Abstract
Salinity is one of the most important abiotic stresses, especially in arid regions. Such devastating constraint is converted mainly to oxidative burst. Thus, plants have to develop strategies to scavenge salt-related regenerated oxidant molecules. In the present work, fully aged plants derived from two Vitis vinifera L. cultivars, the Tunisian autochthonous tolerant genotype Razegui and the salt sensitive Syrah, were analyzed regarding their short term response to 100 mM NaCl, in hydroponic cultures. The ratio [ASA/ASA + DHA] was calculated on the basis of the oxidation of ascorbic acid (ASA) into dehydroascorbic acid (DHA) in leaves. Results proved that oxidative stress was generated. This led to the accumulation of malondialdehyde which referred to a lipid peroxidation mainly in the sensitive Syrah. In order to cope with these oxidative disturbances, trans-resveratrol as well as its glucosides trans-piceid and cis-piceid have been de novo synthesized in the sensitive variety. Razegui stilbene concentrations were presented here for the first time and unexpectedly did not show a very important variation during the salt elicitation.
Keywords: Salt stress, Oxidation, Tolerance, Antioxidant, Resveratrol, Piceid
Introduction
Much attention has been drawn to the antioxidant proprieties of crop plants for their biological benefits for human consumption and also for their involvement in plant’s anti-stress protection. Biotic and abiotic environmental stresses enhance the biosynthesis of antioxidant molecules in crops. The grapevine, as one of the worldwide major economically cultivated crops, is generally planted in areas where it is under permanent stressed, like in soils with salinity levels ranging from 10 to 100 mM (Flowers et al. 1977).
Salt stress, as a devastating constraint, affects different facets of plant’s physiological homeostasis and development processes, causing damages like membrane disorganization (Fabris et al. 2008), cytoplasm alkalinisation (Suhita et al. 2004), reactive oxygen species (ROS) induction (Petrov et al. 2015), photosynthesis deregulation (Dinakar et al. 2012), ionic and ionic related channels and transport perturbations (Ismail et al. 2014a) and tissue proliferation (Wani et al. 2013).
Salinity related ROS generation causes several damages such as lipid peroxidation and changes in the potential redox status (Toumi et al. 2008), and as an ultimate effect, nucleic acid damage. Crop plants have developed several mechanisms to scavenge ROS and to ‘buffer’ their levels according to the cell requiremnets. In this context, and to offset oxidative stress, plant cells employ enzymatic and non-enzymatic antioxidative mechanisms. The first category of mechanisms requires enzymatic systems like superoxide dismutase, catalase, ascorbate peroxidase, glutathione reductase, monodehydroascorbate reductase (Gill and Tuteja 2010). The second category includes molecules like ascorbic acid, glutathione, alkaloids, non-protein amino acids, α-tocopherol and phenolic compounds (Carvalho et al. 2015). In the latter group, stilbenes emerge as an important polyphenol subfamily produced to act against oxidative species (Chong et al. 2009). Stilbenes have the property to be synthesized by few plant families such as Pinaceae, Myrtaceae, Fogacea, Liliaceae, Moraceae, Papilionaceae and Vitaceae (Bavaresco and Fregoni 2001). They can also be accumulated as phytoalexins in plant tissues. In fact, stilbenes can be produced de novo by elicitors and become part of active plant defense mechanisms, initiated in response to chemical elicitors formed either from pathogen’s invasion or from abiotic environmental stress (Pezet et al. 2003).
Resveratrol (3,5,4′-trihydroxystilbene) is a very important stilbene and is widely shown to be a very potent antioxidant and free radical scavenger (Mikulski and Molski 2010). Resveratrol is produced by a stilbene synthase (STS) and is naturally existing as two geometric isoforms (trans and cis). Trans-resveratrol is the most stable isoform but can be transformed into cis-resveratrol by UV treatment (Figueiras et al. 2011). Glycosyltransferase catalyzes resveratrol glycosylation and generates trans- and cis- piceid (resveratrol 3-O-β-glucosides). It is synthesized as a response to pathogen infection (Boubakri et al. 2013) as well as abiotic stresses such as UV irradiation (Maurer et al. 2017), drought (Villangó et al. 2016) and salinity (Ismail et al. 2012, 2014b; Kostopoulou et al. 2014; Caddeo et al. 2017). However, in plant cells, excess trans-resveratrol accumulation can induce the oxidative burst and act as programmed cell death trigger (Chang et al. 2011). In this respect, trans-resveratrol must be under control to avoid high concentration. This idea is supported by the finding that WRKY8 transcription factor keeps resveratrol under negative regulation through the decrease of the stilbene synthase gene expression (Jeandet et al. 2019).
Grapevine is recognized as one of the few worldwide highly cultivated crop plants that produce considerable amounts of resveratrol in different organs such as berries and leaves (Griesser et al. 2015; Pugajera et al. 2018). Razegui is a Tunisian autochthonous grapevine variety that is cultivated in harsh environmental conditions where different abiotic stresses, mainly drought and salinity, exist or co-exist permanently or periodically. The native Razegui has been classified as a salt-tolerant genotype (Hanana et al. 2008; Jellouli et al. 2008; Daldoul et al. 2010).
While the secondary metabolism of several Tunisian autochthonous grape varieties were studied (Souid et al. 2008), Razegui’s relative phenolic profiles remain yet to be elucidated, especially in comparison with the well-known international varieties. Regarding the emerging value of stilbenes in the stress defense, the objective of this study was to analyze the salt cultivars-differential responses mainly through the accumulation of resveratrol and piceid in two Vitis vinifera varieties, Syrah and Razegui. Thus, in the present work, we established for the first time the stilbene profile of the salt-tolerant Tunisian autochthonous genotype Razegui.
Materials and methods
Plant material
Two V. vinifera cultivars: the Tunisian autochthonous Razegui and the French introduced Syrah were used in this work. The plants were obtained from dormant cuttings rooted in sand substrate in growth room (T: 25 °C day/20 °C night, humidity: 60–70%, photoperiod: 16/8) before they were transplanted into perlite and cultivated in a greenhouse under controlled conditions (T: 24 °C, humidity: 98%, photoperiod 16/8). Six months old plantlets were single potted in the nutrient solution as described by Hewitt (1966). After acclimation, plantlets were subjected to NaCl (100 mM) for a period of 24 h followed directly by a recovery treatment. During the recovery period, the plants were removed from the saline solution and reintroduced in the nutritive solution as described by Hewitt (1966).
ASA redox state measurement
Ascorbic acid (ASA) and dehydroascorbic acid (DHA) amounts were determined as described by Wang et al. (1991). Redox state was measured by the calculation of the ratio [ASA/ASA + DHA] based on the oxidation of ascorbic acid (ASA) into dehydroascorbic acid (DHA).
Lipid peroxidation measurement
Lipid peroxidation was measured via the quantification of malondialdehyde (MDA) according to the method of Heath and Packer (1968). 100 mg of fresh leaf tissue was homogenized with 1.2 mL of trichloroacetic acid 5% (TCA) and 0.12 mL methanolic butylated hydroxytoluene (BHT, 0.5 g L−1). The homogenate was incubated for 30 min at 90 °C, and then centrifuged for 15 min at 9000 rpm. An aliquot of the supernatant was recovered and added to an equal volume of thiobarbituric acid 0.67% (TBA), the mixture was incubated at 95 °C for 30 min before being cooled and O.D. was measured at 600 nm and 532 nm.
Stilbenes extraction
From each variety, 5 g of leaves was weighed and then extracted by 50 mL of MeOH/HCOOH (88/2 v:v) (1:10 w/v). The mix was homogenized using a blender for 10 min and kept for 1 h in the darkness with constant shaking. Samples were then centrifuged for 20 min at 1000 rpm. Supernatants were diluted twice in the MeOH/HCOOH (88/2 v:v). All the solvents used were of HPLC quality and likewise chemicals were of analytical grade (> 99%). Commercial standards of stilbenes and 3-glucoside of quercetin were obtained from Sigma Aldrich.
The obtained solutions were injected into the chromatographic system. The compounds in each sample were identified by comparing their retention time and UV spectra in the 280–306 nm range with those of standards.
Stilbenes were quantified by absorbance at λ = 306 nm and with external calibration by trans-resveratrol.
HPLC–DAD-ESI-MSn analysis of stilbenes
Analyses were carried out on an Agilent 1 100 Series system (Waldbronn, Germany), equipped with DAD (G13115B) and a LC/MSD Trap VL (G2445C VL) electrospray ionisation mass spectrometry (ESI-MSn) system and coupled to an Agilent Chem station (version B.01.03) data-processing station. The mass spectra data were processed with the Agilent LC/MS Trap software (version 5.3). 10 μL of polyphenol extract were injected on a reversed-phase column Zobra Eclipse XDB-C18 (4.6 × 250 mm; 5 μm particle; Agilent Waldbronn, Germany), and thermostated at 40 °C. For detection and quantification of 6 compounds, the chromatograms were recorded at 280, 306 and 320 nm. This was performed with a ternary mobile phase gradient: Pump A: HCOOH/MeCH/H2O (8.5%; 3%; 88.5%), Pump B: HCOOH/MeCH/H2O (8.5%; 50%; 41.5%); Pump C: HCOOH/MeOH/H2O (8.5%; 90%; 90%). The elution gradient used was: 100% A (min 0); 100% A (min 2); 92% A + 8% B (min 5); 14% B + 86% C (min 17); 18% B + 82% C (min 22); 21.5% B + 8.5% C (min 32); 43% B + 57% C (min 62); 100% A (min 70); and 100% A (min 75). The trans isomers of resveratrol and piceid were identified according to their order of elution and retention times of pure standards (Sigma, St. Louis, USA). Also ESI-MSn was used, employing the following parameters: positive ionisation mode; dry gas, N2, 11 mL min−1; drying temperature, 350 °C; nebulizer, 65 psi; capillary, − 2500 V; capillary exit offset, 70 V; skimmer 1, 20 V; skimmer 2, 6 V; compound stability, 100%; scan range, 50–1200 m/z.
Statistical analysis
A one-way analysis of variance (ANOVA) was achieved using STATISTICA program (Stat Soft, France). Means were compared, using Duncan test at a significance level of p ≤ 0.05.
Results
The salinity effects on the redox status
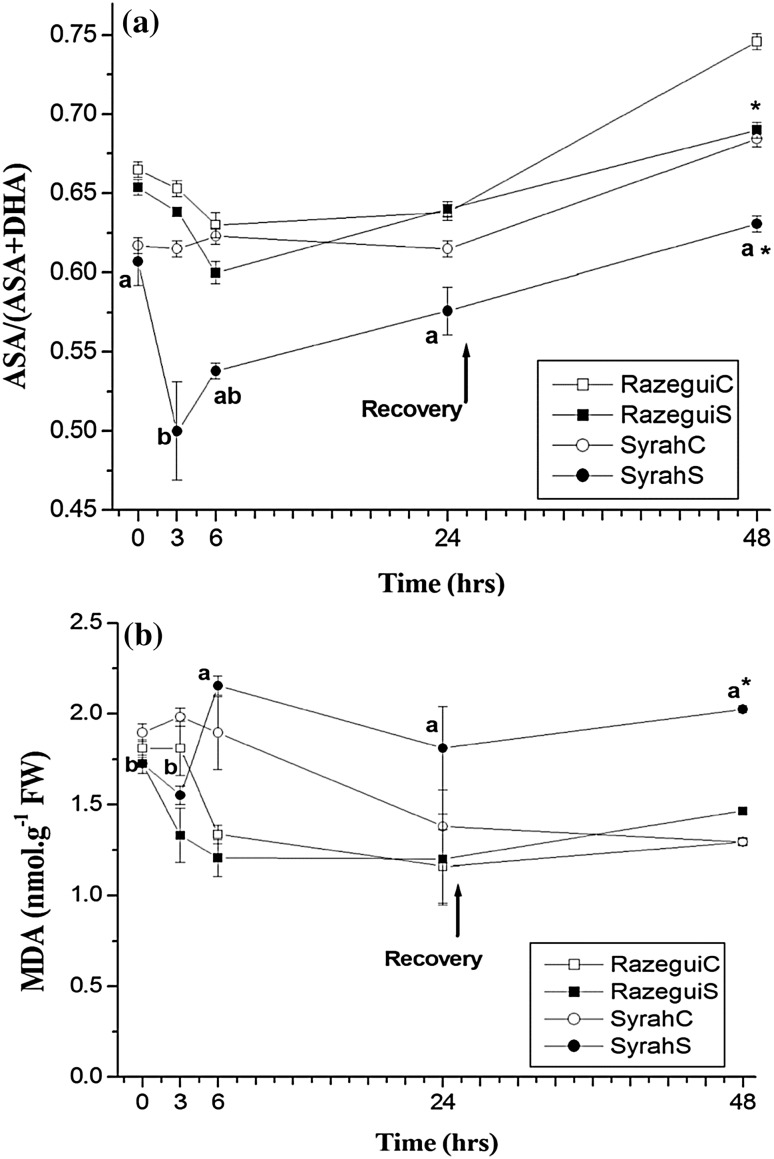
First we quantified the amount of ascorbic acid (ASA) and its oxidative derivative, dehydroascorbic acid (DHA). The ratio [ASA/(ASA + DHA)] gives information about the endogenic redox balance in the analyzed samples. A decrease in the ASA oxidation ratio in stressed leaves was exhibited earlier for Syrah compared to Razegui. In fact, in the Syrah cultivar, we recorded an abrupt decrease of the redox status after 3 h of salt application followed by a gradual correction. Such a singular result, can be a major distinguishing trait of the salt-shock effect amongst genotypes. Razegui and Syrah were expected to normalize the redox status. However, measurements after the recovery period showed that both varieties do not achieve the re-establishment of normalized ASA status (Fig. 1a).
Fig. 1.
Salt induced ROS expressed via measurement of (a) ASA redox status and (b) lipid peroxidation following the accumulation of malondialdehyde (MDA) in: Razegui control (RazeguiC), Razegui stressed (RazeguiS), Syrah control (SyrahC) and Syrah stressed (SyrahS). Evaluation was carried out in leaf samples of plants exposed to a 24 h salt stress treatment (NaCl at 100 mM) in controlled conditions of hydroponic culture, followed by a 24 h recovery phase. Data represent mean values and standard errors of 3 independent experiments. Letter suffixes express significant differences between the values among the salt stress Syrah at p < 0.05 according to Duncan’s multiple range test. No significant difference has been shown among values in the control and Razegui stressed leaves. Asterisk (*) indicates significant difference between control and stressed leaves after recovery
As a second trait for ROS effect evaluation during NaCl stress, we measured the amounts of the malondialdehyde (MDA) as the direct product of the peroxidation of lipid species in the leaves. Surprisingly, MDA amounts were not significantly modified in Razegui stressed plants. The measurements of MDA in Syrah showed that perceptive increase started to be recorded after 6 h of stress (Fig. 1b). During the recovery phase, Syrah failed to normalize the excessive titers of MDA in the leaves, which can lead to presenting the speculation that lipid peroxidation is an irreversible trait under stress in sensitive genotypes, especially if it affects lipids in membranes compartments.
The effects of salinity on the accumulation of stilbenes
Stilbenes were detected in the two V. vinifera cv. genotypes (the introduced Syrah and the autochthonous Razegui) that differ in their sensitivity to osmotic stress. The NaCl sensitivity degree of the studied varieties has been previously characterized proving that Syrah was salt-sensitive and Razegui was salt-tolerant (Jellouli et al. 2008; Daldoul et al. 2010).
Taking into consideration the effect of stilbene compounds on the ROS scavenging and their antioxidant proprieties, trans-resveratrol as well as its two glucosides trans- and cis-piceid were examined. Leaves from both varieties were harvested at four time points, 0 h, 8 h, 16 h and 24 h, during salt (100 mM NaCl) exposure, and after 24 h of recovery period, i.e. the culture of stressed vines were kept out of saline solution for 24 h.
Trans-resveratrol accumulation during salt stress
Table 1 presents the calculated concentrations of stilbene compounds in Syrah and, for the first time, in the autochtonous salt-tolerant Razegui. It shows that trans-resveratrol, trans-piceid and cis-piceid were detected already in leaf extracts from both grape varieties under non-stress condition. However, the cis isomer of resveratrol was not detected in any of the studied varieties.
Table 1.
Leaf stilbene concentrations (mg g−1 DW) in Razegui and Syrah Vitis vinifera before salt treatment. Results are the average ± SE of 3 independent experiments. Different letters in the same row indicate significant differences between different grape varieties according to Duncan test (p < 0.05)
| Razegui | Syrah | |
|---|---|---|
| trans-resveratrol | 0.043b ± 0.002 | 0.637a ± 0.306 |
| trans-piceid | 0.553a ± 0.148 | 1.176a ± 0.486 |
| cis-piceid | 1.35 × 10−8b ± 0.03 × 10−8 | 0.211a ± 0.048 |
| Total stilbenes | 0.597b ± 0.148 | 2.024a ± 0.294 |
Leaf extracts of Razegui variety contained low concentrations of trans-resveratrol and resveratrol glucosides. In contrast, a significant higher concentration of stilbenes was detected in Syrah.
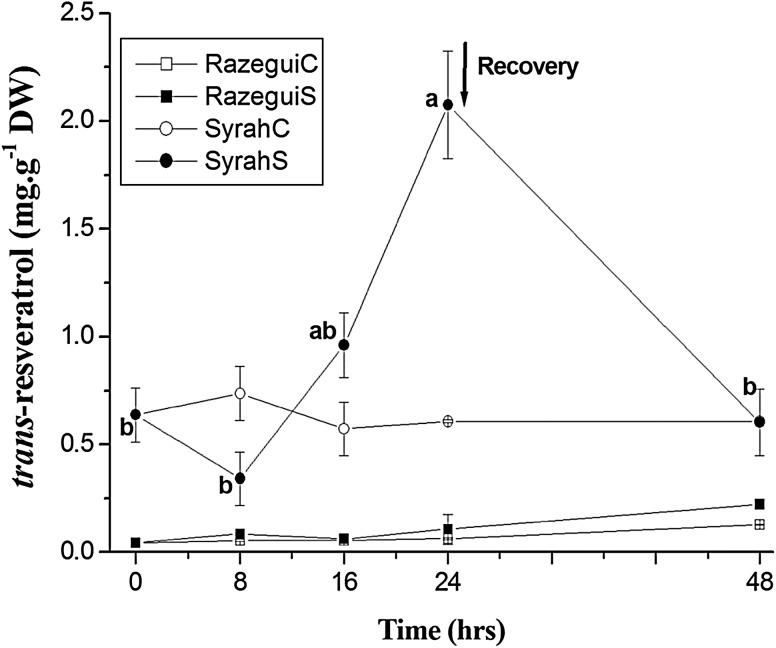
The biosynthesis of each stilbene compound during salt treatment was correlated with the tolerance degree of each variety. In fact, trans-resveratrol, which was supposed to be accumulated as a secondary signal of the elicitor-triggered hypersensitive response (Guerrero et al. 2010), did not show any significant change in quantity in Razegui stressed leaves. However, for the salt-sensitive Syrah, an unexpected decrease in trans-resveratrol concentration was found through 8 h of elicitation. A gradual significant increase of trans-resveratrol concentration was then observed, reaching 2.075 mg g−1 DW at 24 h of salt stress which is 3.42-fold higher than the respective control content (Fig. 2).
Fig. 2.
Time course of the trans-resveratrol accumulation in: Razegui control (RazeguiC), Razegui stressed (RazeguiS), Syrah control (SyrahC) and Syrah stressed (SyrahS). Evaluation was carried out in leaf samples of plants exposed to a 24 h salt stress treatment (NaCl at 100 mM) in controlled conditions of hydroponic culture, followed by a 24 h recovery phase. Data represent mean values and standard errors of 3 independent experiments. Letter suffixes express significant differences between the values among the salt stress Syrah at p < 0.05 according to Duncan’s multiple range test. No significant difference has been shown among the control and Razegui stressed leaves
During the 24 h of post-treatment period, trans-resveratrol concentration decreased in Syrah leaves which proved that its accumulation was induced by the salt stress (Fig. 2).
Trans-piceid and cis-piceid accumulation during salt stress
Piceid is a 3-O-β-glucoside of resveratrol derivative induced by glycosylation. In stilbene-producing plant species such as V. vinifera, large amounts of piceid (tans- and cis-isomers) accumulate to ensure their storage, transport and protection from peroxidative degradation (Chong et al. 2009). In fact, in Razegui and Syrah leaves, the sum of initial trans-piceid and cis-piceid exceeded their respective free aglycone content, and Syrah seemed to be among genotypes with higher basal levels of pre-formed piceid isomers (Table 1).
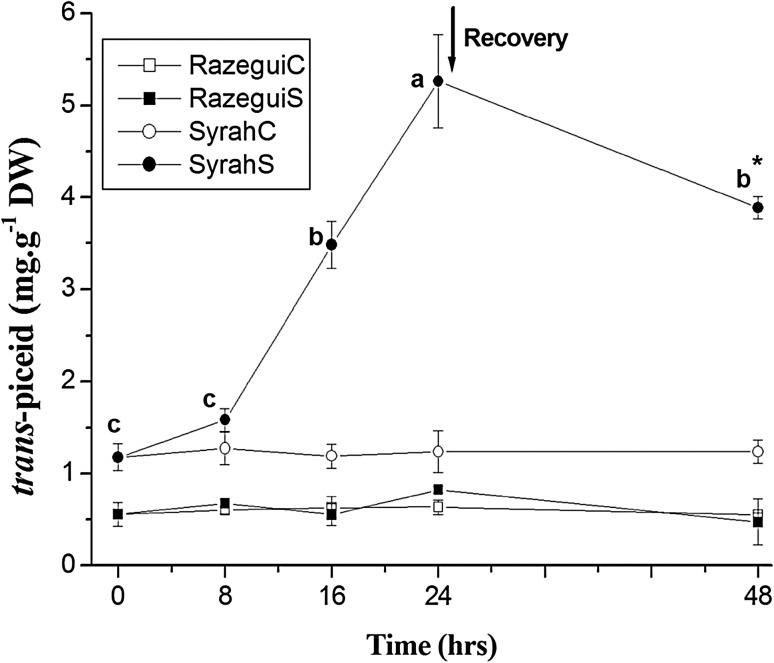
During the treatment, the two studied varieties exhibited different behaviors according to the trans- and cis- piceid accumulation. The salt-sensitive Syrah gradually accumulated trans-piceid during the 24 h of salt stress (Fig. 3) with a final concentration of 4.24-fold higher than respective control. However, the trans-piceid concentration was not significantly modified during the stress treatment and after recoverment in Razegui leaves. It should be noted that, in Syrah leaves, even during salt treatment, the trans-piceid concentrations were markedly higher than those of its free aglycone trans-resveratrol.
Fig. 3.
Time course of the trans-piceid accumulation in: Razegui control (RazeguiC), Razegui stressed (RazeguiS), Syrah control (SyrahC) and Syrah stressed (SyrahS). Evaluation was carried out in leaf samples of plants exposed to a 24 h salt stress treatment (NaCl at 100 mM) in controlled conditions of hydroponic culture, followed by a 24 h recovery phase. Data represent mean values and standard errors of 3 independent experiments. Letter suffixes express significant differences between the values among the salt stress Syrah at p < 0.05 according to Duncan’s multiple range test. No significant difference has been shown among the control and Razegui stressed leaves, and Syrah control leaves. Asterisk (*) indicates significant difference between control and stressed leaves after recovery
After 24 h of recoverment, the trans-piceid concentration in Syrah leaves decreased but was still significantly higher than control leaves (Fig. 3).
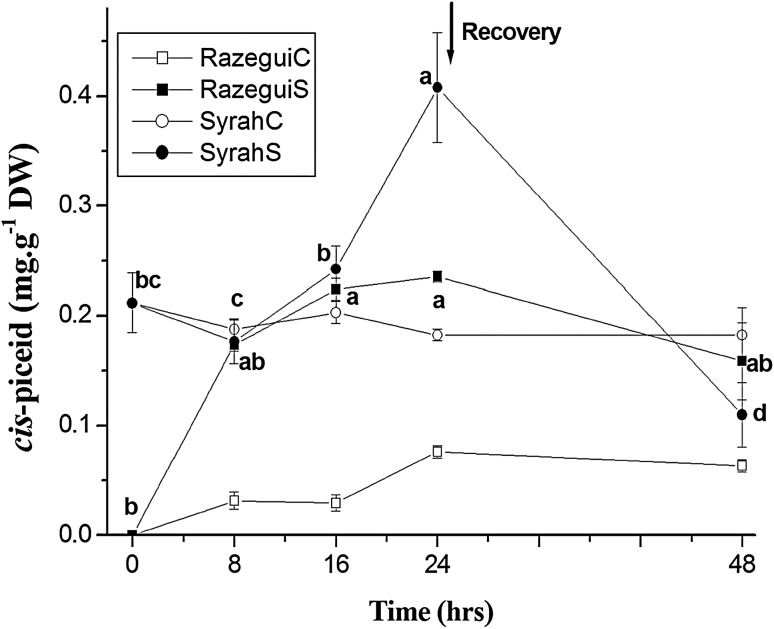
Although low in concentration, cis-piceid accumulation as a response to salinity in the tolerant as well as in the sensitive grapevine leaves was significant (Fig. 4).
Fig. 4.
Time course of the cis-piceid accumulation in: Razegui control (RazeguiC), Razegui stressed (RazeguiS), Syrah control (SyrahC) and Syrah stressed (SyrahS). Evaluation was carried out in leaf samples of plants exposed to a 24 h salt stress treatment (NaCl at 100 mM) in controlled conditions of hydroponic culture, followed by a 24 h recovery phase. Data represent mean values and standard errors of 3 independent experiments. Letter suffixes express significant differences between the values among the salt stress treatments at p < 0.05 according to Duncan’s multiple range test. No significant difference has been shown between cis-piceid values among the control leaves
In order to better visualize the genotypic differences according to the studied stilbene biosynthesis, mainly for the piceid isomers accumulation during salt stress, we calculated the ratio of trans-resveratrol, trans-piceid and cis-piceid as a percentage of the total stilbene value in each variety. The results are summarized in Table 2 where significant differences were shown.
Table 2.
The percentage content of trans-resveratrol, trans-piceid and cis-piceid in Syrah and Razegui stressed leaves. Results are the average ± SE of three independent experiments. Different letters in the same row indicate significant differences between time points according to Duncan test (p < 0.05)
| 0 h | 8 h | 16 h | 24 h | |
|---|---|---|---|---|
| Razegui | ||||
| trans-resveratrol | 7.598a ± 2.253% | 8.387a ± 2.260% | 6.923a ± 1.701% | 5.955a ± 1.330% |
| trans-piceid | 92.402a ± 2.254% | 62.893b ± 3.616% | 63.899b ± 2.923% | 64.563b ± 3.636% |
| cis-piceid | 2.057 × 10−6b ± 6.7 × 10−7% | 28.719a ± 1.651% | 29.177a ± 1.334% | 29.481a ± 1.660% |
| Syrah | ||||
| trans-resveratrol | 28.734a ± 5.260% | 14.178b ± 3.963% | 12.840b ± 3.698% | 20.145ab ± 5.515% |
| trans-piceid | 48.926b ± 10.498% | 58.918a ± 5.465% | 59.837a ± 5.839% | 54.822ab ± 6.720% |
| cis-piceid | 22.340b ± 4.794% | 26.903a ± 2.465% | 27.322a ± 2.666% | 25.033a ± 3.068% |
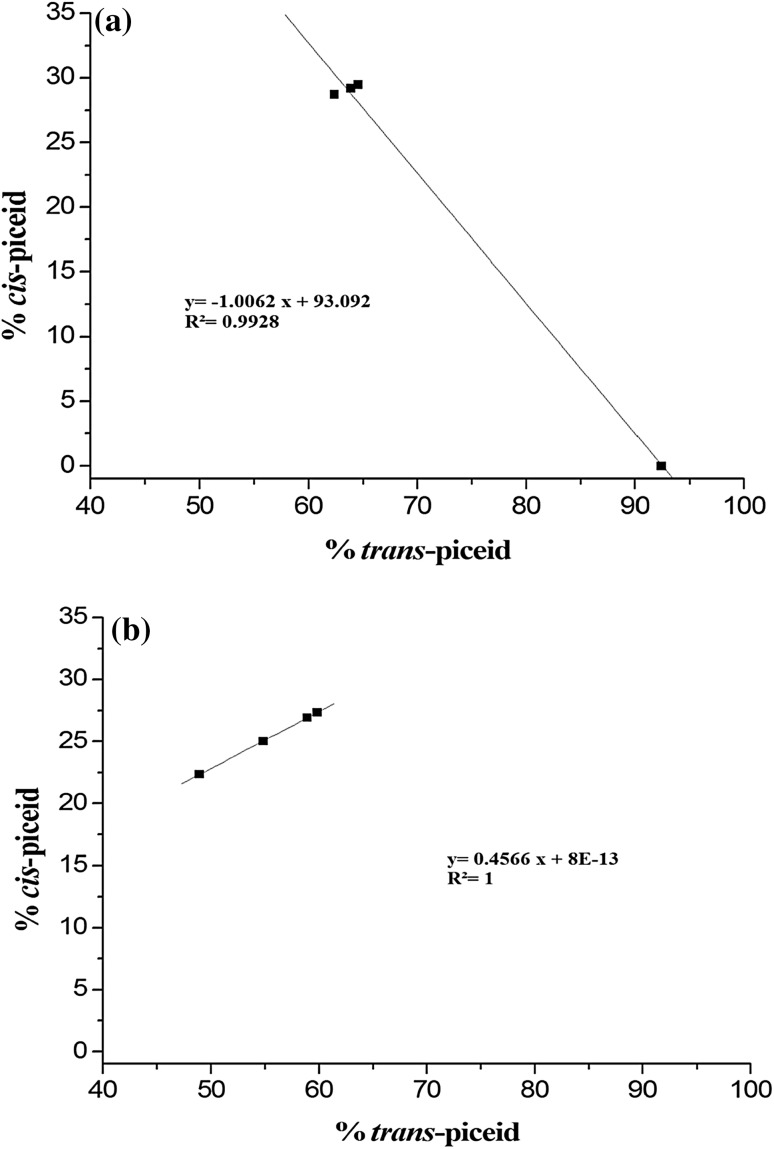
During experimentation, we noted that the percentage of trans-piceid increased in Razegui stressed leaves while cis-piceid percentage decreased. The regression curve (Fig. 5A) proved that these two parameters were negatively correlated (r2 = 0.9928). In contrast, Fig. 5B proved that in Syrah stressed leaves, the percentages of trans- and cis-piceid were positively correlated (r2 = 1).
Fig. 5.
The correlation plot of trans-piceid and cis-piceid percentages (a) in Razegui stressed leaves and (b) in Syrah stressed leaves
Discussion
Higher plants response to abiotic stresses is monitored through the control of complex endogenous and exogenous parameters, depending on the nature and the intensity of the constraint as well as the intrinsic genotype potentialities. Salt stress is a widely occurring abiotic stress that induces severe damages (Rhoades and Loveday 1990; Xiong and Zhu 2002; Sairam and Tyagi 2004). Salt stress, as a multigenic trait, results in induction of multiple pathways in the cells, and mainly develops osmotic and toxic constraints which can generate severe damages (Liang et al. 2018). The generation of oxidative stress is considered to be one of the major ‘obligatory’ fates of salt stress, and for this reason, the evolution as well as the efficiency of scavenging of reactive species like hydrogen peroxide, superoxide, hydroxyl peroxide, are highly related to the acquisition of efficient resistance response to salinity (Bose et al. 2014; Kao 2017). The present work aimed to distinguish the tolerance indicators in Razegui and Syrah cultivars under salt treatment (NaCl 100 mM). The hydroponic culture technique was chosen for this study to optimize nutrient availability and uptake by roots for all different samples (Gibeaut et al. 1997). We analyzed two parameters, namely ASA redox state and lipid peroxidation, which are tightly related to the development of oxidation. The results revealed that, unlike the sensitive cultivar Syrah, the tolerant cultivar Razegui recorded indeed lower levels of oxidative damages as well as a better recovery potential after removing the stressful agent from the nutritive solution.
The two studied varieties reveal an intrinsic capacity for trans-resveratrol production before applying the salt stress. In fact, many authors proved that grapevine varieties synthesize stilbene compounds with genotype specific concentrations under natural environmental conditions in the absence of any stress (Ali and Strommer 2003; Sun et al. 2006). Also, Guerrero et al. (2010) demonstrated that Syrah was notable for its capacity to produce initial high concentration of trans-resveratrol without any applied stress, which qualifies Syrah as a potential source of stilbenes.
After 8 h of elicitation and when oxidative stress has been already proved, the two varieties exhibited different responses in their ability to synthesize trans-resveratrol (Fig. 2). In fact, an unexpected decrease in trans-resveratrol concentration was shown in the sensitive variety Syrah leaves when compared with respective controls. A similar time lapse was reported in the work of Sgarbi et al. (2003) and has been explained as a lack of the activation of stilbene synthase, the enzyme used in the biosynthesis of resveratrol, which proves the inefficiency of some genotypes to produce the trans-resveratrol after few hours of elicitation. This must be owed to a delay in signal transduction pathway related to Syrah because of its sensitivity to the salt stress. In this same perspective, Qiao et al. (2010) showed the effect of harpin elicitor on the transcription of stilbene synthase which was earlier and reached a higher amplitude in the tolerant Vitis rupestris as compared with the sensitive V. vinifera cv. ‘Pinot noir’. Figure 2 showed that during 8 h of elicitation trans-resveratrol concentration was still insignificantly modified in Razegui stressed leaves. To explain this apparent contradiction between Syrah and Razegui behaviors, we suggested that it must have been due to the intrinsic resveratrol-synthesizing capacity of each variety at a short time of elicitation. In fact, the sensitive Syrah is originated in Europe where no osmotic stresses (drought and salinity) seem to seriously damage vine. On the other hand, Razegui is an autochthonous genotype originating in Tunisia where diverse stresses take place due to the severe climatic conditions in that area.
Moreover, it has been proved that Razegui was very well adapted to the salt stress. In fact, it displayed high transcript levels of Mitogen-activated protein kinases VvMAP genes to induce a rapid signal transmission of the extracellular stress (Daldoul et al. 2011). Salt stress induced the Vvrd22 genes in Razegui cultivar whereas Syrah showed no variation of Vvrd22 (Hanana et al. 2008). Furthermore, a higher capacity of turgescence adjustment and ionic homeostasis maintain were accorded to Razegui when compared to Syrah (Daldoul et al. 2010).
In the present work, Syrah was most affected by ROS, and accumulated higher trans-resveratrol concentrations than Razegui in order to reduce the degree of damage against oxidative stress.
It is well known that trans-resveratrol is a potent antioxidant. It modulates lipid metabolism (Frémont et al. 1999) and prevents the cell from apoptosis (Curti et al. 2017). Trans-resveratrol is accumulated under the oxidative burst caused by several abiotic stresses especially salinity and UV radiation which have similar effects in enhancing the biosynthesis of non-enzymatic antioxidants (Agati et al. 2011). However, the resveratrol role in the Vitaceae stress adaptation is still a subject of debate. For example, Ismail et al. (2012) and Ismail et al. (2014a) studied the effect of the salt stress on the American Vitis species that are the salt tolerant V. rupestris and the salt sensitive Vitis riparia. They found that, unlike V. riparia, the salt tolerance of V. rupestris was linked with a suspension of the oxidative burst and a reduction of the trans-resveratrol accumulation.
It was shown that the abundance of trans-resveratrol induced in response to UV-C stress in Vitis sylvestris, the ancestor of cultivated grapevine, was genotype dependant (Duan et al. 2015). Moreover, two contrasting responses were observed after UV radiation in V. vinifera varieties. In fact, UV elicitation induced an increase of the resveratrol concentration in V. vinifera leaves of ‘Hongbaladuo’ (Xi et al. 2015) and in suspension cells of ‘Cabernet sauvignon’ (Xu et al. 2015) but led to the decrease of the trans-resveratrol level in V. vinifera ‘Pinot noir’ and ‘Chardonney’ (Borie et al. 2004).
It is worth noting that excessive accumulation of trans-resveratrol from oxidative stress increased the cell damage and provoked the cell death (Chang et al. 2011). The present work shows that salt stress led to a low trans-resveratrol concentration in the salt-tolerant Razegui leaves. Therefore, the trans-resveratrol biosynthesis in Razegui would be under regulated which avoids high concentration and prevents damage effects. Indeed, a recent study from Jiang et al. (2019) revealed that the WRKY8 transcription factor acts as a negative regulator of resveratrol biosynthesis through the decrease of the stilbene synthase gene expression.
During the recovery period, the decrease of the trans-resveratrol concentration in Syrah leaves indicates that its accumulation was linked to the stress conditions and that trans-resveratrol was accumulated in response to the oxidative burst. This is of great importance because it can make it possible, through the nutraceutical industry, to use stressed Syrah leaves as a natural source of trans-resveratrol known for its benefits to human health.
Trans-piceid and cis-piceid are the most important resveratrol glucosides in many plant species especially V. vinifera. Results of the present work indicated that the trans-piceid concentrations were markedly higher than those of its free aglycone trans-resveratrol in all examined samples. This result was in agreement with those found by Vitrac et al. (2002) and Chong et al. (2009) in showing that significant proportion of total stilbenes may accumulate as glucosides in stilbene-producing plant species. Trans-piceid has antioxidant activity and acts as a free radical scavenger and inhibitor of ROS damage such as lipid peroxidation (Fabris et al. 2008). However, it is harder for the cell to use trans-piceid than the free aglycone (Su et al. 2013) which justifies the trans-piceid higher concentration.
The accumulation of trans-piceid during salt stress has been significantly affected by genotype. In fact, trans-piceid concentration significantly increased in response to salt stress in leaves of Syrah but not in Razegui. These results are in accordance with those of Ismail et al. 2015 proving that salt stress led to a low concentration of trans-piceid in V. rupestris compared to the salt-sensitive V. riparia. Moreover, it was shown that under an osmotic stress caused by the water deficit, V. vinifera ‘Cabernet sauvignon’ accumulated high trans-piceid concentration, however ‘Chardonney’ was not able to do so (Deluc et al. 2011). In this respect, it is proposed that the salt tolerance displayed by Razegui variety could be associated to the accumulation of antioxidant, other than trans-resveratrol and trans-piceid, to scavenge the salt generated ROS. In fact, Daldoul et al. (2010) proved that Razegui accumulated higher concentration of proline when compared to Syrah under salt stress. This suggested explanation is justified by previous work proving the free radical scavenger role of proline (Hossain and Fujita, 2010). As far as the authors are aware, no previous works were published on the antioxidant molecules accumulated by Razegui under oxidative stress. Thus, further experiments should be carried out to determine the antioxidant strategy involved in ROS scavenging via enzymatic and non enzymatic mechanisms in the autochthonous salt-tolerant Razegui.
On the other hand, in the salt sensitive Syrah stressed leaves, the trans-piceid increase must be correlated with the degree of oxidative load generated in the absence of any efficient defense strategy and/or to a low stress perception. In this case, and in accordance to Regev-Shoshani et al. (2003), trans-piceid must be accumulated as a storage form that is more stable and less cytotoxic than the free aglycone and to be used as a pool of trans-resveratrol. However, other previous works did not agree with the idea that piceid functions as a precursor for later release of resveratrol. As a matter of fact, Duan et al. (2015) proved that for some genotypes, the majority of induced bioactive stilbenes must be synthesized de novo, after plant tissue’s exposition to stress, rather than released from a glycosylated precursor.
The initially abundance of cis-piceid differs strongly between the studied varieties (Table 1). cis-piceid is lower in antioxidant activity than trans-resveratrol and trans-piceid. It also doesn’t have the same ability to scavenge free radicals in plant tissues (Mikulski and Molski 2010) which would justify its low concentrations in vine leaves when submitted to the salt treatment (Fig. 4).
In the present study, we did not detect any cis-resveratrol in leaf extracts (Goldberg et al. 1995). The lack of cis-resveratrol must have been due to the absence of UV owing to the deficiency of sunlight exposure needed for its formation or its conversion from the trans- isomer (Sato et al. 1997, Romero-Pérez et al. 1999; Camont et al. 2009; Lachman et al. 2016). In fact, the studied varieties were grown under greenhouse that kept grapevine leaves away from exposure to the sunlight. So, we suggested that trans-resveratrol was preferably glycosylated into trans-piceid by glycosyltransferase than isomerized into cis-piceid by cis-isomerise (Fig. 6). These results comply with those of Jeandet et al. 1997. When considering all the mentioned data regarding the stilbenes ratios (Table 2), we discovered that during the salt treatment, the accumulation of trans-piceid and cis-piceid have been negatively correlated (r2 = 0.9928) only in the tolerant Razegui leaves (Fig. 5A) where trans-piceid seemed to be converted to cis-piceid, the less hydrophilic and bioactive (Camont et al. 2009). The cis-piceid ratio and the trans-piceid ratio were positively correlated (r2 = 1) in the sensitive Syrah stressed leaves (Fig. 5B) which still accumulated trans-piceid because of its bioactive antioxidant property.
Fig. 6.
The most common modification of trans-resveratrol and its glucosides trans-piceid and cis-piceid
Conclusion
In the current work, it can be concluded that the effect of salinity (100 mM NaCl) on two contrasting V. vinifera L. cultivars (Tunisian autochthonous Razegui and European Syrah) depended on their genotype intrinsic potentialities. Our work confirmed that, salt elicitation led to an oxidative stress and consequently to a lipid peroxidation, essentially in Syrah variety which presented deficiency regarding salinity resistance. In addition, under such conditions, this sensitive cultivar accumulated high concentrations of the antioxidant stilbenes trans-resveratrol and its glucosides trans- and cis-piceid. Razegui showed moderate stilbene concentrations, which exhibited better homeostasis under such abiotic stress.
Acknowledgements
The authors would like to express their gratitude to King Khalid University, Saudi Arabia for providing administrative and technical support. The authors thank Prof. K.A Roubelakis and Dr. Panagiotis Moschou for excellent assistance.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Imen Souid and Imene Toumi have contributed equally to the accomplishment of the actual work and manuscript.
References
- Agati G, Biricolti S, Guidi L, Ferrini F, Fini A, Tattini M. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J Plant Physiol. 2011;16:204–212. doi: 10.1016/j.jplph.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Ali A, Strommer J. A simple extraction and chromatographic system for the simultaneous analysis of anthocyanins and stilbenes of Vitis species. J Agric Food Chem. 2003;51:7246–7251. doi: 10.1021/jf030435g. [DOI] [PubMed] [Google Scholar]
- Bavaresco L, Fregoni C. Molecular biology and biotechnology of the grapevine. In: Roubelakis-Angelakis KA, editor. Physiological role and molecular aspects of grapevine stilbenic compounds. Dordrecht: Kluwer Academic Publishers; 2001. pp. 153–182. [Google Scholar]
- Borie B, Jeandet P, Parize A, Bessis R, Adrian M. Resveratrol and stilbene synthase mRNA production in grapevine leaves treated with biotic and abiotic phytoalexin elicitors. Am J Enol Vitic. 2004;55:60–64. [Google Scholar]
- Bose J, Rodrigo-Moreno A, Shabala S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot. 2014;65:1241–1257. doi: 10.1093/jxb/ert430. [DOI] [PubMed] [Google Scholar]
- Boubakri H, Poutaraud A, Wahab MA, Clayeux C, Baltenweck-Guyot R, Steyer D, Marcic C, Mliki A, Soustre-Gacougnolle I. Thiamine modulates metabolism of the phenylpropanoid pathway leading to enhanced resistance to Plasmopara viticola in grapevine. BMC Plant Biol. 2013;13:31–46. doi: 10.1186/1471-2229-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddeo C, Puccib L, Gabrieleb M, Carbonec C, Fernàndez-Busquetsd X, Valentia D, Ponsg R, Vassalloh A, Faddaa AM, Manconia M. Stability, biocompatibility and antioxidant activity of PEG-modified liposomes containing resveratrol. Int J Pharm. 2017;538:40–47. doi: 10.1016/j.ijpharm.2017.12.047. [DOI] [PubMed] [Google Scholar]
- Camont L, Cottart CH, Rhayem Y, Nivet-Antoine V, Djelidi R, Collin F, Beaudeux JL, Bonnefont-Rousselot D. Simple spectrophotometric assessment of the trans-/cis-resveratrol ratio in aqueous solutions. Anal Chim Acta. 2009;634:121–128. doi: 10.1016/j.aca.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Carvalho LC, Vidigal P, Amâncio S. Oxidative stress homeostasis in grapevine (Vitis vinifera L.) Front Environ Sci. 2015;3:20–35. doi: 10.3389/fenvs.2015.00020. [DOI] [Google Scholar]
- Chang X, Heene E, Qiao F, Nick P. The phytoalexin resveratrol regulates the initiation of hypersensitive cell death in Vitis cell. PLoS ONE. 2011;6:1–12. doi: 10.1371/journal.pone.0026405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J, Poutaraud A, Huguene P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009;177:143–155. doi: 10.1016/j.plantsci.2009.05.012. [DOI] [Google Scholar]
- Curti V, Di Lorenzo A, Dacrema M, Xiao J, Nabavi SM, Daglia M. In vitro polyphenol effects on apoptosis: an update of literature data. Semin Cancer Biol. 2017;46:119–131. doi: 10.1016/j.semcancer.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Daldoul S, Guillaumie S, Götz MR, Krczal G, Ghorbel A, Delrot S, Mliki A, Höfer MU. Isolation and expression analysis of salt induced genes from contrasting grapevine (Vitis vinifera L.) cultivars. Plant Sci. 2010;179:489–498. doi: 10.1016/j.plantsci.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Daldoul S, Hoefer M, Mliki A. Osmotic stress induces the expression of VvMAP Kinase Gene in grapevine (Vitis vinifera L.) Journal of Botany. 2011;2012:1–4. doi: 10.1155/2012/737035. [DOI] [Google Scholar]
- Deluc LG, Decendit A, Parastamoulis Y, Mérillon JM, Cushman JC, Cramer GR. Water deficit increases stilbene metabolism in Cabernet sauvignon berries. J Agric Food Chem. 2011;59:289–297. doi: 10.1021/jf1024888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinakar C, Djilianov D, Bartels D. Photosynthesis in desiccation tolerant plants: energy metabolism and antioxidative stress defense. Plant Sci. 2012;182:29–41. doi: 10.1016/j.plantsci.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Duan D, Halter D, Baltenweck R, Tisch C, Tröster V, Kortekamp A, Hugueney P, Nick P. Genetic diversity of stilbene metabolism in Vitis sylvestris. J Exp Bot. 2015;66:3243–3257. doi: 10.1093/jxb/erv137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabris S, Momo F, Ravagnan G, Stevanato R. Antioxidant properties of resveratrol and piceid on lipid peroxidation in micelles and monolamellar liposomes. Biophys Chem. 2008;135:76–83. doi: 10.1016/j.bpc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Figueiras TS, Neves-Petersen MT, Petersen SB. Activation energy of light induced isomerization of resveratrol. J Fluoresc. 2011;21:1897–1906. doi: 10.1007/s10895-011-0886-3. [DOI] [PubMed] [Google Scholar]
- Flowers T, Troke PF, Yeo AR. The mechanisms of salt tolerance in halophytes. Annual Review of Plant Physiology. 1977;28:89–121. doi: 10.1146/annurev.pp.28.060177.000513. [DOI] [Google Scholar]
- Frémont L, Belguendou L, Delpal S. Antioxydant activity of resveratrol and alcohol-free wine polyphenols related to LDL oxidation and polyunsaturated fatty acids. Life Sci. 1999;64:2511–2521. doi: 10.1016/S0024-3205(99)00209-X. [DOI] [PubMed] [Google Scholar]
- Gibeaut DM, Hulett J, Cramer GR, Seemann JR. Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiol. 1997;115:317–319. doi: 10.1104/pp.115.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Goldberg DM, Ng E, Karumanchiri A, Yan J, Soleas GJ. Assay of resveratrol glucosides and isomers in wine by direct-injection high-performance liquid chromatography. J Chromatogr A. 1995;708:89–98. doi: 10.1016/0021-9673(95)00368-W. [DOI] [Google Scholar]
- Griesser M, Weingart G, Schoedl-Hummel K, Neumann N, Becker M, Varmuza K, Liebner K, Schuhmacher R, Forneck A. Severe drought stress is affecting selected primary metabolites, polyphenols, and volatile metabolites in grapevine leaves (Vitis vinifera cv. Pinot noir) Plant Physiol Biochem. 2015;88:17–26. doi: 10.1016/j.plaphy.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Guerrero RF, Puertas B, Fernández MI, Palma M, Cantos-Villar E. Induction of stilbenes in grapes by UV-C: comparison of different subspecies of Vitis. Innovative Food Science and Emerging Technologies. 2010;11:231–238. doi: 10.1016/j.ifset.2009.10.005. [DOI] [Google Scholar]
- Hanana M, Deluc L, Fouquet R, Daldoul S, Léon C, Barrieu F, Ghorbel A, Mliki A, Hamdi S. Identification and characterization of ‘rd22’ dehydration responsive gene in grapevine (Vitis vinifera L.) CR Biol. 2008;331:569–578. doi: 10.1016/j.crvi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplast: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition. CAB Technical Communication. No. 22 (2nd edn), pp. 297–315
- Hossain MA, Fujita M. Evidence for a role of exogenous glycinebetaine and proline in antioxidant defense and methylglyoxal detoxification systems in mung bean seedlings under salt stress. Physiol Mol Biol Plants. 2010;16:19–29. doi: 10.1007/s12298-010-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail A, Riemann M, Nick P. Jasmonate pathway mediates salt tolerance in grapevines. J Exp Bot. 2012;63:2127–2139. doi: 10.1093/jxb/err426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail A, Seo M, Takebayashi Y, Kamiya Y, Eiche E, Nick P. Salt adaptation requires efficient fine-tuning of jasmonate signalling. Protoplasma. 2014;251:881–898. doi: 10.1007/s00709-013-0591-y. [DOI] [PubMed] [Google Scholar]
- Ismail A, Takeda S, Nick P. Life and death under salt stress: same players, different timing? J Exp Bot. 2014;65:2963–2979. doi: 10.1093/jxb/eru159. [DOI] [PubMed] [Google Scholar]
- Ismail A, Seo M, Takebayashi Y, Kamiya Y, Nick P. A balanced JA/ABA status may correlate with adaptation to osmotic stress in Vitis cells. J Plant Physiol. 2015;185:57–64. doi: 10.1016/j.jplph.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Jeandet P, Breuil AC, Adrian M, Weston LA, Debord S, Meunier P, Maume G, Bessis R. HPLC analysis of grapevine phytoalexins coupling array detection and fluorometry. Anal Chem. 1997;69:5172–5177. doi: 10.1021/ac970582b. [DOI] [Google Scholar]
- Jeandet P, Clément C, Cordelier S. Regulation of resveratrol biosynthesis in grapevine: new approaches for disease resistance? J Exp Bot. 2019;70:375–378. doi: 10.1093/jxb/ery446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellouli N, Ben Jouira H, Skouri H, Ghorbel A, Gourgouri A, Mliki A. Proteomic analysis of Tunisian grapevine cultivar Razegui under salt stress. J Plant Physiol. 2008;165:471–481. doi: 10.1016/j.jplph.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Jiang J, Xi H, Dai Z, Lecourieux F, Yuan L, Liu X, Patra B, Wei Y, Li S, Wang L. VvWRKY8 represses stilbene synthase genes through direct interaction with VvMYB14 to control resveratrol biosynthesis in grapevine. J Exp Bot. 2019;70:715–729. doi: 10.1093/jxb/ery401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao CH. Mechanisms of salt tolerance in rice plants: reactive oxygen species scavenging-systems. J Taiwan Agric Res. 2017;66:1–8. [Google Scholar]
- Kostopoulou Z, Therios I, Molassiotis A. Resveratrol and its combination with α-tocopherol mediate salt adaptation in citrus seedlings. Plant Physiol Biochem. 2014;78:1–9. doi: 10.1016/j.plaphy.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Lachman J, Kotíkovál Z, Hejtmánková A, Pivec V, Pšeničnaja O, Šulc M, Střalková R, Dědina M. Resveratrol and piceid isomers concentrations in grapevine shoots, leaves, and tendrils. Hort Sci. 2016;43:25–32. [Google Scholar]
- Liang W, Ma X, Wan P, Liu L. Plant salt-tolerance mechanism. Biochem Biophys Res Commun. 2018;495:286–291. doi: 10.1016/j.bbrc.2017.11.043. [DOI] [PubMed] [Google Scholar]
- Maurer LH, Bersch AM, Santos RO, Trindade SC, Costa EL, Peres MM, Malmann CA, Schneider M, Bochi VC, Sautter CK, Emanuelli T. Postharvest UV-C irradiation stimulates the non-enzymatic and enzymatic antioxidant system of ‘Isabel’ hybrid grapes (Vitis labrusca × Vitis vinifera L.) Food Res Int. 2017;102:738–747. doi: 10.1016/j.foodres.2017.09.053. [DOI] [PubMed] [Google Scholar]
- Mikulski D, Molski M. Quantitative structure–antioxidant activity relationship of trans-resveratrol oligomers, trans-4,4′-dihydroxystilbene dimer, trans-resveratrol-3-O-glucuronide, glucosides: trans-piceid, cis-piceid, trans-astringin and trans-resveratrol-4′-O-β-D-glucopyranoside. Eur J Med Chem. 2010;45:2366–2380. doi: 10.1016/j.ejmech.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Petrov V, Hille J, Mueller-Roeber B, Gechev TS. ROS-mediated abiotic stress-induced programmed cell death in plants. Front Plant Sci. 2015;6:1–16. doi: 10.3389/fpls.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet R, Perret C, Jean-Denis JB, Tabacchi R, Gindro K, Viret O. δ-vinifrein a resveratrol dehydrodimer: one of the major stilbenes synthesized by stressed grapevine leaves. J Agric Food Chem. 2003;51:5488–5492. doi: 10.1021/jf030227o. [DOI] [PubMed] [Google Scholar]
- Pugajera I, Perkons I, Górnaś P. Identification and determination of stilbenes by Q-TOF in grape skins, seeds, juice and stems. J Food Compos Anal. 2018;74:44–52. doi: 10.1016/j.jfca.2018.09.007. [DOI] [Google Scholar]
- Qiao F, Chang XL, Nick P. The cytoskeleton enhances gene expression in the response to the Harpin elicitor in grapevine. J Exp Bot. 2010;61:4021–4031. doi: 10.1093/jxb/erq221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev-Shoshani G, Shoseyov O, Bilkis I, Kerem Z. Glycosylation of resveratrol protects it from enzymic oxidation. Biochem J. 2003;374:157–163. doi: 10.1042/bj20030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades JD, Loveday J. Salinity in irrigated agriculture. In: Steward BA, Nielsen DR, editors. Irrigation of agricultural crops. Agronomists, monograph. Reston: American Society of Civil Engineers; 1990. pp. 1089–1142. [Google Scholar]
- Romero-Pérez AI, Ibern-Gómez M, Lamuela-Raventós RM, de La Torre-Boronat MC. Piceid, the major resveratrol derivative in grape juices. J Agric Food Chem. 1999;47:1533–1536. doi: 10.1021/jf981024g. [DOI] [PubMed] [Google Scholar]
- Sairam RK, Tyagi A. Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci. 2004;86:407–421. [Google Scholar]
- Sato M, Suzuki Y, Okuda T, Yokotsuka K. Contents of resveratrol, piceid, and their isomers in commercially available wines made from grapes cultivated in Japan. Biosci Biotechnol Biochem. 1997;61:1800–1805. doi: 10.1271/bbb.61.1800. [DOI] [PubMed] [Google Scholar]
- Sgarbi E, Fornasiero RB, Lins AP, Bonatti PM. Phenol metabolism is differentially affected by ozone in two cell lines from grape (Vitis vinifera L.) leaf. Plant Sci. 2003;165:951–957. doi: 10.1016/S0168-9452(03)00219-X. [DOI] [Google Scholar]
- Souid I, Hassene Z, Sánchez-Palomo E, Pérez-Coello MS, Ghorbel A (2008) Aroma potential of three autochthonous grapevine varieties from Tunisia. J Int Sci Vigne Vin 42: 231-239. 10.20870/oeno-one.2008.42.4.813
- Su D, Cheng Y, Liu M, Liu D, Cui H, Zhang B, Zhou S, Yang T, Mei Q. Comparision of piceid and resveratrol in antioxidation and antiproliferation activities In Vitro. PLoS ONE. 2013;8:1–13. doi: 10.1371/journal.pone.0054505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A. Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate and abscisic acid-induced stomatal closure. Plant Physiol. 2004;134:1536–1545. doi: 10.1104/pp.103.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Ribes AM, Leandro MC, Belchior AP, Spranger MI. Stilbenes: quantitative extraction from grape skins, contribution of grape solids to wine and variation during wine maturation. Anal Chim Acta. 2006;563:382–390. doi: 10.1016/j.aca.2005.12.002. [DOI] [Google Scholar]
- Toumi I, Gargouri M, Nouairi I, Moschou PN, Ben Salem-Fnayou A, Mliki A, Zarrouk M, Ghorbel A. Water stress induced changes in the leaf lipid composition of four grapevine genotypes with different drought tolerance. Biol Plant. 2008;52:161–164. doi: 10.1007/s10535-008-0035-2. [DOI] [Google Scholar]
- Villangó SZ, Szekeres A, Bencsik O, Láposi R, Pálfi Z, Zsófi ZS. The effect of postveraison water deficit on the phenolic composition and concentration of the Kékfrankos (Vitis vinifera L.) berry. Sci Hortic. 2016;209:113–116. doi: 10.1016/j.scienta.2016.06.010. [DOI] [Google Scholar]
- Vitrac X, Monti JP, Vercauteren J, Deffieux G, Mérillon JM. Direct liquid chromatographic analysis of resveratrol derivatives and flavononols in wines with absorbance and fluorescence detection. Anal Chim Acta. 2002;485:103–110. doi: 10.1016/S0003-2670(01)01498-2. [DOI] [Google Scholar]
- Wang SY, Jiao HJ, Faust M. Changes in ascorbate, glutathione, and related enzyme activities during thidiazuron-induced bud break of apple. Physiol Plant. 1991;82:231–236. doi: 10.1111/j.1399-3054.1991.tb00086.x. [DOI] [Google Scholar]
- Wani AS, Ahmad A, Hayat C, Fariduddin Q. Salt-induced modulation in growth, photosynthesis and antioxidant system in two varieties of Brassica juncea. Saudi J Biol Sci. 2013;20:183–193. doi: 10.1016/j.sjbs.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi HF, Ma L, Wang LN, Li SH, Wang LJ. Differential response of the biosynthesis of resveratrols and flavonoids to UV-C irradiation in grape leaves. N Z J Crop Hortic Sci. 2015;43:1–10. doi: 10.1080/01140671.2014.989862. [DOI] [Google Scholar]
- Xiong L, Zhu J. Salt tolerance. Arabidopsis Book. 2002;1:e0048. doi: 10.1199/tab.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, Cheng Zhan JI, Huang WD. Effects of ultraviolet C, methyl jasmonate and salicylic acid, alone or in combination, on stilbene biosynthesis in cell suspension cultures of Vitis vinifera L. cv. Cabernet sauvignon. Plant Cell, Tissue Organ Cult. 2015;122:197–211. doi: 10.1007/s11240-015-0761-z. [DOI] [Google Scholar]