Abstract
Nintedanib has been shown to significantly reduce the annual rate of decline in the forced vital capacity (FVC) in patients with idiopathic pulmonary fibrosis (IPF) in previous randomized trials. A 71-year-old man developed exertional dyspnea and was diagnosed with IPF. Four months after treatment with nintedanib, high-resolution computed tomography findings revealed reduced areas of ground-glass opacity and consolidation; 13 months after treatment, the FVC showed improvement from 3.07 to 3.43 L, and the serum Krebs von den Lungen (KL)-6 concentration showed a decline to normal levels. We herein report a patient with IPF who was considered a super responder to nintedanib.
Keywords: nintedanib, idiopathic pulmonary fibrosis, KL-6, high-resolution computed tomography
Introduction
Idiopathic pulmonary fibrosis (IPF) is the most frequently occurring form of idiopathic interstitial pneumonias in adults (1, 2). The progression of this disease is usually chronic, with the occasional occurrence of severe acute respiratory worsening, referred to as acute exacerbation (3). At present, nintedanib or pirfenidone is recommended for the treatment of IPF (1, 2).
Nintedanib is a tyrosine kinase inhibitor that targets the fibroblast, platelet-derived, and vascular endothelial growth factor receptors (2). Treatment with nintedanib has been reported to reduce the annual decline in the forced vital capacity (FVC) by approximately 50% over the course of 1 year (4). Furthermore, treatment with nintedanib has the potential to reduce the incidence of acute exacerbation (5).
Flaherty et al. reported that 158 of 638 patients (24.8%) treated with nintedanib and 38 of 423 patients (9.0%) treated with placebo in the INPULSIS trials had an improvement or no decline in the FVC in the course of 52 weeks of treatment (6). They also reported that some IPF patients treated with nintedanib experienced an annual improvement of >200 mL in the FVC. To our knowledge, no previous case reports have described nintedanib super-responders. Furthermore, data on the serial changes in findings from high-resolution computed tomography (HRCT) and from the analysis of biomarkers during treatment in nintedanib responders are insufficient at present.
We herein report the first case of IPF in which treatment with nintedanib yielded remarkable improvements in FVC, HRCT findings, and serum Krebs von den Lungen (KL)-6 concentrations.
Case Report
A 71-year-old man with type 2 diabetes for 10 years had been prescribed oral drugs at a local clinic. Six months prior to presentation, he developed exertional dyspnea without symptoms of a fever, cough, or sputum production. He was then referred to a pulmonary physician who was a specialist in interstitial lung disease. The patient had no history of exposure to asbestos and had previously smoked two packs of cigarettes a day for 26 years. There was no history of bird rearing or drug allergies and no family history of interstitial lung diseases. No conditions were identified that might have caused interstitial lung disease, such as collagen vascular disease or chronic hypersensitivity pneumonia. His vital signs were unremarkable.
The patient’s resting and ambulatory percutaneous oxygen saturation percentages at room atmosphere were 96% and 87%, respectively, and an arterial blood gas analysis indicated a partial pressure of arterial oxygen (PaO2) of 89.4 Torr. Fine crackles were audible on his back. HRCT of the chest revealed the presence of reticular abnormality and honeycombing, particularly in the subpleural lower lobes, with mixed ground-glass opacity and consolidation. There were no features listed as inconsistent with the usual interstitial pneumonia (UIP) pattern according to the 2011 international guidelines of IPF (1). The serum lactate dehydrogenase concentration was elevated at 314 IU/L (normal range: 120-220 IU/L), and the serum KL-6 concentration was also elevated at 515 U/mL (normal range: <500 U/mL). The level of C-reactive protein was normal. All of the examined autoantibodies were negative. Pulmonary function test results showed an FVC of 3.07 L (89.8% of the predicted value) and a diffusing capacity of the lung for carbon monoxide (DLco) of 8.49 mL/min/torr (49.6% of the predicted value). Based on a diagnosis of IPF according to the 2011 international guidelines (1), treatment with 150 mg nintedanib administered twice daily was initiated.
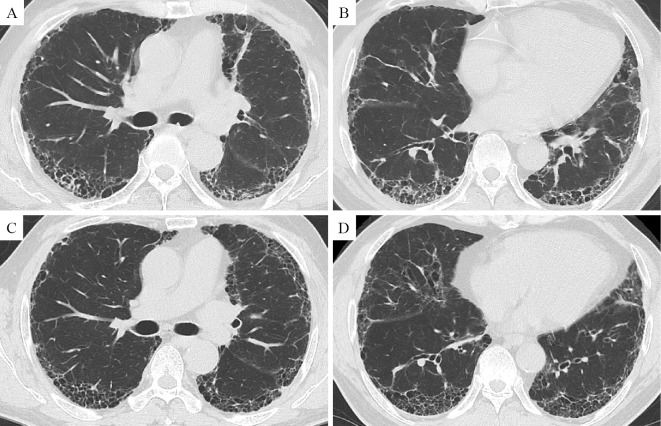
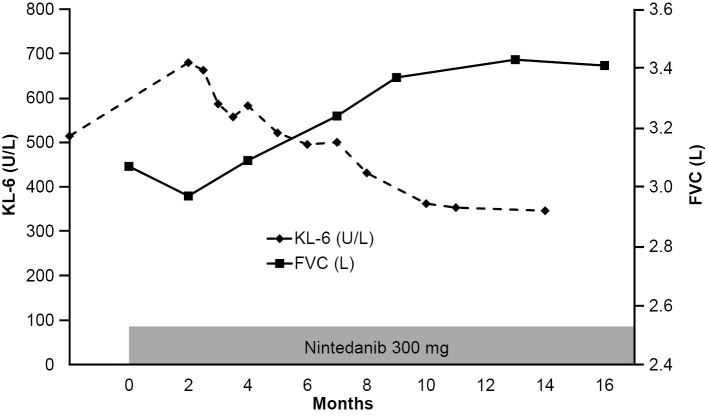
Four months after treatment with nintedanib, HRCT images showed reduced areas of ground-glass opacity and consolidation (Fig. 1). These HRCT findings did not change, even at nine months later. Thirteen months after treatment, the FVC had improved from 3.07 to 3.43 L, a percentage improvement of 11.7% (Fig. 2). The serial changes in the serum KL-6 concentration are shown in Fig. 2. Even though the KL-6 concentration increased to 680 IU/mL when tested 2 months after the initiation of treatment with nintedanib, the concentrations gradually decreased and were normalized after 8 months. The PaO2 was slightly improved to 94.3 Torr on room atmosphere. During the treatment, we noted no elevation of the liver enzymes. Diarrhea continued but was tolerable with loperamide hydrochloride treatment.
Figure 1.
HRCT images at the carina level (A) and at the lower lobe level (B) two months before initiating nintedanib therapy. HRCT images at the carina level (C) and at the lower lobe level (D) four months after starting treatment. HRCT images showed reductions in the areas of ground-glass opacity and consolidation compared with those in the images obtained before treatment.
Figure 2.
Changes in the FVC and KL-6 concentration during the treatment period. The FVC slowly improved by up to 11.7%, while the KL-6 concentration decreased to normal levels.
Discussion
In the present case, the positive effects of nintedanib were clearly demonstrated by clinical parameters, including the FVC, HRCT findings, and serum KL-6 concentration. The FVC is accepted as the most reliable parameter of the disease status and has been used as an endpoint for several clinical trials in patients with IPF (4-9). In this case, the FVC improved by up to 11.7% when tested approximately 1 year after the initiation of treatment with nintedanib. Given these changes in the FVC, this patient was regarded as a super-responder to nintedanib.
CT may be useful for assessing the treatment efficacy of anti-fibrotic agents. In a previous case report involving a super-responder to pirfenidone, HRCT images showed improvement in the ground-glass opacity (10). Iwasawa et al. further reported a computer-based volumetric CT analysis method that classified the CT lung field into normal, ground-glass opacities, consolidation, emphysema, and fibrosis patterns (11). These authors also reported that the volume change of a fibrous pattern lesion was significantly less (p=0.006) in the patients treated with pirfenidone than in the control group. In the present case, the HRCT images showed a reduction in the areas of ground-glass opacity and consolidation. Changes in the HRCT findings of nintedanib responders may resemble those of pirfenidone responders.
KL-6 is one of the most useful serum biomarkers in patients with IPF, along with surfactant protein D, surfactant protein A, and matrix metalloproteinase-7 (12). Wakamatsu et al. reported that patients with increased serum concentrations of KL-6 during follow-up had a significantly higher decline in the predicted percent FVC than those with no increase in KL-6 (p=0.0001) (13). Sokai et al. reported that serial changes in serum KL-6 concentration in patients with IPF represented a stronger predictor of mortality than that of KL-6 when measured at a single time point (14). These present and previous findings therefore suggest that serum KL-6 may be a useful biomarker involved with the serial changes in the FVC.
IPF progresses slowly in many patients but rapidly in others (2, 15, 16). In the INPULSIS trials, the FVC declined at a significantly slower rate in patients treated with nintedanib for 6 to 12 weeks of treatment than it did in the placebo group (4). In the present patient, a transient decline in the FVC and an elevation of the serum concentrations of KL-6 were observed 2 months after the initiation of nintedanib. This might have been caused by the rapid progression of the disease that had occurred before the initiation of nintedanib. In this patient, the FVC gradually improved by 11.7%, and the KL-6 concentration decreased to normal levels with continued nintedanib treatment. It is important to determine whether or not nintedanib should be continued in patients who experience worsening disease at 6 to 12 weeks after the start of treatment.
In the present case, the diagnosis of IPF was determined according to the 2011 international guidelines (1). The clinical and radiological findings were consistent with those of IPF. However, pathological evidence for UIP was not available. Consequently, we cannot definitively rule out a diagnosis of fibrotic non-specific interstitial pneumonia or chronic hypersensitivity pneumonia.
In conclusion, we encountered a patient with IPF who was classified as a super-responder to nintedanib. The FVC, the findings of CT images, and serum concentrations of KL-6 may be useful for assessing the efficacy of nintedanib. Some novel agents were reported to be effective in phase 2 trials for IPF (2, 8, 9). Further research is therefore highly warranted for identifying predictive factors that may aid in the selection of the appropriate treatment for individual patients with IPF.
Author's disclosure of potential Conflicts of Interest (COI).
Hirotsugu Ohkubo: Honoraria, Boehringer Ingelheim and SHIONOGI; Research funding, Boehringer Ingelheim. Akio Niimi: Honoraria, AstraZeneca, Boehringer Ingelheim and MSD; Research funding, Boehringer Ingelheim, KYORIN Pharmaceutical, Astellas Pharma and Kyowa Medex.
References
- 1.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788-824, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med 378: 1811-1823, 2018. [DOI] [PubMed] [Google Scholar]
- 3.Marchioni A, Tonelli R, Ball L, et al. Acute exacerbation of idiopathic pulmonary fibrosis: lessons learned from acute respiratory distress syndrome? Crit Care 22: 80, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richeldi L, du Bois RM, Raghu G, et al. INPULSIS Trial Investigators: efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370: 2071-2082, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Azuma A, Taniguchi H, Inoue Y, et al. Nintedanib in Japanese patients with idiopathic pulmonary fibrosis: a subgroup analysis of the INPULSISⓇ randomized trials. Respirology 22: 750-757, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Flaherty KR, Kolb M, Vancheri C, et al. Stability or improvement in forced vital capacity with nintedanib in patients with idiopathic pulmonary fibrosis. Eur Respir J 52: 1702593, 2018. [DOI] [PubMed] [Google Scholar]
- 7.King TE Jr, Bradford WZ, Castro-Bernardini S, et al. ASCEND Study Group A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370: 2083-2092, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Raghu G, van den Blink B, Hamblin MJ, et al. Effect of recombinant human pentraxin 2 vs placebo on change in forced vital capacity in patients with idiopathic pulmonary fibrosis: a randomized clinical trial. JAMA 319: 2299-2307, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maher TM, van der Aar EM, Van de Steen O, et al. A safety, tolerability, pharmacokinetics, and pharmacodynamics of GLPG1690, a novel autotaxin inhibitor, to treat idiopathic pulmonary fibrosis (FLORA): a phase 2a randomised placebo-controlled trial. Lancet Respir Med S2213-2600: 30181-30184, 2018. [DOI] [PubMed] [Google Scholar]
- 10.Miyamoto A, Morokawa N, Takahashi Y, et al. Marked improvement with pirfenidone in a patient with idiopathic pulmonary fibrosis. Intern Med 55: 657-661, 2016. [DOI] [PubMed] [Google Scholar]
- 11.Iwasawa T, Ogura T, Sakai F, et al. CT analysis of the effect of pirfenidone in patients with idiopathic pulmonary fibrosis. Eur J Radiol 83: 32-38, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Hamai K, Iwamoto H, Ishikawa N, et al. Comparative study of circulating MMP-7, CCL18, KL-6, SP-A, and SP-D as disease markers of idiopathic pulmonary fibrosis. Dis Markers 2016: 4759040, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wakamatsu K, Nagata N, Kumazoe H, et al. Prognostic value of serial serum KL-6 measurements in patients with idiopathic pulmonary fibrosis. Respir Investig 55: 16-23, 2017. [DOI] [PubMed] [Google Scholar]
- 14.Sokai A, Tanizawa K, Handa T, et al. Importance of serial changes in biomarkers in idiopathic pulmonary fibrosis. ERJ Open Res 3: 00019-2016, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet 378: 1949-1961, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 183: 431-440, 2011. [DOI] [PubMed] [Google Scholar]