Abstract
Organisms use circulating diuretic hormones to control water balance (osmolarity), thereby avoiding dehydration and managing excretion of waste products. The hormones act through G-protein-coupled receptors to activate second messenger systems that in turn control the permeability of secretory epithelia to ions like chloride. In insects, the chloride channel mediating the effects of diuretic hormones was unknown. Surprisingly, we find a pentameric, cys-loop chloride channel, a type of channel normally associated with neurotransmission, mediating hormone-induced transepithelial chloride conductance. This discovery is important because: 1) it describes an unexpected role for pentameric receptors in the membrane permeability of secretory epithelial cells, and 2) it suggests that neurotransmitter-gated ion channels may have evolved from channels involved in secretion.
Subject terms: Molecular evolution, Neurophysiology
Introduction
Organisms have evolved diverse mechanisms to solve the problems of osmoregulation and excretion. In insects, urine is excreted through Malpighian tubules (MTs), the primary renal epithelium. MTs regulate the net flux of ions from the surrounding hemolymph into the tubule lumen, establishing the osmotic gradient that drives fluid secretion. In the prevailing model, cation and anion transport are, for the most part, spatially segregated within the Drosophila MT: cation transport is restricted to principal cells and anion transport is primarily restricted to stellate cells1,2. In principal cells, the apically localized V-type H+ ATPase energizes transepithelial secretion, providing electrogenic transport of H+ into the lumen, while alkali-metal cation/H+ antiporters are thought to recycle the extruded H+ in exchange for Na+ and K+3. Stellate cells appear to be the primary source of anion permeability, mainly chloride1,2,4 however less is known about the specific proteins involved in regulating chloride transport in these tissues.
Much of our knowledge concerning transepithelial chloride secretion in the MTs stems from research characterizing the diuretic effects of the leucokinin neuropeptides and the biogenic amine tyramine. Both tyramine and the leucokinins stimulate diuresis by increasing net chloride transport into the lumen1,2,5. They appear to act through distinct G-protein coupled receptors (GPCRs) that converge on the same second messenger pathway; the diuretic action of both secretory hormones is dependent on a rise in intracellular calcium levels specifically in stellate cells2,6–8. In contrast to the insect Aedes, where evidence points to a paracellular route for chloride secretion, in Drosophila, leucokinin stimulation activates a transcellular pathway for chloride flux9–11. A ClC chloride channel, ClC-a, is expressed in the basolateral and apical membranes of stellate cells and is required for the leucokinin-mediated increase in cytoplasmic chloride levels and secretion6. However, its role in chloride exit through the apical membrane is unclear.
Here we describe a role for a pentameric chloride channel encoded by the secCl (CG7589) gene in hormone-induced transepithelial chloride conductance. We previously identified secCl (CG7589) and pHCl-2 (CG11340) as highly divergent putative anion-selective Cys-loop pLGIC subunits in Drosophila melanogaster12. We demonstrate that secCl forms a homomeric chloride channel that is open in the absence of any ligand and is expressed in the apical membrane of stellate cells. Moreover, loss of secCl eliminates the effects of diuretic hormones and has a penetrant lethal phenotype in adults, demonstrating that secCl plays a significant role in secretion. These results suggest that in Drosophila MTs, a member of the pentameric, cys-loop ligand-gated ion channel family mediates transcellular chloride secretion without direct interaction with a ligand.
Results
secCl forms a constitutively open homomeric channel
secCl (CG7589) belongs to a subfamily of divergent Cys-loop LGICs in the Drosophila genome that also comprises pHCl-2 and CG692712–15. These putative channel subunits are both specific to and conserved among arthropods.
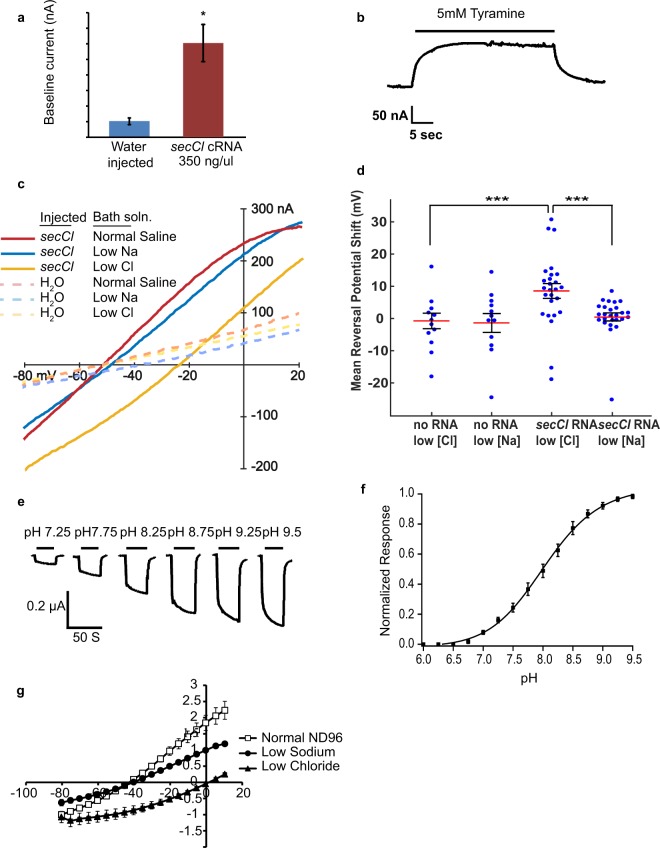
To determine if secCl can form a functional channel, we isolated a secCl cDNA from wild-type flies that encodes a protein of 510 amino acids with the characteristic features of Cys-loop LGICs (Supplemental Figure S1) and no evidence of RNA editing16. We injected Xenopus oocytes with secCl cRNA and clamped the oocytes at −80 mV. Application of 1 mM acetylcholine, GABA, glutamate, glycine, histamine, serotonin, dopamine, nicotine, tyramine and octopamine to oocytes expressing secCl did not induce changes in holding current, indicating that none of these putative neurotransmitters gate secCl (data not shown). Even though secCl displays greatest homology to a class of pH-sensitive Cys-loop LGICs14,15,17, secCl -expressing oocytes were not sensitive to changes in pH (pH 6–pH 9.5). However, the holding currents of oocytes injected with secCl cRNA were significantly higher than water injected controls (302 nA ± 59.6 vs. 38.9 nA ± 10.7; p < 0.002, two tailed t-test) (Fig. 1a), indicating that an open ion channel is formed in secCl -expressing oocytes. Furthermore, exposure to 5 mM tyramine elicited a rapid and reversible, albeit modest, decrease in baseline current in secCl expressing oocytes (Fig. 1b), but had no effect on water injected oocytes. These results suggest that secCl forms a constitutively open ion channel that is weakly blocked by 5 mM tyramine.
Figure 1.
secCl forms a constitutively open homomeric chloride channel. (a) Bars indicate baseline currents of oocytes clamped at −80 mV that were injected with either water (n = 5) or secCl cRNA (n = 10). Error bars represent standard error of the mean. *P < 0.002 (two-tailed t-test). (b) Sample trace from an oocyte clamped at −80mV expressing secCl. The upward deflection corresponds to channels closing or being blocked upon treatment with 5 mM tyramine. (c) Sample current-voltage (IV) curves from an oocyte injected with secCl cRNA (solid traces) and a control oocyte injected with water (dashed traces). “Normal Saline” (ND96: 96 mM Na+ and 103.6 Cl−) “low Na” (6 mM Na+ and 103.6 mM Cl−) and”Low Cl” (96 mM Na+ and 13.6 mM Cl−). (d) Reversal potential shifts of secCl-expressing and control oocytes in low-chloride and low-sodium buffers relative to normal saline. ***P < 0.0001. (e–g) Coexpression of secCl with CG6927 results in a heteromeric pH-sensitive chloride channel. (e) Representative traces from an oocyte clamped at −80 mV expressing secCl and CG6927, showing responses to changes in pH. Control buffer was ND96 at pH 6.0. (f) The response profile of the channel to changes in pH. The responses were normalized to the maximum current response of each oocyte. The curve represents the fit to the Hill equation (n = 5). (g) Representative traces of the current voltage relationship in “normal” (n = 6), “low sodium” (n = 4), and “low chloride” (n = 4) buffer. Error bars represent standard error of the mean.
secCl contains a motif that is highly predictive of chloride selectivity among Cys-loop LGICs17–19 (Supplemental Figure S1). In oocytes injected with secCl cRNA we observed a significant 8.5 ± 2.5 mV average positive shift in reversal potential in low-chloride buffer (13.6 mM Cl) relative to normal saline (103.6 mM Cl) compared to an insignificant 0.5 mV shift in low-sodium buffer (6 mM Na) relative to normal saline (96 mM Na) (Fig. 1c,d). In water-injected control oocytes the shifts were insignificant in both low chloride and low sodium buffers: −1.6 ± 2.5 mV (low Cl) and −1.3 ± 2.9 mV (low Cl). A reversal potential shift specific to low-chloride buffer is characteristic of chloride-selective channels. Although the mean and maximum (30.6 mV) shifts in reversal potential seen in RNA-injected oocytes were less than the 52 mV shift predicted by the Nernst equation for a chloride-selective channel, this was because: 1) not all RNA-injected oocytes were expressing secCl, and 2) we were unable to completely isolate secCl currents from endogenous oocyte currents that also contribute to the reversal potential.
Interestingly, CG6927, the third gene in the clade that includes secCl and pHCl-2, does not form a homomeric channel when expressed in oocytes but forms a pH sensitive, chloride-selective channel when co-expressed with secCl (Fig. 1d–f). Although CG6927 is apparently not co-expressed with secCl in vivo15,20, the two subunits can form a heteromeric channel in oocytes that shows the expected shift in reversal potential (53.0 ± 1.5 mV) in low chloride buffer, supporting the conclusion that secCl is a functional chloride channel subunit.
secCl is expressed in secretory tissues
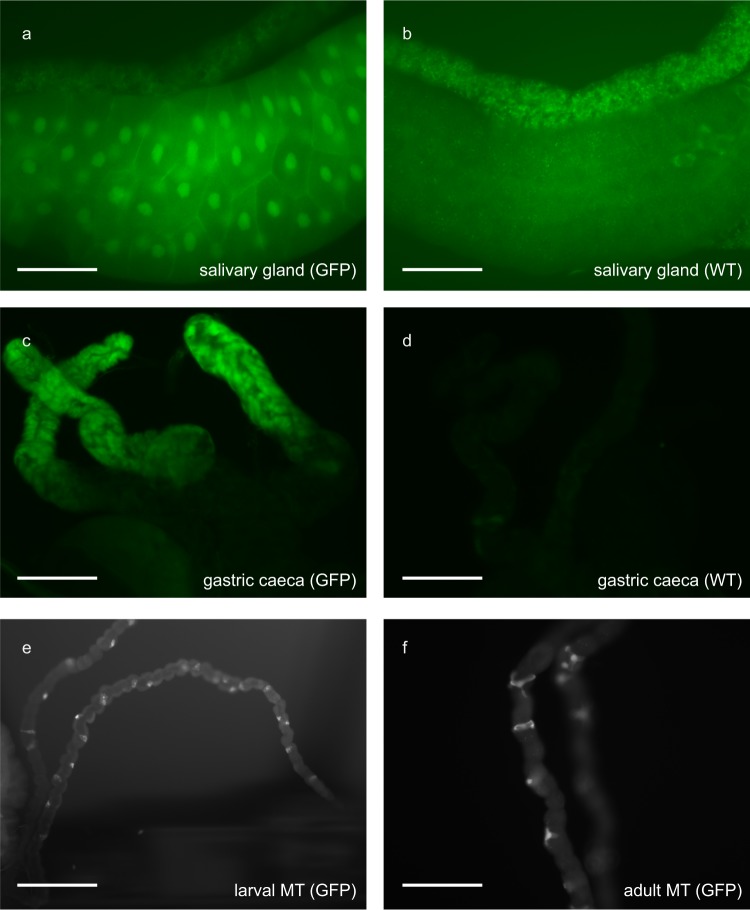
Cys-loop LGICs are typically expressed in the nervous system and in muscle cells where they initiate rapid, ionotropic, post-synaptic communication in response to the presynaptic release of neurotransmitters21. In contrast, tissues imaged from flies bearing a secCl promoter-GFP construct showed fluorescence in the salivary glands and gastric caeca of third instar larvae (Fig. 2a–d), as well as in the stellate cells of initial/transitional and main segments of MTs in third instar larvae and adults (Fig. 2e,f), consistent with previous reports15. The GFP in the salivary glands localized to the nucleus for reasons that were unclear.
Figure 2.
secCl gene expression patterns. Expression patterns of a secCl promoter-GFP fusion transgene. (a–d) Tissues of third instar larvae with (a,c; GFP) and without (b,d; WT) transgene. (a–b) Salivary glands. (c,d) Gastric cecae. (e,f) Main segments of MTs of third instar larvae (e) and adult (f) showing expression in stellate cells. Scale bars represent 100 μm.
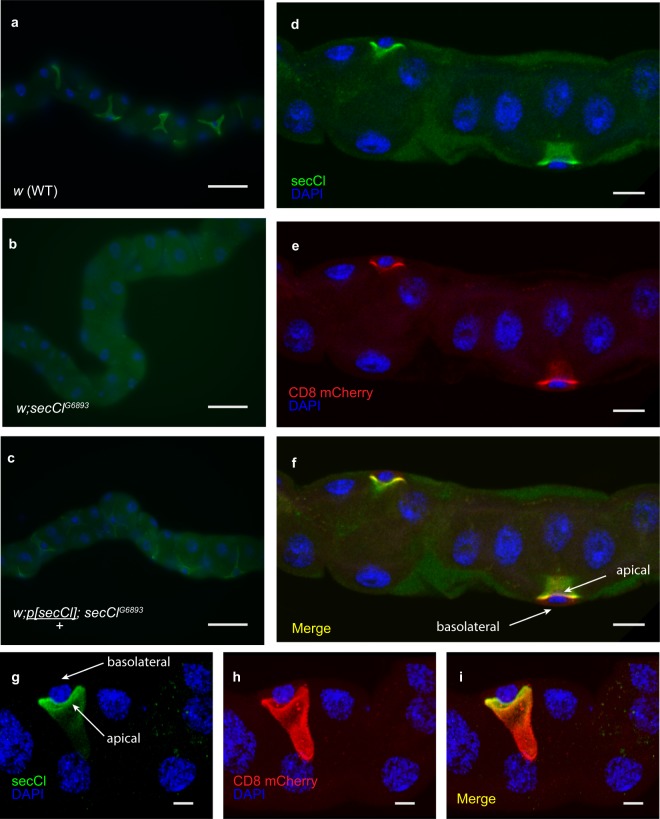
To determine the subcellular localization of secCl in polarized epithelia, we generated polyclonal antibodies raised against the unique intracellular loop. Immunostaining was detected exclusively in stellate cells of MTs (Fig. 3a). No immunostaining was detected in the MTs of homozygous secClG6893/G6893 individuals (Fig. 3b), and the stellate cell-specific signal was restored in MTs from secClG6893/G6893 homozygous mutants bearing a wild-type transgene for secCl (Fig. 3c), thus confirming that the signal observed in wild-type corresponds to secCl protein, and that secCl is dramatically reduced in the secClG6893/G6893 homozygous mutants. Next we immunostained secCl in stellate cells expressing CD8-mCherry, a marker of apical membranes in polarized epithelia22. The secCl and CD8 staining were co-localized to the lumenal side of the nucleus (Fig. 3d–i), confirming that secCl is localized to the apical membrane in stellate cells.
Figure 3.
secCl protein is expressed in the apical membrane of stellate cells. Immunostaining of adult MTs using anti-secCl (green). Nuclei are labelled with DAPI (blue). (a) secCl is expressed in the stellate cells but not the principal cells of MTs. (b) Immunostaining is not observed in homozygous mutant secClG689/G68933 MTs. (c) Immunostaining is restored to stellate cells in homozygous mutant secClG6893/G6893 bearing a single copy of a wild-type secCl transgene, p[secCl]. (d) An optical cross-section of MTs stained with anti-secCl showing localization to apical membrane (luminal side of the nucleus). (e) Expression of the transgenic mCherry-tagged CD8 (red), which is a marker of the apical membrane in polarized epithelia. (f) Merge of (d) and (e) showing co-localization of secCl and CD8 (yellow). (g–i) A z-stack rendered in 3D and rotated showing single stellate cell. Colors as in (d–f). Scale bars in (a–c) represent 50 μm; (d–f) 10 μm; (g–i) 5 μm.
secCl regulates fluid secretion in the Malpighian tubules
The tissues where we found secCl expression, midgut, salivary glands and MTs, are all secretory tissues that rely on ion transport to facilitate digestion, generate saliva and regulate urine production respectively23–25. To determine if secCl is necessary for secretion, we used the Ramsey assay to determine fluid secretion rates (FSR) in the MTs3. We first measured the basal output of explanted MTs. In standard Drosophila saline, wild-type MTs spontaneously secreted fluid at 0.43 ± 0.02 nL min−1 compared to 0.48 ± 0.02 nl min−1 from secClG6893/G6893 MTs, which lack secCl protein. Thus, loss of secCl does not impair basal FSRs (Fig. 4a).
Figure 4.
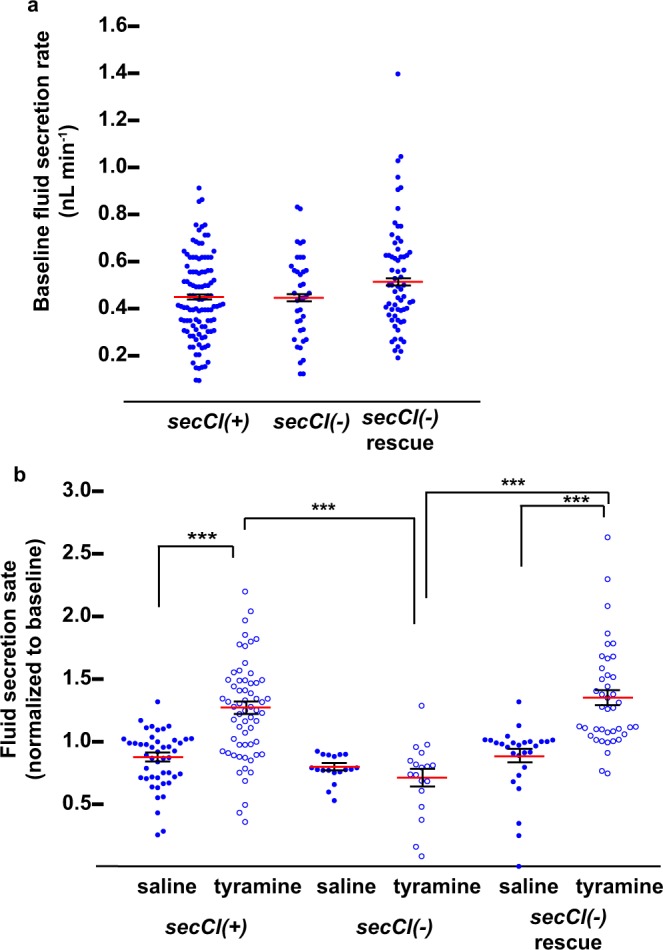
secCl is required for the tyramine-mediated diuretic response. Fluid secretion assays conducted on tubules with the genotypes: w (secCl(+)), w; secClG6893/G6893 (secCl(−)) and w; p[secCl], secClG6893/G6893 (secCl(−) rescue). (a) Basal fluid secretion rate (FSR) in Drosophila saline42 over 40 minutes. (b) Change in FSR in response to 2.9 μM tyramine or Drosophila saline (mock treatment) normalized to the first 40 minute interval. Red horizontal bar indicates the mean and error bars represent standard error of the mean. *** indicates p < 10−5. Significance was estimated by ANOVA and pairwise comparisons by Tukey’s HSD.
We next asked whether secCl mutants affected diuretic stimulation of secretion. The biogenic amine tyramine and the neuropeptide leucokinin stimulate fluid secretion by increasing transepithelial chloride conductance, a process that is dependent on intracellular calcium signaling specifically in stellate cells2,6,8,26. Exposure to 2.9 μM tyramine increased FSRs in wild-type by 55.8 ± 7.93% compared to mock-treated controls, whereas the output of MTs from secClG6893/G6893 loss-of-function mutants in response to tyramine were indistinguishable from mock-treated controls (Fig. 4b,c). Moreover, the diuretic effects of tyramine were restored in secClG6893/G6893 MTs expressing a wild-type secCl transgene, as fluid secretion increased by 53.0 ± 6.48% in response to 2.9 μM tyramine compared to mock-treated controls (Fig. 4b,c). Thus, secCl is required for the diuretic response mediated by tyramine.
secCl is necessary for chloride currents in response to the diuretic hormone tyramine
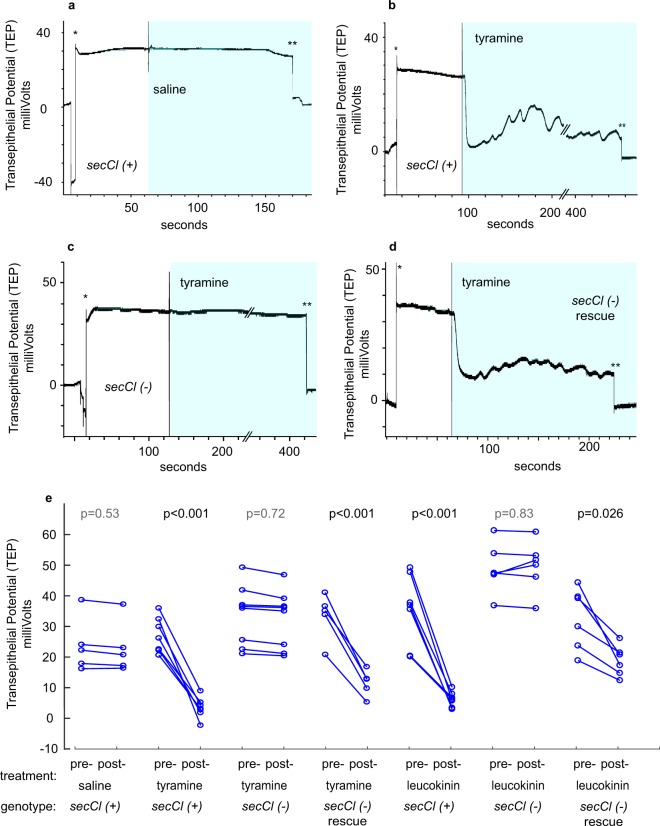
In the resting state, the transepithelial potential (TEP) of the Drosophila Malpighian tubule is positive, reflecting the accumulation of positive charge in the lumen, ultimately attributable to the activity of a vacuolar-type proton ATPase (V-ATPase). Positive charge accumulates because the rate of flow of a negative counter ion, chloride, is limiting. In response to the diuretic hormones tyramine and leucokinin, the transcellular chloride conductance through the stellate cells increases. The increased chloride flux neutralizes positive charge in the lumen and collapses the TEP. Increased ion flux into the lumen in turn increases osmotically driven secretion. Absence of secCl could prevent diuretic-induced secretion by: 1) reducing activity of the V-ATPase such that chloride conductance is not limiting for secretion, or 2) by preventing the increase in chloride conductance in response to diuretic hormone. In the first case we would predict that the resting TEP would be reduced in the secCl mutant relative to wild type, whereas in the second case we would predict that the resting TEP would be normal (positive) but would fail to collapse in response to diuretic hormone. We compared TEP in wild type to secClG6893/G6893 before and after treatment with 2.2 μM tyramine (Fig. 5a–e). Representative traces in Fig. 5 show the expected positive TEP prior to the exposure to tyramine and the collapse of the TEP to near zero potential after perfusion with tyramine. In contrast, the secCl loss-of-function mutant has a normal (positive) resting TEP but fails to depolarize upon perfusion with tyramine. We rescued the depolarization in response to tyramine by introducing the secCl transgene into the secClG6893/G6893 background (Fig. 5d,e). We likewise observed that treatment with the peptide hormone leukokinin resulted in a consistent decrease in TEP in the rescued strain, but not in the secClG6893/G6893 mutant. Thus, secCl is necessary for the increase in chloride conductance in response to diuretic hormones (Fig. 5e).
Figure 5.
secCl is necessary for the decrease in transepithelial potential in response to tyramine and leucokinin. Responses of the transepithelial potential (TEP) to tyramine. (a–d) Recordings of TEP in isolated Malpighian tubules. ‘*’ indicates penetration of the electrode into the lumen and ‘**’ indicates exit of electrode. The shaded area indicates the period of perfusion with saline or 2.9 μM tyramine as indicated. The beginning of perfusion was marked by a recording artifact. (a) The TEP of control w (secCl(+)) tubules did not respond to perfusion with saline. (b) In response to tyramine, the TEP of control tubules dropped to near zero mV and began to oscillate. (c) Tubules from w; secClG6893/G6893 (secCl(−)) flies did not respond to tyramine with a voltage drop. (d) Rescuing the secClG689/G68933 with a wild-type transgene (w; p[secCl]/+; secClG6893/G6893) restored the voltage drop and oscillations in response to tyramine. (e) P-values indicate the significance of the median change in absolute TEP pre- vs post-treatment (15 seconds after perfusion with saline control, 2.9 μM tyramine or 5 μM leucokinin) by Mann-Whitney U test.
Loss of secCl is lethal
We observed that the secClG6893 allele was recessive and semi-lethal as the emergence of homozygous secClG6893/G6893 adult progeny arising from heterozygous parents was 94% less than would be expected by Mendelian ratios (Table 1). Flies transheterozygous for secClG6893 and a deficiency chromosome (secClDf), which contains a deletion that spans over 100 kb and removes the entire secCl gene, display a similar degree of lethality (94%) as secClG6893/G6893, suggesting that the secClG6893 allele is null for secCl function. Moreover, introducing a single copy of a wild-type secCl transgene into the genetic background of secClG6893/G6893 rescued 89–100% of the lethality, confirming that the lethal phenotype is due to loss of secCl. Restoring secCl specifically to stellate cells by using c724-Gal4 to drive UAS::secCl in a secClG6893/G6893 mutant background did not rescue the lethality (Table 1), indicating that secCl expression in the stellate cells of the MTs is not sufficient for viability.
Table 1.
Lethal phenotype of secClG6893 loss-of-function allele and secCl RNAi.
| Genetic Cross: | # Of Homozygous Mutants Or RNAi (Obs/Exp) No Rescue 1 | % Surv-IvaL2 | # Of Homozygous Mutants (Obs/Exp) Rescued1 | % Rescued Survival3 |
|---|---|---|---|---|
| 29/458 | 6% | — | — | |
| 18/308 | 6% | — | — | |
| 47/235 | 20% | 208/235 | 89% | |
| 37/243 | 16% | 260/243 | 106% | |
| 59/163 | 36% | 13/54 | 24% | |
| 119/1044 | 114% |
1The expected values are calculated from Mendelian ratios.
2Percent survival was calculated as the observed/expected ratio in column 2.
3Rescue efficiency was calculated as the observed/expected ratio in column 4.
4Viability after targeted knockdown of SecCl by RNAi driven by c724-GAL4. Expected survival is based on total progeny bearing the UAS-secCl RNAi transgene. Data also includes progeny from crosses: w; GAL4/CyO; Pri/+ x w/Y; S/+; RNAi/TM3, Ser and w; S/+; RNAi/TM3, Ser x w/Y; GAL4/Cyo; Pri/+.
Loss of secCl in the MTs increases adult resistance to desiccation but not to salt stress
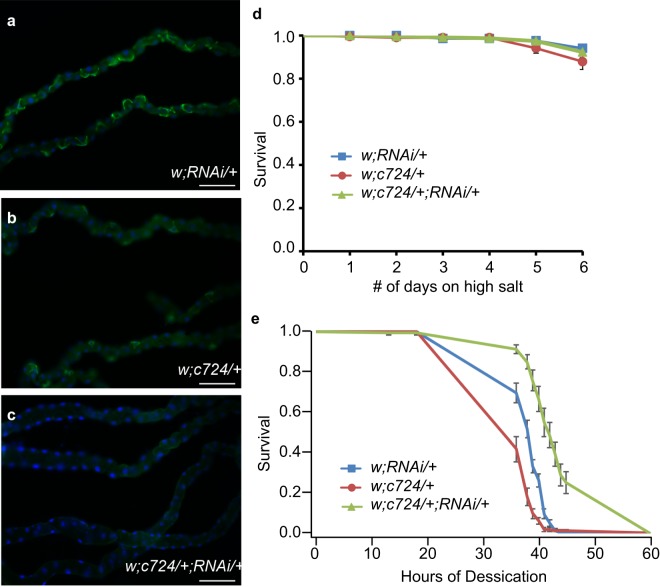
Because secCl expression in MTs was necessary for hormone-induced diuresis but not viability, we investigated the role of hormone-induced diuresis in Drosophila physiology. We hypothesized that diuresis is important for osmoregulation, which can be tested by raising flies on a salt-rich diet27–29. We used c724-Gal4 to drive expression of a UAS- secCl -RNAi construct to knock down secCl specifically in stellate cells. Immunostaining showed that SecCl was expressed in MTs from control flies expressing either the Gal4 or UAS-RNAi alone (Fig. 6a,b), but was undetectable in the MTs from individuals in which the RNAi was driven by the Gal4 construct (Fig. 6c), thus verifying the efficacy of the knock down. Targeted knockdown of secCl exclusively in the stellate cells did not result in a reduction of adult viability (Table 1), nor did it affect adult survival on a diet containing 0.5 M extra NaCl (Fig. 6d), suggesting that loss of secCl in MTs does not compromise the osmoregulatory processes required to survive, even under conditions of high salt stress.
Figure 6.
Genetic knockdown of secCl in stellate cells does not alter viability on a high-salt diet but does increase resistance to desiccation. (a–c) Immunostaining of adult MTs using anti-secCl (green). Nuclei are labelled with DAPI (blue). secCl expression is observed in MTs from undriven RNAi (a) and c724-Gal4 (b) control lines. secCl protein is not detected in MTs where the RNAi is driven in stellate cells (c). (d) Survival rates of w; RNAi/+, w; c724/+ and w; c724/+; RNAi/+ maintained on a NaCl rich diet over six days. N = 11–12 vials of 20 flies (see methods) for each genotype. Survival rates of w; RNAi/+, w; c724/+ and w; c724/+; RNAi/+ under conditions of desiccation stress over the course of 60 hours. N = 11–14 vials of 10 flies (see methods) per genotype. Scale bars represent 100 μm. For (d) and (e), error bars represent standard error of the mean. For (e) survival rates for w; c724/+; RNAi/+ are significantly different from w; RNAi/+ and w; c724/+ at all points between18–45 hours (p < 0.002, two tailed t-test).
Since stimulated diuresis is reduced in secCl mutants, we hypothesized that removing secCl from the MTs might lead to a reduction in overall fluid loss, which would increase resistance to desiccation. To test this hypothesis, we compared the survival of adult flies with secCl RNAi knock-down in the stellate cells with the corresponding controls (Fig. 6e). Flies with secCl knocked down in the MT stellate cells exhibited significantly prolonged survival compared to controls under conditions of desiccation, suggesting that loss of secCl in the MTs increases resistance to desiccation.
Discussion
The family of pentameric (Cys-loop) ligand-gated ion channels mediates fast, ionotropic neurotransmission in all bilateria studied. However, it is becoming increasingly clear that this family of ion channels has functions beyond ligand-gated neurotransmission. We previously showed that pHCl-2, which forms a proton gated anion channel, functions in the principal cells of MTs to modulate urine secretion17. Here we present evidence that a related channel subunit, secCl, forms a channel that mediates chloride flux in Malpighian tubules in response to diuretic hormones.
secCl is required for hormone-mediated diuresis in the Malpighian tubules
Transepithelial chloride secretion in the MTs is generally thought to occur through or around stellate cells. Chloride channels have been identified in excised apical membrane patches of stellate cells and chloride conductance “hotspots” identified by vibrating probe analysis have been shown to localize at stellate cells2, suggesting a transcellular route for chloride secretion. The diuretic actions of tyramine and leukokinin are mediated by a rise in transepithelial chloride secretion in stellate cells2,8,26. Signalling by both hormones requires the coupled action of phospholipase C (PLC) and inositol triphosphate (IP3) to increase intracellular calcium levels in stellate cells30 and the two signalling pathways display cross-desensitization at the level of intracellular calcium signaling5,31. However, the specific link between intracellular calcium signalling and increased chloride conductance has remained elusive.
We have shown that secCl expression is necessary for tyramine-mediated diuresis and that its expression in stellate cells is sufficient to restore diuresis in the mutant background. Thus, the secCl channel is a central element in the path from endocrine signalling to transepithelial chloride permeability (Fig. 7). Secretion in MTs is powered by an electrochemical proton gradient generated by a V-ATPase in principal cells. The lumen-positive apical electrochemical proton gradient is used to drive sodium and potassium transport into the lumen along with chloride counter ions. The resulting osmotic gradient drives fluid secretion. Basal FSR is limited by the chloride flux as indicated by: 1) the positive basal transepithelial potential, 2) the increase in chloride conductance when secretion is stimulated with diuretic hormones, and 3) the coincident collapse of the TEP to near zero potential as chloride conductance ceases to be limiting. Because TEP did not collapse in hormone-treated secCl mutants, we conclude that secCl is necessary specifically for the increase in chloride conductance. One possibility is that secCl is the chloride channel in the apical membrane of stellate cells that mediates the increase in chloride conductance in response to tyramine and leucokinin hormones.
Figure 7.
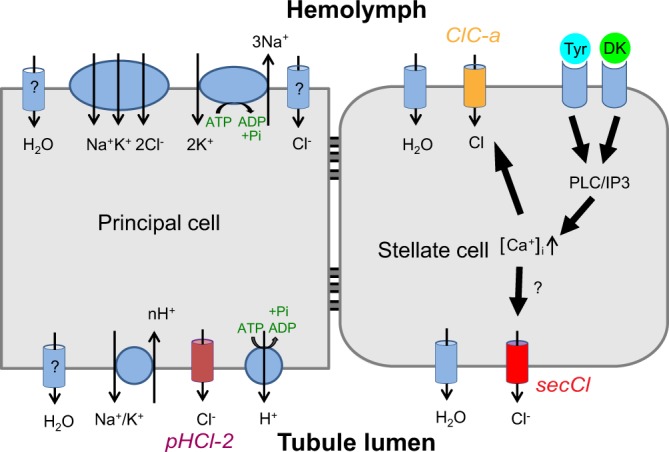
Model: The role of secCl within the tyramine/leucokinin diuretic pathway. A model describing the role of secCl within the signaling pathways of the diuretic hormones tyramine (Tyr) and Drosophila leucokinin (DK). Diuretic hormones bind to their respective GPCRs on the basolateral membrane of stellate cells, which triggers the release of calcium from intracellular stores via the PLC/IP3 pathway. Through an unknown mechanism, this rise in intracellular [Ca++] results in the activation of ClC-a (orange), and secCl (red). ClC-a, localized to the basolateral membrane, provides necessary Cl− entry into the cell from the hemolymph and secCl, localized to the apical membrane, provides a route for Cl− exit into the lumen. FSRs increase as a consequence of increased chloride secretion.
secCl shares its hormone-induced secretion phenotype with another chloride channel, ClC-a, raising the question of what may be their respective roles6. Like secCl, ClC-a is expressed exclusively in stellate cells and is necessary in stellate cells for leucokinin-induced fluid secretion, a rise in intracellular chloride and TEP collapse. Unlike secCl however, ClC-a is localized to both the basolateral and apical plasma membranes with apparently greater accumulation at the basolateral membrane. These results are consistent with models in which: 1) ClC-a is necessary for secCl function, 2) secCl is necessary for ClC-a function, or 3) secCl and ClC-a act additively. The third model seems the least plausible based on biophysical principles; TEP should be highly sensitive to membrane conductance. Thus, if the secCl and ClC-a channels acted in parallel, in the absence of one channel we should see a change in TEP upon diuretic stimulation as a result of increased conductance through the other channel. Instead, we see no change in TEP in response to tyramine or leucokinin in the secCl mutant. The first two models appear equally plausible. Both pLGIC- and ClC-type channels have been implicated in vesicle trafficking and either could be responsible for correct trafficking of the other channel to the plasma membrane32,33. Alternatively, ClC-a may be primarily necessary for chloride entry into stellate cells via the basolateral membrane, whereas secCl is primarily necessary for the exit of chloride through the apical membrane. Thus, both channels would be necessary for transepithelial chloride flux, TEP collapse and diuresis.
In a model where secCl is the apical membrane chloride channel or is necessary for its expression, secCl could be constitutively active or co-regulated by the GPCR pathway that controls basolateral chloride influx in response to tyramine and leucokinin. For example, calcium-activated, protein kinase C-mediated phosphorylation of the intracellular loop is a known mechanism of pLGIC regulation34 that could gate the secCl channel or regulate its transport to the apical membrane.
The secCl-mediated secretion in Drosophila physiology
secCl -mediated secretion is essential for viability as shown by the high lethality of flies lacking secCl. However, diuretic hormone-induced secretion in MTs is not necessary for viability in a laboratory setting, as secCl RNAi knock-down exclusively in stellate cells did not result in adult lethality (consistent with the viability of ClC-a mutants) nor did expression of secCl specifically in stellate cells rescue the lethality of the secCl mutant. Apparently secCl’s roles in the salivary glands and/or midgut are critical to viability, possibly because secretion in these tissues is essential for digestion. Instead, flies in which secCl was exclusively knocked down in stellate cells exhibited prolonged survival under conditions of desiccation, indicating that secCl’s role in hormone-induced diuresis is important for water balance.
secCl is not primarily a neurotransmitter receptor
secCl was previously identified, along with pHCl-2, as a highly divergent Cys-loop pLGIC subunit that is arthropod specific13,35. Although relatively divergent, secCl has the structural characteristics of pLGIC neurotransmitter-gated channel subunits. However, we found that secCl forms a homomeric channel in Xenopus oocytes that is constitutively ion permeable in the absence of any obvious ligand. Moreover promoter-GFP expression (here and in15), immunocytochemistry and RNA expression data20,36 show that secCl, like pHCl-2, is primarily expressed in non-innervated, secretory tissues: salivary glands, gastric caeca of third instar larvae, as well as in stellate cells of larval and adult MTs. The gastric caeca and salivary glands are secretory tissues of the digestive system and the MTs are part of the excretory system. The secCl-expressing tissues do not receive synaptic input from the nervous system and we confirmed that secCl is localized to the apical membrane of MT stellate cells and therefore not directly exposed to hormones dispersed in haemolymph. Thus, secCl is unlikely to be directly gated by a neuroendocrine ligand.
Is secCl’s role in secretion an ancestral function of pLGICs in metazoa?
secCl and the other channel subunits in its Drosophila clade, CG6927 and pHCl-2, are highly divergent relative not only to other pLGICs in Drosophila but also relative to the diverse pLGIC complements of other metazoan phyla: e.g. nematodes, molluscs and chordates12,13,35. Assuming that pLGIC subunits evolve at roughly equivalent rates, secCl and pHCl-2 would thus represent an ancestral clade retained in Drosphila but lost in other phyla. pLGICs are present in eubacteria, archaebacteria and unicellular eukaryotes and therefore predate metazoa and the evolution of nervous systems37,38. We propose that secCl and pHCl-2 may represent an ancestral function of pLGICs in metazoa prior to the evolution of complex nervous systems and the adaptation of pLGICs to their role as neurotransmitter receptors.
Methods
Cloning secCl cDNA
Whole RNA was purified from adult Oregon-R flies. First strand cDNA was synthesized using oligo (dT) primers and AMV (avian myeloblastosis virus) reverse transcriptase (Invitrogen). The secCl ORF was amplified by PCR (all primer sequences listed in Supplemental Data S2) and sub-cloned into the pDONR201 vector via the Gateway BP recombination system (Invitrogen) and verified by sequencing.
secCl expression in Xenopus oocytes and electrophysiology
secCl cDNA was subcloned into a modified pT7 Xenopus expression vector using EcoRV and Xba1 cloning sites. The resulting pT7-secCl construct was linearized with BamH1 prior to synthesizing capped RNA (cRNA) by in-vitro transcription using the mMESSAGE mMACHINE T7 kit (Ambion). Oocytes were harvested from mature Xenopus laevis and injected with cRNA according to standard procedures39 and Two Electrode Voltage Clamp (TEVC) recordings were performed in ND96 buffer (‘Normal Saline’) as described in Feingold et al.17. For ion substitutions experiments, we substituted sodium acetate for sodium chloride (“Low Cl”) and cesium chloride for sodium chloride (“Low Na”). All animal care protocols comply with McGill’s animal care Standard Operating Procedures; protocol number 2006–5284. All animal care procedures were approved by the MGill Facility Animal Care Committee (FACC).
Fly strains and maintenance
All Drosophila strains were maintained at room temperature and raised in standard cornmeal yeast sugar agar medium supplemented with dry yeast. As an apical membrane marker we used w*; P{UAS-mCD8.ChRFP}2 (Bloomington Drosophila Stock Center (BDSC) # 27391) driven in stellate cells by the tissue specific driver c724-Gal4 (a gift from Julian Dow)40. We used two secCl mutant backgrounds: the secCl deficiency, w1118; Df(3 L)Exel16132/TM6B,Tb (BDSC) # 7611), and w*; P{EP} secClG6893/G6893, which was derived from, y1,w*; p{EP} secClG6893/TM3,Sb1,Ser1 (BDSC # 27218) and has a P-element inserted in the first intron of secCl. To achieve tissue-specific knockdown of secCl, we used c724-Gal4 to drive w*; P{TRIP.JF02437}attP2, a UAS- secCl -RNAi strain generated from y1,v1; P{TRIP.JF02437}attP2 (BDSC #27090).
A secCl rescue transgene, P[secCl] was generated by PCR-amplification of the secCl locus, including the secCl gene as well as flanking sequence spanning 2 kb upstream and 1 kb downstream of the open reading frame, from Oregon-R genomic DNA, followed by subcloning into the pENTR-SD-TOPO vector (Invitrogen) and recombination via Gateway LR into the pTWH destination vector (Drosophila Genomics Resource Center, DGRC), which contains sequences for P-element mediated transgenesis. Transgenic w flies expressing p[secCl] were generated by BestGene Inc. (www.thebestgene.com, California) and an insertion that mapped to chromosome 2 was used for all experiments. To drive green fluorescent protein (GFP) expression from the secCl promoter, a genomic region including 2 kb of upstream DNA and the first exon of secCl was amplified and cloned into the pTWG destination vector (DGRC) by LR recombination using the intermediate pENTR-TOPO-SD Gateway system. Mutation of two ATG sites prevent translation of the in-frame secCl signal sequence. Transgenics were generated by BestGene Inc., as above. For tissue specific expression under the GAL4/UAS system, the secCl cDNA was cloned downstream of the UAS promoter in the pTWH vector (DGRC, not HA-tagged), and the resulting construct was injected into yw embryos41.
Immunohistochemistry
To raise polyclonal antibodies, the M3-M4 intracellular loop of secCl cDNA was amplified and the PCR product subcloned into the pEColi-Nterm 6xHN vector using the In-Fusion kit (Clontech). The peptide was expressed in BL21 cells and purified using Talon His-affinity binding columns (Clontech) and used to immunize rats. Antiserum was pre-absorbed to homozygous secClG6893 third instar larvae fixed in 4% (vol vol−1) formaldehyde/PBS and used at a final concentration of 1:1000. antiRat-Alexa Fluor 488 secondary antibody (Invitrogen) was pre-absorbed to fixed wild-type (Oregon-R) embryos and used at a concentration of 1:1000. Immunostaining was performed as described in Feingold et al.17.
Malpighian tubule dissection and Ramsay fluid secretion assays
MT dissections and Ramsay fluid secretion assays were carried out as described2,42,43 on adult females 3–10 days post eclosion. 1.5 minutes prior to the end of the first 40-minute interval, the bathing droplet (18 μl) was spiked with 2 μl Drosophila saline or 29 μM tyramine in Drosophila saline.
Viability assays on high salt diet
Flies were raised on a standard diet except water was replaced with a 0.5 M NaCl solution as described by Huang et al.27. Viability of flies in vials with high-salt food was scored every 24 hours for 6 days. On day 3, flies were transferred into vials containing fresh, high-salt food.
Desiccation assays
Adult females 3–10 days post eclosion were placed in empty vials in groups of 10 individuals. Survival was scored at time intervals, as indicated, until 100% mortality was reached.
Transepithelial potential assays
Malpighian tubules from adult females 3–10 days post eclosion were dissected in Drosophila saline and mounted on glass coverslips coated with 100 μg/ml poly-L lysine. A sharp electrode (R = 20–40 MΩ) filled with 3 M KCl pulled from theta septum borosilicate glass (Warner Instruments, Hamden, CT, USA) was used to impale the tubule using a PCS-5000 micromanipulator (EXFO Burleigh, Mississauga, ON, Canada)5. Only tubules with TEP > +20 mV were used. An Axopatch 200 A amplifier, a CV 201 A headstage and pClamp 8 software (Axon Instruments, Sunnyvale, CA, USA) were used to record TEPs. 50 μl of 29 μM tyramine (Sigma-Aldrich) in saline or saline alone was then added to the 600 μl bath to achieve a concentration of 2.2 μM. Similarly, leukokinin I (Sigma-Aldrich) was diluted from a 65 μM stock solution. The electrode was removed from the lumen to ensure that the baseline drift was <3 mV.
Statistical analysis
All data are presented as the mean ± SEM. Data were analyzed by two-tailed t-test in Igor-Pro (Wavemetrics) and ANOVA (Matlab, MathWorks).
Supplementary information
Acknowledgements
This research was supported by Natural Science and Engineering Research Council grant CRDPJ 401634-10 and a research contract with Chemtura Canada Co. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study. We thank Scott De Vito for help with immunostaining
Author Contributions
D.F., M.J.O., L.A.N. and J.A.D. designed experiments. D.F., L.K., T.S., L.A.N., J.A.D. performed experiments. D.F., L.K., L.A.N. and J.A.D. analysed data. D.F. and L.K. wrote the manuscript with editing by P.D., M.J.O., L.A.N. and J.A.D.
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-42849-9.
References
- 1.O’Donnell MJ, Dow JA, Huesmann GR, Tublitz NJ, Maddrell SH. Separate control of anion and cation transport in malpighian tubules of Drosophila melanogaster. J. Exp. Biol. 1996;199:1163–75. doi: 10.1242/jeb.199.5.1163. [DOI] [PubMed] [Google Scholar]
- 2.O’Donnell MJ, et al. Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am. J. Physiol. 1998;274:R1039–R1049. doi: 10.1152/ajpregu.1998.274.4.R1039. [DOI] [PubMed] [Google Scholar]
- 3.Beyenbach KW, Skaer H, Dow JAT. The Developmental, Molecular, and Transport Biology of Malpighian Tubules. Annu. Rev. Entomol. 2010;55:351–74. doi: 10.1146/annurev-ento-112408-085512. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor KR, Beyenbach KW. Chloride channels in apical membrane patches of stellate cells of Malpighian tubules of Aedes aegypti. J. Exp. Biol. 2001;204:367–378. doi: 10.1007/978-1-4615-1321-6_46. [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal EM. Regulation of chloride permeability by endogenously produced tyramine in the Drosophila Malpighian tubule. Am. J. Physiol. Cell Physiol. 2003;284:718–728. doi: 10.1152/ajpcell.00359.2002. [DOI] [PubMed] [Google Scholar]
- 6.Cabrero P, et al. Chloride channels in stellate cells are essential for uniquely high secretion rates in neuropeptide-stimulated Drosophila diuresis. Proc. Natl. Acad. Sci. USA. 2014;111:14301–6. doi: 10.1073/pnas.1412706111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollock VP, et al. NorpA and itpr mutants reveal roles for phospholipase C and inositol (1,4,5)- trisphosphate receptor in Drosophila melanogaster renal function. J. Exp. Biol. 2003;206:901–911. doi: 10.1242/jeb.00189. [DOI] [PubMed] [Google Scholar]
- 8.Rosay P, et al. Cell-type specific calcium signalling in a Drosophila epithelium. J. Cell Sci. 1997;110(Pt 1):1683–92. doi: 10.1242/jcs.110.15.1683. [DOI] [PubMed] [Google Scholar]
- 9.Beyenbach KW, Piermarini PM. Transcellular and paracellular pathways of transepithelial fluid secretion in Malpighian (renal) tubules of the yellow fever mosquito Aedes aegypti. Acta Physiol. (Oxf) 2011;202:387–407. doi: 10.1111/j.1748-1716.2010.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyenbach KW, Baumgart S, Lau K, Piermarini PM, Zhang S. Signaling to the apical membrane and to the paracellular pathway: changes in the cytosolic proteome of Aedes Malpighian tubules. J. Exp. Biol. 2009;212:329–340. doi: 10.1242/jeb.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pannabecker TL, Hayest TK, Beyenbach KW. Regulation of epithelial shunt conductance by the peptide leucokinin. J. Membr. Biol. 1993;132:63–76. doi: 10.1007/BF00233052. [DOI] [PubMed] [Google Scholar]
- 12.Dent JA. Evidence for a diverse Cys-loop ligand-gated ion channel superfamily in early bilateria. J. Mol. Evol. 2006;62:523–35. doi: 10.1007/s00239-005-0018-2. [DOI] [PubMed] [Google Scholar]
- 13.Jones AK, Sattelle ÆDB. The cys-loop ligand-gated ion channel superfamily of the honeybee, Apis mellifera. Inertebrate Neurosci. 2006;6:123–132. doi: 10.1007/s10158-006-0026-y. [DOI] [PubMed] [Google Scholar]
- 14.Mounsey KE, et al. Molecular characterisation of a pH-gated chloride channel from Sarcoptes scabiei. Invertebr. Neurosci. 2007;7:149–156. doi: 10.1007/s10158-007-0050-6. [DOI] [PubMed] [Google Scholar]
- 15.Remnant, E. J. et al. Evolution, Expression, and Function of Nonneuronal Ligand-Gated Chloride Channels in Drosophila melanogaster. G36, 2003–2012 (2016). [DOI] [PMC free article] [PubMed]
- 16.Semenov EP, Pak WL. Diversification of Drosophila chloride channel gene by multiple posttranscriptional mRNA modifications. J. Neurochem. 1999;72:66–72. doi: 10.1046/j.1471-4159.1999.0720066.x. [DOI] [PubMed] [Google Scholar]
- 17.Feingold D, Starc T, Donnell MJO, Nilson L, Dent JA. The orphan pentameric ligand-gated ion channel pHCl-2 is gated by pH and regulates fluid secretion in Drosophila Malpighian tubules. J. Exp. Biol. 2016;219:2629–2638. doi: 10.1242/jeb.141069. [DOI] [PubMed] [Google Scholar]
- 18.Galzi J-L, et al. Mutations in the channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature. 1992;359:500–505. doi: 10.1038/359500a0. [DOI] [PubMed] [Google Scholar]
- 19.Gunthorpe MJ, Lummis SCR. Conversion of the Ion Selectivity of the 5-HT 3A Receptor from Cationic to Anionic Reveals a Conserved Feature of the Ligand- gated Ion Channel Superfamily. J. Biol. Chem. 2001;276:10977–10983. doi: 10.1074/jbc.M009575200. [DOI] [PubMed] [Google Scholar]
- 20.Graveley B, Brooks A, Carlson J. The developmental transcriptome of Drosophila melanogaster. Nature. 2011;471:473–479. doi: 10.1038/nature09715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sine SM, Engel AG. Recent advances in Cys-loop receptor structure and function. Nature. 2006;440:448–455. doi: 10.1038/nature04708. [DOI] [PubMed] [Google Scholar]
- 22.Rousso T, Shewan AM, Mostov KE, Schejter ED, Shilo BZ. Apical targeting of the formin diaphanous in Drosophila tubular epithelia. Elife. 2013;2013:1–19. doi: 10.7554/eLife.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dow AT. Extremely high pH in biological systems: a model for carbonate transport. Am. J. Physiol. 1984;246:633–636. doi: 10.1152/ajpregu.1984.246.4.R633. [DOI] [PubMed] [Google Scholar]
- 24.Shanbhag S, Tripathi S. Epithelial ultrastructure and cellular mechanisms of acid and base transport in the Drosophila midgut. J. Exp. Biol. 2009;212:1731–1744. doi: 10.1242/jeb.029306. [DOI] [PubMed] [Google Scholar]
- 25.Phillips J. Comparative Physiology of Insect Renal-Function. Am. J. Physiol. 1981;241:R241–257. doi: 10.1152/ajpregu.1981.241.5.R241. [DOI] [PubMed] [Google Scholar]
- 26.Terhzaz S, et al. Isolation and characterization of a leucokinin-like peptide of Drosophila melanogaster. J. Exp. Biol. 1999;202:3667–3676. doi: 10.1242/jeb.202.24.3667. [DOI] [PubMed] [Google Scholar]
- 27.Huang X, et al. The Drosophila inebriated-encoded neurotransmitter/osmolyte transporter: Dual roles in the control of neuronal excitability and the osmotic stress response. Genetics. 2002;160:561–569. doi: 10.1093/genetics/160.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keyser P, Borge-Renberg K, Hultmark D. The Drosophila NFAT homolog is involved in salt stress tolerance. Insect Biochem. Mol. Biol. 2007;37:356–362. doi: 10.1016/j.ibmb.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Stergiopoulos K, Cabrero P, Davies S-A, Dow JAT. Salty dog, an SLC5 symporter, modulates Drosophila response to salt stress. Physiol. Genomics. 2009;37:1–11. doi: 10.1152/physiolgenomics.90360.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabrero P, Richmond L, Nitabach M, Davies SA, Dow JAT. A biogenic amine and a neuropeptide act identically: tyramine signals through calcium in Drosophila tubule stellate cells. Proc. Biol. Sci. 2013;280:20122943. doi: 10.1098/rspb.2012.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blumenthal EM. Characterization of transepithelial potential oscillations in the Drosophila Malpighian tubule. J. Exp. Biol. 2001;204:3075–84. doi: 10.1242/jeb.204.17.3075. [DOI] [PubMed] [Google Scholar]
- 32.Piwon N, Günther W, Schwake M, Bösl MR, Jentsch TJ. ClC-5 Cl–channel disruption impairs endocytosis in a mouse model for Dent’s disease. Nature. 2000;408:369–373. doi: 10.1038/35042597. [DOI] [PubMed] [Google Scholar]
- 33.Patton A, et al. Endocytosis function of a ligand-gated ion channel homolog in Caenorhabditis elegans. Curr. Biol. 2005;15:1045–1050. doi: 10.1016/j.cub.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 34.Talwar S, Lynch JW. Phosphorylation mediated structural and functional changes in pentameric ligand-gated ion channels: Implications for drug discovery. Int. J. Biochem. Cell Biol. 2014;53:218–223. doi: 10.1016/j.biocel.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 35.Dent, J. A. The Evolution of Pentameric Ligand-Gated Ion Channels. In Insect Nicotinic Acetylcholine Receptors (ed. Thany, S. H.) 1-12 (Landes Bioscience, 2009).
- 36.Chintapalli VR, Wang J, Dow JAT. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 2007;39:715–20. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 37.Tasneem A, Iyer LM, Jakobsson E, Aravind L. Identification of the prokaryotic ligand-gated ion channels and their implications for the mechanisms and origins of animal Cys-loop ion channels. Genome Biol. 2005;6:R4. doi: 10.1186/gb-2004-6-1-r4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaiteh M, Taly A, Henin J. Evolution of pentameric ligand-gated ion channels: Pro-loop receptors. PLoS One. 2016;11:1–24. doi: 10.1371/journal.pone.0151934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldin AL. Maintenance of Xenopus laevis and oocyte injection. Methods Enzymol. 1992;207:266–279. doi: 10.1016/0076-6879(92)07017-I. [DOI] [PubMed] [Google Scholar]
- 40.Sozen MA, Armstrong JD, Yang M, Kaiser K, Dow JT. Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc. Natl. Acad. Sci. USA. 1997;94:5207–5212. doi: 10.1073/pnas.94.10.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubin GM, Spradling AC. Genetic-Transformation of Drosophila With Transposable Element Vectors. Science (80-.) 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 42.Chahine S, Seabrooke S, Michael J, Donnell O. Effects of genetic knock‐down of organic anion transporter genes on secretion of fluorescent organicions by Malpighian tubules of Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2012;81:228–240. doi: 10.1002/arch.21066. [DOI] [PubMed] [Google Scholar]
- 43.Dow JAT, et al. Drosophila melanogastor: A Novel Phenotype for Studies of Fluid Secretion and its Control. J. Exp. Biol. 1994;197:421–428. doi: 10.1242/jeb.197.1.421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.