Abstract
A 79-year-old Japanese woman presented with consciousness disturbance, hypercalcemia, and hypermagnesemia. She had rheumatoid arthritis and osteoporosis. Three months before admission, she was treated with oral calcitriol for osteoporosis. Two months before admission, she was treated with magnesium oxide for constipation. One month before admission, she underwent articular femoral bone replacement. Two weeks postoperatively, consciousness disturbance and elevated serum calcium levels were observed, and she was transferred to our hospital. On admission, her laboratory data showed elevated serum magnesium and creatinine levels. This is a rare case of coexistent hypercalcemia and hypermagnesemia associated with consciousness disturbance and acute kidney injury.
Keywords: hypercalcemia, hypermagnesemia, acute kidney injury (AKI), hyperparathyroidism
Introduction
Hypercalcemia is caused by primary hyperparathyroidism, malignancies, and medications such as calcium (Ca) and 1,25-dihydroxycholecalciferol. Hypermagnesemia can be attributed to the administration of magnesium (Mg), especially in patients with renal failure. In patients with hypermagnesemia, the serum calcium level is usually decreased or unchanged (1). We herein report an atypical case of hypermagnesemia in acute kidney injury (AKI) caused by hypercalcemia, induced by the administration of activated vitamin D3 and magnesium oxide.
Case Report
A 79-year-old woman presented with consciousness disturbance and AKI. She had a history of rheumatoid arthritis and had been treated with 5 mg/day of prednisolone. Three months and 2 months before presentation, she was treated with calcitriol 0.25 μg/day and magnesium oxide powder 3 g/day for osteoporosis and constipation, respectively. One month before admission, she had undergone artificial femoral head replacement for left femoral bone head fracture. Two weeks after the operation, she developed consciousness disturbance and was transferred to the emergency clinic at our hospital. On presentation, her temperature was 36.6°C. Her blood pressure and heart rate were 116/84 mmHg and 80/min, respectively. Her state of consciousness was stuporous. Her respiratory movement was impaired, with reduced oxygen saturation (90% at room air); nasal oxygen supplementation was started at 2 L/min. Neurological examinations did not reveal any abnormalities, including deep tendon reflexes. Chest and abdomen examinations showed no remarkable findings. Laboratory values showed a red blood cell count of 232×104/μL (reference range: 400-552) and a hemoglobin level of 7.2 g/dL (13.2-17.2).
Her C-reactive protein level was 2.6 mg/dL (<0.3), and her albumin level was 2.6 g/dL (3.9-4.9); the sodium, potassium, and chloride levels were 132 (136-145), 4.9 (3.4-4.5), and 97 (100-108) mmol/L, respectively. The serum Ca, phosphorus, and Mg levels were 14.9 (8-10.4), 4.1 (2.5-4.5), and 5.6 (1.9-2.5) mg/dL, respectively. Her serum creatinine, blood urea nitrogen, and uric acid levels were elevated at 3.98 (0.6-1.1), 76 (8-20), and 8.6 (3.4-7.8) mg/dL, respectively. Her estimated glomerular filtration rate (eGFR) was decreased to 9.2 mL/min/1.73 m2. Intact parathyroid hormone (PTH) and parathyroid hormone related protein (PTHrP) were 99.6 pg/mL (10.3-65.9) and <1.0 pmol/L, respectively. A urinalysis revealed no remarkable findings. An initial arterial blood gas analysis with inhalation of O2 at 2 L/min revealed the following: pH 7.423, partial pressure oxygen (pO2) 69.0 torr, carbon dioxide partial pressure (pCO2) 47.7 torr, HCO3 30.6 mEq/L, base excess 6.1 mEq/L, and O2 saturation 94.2%. Abdominal computed tomography (CT) revealed bilateral cortical nephrocalcinosis (Fig. 1).
Figure 1.
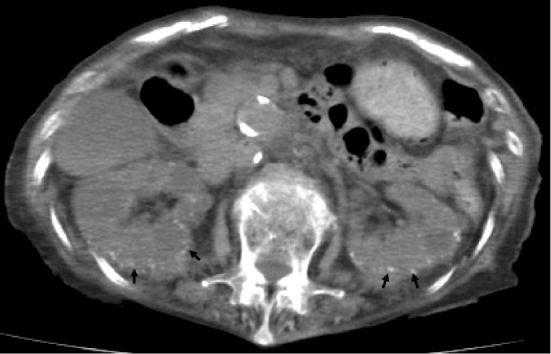
A computed tomography scan showing cortical nephrocalcinosis in the bilateral kidneys (arrows).
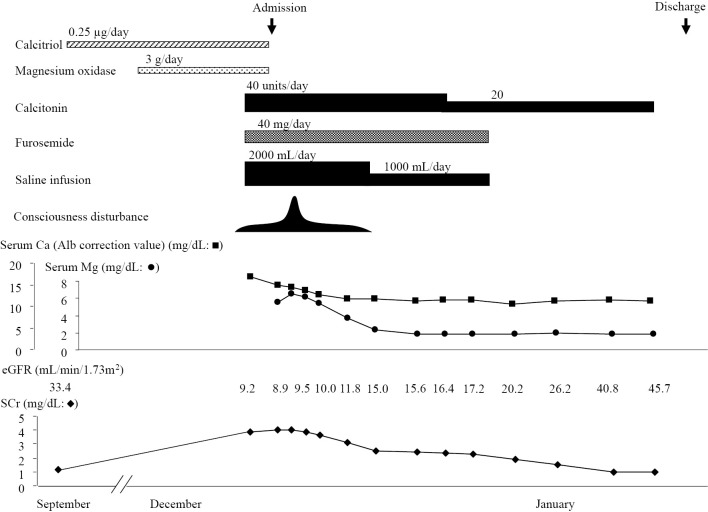
She was diagnosed with AKI due to hypercalcemia and hypermagnesemia, thought to be induced by the administration of supplements combined with subclinical hyperparathyroidism. Therefore, calcitriol and magnesium oxide were stopped and intravenous eel calcitonin (40 U/day), intravenous saline infusion, and intravenous furosemide (40 mg/day) were started. On the second hospital day, her serum Ca level decreased to 14.3 mg/dL, serum Mg level increased to 6.5 mg/dL, and consciousness disturbance progressed intermittently (JCS III-100). However, on the 4th hospital day, both the serum Ca and Mg levels decreased, and her conscious disturbance slightly improved subsequently (JCS II-20). On the 7th hospital day, the levels of Ca and Mg normalized, and her consciousness levels improved (Fig. 2). Calcitonin was tapered, and both intravenous saline infusion and intravenous diuretics were stopped. Her clinical course showed good progression, and she was discharged on hospital day 35. Unfortunately, she died suddenly two months after discharge from an unknown cause.
Figure 2.
The clinical course of the patient.
Discussion
Hypercalcemia is common and can occur in several clinical situations, including malignancy, hyperparathyroidism, and vitamin D3 excess (2). It has been reported that the first- and second-most common causes are malignancy and hyperparathyroidism, respectively, and that these 2 diagnoses account for over 80% of hypercalcemia cases (2). In the present case, the serum PTHrP level was within the normal range. In addition, no obvious metastatic lesions in the bone were seen. However, although her serum calcium level was elevated, her intact PTH level was slightly higher than the reference range. CT showed no remarkable findings in the neck, including the thyroid and parathyroid. However, because her intact PTH was not suppressed by hypercalcemia and her clinical course improved after stopping calcitriol treatment, we speculated that she had hyperparathyroidism, and that the subsequent administration of calcitriol had led to hypercalcemia. To our knowledge, only one report on the association of hypermagnesemia and hypercalcemia has been published (1). The previously reported patient underwent hemodialysis and was suspected of having hyperparathyroidism, as in the current case. However, this association might be more common than is currently recognized, and its epidemiology should be further investigated.
Mg homeostasis is regulated by the balance between gastrointestinal absorption and renal excretion. In healthy individuals without renal dysfunction, the maximal renal excretion of Mg can exceed 6 g daily, so the levels of serum Mg are well controlled within the normal range (3). Even in patients with renal failure, the serum Mg levels can usually be normalized with the down-regulation of gastrointestinal Mg absorption (4). Therefore, symptomatic hypermagnesemia is an extremely rare clinical event and is generally observed in patients receiving Mg compounds in the presence of renal dysfunction and/or small bowel hypomotility (4). In addition, it has been reported that hypermagnesemia itself might contribute to intestinal smooth muscle dysfunction (5). The eGFR that tends to lead to hypermagnesemia is reportedly ≤30 mL/min (6), and an eGFR of <30 mL/min/1.73 m2 in elderly people in Japan who have been prescribed magnesium oxide is associated with a risk of hypermagnesemia (7). Hypermagnesemia usually results from iatrogenic causes, such as intravenous Mg administration, oral ingestion of Mg-containing antacids or cathartics, and procedure-related retroperitoneal or peritoneal leakage of Mg-containing preparations (5). The administration of calcitriol, activated vitamin D3 given as a therapeutic agent for osteoporosis, is known to result in increased gastrointestinal Mg absorption and decreased renal Mg excretion, leading to elevated Mg levels (6). In the present case, the use of calcitriol might have led to acute renal injury and severe hypercalcemia, and in this situation, the subsequent administration of magnesium oxide might have resulted in hypercalcemia and hypermagnesemia. Therefore, as the incidence of complications associated with osteoporosis and constipation is high in elderly people, it is necessary to pay close attention to this vicious circle.
The clinical manifestation of hypermagnesemia is characterized by neuromuscular, respiratory, and cardiovascular complications. In general, saline diuresis and loop diuretics can be used to enhance Mg excretion. Because Mg acts as a Ca channel blocker, intravenous Ca is sometimes used in the treatment of hypermagnesemia. In the present case, there were fewer clinical manifestations than expected based on the severity of her hypercalcemia and hypermagnesemia. We suspect that, in this patient, hypercalcemia and hypermagnesemia might have masked each other’s manifestations.
Cortical nephrocalcinosis is rare (2.4%) and usually results from severe destructive disease of the cortex (8). This has been described in chronic glomerulonephritis, although often in the presence other factors, such as increased calcium ingestion (9); acute cortical necrosis (10); chronic pyelonephritis, which may be focal and therefore asymmetric (11); and trauma (12). Conversely, primary hyperparathyroidism is a known risk factor for nephrolithiasis, and in most published series of patients presenting with nephrolithiasis, the prevalence of concurrent primary hyperparathyroidism is 2-8% (13). Therefore, in the present case, although the onset of cortical calcification is unclear, it is likely that hypercalcemia caused by primary hyperparathyroidism resulted in cortical nephrocalcinosis. Chronic kidney disease, which often causes AKI, is common in elderly patients (14). Therefore, to prevent the development of AKI in such patients with underlying diseases, the eGFR should be monitored to determine their renal function, and the serum levels of Ca and Mg should be measured when vitamin D3, Ca, and Mg are being administered.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Matsuo H, Nakamura K, Nishida A, Kubo K, Nakagawa R, Sumida Y. A case of hypermagnesemia accompanied by hypercalcemia induced by a magnesium laxative in a hemodialysis patient. Nephron 71: 477-478, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care 35: 215-237, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fassler CA, Rodriguez RM, Badesch DB, Stone WJ, Marini JJ. Magnesium toxicity as a cause of hypotension and hypoventilation, occurrence in patients with normal renal function. Arch Intern Med 145: 1604-1606, 1985. [PubMed] [Google Scholar]
- 4.Van Hook JW. Endocrine crisis. Hypermagnesemia. Crit Care Clin 7: 215-223, 1991. [PubMed] [Google Scholar]
- 5.Kontani M, Hara A, Ohta S, Ikeda. Hypermagnesemia induced by massive cathartic ingestion in an elderly woman without pre-existing renal dysfunction. Intern Med 44: 448-452, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Nishikawa M, Shimada N, Kanzaki M, et al. The characteristics of patients with hypermagnesemia who underwent emergency hemodialysis. Acute Med Surg 5: 222-229, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horibata K, Tanoue A, Ito M, Takemura Y. Relationship between renal function and serum magnesium concentration in elderly outpatients treated with magnesium oxide. Geriatr Gerontol Int 16: 600-605, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira B, Kleta R, Bockenhauer D, Walsh SB. Genetic, pathophysiological, and clinical aspects of nephrocalcinosis. Am J Physiol Renal Physiol 311: F1243-F1252, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Arons WL, Christensen WR, Sosman MC. Nephrocalcinosis visible by X-ray associated with chronic glomerulonephritis. Ann Intern Med 42: 260-282, 1955. [DOI] [PubMed] [Google Scholar]
- 10.Cramer GG, Fuglestad JR. Cortical calcification in renal cortical necrosis. Am J Roentgenol Radium Ther 95: 344-348, 1965. [DOI] [PubMed] [Google Scholar]
- 11.Ekengren K. Renal cortical calcification in pyelonephritis. Ann Radiol 16: 223-226, 1973. [Google Scholar]
- 12.Calviño J, Romero R, Blanco M, Lens XM, Novoa D, Sanchez-Guisande D. Cortical nephrocalcinosis induced by extracorporeal shock wave lithotripsy. Nephron 81: 242-243, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Pak CY. Etiology and treatment of urolithiasis. Am J Kidney Dis 18: 624-637, 1991. [DOI] [PubMed] [Google Scholar]
- 14.Hatakeyama Y, Horino T, Nagata K, Matsumoto T, Terada Y, Okuhara Y. Transition from acute kidney injury to chronic kidney disease: a single-centre cohort study. Clin Exp Nephrol 2018. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]