Abstract
Transgenic crops producing insecticidal proteins from Bacillus thuringiensis (Bt) are cultivated extensively, but rapid evolution of resistance by pests reduces their efficacy. We report a 3,370-bp insertion in a cadherin gene associated with resistance to Bt toxin Cry1Ac in the pink bollworm (Pectinophora gossypiella), a devastating global cotton pest. We found the allele (r15) harboring this insertion in a field population from China. The insertion is a miniature inverted repeat transposable element (MITE) that contains two additional transposons and produces two mis-spliced transcript variants (r15A and r15B). A strain homozygous for r15 had 290-fold resistance to Cry1Ac, little or no cross-resistance to Cry2Ab, and completed its life cycle on Bt cotton producing Cry1Ac. Inheritance of resistance was recessive and tightly linked with r15. For transformed insect cells, susceptibility to Cry1Ac was greater for cells producing the wild-type cadherin than for cells producing the r15 mutant proteins. Recombinant cadherin protein occurred on the cell surface in cells transformed with the wild-type or r15A sequences, but not in cells transformed with the r15B sequence. The similar resistance of pink bollworm to Cry1Ac in laboratory- and field-selected insects from China, India and the U.S. provides a basis for developing international resistance management practices.
Subject terms: Pathogens, Molecular biology
Introduction
Crops genetically engineered to produce insecticidal proteins from Bacillus thuringiensis (Bt) have been widely adopted for pest control, with a cumulative total of over 930 million hectares of Bt crops planted globally from 1996 to 20171. These Bt crops kill some major pests, but cause little or no harm to humans and most other organisms2–6. The benefits of Bt crops include pest suppression, decreased insecticide use, and enhanced biological control2–4. However, increasingly rapid evolution of resistance by insect pests has reduced these benefits7–10.
The most common mechanism of resistance to Bt toxins is reduced binding of toxins to midgut receptor proteins including cadherin, alkaline phosphatase, aminopeptidase N, and ATP-binding cassette transporters11–17. The insertion of transposons, which can confer resistance to chemical insecticides, can also cause resistance to Bt toxins by disrupting genes encoding Bt receptor proteins18–26.
This study focuses on a novel cadherin allele that harbors a transposon and is linked with resistance to Bt toxin Cry1Ac in pink bollworm (Pectinophora gossypiella), a devastating global pest of cotton. Pink bollworm has been exposed extensively to Bt cotton producing Cry1Ac in the world’s top three cotton-producing countries: China, India, and the U.S.27–29. In China and the U.S., Cry1Ac has remained effective against pink bollworm for the past two decades7,27,29. In India, however, this pest evolved practical resistance to Bt cotton producing Cry1Ac alone, then to Bt cotton producing both Cry1Ac and Cry2Ab7,28,30–32.
Previous work shows that resistance to Cry1Ac in pink bollworm is associated with mutations in the PgCad1 gene, which encodes a cadherin protein that binds Cry1Ac in susceptible larvae30,33–35. Fourteen PgCad1 resistance alleles have been identified: r1-r4 in lab-selected strains from the U.S.33,36, r5-r12 in field-selected populations from India30, and r13-r14 in a field-selected strain from the Yangtze River Valley of China35.
In this study, we detected a novel PgCad1 allele (r15) while screening pink bollworm collected in 2013 from field populations in the Yangtze River Valley of China. Here we characterize this allele in terms of its DNA sequence, including insertion of three nested transposons. We also analyzed the resistance in a strain homozygous for this allele, including inheritance, cross-resistance to Cry2Ab, as well as survival and other life history traits on Bt cotton. In addition, we used transfected insect cells to determine the effects of the mutant allele on cadherin protein movement within cells and the susceptibility of cells to Cry1Ac.
Results
Identification of the r15 cadherin allele
We started pink bollworm strain JL46 by pairing a field-collected male (#46) from Jianli in the Yangtze River Valley with a resistant female (cadherin genotype r1r1) from the lab-selected AZP-R strain from Arizona, U.S.A. Survival of their F1 offspring was 47% at the diagnostic concentration of Cry1Ac (10 μg Cry1Ac protoxin per ml diet), suggesting that the field-collected male carried one recessive resistance allele at the cadherin locus. We used a series of crosses, DNA screening, and selection with Cry1Ac to generate strain JL46 (Fig. S1). We discovered that strain JL46 had a novel cadherin allele, which we name r15, following the nomenclature convention for PgCad1 resistance alleles30,33,35.
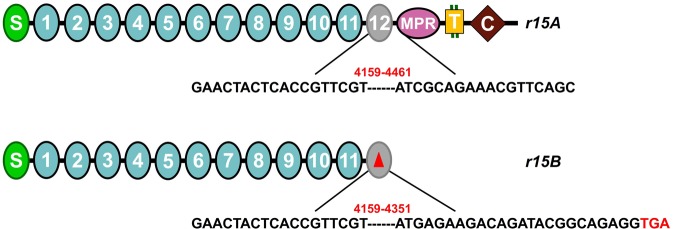
Sequencing cDNA of PgCad1 from JL46 revealed that r15 has two different transcripts. Transcript r15A (GenBank Acc# KY814704) has a 303-bp deletion (4,159–4,461) encoding a PgCad1 protein that lacks 101 amino acids, 88 from cadherin repeat twelve (CR12) and 13 from the membrane proximal region (MPR) (Figs 1 and S2). Transcript r15B (GenBank Acc# KY814705) has a 193-bp deletion (4,159–4,351) that creates a frameshift and introduces a premature stop codon at amino acid 1,394 (Figs 1 and S2). Sequencing of corresponding gDNA showed r15 has a 3,370 bp insertion (1,166–4,535) in exon 28 (GenBank Acc# KY814708), which is not in the wild-type (s) allele (GenBank Acc# MH266779) (Fig. 2). Alignment of cDNA sequences indicates that the 3,370-bp insertion causes skipping during pre-mRNA splicing that yields two aberrant transcripts: r15A missing exons 28 and 29, and r15B missing only exon 28 (Fig. S3).
Figure 1.
Predicted cadherin protein in pink bollworm strain JL46. The amino-terminal membrane signal sequence (S), cadherin repeats (1–12), membrane proximal region (MPR), transmembrane region (T), and cytoplasmic domain (C) are shown. Red numbers indicate deletions in the cDNA from r15A (303 bp) and r15B (193 bp). The red triangle indicates the truncation of the protein predicted from r15B because of the premature stop codon (red letters TGA).
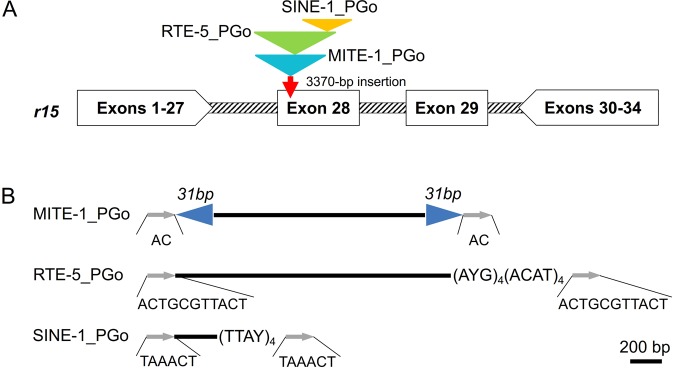
Figure 2.
Map of the PgCad1 r15 mutation. (A) r15 allele of PgCad1. The 3,370-bp insertion in exon 28 contains remnants of three nested transposons, each shown as a colored triangle. (B) Details of the three nested transposons. The primary structure of each transposon is drawn to scale. Gray arrows show target site duplications (TSDs) (sequences below the arrows). Blue arrowheads represent the 31-bp terminal inverted repeats (TIRs). (AYG)4(ACAT)4 in RTE-5_PGo and (TTAY)4 in SINE-1_PGo are microsatellite repeats.
Allele-specific PCR
Based on the 3,370-bp insertion in PgCad1 gDNA in the r15 allele relative to the s allele from the susceptible strain APHIS-S, we designed one pair of primers that amplified gDNA from r15 but not from s, and another pair that amplified gDNA from s, but not from r15 (Table S1). By testing individuals with both pairs of primers, we could distinguish among the three genotypes r15r15, ss, and r15s (Fig. S4). All of the larvae tested had the expected bands indicating r15r15 for JL46, ss for APHIS-S, and r15s for their F1 progeny (total n = 90 larvae, 30 of each type).
Characterization of three nested transposons in r15
By direct sequencing of the 3,370-bp insertion in r15, we discovered three new transposable elements (TEs) in exon 28 of PgCad1 (Figs 2 and S5). Based on their similarity to known TEs, we name these MITE1_PGo (a miniature inverted repeat transposable element), RTE-5_PGo (a retrotransposable element type 5), and SINE-1_PGo (a short interspersed nuclear element). These three TEs are nested: RTE-5_PGo within MITE1_PGo and SINE-1_PGo within RTE-5_PGo (Fig. 2).
MITE1_PGo (1,342 bp), which apparently was inserted first into exon 28, is AT rich (71%). It has 31-bp terminal inverted repeats (TIRs) (left TIR = ATATGGGCTATTATTTTATTTTGGGTCCCAT) flanked by 2-bp (AC) target site duplications (TSDs), and lacks coding potential (Figs 2 and S5).
RTE-5_PGo is 1,751 bp (1,941–3,680, 3,964–3,974) and flanked by 11-bp TSDs (ACTGCGTTACT) (Figs 2 and S5). A CENSOR search37 against the Repbase (http://www.girinst.org/repbase/index.html) shows that the first 503 bp of this TE corresponds to an apurinic-apyrimidic endonuclease (AP ENDO) domain and shares 76% identity with RTE-4_PPo (431–935) from Papilio polytes38 (Table S2). The final 982 bp of this insert corresponds to an apparent reverse transcriptase (RT) domain, sharing 77% identity with RTE-5_DPl (1,719–2,719) from Danaus plexippus39 (Table S2). Further alignments show that sequences corresponding to bases 243–612 and 634–1,611 (Fig. S6) share 71% and 75% identity with RTE-5_DPl (361–731 and 1,719–2,714), respectively. This indicates an internal deletion of about 1,000 bp occurs within this TE. It no longer has an intact open reading frame (ORF) because indels and other mutations yield frameshifts and pre-mature stop codons. Nonetheless, it does encode an interrupted and internally deleted endonuclease-reverse transcriptase with 50% amino acid identity within the putative AP ENDO domain and 74% identity within the RT domain from RTE-5_DPl (Fig. S7), as well as 80% identity (data not shown) in the RT domain with the Bombyx mori non-LTR retrotransposon BmRTE-d08 (GenBank accession nos. FJ265549.1). Its 3′ untranslated region (UTR) is short and ends with four AYG trinucleotide repeats followed by four ACAT tetramer repeats (Figs 2 and S5), the typical features found in the members of the RTE clade40.
SINE-1_PGo is 283 bp (3,681–3,963) (Figs 2 and S5). This TE is flanked by 6-bp (TAAACT) TSDs, lacks coding potential, and has four TTAY repeats at its 3′ end (Figs 2 and S5).
Inheritance of Cry1Ac resistance
Based on the concentration of Cry1Ac killing 50% of larvae (LC50), JL46 had 290-fold resistance relative to APHIS-S (Table 1). The responses to Cry1Ac for F1 larvae from the two reciprocal crosses between JL46 and APHIS-S were similar (Table 1), which indicates inheritance of resistance to Cry1Ac was autosomal (no sex linkage or maternal effects). Survival at the diagnostic concentration was 83% for JL46 and 0% for both APHIS-S and F1 progeny from the two reciprocal crosses (n = 72 larvae for each strain and reciprocal cross). At this concentration of Cry1Ac, the value for the dominance parameter h was 0 for both reciprocal crosses, indicating inheritance of resistance was completely recessive.
Table 1.
Responses to Cry1Ac of pink bollworm larvae from a resistant strain (JL46), a susceptible strain (APHIS-S), and their hybrid F1 progeny.
| Strain | Slope (SE)a | LC50 (95% FL)b | RRc |
|---|---|---|---|
| APHIS-S | 3.78 (0.336) | 0.097 (0.048–0.132) | |
| JL46 | 2.41 (0.506) | 28.0 (22.4–37.3) | 290 |
| JL46♀ × APHIS-S♂ | 3.34 (0.305) | 0.448 (0.397–0.497) | 4.6 |
| JL46♂ × APHIS-S♀ | 2.23 (0.223) | 0.548 (0.463–0.648) | 5.6 |
aSlope of the concentration-mortality line with its standard error in parentheses.
bConcentration killing 50% with 95% fiducial limits in parentheses, in μg Cry1Ac per ml diet.
cResistance ratio, the LC50 for JL46, JL46♀ × APHIS-S♂ or JL46♂ × APHIS-S♀ divided by the LC50 for APHIS-S.
Little or no cross-resistance to Cry2Ab
The LC50 of Cry2Ab was 30% higher for JL46 than APHIS-S, but this difference between strains is not statistically significant based on the conservative criterion of non-overlap of the 95% fiducial limits of the LC50 values (Table S3). Hence, JL46 had little or no cross-resistance to Cry2Ab.
Genetic linkage between resistance to Cry1Ac and PgCad1
We used genetic linkage analysis to test the hypothesis that Cry1Ac resistance in JL46 is linked with the r15 allele (Table S4). We generated five backcross families, each from a single-pair cross between a JL46 female and an F1 male (JL46 × APHIS-S). For the backcross progeny on untreated diet, the mean percentage of larvae that were r15r15 (49%) did not differ significantly from the 50% expected under random segregation (one-sample t-test, df = 4, P = 0.56, Table S4). For the backcross progeny on diet treated with the diagnostic concentration of Cry1Ac, all 105 survivors were r15r15. The proportion of r15r15 survivors was significantly higher on treated diet than that on control diet (Fisher’s exact test, P < 10−18). These results indicate Cry1Ac resistance is tightly linked with r15 in JL46.
Survival and other life history traits on Bt and non-Bt cotton
Larval survival on Bt cotton bolls was significantly higher for JL46 (13.0%) than APHIS-S (0.0%) (t-test, t = 28.7, df = 4, P < 0.0001, Table S5). However, on non-Bt cotton bolls, larval survival did not differ significantly between JL46 (27.1%) and APHIS-S (31.1%) (t-test, t = −2.7, df = 4, P = 0.052, Table S5). Relative survival, calculated as larval survival on Bt cotton divided by larval survival on non-Bt cotton, was significantly higher for JL46 (48.0%) than for APHIS-S (0.0%) (t-test, t = 14.0, df = 4, P < 0.0001).
On non-Bt cotton bolls, pupal weight did not differ significantly between JL46 and APHIS-S, but the time to develop from neonate to pupa was significantly longer for JL46 (16.7 days) than that for APHIS-S (15.0 days) (Table 2). For JL46, the time to pupation was also significantly longer on Bt cotton (21.8 days) than on non-Bt cotton (16.7 days), and pupal weight on Bt cotton (11.9 mg) was significantly less than on non-Bt cotton (14.3 mg) (Table 2). For JL46, both survival from neonate to adult and the proportion of adults that were female were significantly lower on Bt cotton than that on non-Bt cotton (Fisher’s exact test, P < 0.0001 for each trait). By contrast, no difference occurred between Bt cotton and non-Bt cotton for eggs laid per female (t-test, t = 1.6, df = 5, P = 0.17) and hatching rate of eggs (t-test, t = 0.21, df = 12, P = 0.84) (Table 3). Based on all of the above measures, the net reproductive rate41 for JL46 was four times higher on non-Bt cotton than that on Bt cotton, indicating incomplete resistance of JL46 to Bt cotton.
Table 2.
Time to pupation and pupal weight for pink bollworm on Bt and non-Bt cotton bolls.
| Strain | Cotton type | Number of pupae | Time to pupation (days) | Pupal wt. (mg) |
|---|---|---|---|---|
| APHIS-S | Non-Bt | 70 | 15.0 ± 0.2a | 13.7 ± 0.4a |
| JL46 | Non-Bt | 60 | 16.7 ± 0.2b | 14.3 ± 0.5a |
| JL46 | Bt | 23 | 21.8 ± 0.5c | 11.9 ± 0.6b |
Values are means ± SE. Different lower case letters within columns indicate significant differences between treatments based on ANOVA followed by Tukey’s HSD.
Table 3.
Life history traits of resistant pink bollworm strain JL46 on Bt and non-Bt cotton bolls.
| Trait | Bt | Non-Bt | Bt/non-Bt |
|---|---|---|---|
| Neonate to adult survival | 0.11 | 0.23 | 0.48 |
| Proportion of females | 0.36 | 0.44 | 0.82 |
| Eggs per female | 133 ± 30 | 209 ± 34 | 0.64 |
| Hatch rate | 0.83 ± 0.04 | 0.82 ± 0.02 | 1.01 |
| Net reproductive ratea | 4.4 | 17.3 | 0.25 |
aNet reproductive rate = neonate to adult survival × proportion of females × eggs per female × hatch rate41.
PgCad1 cellular trafficking and susceptibility to Cry1Ac in transformed cells
We used Hi5 cells transfected with expression vectors to produce recombinant PgCad1 proteins fused with a green fluorescent protein (sPgCad1-GFP, r15APgCad1-GFP, or r15BPgCad1-GFP) (Fig. 3). Transfection efficiencies (mean % ± SD) did not differ significantly between sPgCad1-GFP (69 ± 15%), r15APgCad1-GFP (72 ± 7%), and r15BPgCad1-GFP (61 ± 8%) (one-way ANOVA, P = 0.47).
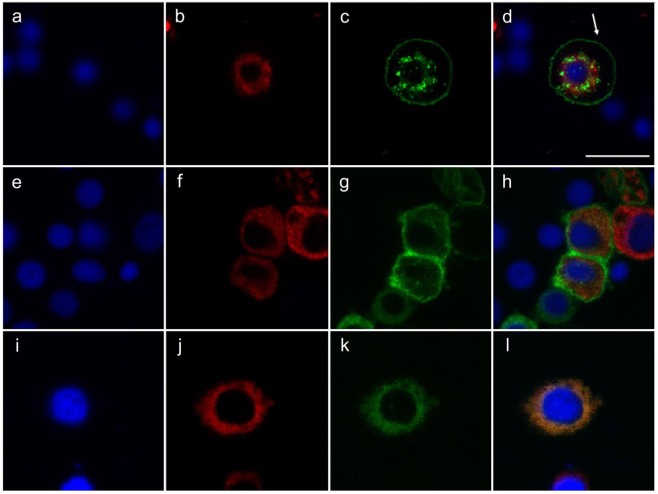
Figure 3.
Cellular localization of PgCad1 proteins within Hi5 cells. Hi5 cells transfected with pIE2-sPgCad1-GFP (a–d), pIE2-r15APgCad1-GFP (e–h), or pIE2-r15BPgCad1-GFP (i–l). Nuclei stained with Hoechst 3342 are shown in blue, dsRED-labeled endoplasmic reticulum shown in red, and GFP-labeled PgCad1 fusion proteins are shown in green. Superimposed images from (a–c) are shown in (d), from (e–g) in (h), and from (i–k) in (l). The arrow in (d) indicates cell membrane. Bar = 20 μm.
Hi5 cells producing PgCad1 proteins were co-transfected the pIE2-DsRed2-ER vector that specifically labels the endoplasmic reticulum (ER) with a red fluorescent protein (Fig. 3). Whereas sPgCad1-GFP and r15APgCad1-GFP localized primarily with the cell membrane (Fig. 3a–h), r15BPgCad1-GFP localized with the ER (Fig. 3i–l). Immunoblots with transfected cell extracts confirmed that the recombinant fusion proteins produced by the transformed cells had the expected molecular weights (sPgCad1-GFP = 253 kDa; r15APgCad1-GFP = 240 kDa; and r15BPgCad1-GFP = 209 kDa) (Fig. S8).
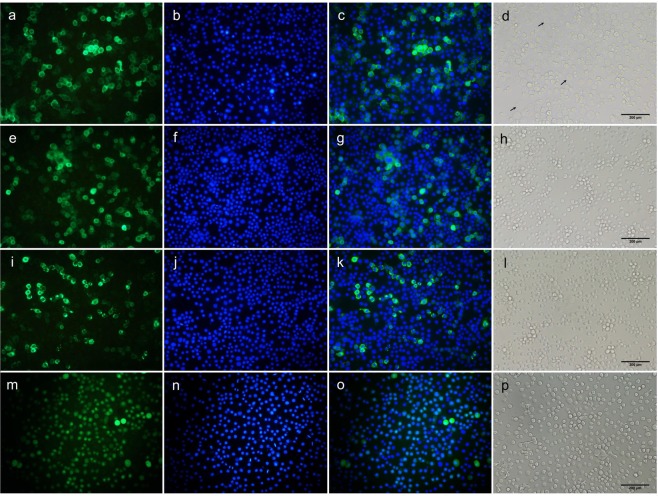
Treatment of the transformed Hi5 cells with Cry1Ac caused swelling and cell lysis in cells producing sPgCad1-GFP (Fig. 4a–d) but not in cells producing r15APgCad1-GFP (Fig. 4e–h), r15BPgCad1-GFP (Fig. 4i–l), or GFP (Fig. 4m–p). The concentration of Cry1Ac causing swelling in 50% of the sPgCad1-GFP cells (EC50) was 7.3 μg/ml (95% FL = 6.2 to 8.4). In contrast, no swelling was observed for cells producing r15APgCad1-GFP or r15BPgCad1-GFP at the highest concentration tested (40 μg Cry1Ac/ml).
Figure 4.
Susceptibility to Cry1Ac of Hi5 cells producing PgCad1 proteins. Hi5 cells transfected with pIE2-sPgCad1-GFP (a–d), pIE2-r15APgCad1-GFP (e–h), pIE2-r15BPgCad1-GFP (i–l) or the empty vector pIE2-GFP (m–p) were treated with Cry1Ac (10 μg Cry1Ac per ml for cells producing sPgCad1-GFP and 40 μg Cry1Ac per ml for r15A- and r15BPgCad1-GFP and GFP cells) and observed for swelling using fluorescence microscopy. Nuclei stained with Hoechst 3342 are shown in blue and PgCad1-GFP fusion proteins are shown in green. Superimposed images from (a,b) are shown in (c), from (e,f) in (g), from (i,j) in (k) and from (m,n) in (o). Arrows in (d) indicate representative swollen cells. Bars shown in (d,h,l,p) = 200 μm.
Discussion
Understanding the genetic basis of pest resistance is increasingly important for sustaining the efficacy of Bt crops29,42. Here, we discovered and characterized a novel mutant cadherin allele (r15) of the pink bollworm gene PgCad1 from a field population in China. The r15 allele contains a 3,370-bp insertion in exon 28 that results in disrupted pre-mRNA splicing and produces two aberrant transcript variants (r15A and r15B). Both variants result from exon skipping, with the r15A transcript lacking exons 28 and 29, and the r15B transcript missing only exon 28.
Previous results provide two other examples of transposable element insertion into PgCad1 associated with mis-splicing and resistance to Cry1Ac26,30. In the r3 allele found in some laboratory-selected strains from Arizona, insertion of a 4,739-bp intact, active non-LTR retrotransposon causes loss of exon 2126. The r5 allele detected in a field-selected larva from India harbors a 3,120-bp insertion that shares sequence similarity with several transposable elements30.
Mis-splicing of cadherin pre-mRNA that does not involve TE insertion is also associated with resistance to Cry1Ac in pink bollworm from the U.S., India, and China30,33,35. For example, the 126-bp r2 deletion from Arizona leads to mis-splicing and complete loss of exon 1633. In India, the PgCad1 r10 allele results in three alternatively spliced transcripts30. Such alternative exon usage is also present in pink bollworm from China, as shown here with the r15A and r15B transcript variants. The PgCad1 r13 mutation from China also involves mis-splicing, in which the 314-bp deletion results in complete loss of exon 3135.
Although the r15 mutation is novel, the resistance in JL46 is similar to that of other Cry1Ac-resistant strains from the U.S. and China that have cadherin resistance alleles (e.g., r1–r4 and r13)33,35,36,41,43,44. Like JL46, these strains have recessive inheritance of high levels of resistance to Cry1Ac and little or no cross-resistance to Cry2Ab. Although their larvae can survive on Bt cotton producing Cry1Ac, the resistance is incomplete, as indicated by reduced performance on Bt cotton relative to non-Bt cotton. Here we found the net reproductive rate on Bt cotton relative to non-Bt cotton for JL46 (0.25) was similar to that of the AQ47 strain from China (0.16) that harbors the r13 PgCad1 allele35 and with U.S. resistant strains carrying r1-r3 alleles (0.35)43.
Similar to previous results with the r13 allele of PgCad1 and the mHaCad allele of Helicoverpa armigera35,45, our data with the r15B protein suggest that failure of cadherin to move to the cell membrane causes resistance. By contrast, we found that the r15A protein did occur on the cell membrane, which implies a different mechanism of resistance, such as reduced toxin binding.
Pink bollworm resistance to Bt cotton remains a threat, especially where non-Bt cotton refuges are scarce. In India, farmers have generally not planted such refuges, pink bollworm resistance to Bt cotton producing Cry1Ac and Cry2Ab is a serious problem, and no Bt traits are currently available to control resistant insects28,30–32. In the Yangtze River Valley of China, the frequency of pink bollworm resistance to Cry1Ac initially went up, then declined from 2011–2015, corresponding with increased abundance of non-Bt cotton generated by the rise in planting of F2 hybrid cotton29. Nonetheless, the continued presence of cadherin resistance alleles in field populations demonstrated here and previously35 suggests that resistance could rise again in this region if refuges are not sufficiently abundant. The similar resistance of pink bollworm to Cry1Ac in lab- and field-selected strains from China, India and the U.S. provides a basis for developing resistance management practices that extends beyond national borders.
Materials and Methods
Insects and toxins
We used three strains of pink bollworm: JL46 from Jianli, Hubei Province of China; and AZP-R and APHIS-S from Arizona in the U.S. APHIS-S is a susceptible strain reared in the laboratory in Arizona and then China without exposure to Bt toxins35,46,47. AZP-R is a Cry1Ac-resistant strain that originated from pooled survivors from 10 populations derived in 1997 from Arizona cotton fields48. As part of an F1 screen used for Cry1Ac resistance monitoring in 2013, the JL46 strain was created by a single-pair cross between a field-collected male (#46) from Jianli and a resistant female (genotype r1r1) from AZP-R. From the F1 progeny of that cross, we selected for survivors on 10 μg Cry1Ac per mL diet and used DNA-based screening from F2 single pair crosses to eliminate individuals harboring the r1 cadherin allele (Fig. S1). The end result was the homozygous r15r15 JL46 strain.
All three strains were maintained at 29 ± 1 °C, 50 ± 10% relative humidity (RH) and a photoperiod of 16:8 (L:D) as previously described47. Larvae were reared on wheat germ diet46 and AZP-R and JL46 were selected every fifth generation on 10 μg Cry1Ac protoxin per mL diet (n = 960 larvae per strain per selection). For all exposure of larvae to toxins in diet for bioassays and selection, we used the protoxin form of Cry1Ac and Cry2Ab purchased from Zhongbao Biotechnology Company, Beijing, China. For cell assays, we used activated Cry1Ac toxin purchased from Marianne Pusztai-Carey (Case Western Reserve University, Cleveland, OH), as described previously35.
PgCad1 cloning
We cloned and sequenced full-length complementary DNA (cDNA) and partial genomic DNA (gDNA) of PgCad1 from fourth instar larvae of JL46 strain survivors on a diagnostic concentration of Cry1Ac (e.g. 10 μg Cry1Ac protoxin per mL diet). Total RNA and gDNA were extracted from fourth instar individual larvae (n = 8) using TaKaRa MiniBEST Universal RNA Extraction Kit (TaKaRa). M-MLV Reverse Transcriptase (Promega) was used for first strand cDNA synthesis and full-length PgCad1 cDNA was PCR amplified using primers F1 + R1 and F2 + R2 (Table S1) Primers were designed based on full-length cDNA sequence of BtR-s allele (Genbank Accession Number AY198374.1) from APHIS-S. For cloning gDNA flanking the r15 mutation site, we used gF46 + gR46 (Table S1) and LA-Taq (TAKARA, Dalian, China) to PCR amplify the PgCad1 partial gDNA fragment. PCR conditions were: 94 °C for 2 min, followed by 32 cycles at 98 °C for 10 s, 60 °C for 30 s, and 72 °C for 3 min, and a final extension at 72 °C for 7 min. PCR products were cloned into pGEM®-T Easy Cloning Vector (Promega) cloning vector and DNA sequencing was performed as previously described35.
Bioassays
Diet bioassays were conducted to compare larval susceptibility of JL46 and APHIS-S strains to both Cry1Ac and Cry2Ab. The concentrations of Cry1Ac (in μg Cry1Ac protoxin per ml diet) were 0 (control), 0.05, 0.1, 0.15, 0.2, 0.25 and 10 for APHIS-S (n = 120 larvae per treatment); 0 (control), 1.25, 2.5, 5, 10, 20 and 40 for JL46 (n = 72 larvae per treatment); and 0 (control), 0.125, 0.25, 0.5, 1, 2 and 4 for the F1 offspring from both APHIS-S × JL46 reciprocal crosses (n = 96 larvae per treatment). The concentrations of Cry2Ab (in μg Cry2Ab per ml diet) were 0 (control), 0.03, 0.06, 0.12, 0.24, 0.48, 0.96 and 1.92 for APHIS-S (n = 72 larvae per treatment); and 0 (control), 0.05, 0.1, 0.2, 0.4, 0.8 and 1.6 for JL46 (n = 72 larvae per treatment) for JL46.
Boll bioassays were conducted to observe specific life history traits for JL46 and APHIS-S reared from larvae on either non-Bt cotton bolls (Simian-3) or bolls from Cry1Ac Bt cotton (GuoXin H318). We tested 10–12 Bt cotton bolls and 13–15 non-Bt cotton bolls in three replicated tests for each strain (n = 300 per boll type per replicate). Neonates were transferred to bolls using a fine brush and we counted and recorded the number of entry holes, exit holes, the date of pupation, pupal weight, and sex ratio as described previously35.
Inheritance of resistance to Cry1Ac
We set up two reciprocal mass crosses including 20 female APHIS-S × 20 male JL46 and 20 female JL46 × 20 male APHIS-S in 2 L cylindrical boxes. Adults were allowed to mate and lay eggs. Newly emerged F1 neonates were tested in bioassays using the Cry1Ac concentration series outlined above for reciprocal crosses. Neonates from JL46 and APHIS-S were included as reference strains. Dominance (h), which varies from 0 for completely recessive resistance to 1 for completely dominant resistance, was determined from survival at 10 μg Cry1Ac per mL diet adjusted for control mortality.
Genetic linkage between resistance to Cry1Ac and r15
To test for genetic linkage between resistance to Cry1Ac and r15, we generated F1 progeny from a single-pair cross between a single APHIS-S male and one JL46 female. Then, five F1 males were paired with JL46 resistant females to generate the backcross families. The F2 progeny from these five families were bioassayed on diet with or without 10 μg Cry1Ac per mL diet to determine whether resistance to Cry1Ac is tightly linked with PgCad1 r15. Because crossing over only occurs in male Lepidoptera49, we only used F1 males to produce backcross families and test the tightness of genetic linkage. A total of 90 neonates were bioassayed for each backcross family, including control diet (n ~ 30) and on 10 μg Cry1Ac per mL diet (n ~ 60). We conducted the allele-specific PCR test according to method mentioned above to identify the genotype of fourth instar larvae either from control diet or from treated diet for each backcross family. In total, 258 larvae were genotyped, including 153 on control diet (n = 30, 32, 30, 30 and 31 for each backcross family) and 105 larvae on 10 μg Cry1Ac per mL diet (n = 20, 24, 20, 20 and 21 larvae for each family).
Expression vectors and transfection of cultured insect cells
Total RNA isolated from 4th instar larval midguts from JL46 and APHIS-S was used to make cDNA as indicated above. Amplicons corresponding to full-length r15A and r15B were PCR amplified from JL46 cDNA using high-fidelity thermostable DNA polymerase (Thermo) (see Table S1 for primers). The full-length PgCad1_s was amplified from APHIS-S cDNA. Each cDNA was cloned into the pIE2-EGFP-N1 expression vector containing the green fluorescent protein (GFP) reporter gene50 used to generate the fusion proteins r15APgCad1-GFP, r15BPgCad1-GFP, and sPgCad1-GFP. All PgCad1 PCR products were amplified to include EcoRI and SacII restriction sites for cloning into pIE2-EGFP-N1. Recombinant vectors were used to transfect Hi5 cells (BTI-Tn-5B1–4 cell line provided by Peter Tijssen, University of Quebec, Canada). The endoplasmic reticulum (ER) of insect Hi5 cells was labeled by pDsRed2-ER as previously described45. Transfection and calculation of transfection efficiency were performed as previously described35.
Expression of recombinant PgCad1-GFP in Hi5 cells
Equal amounts (2 μg) of pIE2-sPgCad1-GFP, pIE2-r15APgCad1-GFP, pIE2-r15BPgCad1-GFP or the empty vector pIE2-GFP (as a negative control) were used to transfect Hi5 cells. Cells were seeded, recovered, lysed and finally analyzed by immunoblotting. After cell lysis, the total protein concentration for each sample was determined by using Pierce® BCA protein assay kit (Thermo) following the manufacturer’s instructions and an equal amount of total protein (40 μg) was separated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and successively incubated with primary and secondary antibodies as previously demonstrated35.
Cell toxicity assays
Twenty-four hours post-transfection, we observed cytotoxicity of the cells with and without Cry1Ac using fluorescence microscopy (Nikon). Whereas pIE2-sPgCad1-GFP-transfected cells were treated either with no toxin or with 10 μg per mL Cry1Ac, pIE2-r15APgCad1-GFP and pIE2-r15BPgCad1-GFP and the empty vector pIE2-GFP were either treated with no toxin or with 40 μg per mL Cry1Ac. Toxicity was estimated by the proportion of swollen cells as previously shown50. Each treatment was repeated three times with a total of six fields of vision used to quantify cell mortality.
Statistical analysis
We analyzed larval diet bioassay data with probit regression using IBM SPSS Statistics 22.0 to determine LC50 values and their 95% fiducial limits (FL), and slopes of the concentration-mortality lines and their standard errors (SE)51. We also used probit regression to analyze cell toxicity data to determine the concentration of Cry1Ac causing swelling of 50% of cells (EC50) and its 95% FL. We estimated the dominance parameter h based on survival at the diagnostic concentration adjusted for control mortality as described previously52. In the linkage analysis, for the backcross progeny on untreated diet, we used a one-sample t-test to determine if the observed percentage of larvae that were r15r15 differed significantly from the 50% expected under random segregation. We used Fisher’s exact test to determine if the proportion of larvae that were r15r15 differed significantly between the survivors on treated and untreated diet. For the boll bioassays, we calculated larval survival in each of the three replicates as the number of survivors divided by the number of entry holes. We also calculated relative survival for each of the three replicates as larval survival on Bt cotton divided by larval survival on non-Bt cotton. We used standard t-tests to determine if significant differences occurred between the resistant strain (JL46) and the susceptible strain (APHIS-S) in larval survival on Bt cotton and relative survival, as well as in larval survival, development time, and pupal weight on non-Bt cotton. In addition, we used standard t-tests to determine if significant differences occurred for JL46 between Bt and non-Bt cotton in development time, pupal weight, eggs laid per female, and hatching rate of eggs. For JL46, we also used Fisher’s exact test to determine if survival from neonate to adult and the proportion of adults that were female differed significantly between Bt and non-Bt cotton.
Supplementary information
Acknowledgements
This work was supported by China’s Key Project for Breeding Genetically Modified Organisms (Grant 2016ZX08012-004) and by Agriculture and Food Research Initiative Competitive Grant no. 2018-67013-27821 from the USDA National Institute of Food and Agriculture. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Author Contributions
K.W. designed this study. L.W., J.W., Y.M., S.C. and D.X. performed the experiments. L.W., P.W., K.L., Y.X., X.L. and B.E.T. analyzed and interpreted the data. L.W., K.W., J.A.F., X.L. and B.E.T. wrote the manuscript. All authors have read and approved the final manuscript.
Competing Interests
B.E.T. is co-author of a patent on modified Bt toxins, ‘Suppression of Resistance in Insects to Bacillus thuringiensis Cry Toxins, Using Toxins that do not Require the Cadherin Receptor’ (patent numbers: CA2690188A1, CN101730712A, EP2184293A2,EP2184293A4, EP2184293B1, WO2008150150A2, WO2008150150A3). DuPont Pioneer, Dow AgroSciences, Monsanto, Bayer CropScience, and Syngenta did not provide funding to support this work, but may be affected financially by publication of this paper and have funded other work by B.E.T. Bayer, DuPont, Syngenta and Gowan did not provide funding to support this work, but may be affected financially by publication of this paper and have funded other work by X. Li. J.A.F. is coauthor of a patent “Cadherin Receptor Peptide for Potentiating Bt Biopesticides” (patent numbers: US20090175974A1, US8354371, WO2009067487A2, WO2009067487A3). DuPont Pioneer and Bayer CropScience did not provide funding to support this work, but may be affected financially by publication of this paper and have funded other work by J.A.F.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43889-x.
References
- 1.James, C. Global Status of Commercialized Biotech/GM Crops in 2017: Biotech crop adoption surges as economic benefits accumulate in 22 years. ISAAA Brief No. 53 (ISAAA, Ithaca, NY) (2017).
- 2.Gould, F. et al. National Academies of Sciences, Engineering, and Medicine. Genetically Engineered Crops: Experiences and Prospects. (The National Academies Press, Washington, DC) (2016). [PubMed]
- 3.Lu Y, Wu K, Jiang Y, Guo Y, Desneux N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature. 2012;487:362–365. doi: 10.1038/nature11153. [DOI] [PubMed] [Google Scholar]
- 4.Dively GP, et al. Regional pest suppression associated with widespread Bt maize adoption benefits vegetable growers. Proc Natl Acad Sci USA. 2018;115:3320–3325. doi: 10.1073/pnas.1720692115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comas C, Lumbierres B, Pons X, Albajes R. No effects of Bacillus thuringiensis maize on nontarget organisms in the field in southern Europe: a meta-analysis of 26 arthropod taxa. Transgenic Res. 2014;23:135–143. doi: 10.1007/s11248-013-9737-0. [DOI] [PubMed] [Google Scholar]
- 6.Nicolia A, Manzo A, Veronesi F, Rosellini D. An overview of the last 10 years of genetically engineered crop safety research. Crit Rev Biotechnol. 2014;34:77–88. doi: 10.3109/07388551.2013.823595. [DOI] [PubMed] [Google Scholar]
- 7.Tabashnik BE, Carrière Y. Surge in insect resistance to transgenic crops and prospects for sustainability. Nat Biotechnol. 2017;35:926–935. doi: 10.1038/nbt.3974. [DOI] [PubMed] [Google Scholar]
- 8.Tabashnik BE. Tips for battling billion-dollar beetles. Science. 2016;354:552–553. doi: 10.1126/science.aag101. [DOI] [PubMed] [Google Scholar]
- 9.Smith JL, Lepping MD, Rule DM, Farhan Y, Schaafsma AW. Evidence for field-evolved resistance of Striacosta albicosta (Lepidoptera: Noctuidae) to Cry1F Bacillus thuringiensis protein and transgenic corn hybrids in Ontario, Canada. J Econ Entomol. 2017;110:2217–2228. doi: 10.1093/jee/tox228. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasena DI, et al. Characterization of field-evolved resistance to Bacillus thuringiensis-derived Cry1F delta-endotoxin in Spodoptera frugiperda populations from Argentina. Pest Manag Sci. 2018;74:746–754. doi: 10.1002/ps.4776. [DOI] [PubMed] [Google Scholar]
- 11.Pardo-Lopez L, Soberon M, Bravo A. Bacillus thuringiensis insecticidal three-domain Cry toxins: mode of action, insect resistance and consequences for crop protection. FEMS Microbiol Rev. 2013;37:3–22. doi: 10.1111/j.1574-6976.2012.00341.x. [DOI] [PubMed] [Google Scholar]
- 12.Wu YD. Detection and mechanisms of resistance evolved in insects to Cry toxins from Bacillus thuringiensis. Adv Insect Physiol. 2014;47:297–342. doi: 10.1016/B978-0-12-800197-4.00006-3. [DOI] [Google Scholar]
- 13.Peterson B, Bezuidenhout CC, Van den Berg J. An overview of mechanisms of Cry toxin resistance in lepidopteran insects. J Econ Entomol. 2017;110:362–377. doi: 10.1093/jee/tow310. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, et al. Mutation of an aminopeptidase N gene is associated with Helicoverpa armigera resistance to Bacillus thuringiensis Cry1Ac toxin. Insect Biochem Mol Biol. 2009;39:421–429. doi: 10.1016/j.ibmb.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Xiao Y, et al. Mis-splicing of the ABCC2 gene linked with Bt toxin resistance in Helicoverpa armigera. Sci Rep. 2014;4:6184. doi: 10.1038/srep06184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, et al. Intra- and extracellular domains of the Helicoverpa armigera cadherin mediate Cry1Ac cytotoxicity. Insect Biochem Mol Biol. 2017;86:41–49. doi: 10.1016/j.ibmb.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Qiu L, et al. Downregulation of Chilo suppressalis alkaline phosphatase genes associated with resistance to three transgenic Bacillus thuringiensis rice lines. Insect Mol Biol. 2018;27:83–89. doi: 10.1111/imb.12349. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Schuler MA, Berenbaum MR. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol. 2007;52:231–253. doi: 10.1146/annurev.ento.51.110104.151104. [DOI] [PubMed] [Google Scholar]
- 19.Claudianos C, Brownlie J, Russell R, Oakeshott J, Whyard S. maT–a clade of transposons intermediate between mariner and Tc1. Mol Biol Evol. 2002;19:2101–2109. doi: 10.1093/oxfordjournals.molbev.a004035. [DOI] [PubMed] [Google Scholar]
- 20.Catania F, et al. World-wide survey of an Accord insertion and its association with DDT resistance in Drosophila melanogaster. Mol Ecol. 2004;13:2491–2504. doi: 10.1111/j.1365-294X.2004.02263.x. [DOI] [PubMed] [Google Scholar]
- 21.Schlenke TA, Begun DJ. Strong selective sweep associated with a transposon insertion in Drosophila simulans. Proc Natl Acad Sci USA. 2004;101:1626–1631. doi: 10.1073/pnas.0303793101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aminetzach YT, Macpherson JM, Petrov DA. Pesticide resistance via transposition-mediated adaptive gene truncation in Drosophila. Science. 2005;309:764–767. doi: 10.1126/science.1112699. [DOI] [PubMed] [Google Scholar]
- 23.Darboux I, Charles JF, Pauchet Y, Warot S, Pauron D. Transposon-mediated resistance to Bacillus sphaericus in a field-evolved population of Culex pipiens (Diptera: Culicidae) Cell Microbiol. 2007;9:2022–2029. doi: 10.1111/j.1462-5822.2007.00934.x. [DOI] [PubMed] [Google Scholar]
- 24.Gahan LJ, Gould F, Heckel DG. Identification of a gene associated with Bt resistance in Heliothis virescens. Science. 2001;293:857–860. doi: 10.1126/science.1060949. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Chen H, Wu Y, Yang Y, Wu S. Mutated cadherin alleles from a field population of Helicoverpa armigera confer resistance to Bacillus thuringiensis toxin Cry1Ac. Appl Environ Microb. 2007;73:6939–6944. doi: 10.1128/AEM.01703-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fabrick JA, Mathew LG, Tabashnik BE, Li X. Insertion of an intact CR1 retrotransposon in a cadherin gene linked with Bt resistance in the pink bollworm, Pectinophora gossypiella. Insect Mol Biol. 2011;20:651–665. doi: 10.1111/j.1365-2583.2011.01095.x. [DOI] [PubMed] [Google Scholar]
- 27.Tabashnik BE, et al. Suppressing resistance to Bt cotton with sterile insect releases. Nat Biotechnol. 2010;28:1304–1307. doi: 10.1038/nbt.1704. [DOI] [PubMed] [Google Scholar]
- 28.Dhurua S, Gujar GT. Field-evolved resistance to Bt toxin Cry1Ac in the pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), from India. Pest Manag Sci. 2011;67:898–903. doi: 10.1002/ps.2127. [DOI] [PubMed] [Google Scholar]
- 29.Wan P, et al. Hybridizing transgenic Bt cotton with non-Bt cotton counters resistance in pink bollworm. Proc Natl Acad Sci USA. 2017;114:5413–5418. doi: 10.1073/pnas.1700396114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fabrick JA, et al. Alternative splicing and highly variable cadherin transcripts associated with field-evolved resistance of pink bollworm to Bt cotton in India. PloS One. 2014;9:e97900. doi: 10.1371/journal.pone.0097900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naik CBV, et al. Field-evolved resistance of pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), to transgenic Bacillus thuringiensis (Bt) cotton expressing crystal 1Ac (Cry1Ac) and Cry2Ab in India. Pest Manag Sci. 2018;74:2544–2554. doi: 10.1002/ps.5038. [DOI] [PubMed] [Google Scholar]
- 32.Mathew LG, et al. ABC transporter mis-splicing associated with resistance to Bt toxin Cry2Ab in laboratory- and field-selected pink bollworm. Sci Reports. 2018;8:13531. doi: 10.1038/s41598-018-31840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morin S, et al. Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. Proc Natl Acad Sci USA. 2003;100:5004–5009. doi: 10.1073/pnas.0831036100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fabrick JA, Tabashnik BE. Binding of Bacillus thuringiensis toxin Cry1Ac to multiple sites of cadherin in pink bollworm. Insect Biochem Mol Biol. 2007;37:97–106. doi: 10.1016/j.ibmb.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, et al. Resistance to Bacillus thuringiensis linked with a cadherin transmembrane mutation affecting cellular trafficking in pink bollworm from China. Insect Biochem Mol Biol. 2018;94:28–35. doi: 10.1016/j.ibmb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Fabrick JA, Tabashnik BE. Similar genetic basis of resistance to Bt toxin Cry1Ac in boll-selected and diet-selected strains of pink bollworm. PloS One. 2012;7:e35658. doi: 10.1371/journal.pone.0035658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohany O, Gentles AJ, Hankus L, Jurka J. Annotation, submission and screening of repetitive elements in Repbase: RepbaseSubmitter and Censor. BMC Bioinformatics. 2006;7:474. doi: 10.1186/1471-2105-7-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kojima, K. K. & Jurka, J. Non-LTR retrotransposons from the common mormon genome. Repbase Reports15, 3155–3155, https://www.girinst.org/2015/vol15/issue9/RTE-4_PPo.html (2015).
- 39.Jurka, J. Non-LTR retrotransposons from the monarch butterfly genome. Repbase Reports18, 129–129, https://www.girinst.org/2018/vol18/issue1/RTE-5_DPl.html (2018).
- 40.Malik HS, Eickbush TH. The RTE class of non-LTR retrotransposons is widely distributed in animals and is the origin of many SINEs. Mol Biol Evol. 1998;15:1123–1134. doi: 10.1093/oxfordjournals.molbev.a026020. [DOI] [PubMed] [Google Scholar]
- 41.Tabashnik BE, Dennehy TJ, Carriere Y. Delayed resistance to transgenic cotton in pink bollworm. Proc Natl Acad Sci USA. 2005;102:15389–15393. doi: 10.1073/pnas.0507857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soberon M, et al. Engineering modified Bt toxins to counter insect resistance. Science. 2007;318:1640–1642. doi: 10.1126/science.1146453. [DOI] [PubMed] [Google Scholar]
- 43.Tabashnik BE, et al. Association between resistance to Bt cotton and cadherin genotype in pink bollworm. J Econ Entomol. 2005;98:635–644. doi: 10.1603/0022-0493-98.3.635. [DOI] [PubMed] [Google Scholar]
- 44.Fabrick JA, et al. Multi-toxin resistance enables pink bollworm survival on pyramided Bt cotton. Sci Rep. 2015;5:16554. doi: 10.1038/srep16554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao Y, et al. A single point mutation resulting in cadherin mis-localization underpins resistance against Bacillus thuringiensis toxin in cotton bollworm. J Biol Chem. 2017;292:2933–2943. doi: 10.1074/jbc.M116.768671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartlett, A. C. & Wolf, W. W. Pectinophora gossypiella. Handbook of Insect Rearing. (Moore, R. F. & Singh, P. eds). Amsterdam: Elsevier Scientific Publishing, 415–430, 10.1016/0169-4758(86)90143-2 (1985).
- 47.Liu YB, Tabashnik BE, Meyer SK, Carriere Y, Bartlett AC. Genetics of pink bollworm resistance to Bacillus thuringiensis toxin Cry1Ac. J Econ Entomol. 2001;94:248–252. doi: 10.1603/0022-0493-94.1.248. [DOI] [PubMed] [Google Scholar]
- 48.Tabashnik BE, et al. Frequency of resistance to Bacillus thuringiensis in field populations of pink bollworm. Proc Natl Acad Sci USA. 2000;97:12980–12984. doi: 10.1073/pnas.97.24.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heckel DG, Gahan LJ, Liu YB, Tabashnik BE. Genetic mapping of resistance to Bacillus thuringiensis toxins in diamondback moth using biphasic linkage analysis. Proc Natl Acad Sci USA. 1999;96:8373–8377. doi: 10.1073/pnas.96.15.8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu P, et al. Expression of recombinant and mosaic Cry1Ac receptors from Helicoverpa armigera and their influences on the cytotoxicity of activated Cry1Ac to Spodoptera litura Sl-HP cells. Cytotechnology. 2016;68:481–496. doi: 10.1007/s10616-014-9801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jia, C. S. Calculating the LC50 of insecticides with software SPSS. Chin. Bull. Entomol. 43, 414–417, http://www.cnki.com.cn/Article/CJFDTotal-KCZS200603034.htm (2006).
- 52.Liu, Y. & Tabashnik, B. E. Inheritance of resistance to the Bacillus thuringiensis toxin Cry1C in the diamondback moth. Appl. Environ. Microbiol.63, 2218–2223, http://www.ncbi.nlm.nih.gov/pubmed/16535623 (1997). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.