0.6%) | 0 (0%) | 1 (1.6%) | 1 (0.6%) | |||
| Proteinuria (1+≥ on dipstick test), n (%) | 44 (14%) | 12 (13%) | 9 (15%) | 23 (15%) | 0.91 | ||
| Contrast volume, (mL) | 115±59 | 111±58 | 111±52 | 118±61 | 0.66 | 0.75 | 1.00 |
| Contrast volume- to- creatinine clearance ratio | 2.5±1.9 | 2.3±1.3 | 2.5±2.0 | 2.7±2.2 | 0.24 | 0.81 | 0.75 |
| Contrast nephropathy risk score | 7.58±3.76 | 7.36±3.45 | 7.84±3.26 | 7.61±4.11 | 0.88 | 0.92 | 0.75 |
Data are presented as mean value±standard deviation, and categorical data as number (%) of patients. Continuous variables were compared using sheffe’s test, and categorical variables were compared using the chi-square test. # chi-square test, § 1-h bicarbonate vs. control, using Sheffe’s test (ANOVA).
12-h saline: pre-procedural saline hydration group at a rate of 1mL/kg/h for 12 hours before PCI, 1-h bicarbonate: pre-procedural sodium bicarbonate hydration group at a rate of 3 mL/kg/h for 1 hour before PCI, LVEF: left ventricular ejection fraction, NYHA: New York Heart Association, ACEi: angiotensin converting enzyme inhibitor, ARB: angiotensin-II receptor blocker, HbA1c: hemoglobin A1c, PCI: percutaneous coronary intervention, saline: isotonic sodium chloride. IABP: intra-aortic balloon pump, HDL: high density lipoprotein, LDL: low density lipoprotein, eGFR: estimated glomerular filtration rate
Changes in the renal function
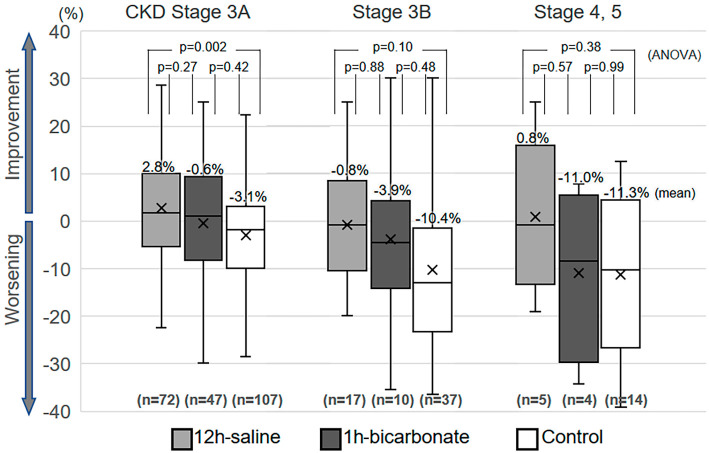
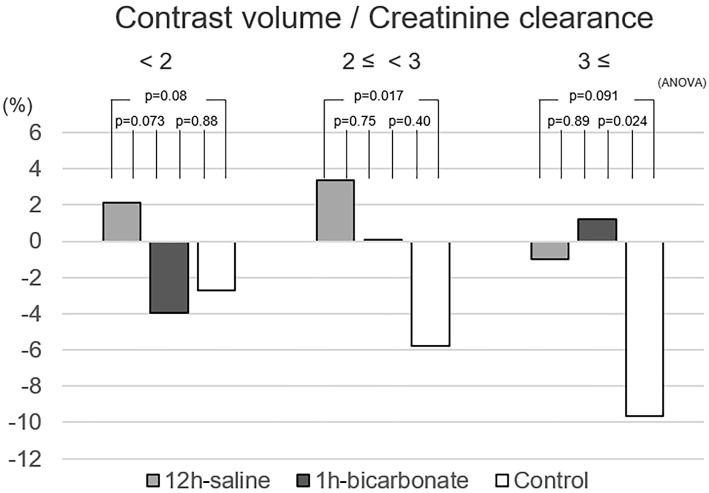
The %-change among all patients showed a Gaussian distribution (Shapiro-Wilk test, p=0.16). The percentage of patients who developed severe renal functional deterioration (%-change ≥25%) was 5.4% (17/313) in all patients, 0% (0/94) in the 12-h saline group, 6.6% (4/61) in the 1-h bicarbonate group, and 8.2% (13/158) in the control group (p=0.019, chi-square test). Box plots of the %-change between the baseline CC and the lowest CC during 3 to 6 months of follow-up (follow-up day, median 175 days, range 90 to 190 days) are shown in Fig. 2. The %-change in the 12-h saline group was significantly higher than in the control group (12-h saline vs. control, 2.01±11.30% vs. −5.56±12.55%, p<0.001, ANOVA). The %-change in the 1-h bicarbonate group was similar to that in other groups (−1.80±14.05%, vs. 12-h saline p=0.18, vs. control p=0.14). Fig. 3 shows box plots of the %-change divided by the CKD stage. The 12-h saline group had a higher mean %-change than the control group in patients with CKD stage 3A (12-h saline vs. control, 2.76±10.44% vs. −3.13±10.41%, p=0.002). The relationship between the %-change and contrast volume-to-CC ratio is shown in Fig. 4. The 12-h saline group had a higher mean %-change than the control group in the intermediate (2 to <3) risk contrast volume-to-CC ratio category (12-h saline vs. control, 3.36±13.12% vs. −5.75±12.55%, p=0.017), while the 1-h bicarbonate group had a higher %-change than the control group in the high (≥3) risk ratio category (1-h bicarbonate vs. control, 1.24±14.07% vs. −9.63±14.03%, p=0.024).
Figure 2.
Box plots of the percent-change in creatinine clearance between the baseline and the lowest value in the three to six months after index PCI. The box plots depict the percent-change in each group. The upper lines represent the 75th percentile, the middle lines the 50th percentile, and the lower lines the 25th percentile. The upper and lower whiskers represent the upper and lower limits. ‘×’ indicates the mean value. PCI: percutaneous coronary intervention, ANOVA: analysis of variance
Figure 3.
Box plots of the percent-change divided by the CKD stage. The box plots depict the percent-change divided by the CKD stage. PCI: percutaneous coronary intervention, CKD: chronic kidney disease, defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2, ANOVA: analysis of variance
Figure 4.
The relationship between the percent-change and contrast volume-to-calculated creatinine clearance ratio in the various hydration methods. ANOVA: analysis of variance
Regression analyses for the %-change in the CC
In univariate models, the %-change was positively correlated with the LVEF, hematocrit, eGFR, and 12-h saline hydration and negatively correlated with age, female sex, NYHA III-IV, loop diuretics, SBP, proteinuria, contrast volume-to-creatinine clearance ratio, and contrast nephropathy risk score (Table 2). In the multivariate model, the %-change correlated positively with 12-h saline and 1-h bicarbonate hydration and negatively with the contrast nephropathy score after adjustment for known persistent risk factors for RD.
Table 2.
Regression Analyses for the %-change in the CC.
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Coefficient | p | Coefficient | p | ||
| Age, (yr) | -0.188 | 0.0008 | |||
| Female | -0.114 | 0.0430 | |||
| LVEF, (%) | 0.125 | 0.0267 | 0.101 | 0.0601 | |
| NYHA functional class III-IV | -0.218 | 0.0001 | |||
| Loop diuretics | -0.179 | 0.0014 | |||
| Hematocrit, (%) | 0.133 | 0.0187 | |||
| Systolic blood pressure, (mmHg) | -0.158 | 0.0051 | |||
| eGFR, (mL/min/1.73 m2) | 0.185 | 0.0010 | |||
| Proteinuria (≥1+, on dipstick test) | -0.181 | 0.0013 | |||
| Contrast volume- to- creatinine clearance ratio ¶ | -0.175 | 0.0018 | |||
| Contrast nephropathy risk score | -0.238 | <0.0001 | -0.222 | <0.0001 | |
| 12-h saline hydration | 0.232 | <0.0001 | 0.262 | <0.0001 | |
| 1-h sodium bicarbonate hydration | 0.029 | 0.6110 | 0.114 | 0.0430 | |
Multivariate model included LVEF, contrast nephropathy score, 12 hours of saline hydration, and 1 hour of sodium bicarbonate hydration. ¶ log conversion to fit regression analyses.
LVEF: left ventricular ejection fraction, NYHA: New York Heart Association, eGFR: estimated glomerular filtration rate
Discussion
This study investigated the preventative effect of preprocedural hydration before PCI on mid-term (3-6 months, median 175 days) persistent RD after exposure to contrast media. The main findings were as follows: (i) 0.9% sodium chloride hydration at a rate of 1 mL/kg/h for 12 hours before and after index PCI showed protective effects on the development of persistent RD, and 154 mEq/L sodium bicarbonate at a rate of 3 mL/kg for 1 hour before and 1 mL/kg/h for 6 hours after index PCI showed no protective effect after adjustment for other clinical predictors, and (ii) the contrast nephropathy score, including the baseline age, renal function, advanced heart failure, anemia, diabetes mellitus, periprocedural hypotension, IABP, and contrast volume, was an independent predictor of persistent RD. This result was consistent with the findings of a previous study (2).
Hydration method
This study was originally conducted to evaluate the effect of 1 hour of bicarbonate prehydration on CI-AKI and persistent RD in patients with acute coronary syndrome, who usually receive insufficient prehydration. A meta-analysis showed that 1 hour of bicarbonate prehydration without N-acetylcysteine decreased the risk of CI-AKI compared with that observed with saline prehydration (21). In recent studies, the combined use of sodium bicarbonate and N-acetylcysteine showed no superiority to sodium bicarbonate alone for the prevention of CI-AKI (10, 22), and the ACC/AHA and ESC PCI guidelines suggest that N-acetylcysteine has no benefit (12, 13). We therefore started 1 hour of bicarbonate prehydration without saline prehydration or N-acetylcysteine to prevent persistent RD in July 2014. In a previous study of persistent RD, all patients received preprocedural hydration, but differences between hydration methods were not mentioned (2). Interestingly, 1 hour of bicarbonate was administered to more patients than 12 hours of saline [12-h saline 2.1% (2/94) vs. 1-h bicarbonate 20% (12/61), p=0.002] and showed a protective effect only in the high-risk contrast volume-to-CC ratio (≥3) category (Fig. 4). The use of bicarbonate hydration offers several possibilities as a preventative preprocedural method of hydration in order to improve the renal function, especially in the high-risk category (contrast volume-to-CC ratio >3), but showed no preventative effect on persistent RD in the regression analysis.
A recent prospective randomized study (AMACING study) showed no beneficial effect of preprocedural saline hydration on CI-AKI or the 30-day renal functional deterioration, but 52% of study participants received a short hydration protocol (4 hours before and after procedures), and the study included lower-risk patients (52% underwent intra-venous contrast administration and 85% underwent non-therapeutic procedures) (11). Therefore, preprocedural hydration may not always be necessary, especially in low-risk patients, such as those with a higher eGFR and those undergoing intravenous or diagnostic procedures. A long saline hydration protocol (12 hours before and after procedures) may be preferable for CKD patients undergoing PCI in order to prevent CI-AKI and persistent RD (7).
Hydration for elective cases
The data in the present study were analyzed in a per-protocol manner. In the 12-h saline group 23% of patients (31/134) received no hydration and were excluded, while the proportion of patients with unstable angina in the 12-h saline group was lower than in the 1-h bicarbonate and control groups [2.1% (2/94) vs. 20% (12/61) vs. 13% (20/158), respectively, p=0.002]. There was therefore a potential selection bias in the 12-h saline group. Subgroup analyses were performed after excluding unstable angina patients (12-h saline n=92, 1-h bicarbonate n=49, and control n=138). Even in the elective cases, there was less deterioration in the renal function in the saline group than in the control group, while the bicarbonate group showed a level of deterioration similar to the other groups (%-change 12-h saline 1.9±11.4% vs. control −5.9±12.5%, p<0.001; vs. 1-h bicarbonate −2.1±13.3%, p=0.18; 1-h bicarbonate vs. control, p=0.18 ANOVA). A univariate regression analysis demonstrated that 12 hours of saline hydration correlated positively with the %-change (r=0.252, p<0.001), while 1 hour of bicarbonate did not (r=0.020, p=0.74). A multiple regression analysis adjusted for persistent risk factors for RD also showed that 12 hours of saline hydration correlated independently with a higher %-change (r=0.285, p<0.001). Although 12 hours of saline hydration may be effective in elective cases, the optimal hydration method for protecting the mid- to long-term renal function in emergent cases remains unclear.
Risk factors for persistent RD
Patients with higher contrast nephropathy risk scores as specified by Mehran et al. (1) were vulnerable to both CI-AKI and persistent RD. A lower EF was shown to be a risk factor only in patients with persistent RD (2). In our study, a lower EF was correlated with persistent RD only in the univariate model. Furthermore, the diabetic parameters (blood glucose and HbA1c), and one of the contrast nephropathy score factors, did not correlate with persistent RD, even in a univariate model. As previously reported (2), an unclear relationship in the regression analyses was observed between persistent RD and the contrast volume. The mean volume of contrast administered in this study was 115 mL, which is about half of the contrast volume (261 mL) administered in the contrast nephropathy score study reported in 2004 (1). This smaller volume of contrast may be one reason for the unclear relationship we observed. Patients with a higher nephropathy score should therefore receive optimal therapy during contrast exposure, including 12 hours of saline hydration before contrast exposure, even those with milder stages of CKD.
Assessment of renal functional deterioration after index PCI
Early reports defined persistent RD as a decline in the CC of 25% between baseline and follow-up, and the incidence of a decline ≥25% was 2.7% (40/1,477) (2). In our study, the incidence of persistent RD was too low [incidence of decline ≥25=5.4% (17/313)] to carry out a logistic multiple regression analysis. However, the %-change showed a normal distribution, and the linear regression models required only two subjects per variable for optimal estimation (20). Even a small decrease in the CC is associated with adverse outcomes in various situations (23, 24). Thus, in the present study, the severity of persistent RD was assessed by the %-change.
The relative change in the CC was used to assess persistent deterioration in the renal function. The absolute change in the serum creatinine value takes advantage of faster recognition after acute kidney injury, especially in advanced CKD patients. However, in the later phases after kidney injury, the absolute change in advanced CKD patients reflects a smaller %-change of CC than in patients with normal to mild CKD (23). The relative change seems to take advantage of the linear relationship with the cardiac prognosis after CI-AKI (25). It is still unclear as to which is the best predictor of a persistent decline in the renal function, namely either absolute or relative change, or the serum creatinine value or calculated creatinine clearance.
Limitations
Several limitations associated with the present study warrant mention. First, this was a single-center, retrospective study. Second, hydration may only be appropriate for patients without heart failure, or well-controlled heart failure, and there may have been selection biases in the hydration groups. Finally, patients undergoing saline hydration may have been more stable than either bicarbonate hydration or no hydration, without the need for emergent PCI. A study population without emergent cases is needed to clarify the effect of 12 hours of saline hydration, while a study population comprising only emergent cases is needed to clarify the effect of 1 hour of bicarbonate hydration on persistent RD.
Conclusion
Hydration with 0.9% saline 12 hours before and after PCI, as recommended by current guidelines, may help prevent renal dysfunction in patients with CKD. Patients with high contrast nephropathy scores may be susceptible to persistent RD. Prospective studies are needed to address the many remaining questions regarding the prevention of contrast-induced acute and persistent kidney damage.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Mehran R, Aymong ED, Nikolsky E, et al. . A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 44: 1393-1399, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Maioli M, Toso A, Leoncini M, Gallopin M, Musilli N, Bellandi F. Persistent renal damage after contrast-induced acute kidney injury: incidence, evolution, risk factors, and prognosis. Circulation 125: 3099-3107, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Généreux P, Palmerini T, Caixeta A, et al. . Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: the residual SYNTAX (Synergy between PCI with Taxus and Cardiac Surgery) score. J Am Coll Cardiol 59: 2165-2174, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SM, Cha R hui, Lee JP, et al. . Incidence and outcomes of contrast-induced nephropathy after computed tomography in patients with CKD: a quality improvement report. Am J Kidney Dis 55: 1018-1025, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Howard DPJ, Banerjee A, Fairhead JF, Hands L, Silver LE, Rothwell PM. Population-based study of incidence, risk factors, outcome, and prognosis of ischemic peripheral arterial events: implications for prevention. Circulation 132: 1805-1815, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trivedi HS, Moore H, Nasr S, et al. . A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract 93: c29-c34, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Krasuski RA, Beard BM, Geoghagan JD, Thompson CM, Guidera SA. Optimal timing of hydration to erase contrast associated nephropathy: the OTHER CAN study. J Invasive Cardiol 15: 699-702, 2003. [PubMed] [Google Scholar]
- 8.Maioli M, Toso A, Leoncini M, et al. . Sodium bicarbonate versus saline for the prevention of contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol 52: 599-604, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Briguori C, Visconti G, Focaccio A, et al. . Renal insufficiency after contrast media administration trial II (REMEDIAL II): renalguard system in high-risk patients for contrast-induced acute kidney injury. Circulation 124: 1260-1269, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Thayssen P, Lassen JF, Jensen SE, et al. . Prevention of contrast-induced nephropathy with N-acetylcysteine or sodium bicarbonate in patients with ST-segment-myocardial infarction a prospective, randomized, open-labeled trial. Circ Cardiovasc Interv 7: 216-224, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Nijssen EC, Rennenberg RJ, Nelemans PJ, et al. . Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open-label, non-inferiority trial. Lancet 6736: 1-11, 2017. [DOI] [PubMed] [Google Scholar]
- 12.Levine GN, Bates ER, Blankenship JC, et al. . 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. J Am Coll Cardiol 58: e44-e122, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Windecker S, Kolh P, Alfonso F, et al. . 2014 ESC/EACTS Guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 35: 2541, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Ohno I, Hayashi H, Aonuma K, et al. . 2012 Guidelines for iodinated contrast in patient with chronic kidney disease. Jpn J Nephrol 54: 393-496, 2012. [Google Scholar]
- 15.Matsuo S, Imai E, Horio M, et al. . Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982-992, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine. Arch Intern Med 160: 2413-2446, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Holtkamp FA, de Zeeuw D, Thomas MC, et al. . An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 80: 282-287, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Morales E, Millet VG, Rojas-Rivera J, et al. . Renoprotective effects of mineralocorticoid receptor blockers in patients with proteinuric kidney diseases. Nephrol Dial Transplant 28: 405-412, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Cockcroft DW, Gault H. Prediction of creatinine clearance from serum creatinine. Nephron 16: 31-41, 1976. [DOI] [PubMed] [Google Scholar]
- 20.Austin PC, Steyerberg EW. The number of subjects per variable required in linear regression analyses. J Clin Epidemiol 68: 627-636, 2015. [DOI] [PubMed] [Google Scholar]
- 21.Navaneethan SD, Singh S, Appasamy S, Wing RE, Sehgal AR. Sodium bicarbonate therapy for prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Am J Kidney Dis 53: 617-627, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Zhao S, Zhong Z, Qi G, Tian W. The efficacy of N-acetylcysteine plus sodium bicarbonate in the prevention of contrast-induced nephropathy after cardiac catheterization and percutaneous coronary intervention: a meta-analysis of randomized controlled trials. Int J Cardiol 221: 251-259, 2016. [DOI] [PubMed] [Google Scholar]
- 23.Weisbord SD, Chen H, Stone RA, et al. . Associations of increases in serum creatinine with mortality and length of hospital stay after coronary angiography. J Am Soc Nephrol 17: 2871-2877, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J. Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol 20: 2617-2624, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 20: 672-679, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]