Abstract
Studies on the genetic diversity and structure in endangered and threatened species are of utmost importance to design and promote effective conservation and management programs. Ephedra foliata, an endemic and threatened species growing naturally in arid and semi-arid regions of north western India, was investigated to estimate genetic variability and population structure using inter-simple sequence repeats (ISSR) and directed amplification of mini-satellite DNA (DAMD) markers. Twenty-five (ISSR 15; DAMD 10) markers produced 449 fragments, of which 382 were polymorphic in nature, revealing 84.59% polymorphism. ISSR markers revealed higher levels of polymorphism, polymorphic information content, marker index, diversity index and effective multiplex ratio than DAMD markers. Higher values of polymorphism, genetic diversity and Shannon information index at species level than at population level revealed that E. foliata possess high genetic diversity. AMOVA revealed much higher variance within populations than among the populations. The three clustering approaches viz., UPGMA, PCoA, and STRUCTURE, grouped the eleven investigated populations into two clusters revealing two genetic populations and the patterns of clustering of populations was in accordance with their geographic distribution, suggesting that these populations have evolved in response to their local environments. The high level of genetic differentiation (GST = 0.31) and moderate gene flow (Nm = 1.11) among populations could be due to geographic isolation, regional climatic conditions, over-exploitation and improper seed setting. To the best of our knowledge, this study is the first endeavour to estimate genetic diversity and population structure of E. foliata using molecular markers.
Electronic supplementary material
The online version of this article (10.1007/s12298-019-00648-6) contains supplementary material, which is available to authorized users.
Keywords: Arid regions, Ephedra foliata, Genetic variability, Molecular markers, Population genetic structure
Introduction
Knowledge and information regarding genetic variability are highly essential for protection and conservation of plant genetic resources. There are innumerable DNA based markers available to estimate the genetic diversity in the plants, however, application and choice of markers largely depend upon the objectives of the study. In the present investigation, Directed Amplification of Minisatellite DNA (DAMD; Heath et al. 1993) and Inter Simple Sequence Repeats (ISSR; Prevost and Wilkinson 1999) methods were used to examine the extent of genetic variability and genetic structure in E. foliata occurring naturally in arid and semi-arid regions of north western parts of India. Though, DAMD and ISSR are dominant markers and have some disadvantages but they are still widely used for determination of polymorphism, and can provide credible discriminating information. These markers are present throughout genomes and are cost effective (Costa et al. 2016). Considering these advantages, these markers have been extensively used either alone or in combination with other markers (Jianfeng et al. 2013; Meena et al. 2016; Singh et al. 2016; Wang et al. 2016; Chen et al. 2017) to estimate the genetic diversity within and between plant species. The genus Ephedra has been analyzed earlier from biogeographic and phylogenetic perspectives using nuclear and plastid markers (Qin et al. 2013; Ickert-Bond and Renner 2016; Wu et al. 2016), but only a few studies are available on the genetic diversity in Ephedra species using single primer amplification reaction (SPAR) methods like RAPD (Takeuchi et al. 2003; Ghafoor et al. 2007) and, RAPD and ISSR (Zhu et al. 2013; Saeed et al. 2015). However, no prior information is available on the application of molecular markers for studying the genetic variability and population genetic structure in E. foliata. The present study, therefore, was aimed to (1) examine the genetic diversity in E. foliata using DAMD and ISSR markers; (2) study genetic variation distribution within and between populations and (3) discuss the possible methods of conservation of the species by utilising the population genetic data.
Ephedra foliata Boiss. et Kotschy ex Boiss. (Ephedraceae) commonly known as ‘Shrubby Horsetail’ is widely distributed in deserts of Africa, Arabian Peninsula and India (Sahni 1990; Freitag and Maier-Stolte 2003). It is a typical component of arid and semi-arid regions of north western parts of India and locally known as ‘Unthphog’ (Bhandari 1978). The plants are dioeciously woody shrubs with trailing or scandent stem 3–5 m long, having subverticillate or fascicled, thin, persistent branches with 2.5–10 cm long internodes. Leaves usually 3, linear setaceous, shortly connate at base, stem performs the function of assimilation and female plants bear semitransparent, nutritious edible berries (female cones) having sweet taste due to the fleshy bracts. These fleshy bracts are important during the food scarcity in arid regions (Kotia 2008). Traditionally, whole plant is used in fever, stomachic, worms, blood purification, asthma, dropsy, snake bite and as cardio tonic (Silori et al. 2005; Quattrocchi 2012). It also possesses hepatoprotective, antimicrobial and antibacterial properties (Alqasoumi and Abdel-Kader 2012; Bissa 2015) and high nutraceutical value (Kamboj 2000). Although, E. foliata is cultivated and harvested to a limited scale on a commercial basis in Gujarat (Gavali and Sharma 2004), but over exploitation, extensive habitat destruction, very slow growth rate, poor regeneration, grazing and other anthropogenic pressures have caused tremendous reduction in its natural populations. Therefore, E. foliata in its natural habitats has now become a rare or endangered species from a vulnerable category (Kharin 2002; Joshi et al. 2013). Presently, E. foliata is considered as a threatened species in India, though it is included in ‘Least Concern’ category of IUCN Red list of threatened species (Lodha et al. 2014; IUCN 2017).
Materials and methods
Plant materials
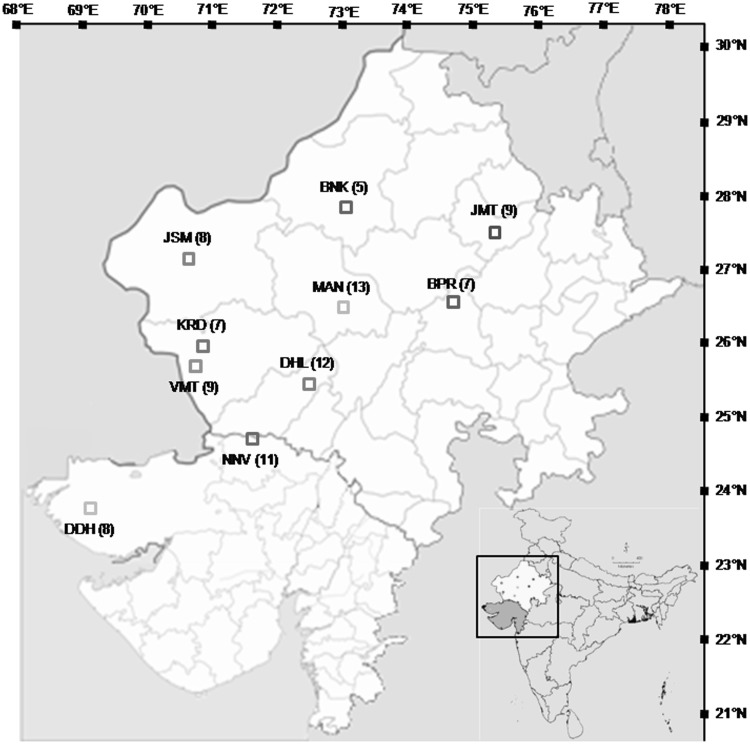
The plants of E. foliata were collected from different locations in arid and semi-arid regions of north western parts (Gujarat and Rajasthan) of India (Table 1, Fig. 1). A total of 95 accessions representing ten natural and one ex situ population covering the maximum distribution range of the species in India were sampled and analyzed. The ex situ population comprised only five accessions comprising two from Botanic garden BSI, Jodhpur; one each from farmer’s backyard, Dhinodhar; Botanic garden NBRI, Lucknow; Botanic garden Lucknow University, Lucknow. Approximately 5 gm fresh and healthy juvenile twigs were stored dry at room temperature over blue self-indicating silica gel (6–20 mesh) in zip-lock plastic bags till the DNA was extracted. Voucher specimens were prepared for all the collected materials and housed at the herbarium of CSIR-National Botanical Research Institute (LWG), Lucknow, India.
Table 1.
Details of the sampling populations: population size, sample size, locality, latitude, longitude, and altitude
| Population name | Accession | Locality | Latitude (°′N) | Longitude (°′E) | Altitude (m) |
|---|---|---|---|---|---|
| BKN (5)a | BKN (1–5)a | Bikaner, RJ | 27°46.340 | 073°03.960 | 292 |
| JSM (18) | JSM (6–13) | Jaisalmer, RJ | 26°58.507 | 070°52.540 | 207 |
| KRD (30) | KRD (14–20) | Kiradu, Barmer, RJ | 25°45.539 | 071°06.679 | 263 |
| VMT (50) | VMT (21–29) | Viratra Mata, Barmer, RJ | 25°28.846 | 071°01.708 | 230 |
| JMT (40) | JMT (30–39) | Jean Mata, Sikar, RJ | 27°27.998 | 075°11.165 | 514 |
| BPR (18) | BPR (40–46) | Budha Pushkar, Ajmer, RJ | 26°30.501 | 074°35.892 | 534 |
| MAN (50) | MAN (47–59) | Mandore, Jodhpur, RJ | 26°23.322 | 073°03.251 | 252 |
| DHL(40) | DHL(60–71) | Dhawala, Jalore, RJ | 25°14.783 | 072°39.655 | 217 |
| NNV (25) | NNV (72–82) | Nainava, Banaskantha, GJ | 24°38.538 | 071°53.458 | 101 |
| DDH (20) | DDH (83–90) | Dinodar Hills, Kutch, GJ | 23°26.545 | 069°19.475 | 105 |
| CLT (5) | CLT (91–95) | Introduced in botanic gardens |
BKN Bikaner, JSM Jaisalmar, KRD Kiradu, VMT Viratra mata temple, JMT Jean mata temple, BPR Budha Pushar region, MAN Mandore, DHL Dhawala, NNV Nainava, DDH Dinodar hills, CLT Cultivated, RJ Rajasthan, GJ Gujarat
aNumbers in parenthesis are approximate number of individuals in that population and number of accessions sampled
Fig. 1.
Geographic locations of Ephedra foliata populations sampled from the arid and semi-arid regions of north western India
Genomic DNA extraction
Total genomic DNA was extracted from the silica dried juvenile twigs following the CTAB method (Doyle and Doyle 1990). Qualitative and quantitative assessment of the extracted genomic DNA was carried out by comparing band intensities with known standards of Lambda DNA EcoRI/Hind III Double Digest on 0.8% agarose gel stained with ethidium bromide and also by UV spectroscopy using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies Inc. USA). The DNA was stored at − 20 °C until further use.
PCR amplification and agarose gel electrophoresis
The DAMD primers already available in public domain (Hu et al. 2011) were custom synthesized from Sigma Aldrich Chemicals Pvt. Ltd, India. A set of 100 anchored ISSR primers was procured from University of British Columbia, Canada. Twenty DAMD and 100 ISSR primers were initially screened with two template DNAs of E. foliata, of which 10 DAMD and 15 ISSR primers were finally selected for profiling of the complete set of 95 accessions on the basis of clear and reproducible banding patterns. PCR amplification was carried out according to Meena et al. (2016).
The amplified PCR products were resolved on 1.5% agarose gel using 1X TBE buffer (Tris–borate 89 mM; 2 mM EDTA, pH 8.3) at a constant voltage of 5 V/cm. After electrophoresis, the gel was stained with ethidium bromide, then visualized and archived using UV Tech Gel Documentation System (UK). The gel profiles were recorded as digital images in Gel Documentation System.
Data analysis
Only distinct, reproducible and clearly separated fragments were scored manually as ‘1’ (presence) and ‘0’ (absence) for both ISSR and DAMD markers. For each marker, the molecular size of each band was estimated on the basis of the corresponding maker lane. The DAMD and ISSR data matrices were computed independently as well as cumulatively (DAMD + ISSR) to calculate total number of polymorphic bands (PB), percentage of polymorphism (P %), polymorphic information content (PIC) and resolving power (RP) according to Botstein et al. (1980) and Prevost and Wilkinson (1999). In order to determine the usefulness of the two methods, diversity index (DI), effective multiplex ratio (EMR) and marker index (MI) were calculated according to Powell et al. (1996).
All statistical analyses regarding the extent of genetic diversity and population genetic structure were carried out using a cumulative data matrix (DAMD + ISSR). Genetic distance among pair of accessions was computed using Jaccard’s coefficient (Jaccard 1908) for Neighbour-Joining (NJ) method, for unweighted pair group method with arithmetic averages (UPGMA) method in the FreeTree program 0.9.1.5 (Pavlicek et al. 1999).
POPGENE version 1.32 software was used to calculate the genetic diversity parameters such as percentage of polymorphic bands (% P), observed number of alleles (Na), effective number of alleles (Ne), Nei’s gene diversity (H), and Shannon information index (I), total genetic diversity (HT), genetic diversity within populations (HS), genetic differentiation (GST) and gene flow (Nm) assuming that all populations were in Hardy–Weinberg equilibrium (Yeh et al. 1999). Genetic distance among pair of populations was also computed using the same program and Nei’s unbiased genetic distance matrix was subjected to construct a UPGMA dendrogram using the PHYLIP program 3.5 (Nei 1978) to infer genetic relationships among populations.
GenAlEx program 6.5 was used to calculate number of private alleles (Pa) among the 11 natural as well as two genetic populations inferred from STRUCTURE analysis and to perform principal coordinate analysis (PCoA) to provide a spatial representation of the relative genetic distances among accessions and to determine the consistency of differentiation among populations (Peakall and Smouse 2012). Using the same program, a Mantel test (Mantel 1967) was also conducted at 9999 permutations to determine the relationship between pair-wise genetic and geographic distances of ten natural populations and to evaluate the isolation by distance (IBD) model. The partitioning of genetic variability among and within populations was computed with analysis of molecular variance (AMOVA) using ARLEQUIN program 3.5 (Excoffier and Lischer 2010). The possible correlation of genetic diversity (H) with rainfall (mm), temperature (°C) and altitude (m) of populations was calculated using SPSS 16.0 (SPSS Inc., USA, 2007).
Bayesian analysis of genetic structure was performed as implanted in STRUCTURE 2.3.4 software (Pritchard et al. 2000; Falush et al. 2003) to estimate the most likely number of population genetic clusters (k), and proportion of assignment of individuals from each of the assumed natural populations to each of the inferred genetic clusters. An admixture model with correlated allele frequencies was used to infer the number of ‘k’ with prior population information. The analysis was performed with a burn-in period of 20,000 and a Markov Chain Monte Carlo (MCMC) replication number set to 50,000. The program was run 10 times for each ‘k’, ranging from 1 to 12. The most appropriate number of genetic clusters ‘k’ was estimated using the criterion described by Evanno et al. (2005), which is based on ∆K. The output from STRUCTURE analysis was the proportion of ancestry membership of each individual of the given population in the inferred genetic cluster calculated by averaging the membership coefficient obtained from 10 runs of the real ‘k’.
Results
Analysis of DAMD and ISSR polymorphism
A total of 202 bands were obtained with 10 DAMD primers with an average 20.2 bands per primer (Table S1). Out of these 202 bands, 161 were polymorphic corresponding to 79.70% polymorphism across 95 accessions of E. foliata. The size of amplified fragments ranged from 160 to 2700 bp. Primer URP9F produced the highest number of the polymorphic bands (31) with 57.14% polymorphism, whereas primer URP30F resulted in least number of polymorphic bands (14) with 93.55% polymorphism. PIC value ranged from 0.13 (URP30F) to 0.27 (M13), with an average of 0.20, while RP value varied from 15.89 (M13) to 43.68 (URP9F) with a mean of 26.75 across all the 10 DAMD primers.
ISSR analysis with 15 primers resulted in a total of 247 bands, with an average of 16.46 bands per primer (Table S1). Out of 247 bands, 221 bands were polymorphic constituting 89.47% polymorphism across 95 accessions of E. foliata. The size of the amplified products varied from 250 to 2500 bp. Primer UBC 835 generated highest number of bands (26), while three primers UBC 807, UBC 812, and UBC 861 resulted in the least number of bands (11). The range of polymorphism varied from 63.64% (UBC 861) to 100% (UBC 835, UBC 836, UBC 840 and UBC 841) across the accessions analyzed. The PIC value varied from 0.11 (UBC 810 and 861) to 0.34 (UBC 891) with a mean of 0.23, while RP value ranged from 12.63 (UBC 807) to 24.55 (UBC 888) with a mean of 19.03. Comparatively, ISSR markers revealed higher polymorphism (89.47%) than DAMD markers (79.70%) across the analyzed accessions of E. foliata. ISSR markers also revealed higher values of DI (0.26), EMR (13.18) and MI (3.49) than the values revealed by DAMD markers (DI = 0.25, EMR = 12.83, MI = 3.17) (Table S1).
Cumulative analysis of DAMD and ISSR primers revealed an average 84.59% polymorphism and mean values for PIC and RP were 0.22 and 22.89, respectively. The genetic distance among accessions ranged from 0.061 (Ef77–Ef78) to 0.447 (Ef22–Ef92), with an average of 0.254 (data not shown).
Analysis of inter- as well as intra-population genetic diversity
Among the analyzed 11 populations of E. foliata, the percentage of polymorphic bands (%P) ranged from 34.30% (BKN) to 50.33% (DHL) with a mean 44.46% polymorphism at population level, however, polymorphism was much higher (85.08%) at the species level (Table 2). At population level, Nei’s gene diversity (H) and Shannon’s information index (I) were lowest in BKN populations (H = 0.13, I = 0.19) and highest in CLT population (H = 0.19, I = 0.27), followed by MAN population (H = 0.18, I = 0.26), though genetic diversity was much higher at species level (H = 0.23, I = 0.35). Similarly, number of alleles were also found minimum in BKN population (Na = 1.43, Ne = 1.22) and the maximum number of alleles were found in DHL (Na = 1.50) and CLT (Ne = 1.32) populations. Further, the highest number of private alleles (Pa) was found in CLT (08) followed by MAN (04) from Rajasthan and DDH (04) from Gujarat populations (Table S2). Total genetic diversity (HT) and genetic diversity within populations (HS) were 0.23 and 0.16, respectively. The proportion of the genetic variations contributed by the differences among populations (GST) was 0.31, thus resulting in 69% of the total genetic variations within the populations. It was consistent with the results of AMOVA, which showed highest genetic variation within populations (73.99), whereas the variance among populations was 26.01% (Table 3). A moderate rate of gene flow among populations was found (Nm = 1.11) (Table 2).
Table 2.
Population genetic parameters of E. foliata populations sampled from arid and semi-arid regions of north western India
| Population | PB | P (%) | Mean Na* | Mean Ne* | Mean H* | Mean I* | H T | H S | G ST | N m |
|---|---|---|---|---|---|---|---|---|---|---|
| BKN (5) | 154 | 34.30 | 1.3430 (0.4752) | 1.2184 (0.3470) | 0.1263 (0.1883) | 0.1878 (0.2722) | ||||
| JSM (8) | 188 | 41.87 | 1.4187 (0.4939) | 1.2534 (0.3604) | 0.1468 (0.1948) | 0.2193 (0.2798) | ||||
| KRD (7) | 199 | 44.32 | 1.4432 (0.4973) | 1.2739 (0.3745) | 0.1571 (0.1994) | 0.2341 (0.2848) | ||||
| VMT (9) | 209 | 46.55 | 1.4655 (0.4994) | 1.2901 (0.3740) | 0.1673 (0.2011) | 0.2489 (0.2880) | ||||
| JMT (10) | 207 | 46.10 | 1.4610 (0.4990) | 1.2865 (0.3725) | 0.1652 (0.2010) | 0.2455 (0.2880) | ||||
| BPR (7) | 171 | 38.08 | 1.3808 (0.4861) | 1.2452 (0.3635) | 0.1405 (0.1956) | 0.2082 (0.2810) | ||||
| MAN (13) | 222 | 49.44 | 1.4944 (0.5005) | 1.3053 (0.3779) | 0.1756 (0.2036) | 0.2609 (0.2908) | ||||
| DHL (12) | 226 | 50.33 | 1.5033 (0.5005) | 1.2999 (0.3746) | 0.1736 (0.2008) | 0.2595 (0.2867) | ||||
| NNV (11) | 200 | 44.54 | 1.4454 (0.4976) | 1.2700 (0.3667) | 0.1560 (0.1981) | 0.2327 (0.2839) | ||||
| DDH (8) | 203 | 45.21 | 1.4521 (0.4983) | 1.2824 (0.3802) | 0.1611 (0.2019) | 0.2393 (0.2877) | ||||
| CLT (5) | 217 | 48.33 | 1.4833 (0.5003) | 1.3227 (0.3948) | 0.1827 (0.2080) | 0.2694 (0.2961) | ||||
| Mean | 199.64 | 44.46 | 1.4446 (0.4953) | 1.2771 (0.3715) | 0.1593 (0.1993) | 0.2369 (0.2854) | ||||
| Total (95) | 382 | 85.08 | 1.8508 (0.3567) | 1.3768 (0.3508) | 0.2284 (0.1808) | 0.3543 (0.2470) | 0.2310 (0.0326) | 0.1593 (0.0176) | 0.3104 | 1.1107 |
Population, population code; PB, number of polymorphic bands; P (%), percentage polymorphic bands; Na, observed number of alleles; Ne, effective number of alleles; H, Nei’s gene diversity; I, Shannon’s information index; HT, total genetic diversity; HS, genetic diversity within populations; GST, the relative magnitude of genetic differentiation among populations; Nm, gene flow among populations; values in parentheses are sample size and SD values
Table 3.
AMOVA analyses of E. foliata carried out using ARLEQUIN program (a) among and within 11 natural populations, (b) among and within two genetic populations as inferred from STRUCTURE analysis
| Source of variations | Degree of freedom | Sum of squares | Variance component | Percentage of variations | F ST |
|---|---|---|---|---|---|
| (a) | |||||
| Among populations | 10 | 2662.156 | 13.33156 | 26.01 | 0.2601 |
| Within populations | 179 | 6788.244 | 37.92315 | 73.99 | |
| Total | 189 | 9450.400 | 51.25471 | 100.00 | |
| (b) | |||||
| Among populations | 1 | 656.814 | 8.50261 | 15.38 | 0.1538 |
| Within populations | 188 | 8793.586 | 46.77439 | 84.62 | |
| Total | 189 | 9450.400 | 55.27700 | 100.00 | |
The population diversity indices were also computed for two genetic clusters (K1 and K2) inferred from STRUCTURE analysis, the levels of polymorphism ranged from 69.04% (K1) to 74.61% (K2) with a mean 71.82% polymorphism at population level, however these levels of polymorphism were much lower than at species level (85.08%) (Table 2). Among the two genetic populations, the effective number of alleles and Nei’s gene diversity were found higher in the cluster K1 (Ne = 1.40, H = 0.23) while observed number of alleles and Shannon’s information index were higher in the cluster K2 (Na = 1.75, I = 0.35). Number of private alleles were higher (35) in K2 than K1 (15) (Table S2). Comparatively, at species level, the two genetic populations revealed higher diversity indices (HT = 0.25, HS = 0.23) than the 11 natural populations (HT = 0.23, HS = 0.16). Similarly, genetic differentiation was higher and gene flow was significantly low in 11 natural populations as compared to two genetic populations (GST = 0.08, Nm = 5.46) (Table 2).
Analysis of population genetic structure
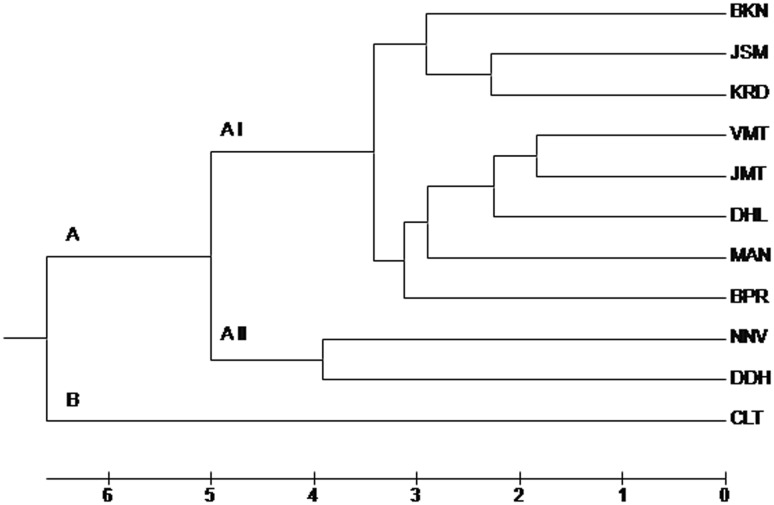
Genetic distance among pair of populations ranged from 0.04 (VMT vs. JMT) to 0.17 (BPR vs. CLT), followed by 0.15 (JSM vs. CLT) (Table S3). UPGMA dendrogram clearly separated out the CLT population from the wild populations that clustered together into two broad clusters (AI and AII). Cluster AI comprised of all the eight populations from Rajasthan while cluster AII grouped together both the populations from Gujarat (NNV and DDH). Cluster AI further divided into two sub-clusters (AIa and AIb). Cluster AIa grouped together BKN, JSM and KRD populations whereas cluster AIb clustered together VMT, JMT, DHL, MAN and BPR populations (Fig. 2). This pattern of clustering of populations was further confirmed in PCoA clustering of accessions.
Fig. 2.
UPGMA dendrogram showing the relationship among eleven populations of Ephedra foliata
The genetic distances among ten natural populations ranged from 0.04 (VMT vs. JMT) to 0.13 (BKN vs. NNV), while corresponding geographic distances of populations ranged from 32.02 km (KRD vs. VMT) to 739.10 km (JMT vs. DDH) (Table S4). Mantel’s test between pair-wise genetic and geographic distances of wild populations revealed a weak but positive correlation (r = 0.375, p = 0.062), indicating that these populations have evolved in response to their local habitat conditions, therefore, geographic isolation has contributed in shaping the present population structure in E. foliata growing wild in arid and semi-arid regions of north western India. Pearson correlation (r) between genetic diversity (H) of populations and rainfall, temperature and altitude of the collection sites did not revealed a significant correlation (data not showed).
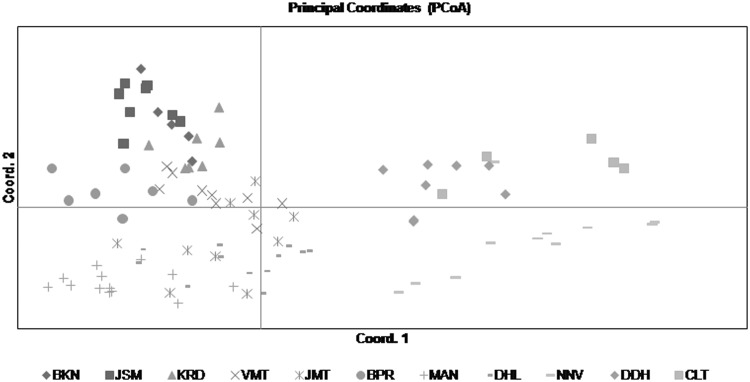
Principal coordinate analysis (PCoA) was performed to provide a spatial representation of the relative genetic distances among accessions and to determine the consistency of differentiation among populations as defined by the cluster analysis. The first three principal coordinates explained 16.75%, 12.93% and 8.09% of total variance, respectively among the accessions representing 11 natural populations (data not shown). PCoA was largely consistent with cluster analysis and clearly separated the 95 accessions of 11 populations (ten natural and one ex situ) into two clusters on the basis of their population identities (Fig. 3). In an agreement with the cluster analysis, accessions from each population formed a separate plot and could be clearly distinguished from those of other populations. The cluster I grouped together 24 accessions from NNV, DDH, and CLT populations, while cluster II grouped all the remaining 71 accessions of eight natural populations from Rajasthan.
Fig. 3.
Principal coordinate analysis showing the spatial differentiation among 95 accessions of ten natural and one ex situ populations of Ephedra foliata
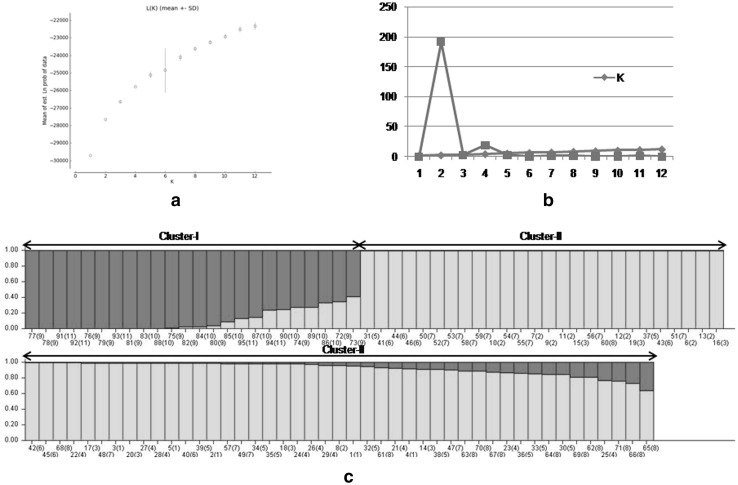
The Bayesian model based STRUCTURE analysis applied to infer genetic structure among E. foliata accessions revealed that maximum likelihood of clustering was obtained when samples were grouped into two clusters (Fig. 4). Evanno test detected two genetic clusters (k = 2) within the 11 assumed natural populations showing an average ancestry membership participation of ≥ 74% to one of the two inferred clusters (Table S5). Cluster I contained 24 accessions, all from three populations including two from Gujarat (NNV and DDH) and one ex situ population (CLT) with an ancestry membership coefficient ≥ 89% (59.2–99.9%), whereas cluster II contained remaining 71 accessions from eight populations from Rajasthan, with an average ancestry membership coefficient ≥ 95% (63.6–99.8%) (Table S6). Nine accessions [Ef72-Ef74 (NNV), Ef86, Ef87, Ef89, Ef90 (DDH) and Ef94, Ef95 (CLT)] of cluster I showed lower values of ancestry membership coefficient than the average (89%). In cluster II, fifty individuals showed ancestry membership higher than the average 95% (95.6–99.8%). This confirms that E. foliata growing in arid and semi-arid parts of Rajasthan and Gujarat, India has two types of genetic populations or gene pools. The assignment of the assumed natural populations revealed a strong population genetic structure among them. The Bayesian clustering analysis further corroborated with the UPGMA and PCoA clustering patterns of detecting two groups of populations (Figs. 2, 3).
Fig. 4.
Genetic structure of ten natural and one ex situ populations of Ephedra foliata sampled from north western arid and semi-arid regions using the Bayesian clustering software STRUCTURE ver. 2.3.4. a The probable K value estimated by the likelihood of the probability of data L(K), b value of ∆K estimated by Evanno test, c bar plots represents accessions arranged according to its most likely ancestry. Each colour represents the most likely ancestry of the cluster from which the genotype was derived (colour figure online)
Analysis of molecular variance (AMOVA) performed to measure population differentiation to further evaluate the genetic structure in 11 natural populations of E. foliata revealed that majority of the variance was restricted within populations (73.99%), whereas 26.01% variance was partitioned among populations with a FST value of 0.26 (Table 3). The estimates of differentiation evaluated among the two genetic populations inferred from clustering analyses (PCoA and Bayesian) methods revealed even higher variance within populations (84.62%) and lower among populations (15.38%) with lower values of FST (0.15).
Discussion
The present study was undertaken to analyze the genetic variability and population structure in representing eleven populations of E. foliata (ten natural and one ex situ) sampled from arid and semi-arid north western parts of India. ISSR markers revealed higher levels of polymorphism, PIC, MI, DI and EMR than DAMD markers and proved more informative for analyzing the genetic diversity in E. foliata accessions and is congruent with Meena et al. (2016) and Singh et al. (2016). Cumulative data (DAMD and ISSR primers) revealed high polymorphism (P = 85.08%) across E. foliata accessions. The level of percentage of polymorphism found in the present study is comparable with the earlier studies carried out in Ephedra species using RAPD (P = 85%; Takeuchi et al. 2003), ISSR (P = 72%; Zhu et al. 2013), RAPD and ISSR (P = 62%; Saeed et al. 2015), and ISSR and DAMD (P = 90.84%; Meena et al. 2016) and is also higher than the levels found in a meta-analysis of the genetic diversity based on the allozyme analysis of 55 out-crossing endemic gymnosperms (P = 70.9%; Hamrick et al. 1992). This level of polymorphism is also comparable with the levels revealed by ISSR and DAMD, and RAPD and ISSR in two important tree species i.e. Prosopis cineraria (P = 95.18%) and Commiphora wightii (P = 86.72%) occuring naturally in arid and semi-arid regions of India (Sharma et al. 2011; Harish et al. 2013). At population level, BKN population showed lowest parameters of genetic diversity (P = 34.30%, H = 0.13, I = 0.19) followed by BPR population (P = 38.08%, H = 0.14, I = 0.21), while MAN population showed highest diversity parameters (P = 49.44%, H = 0.18, I = 0.26). The different levels of genetic diversity were probably due to the locations of sampled populations. The BKN population was found to be the most fragmented, as heavy grazing and cutting of trees by local inhabitants has impacted adversely the natural regeneration process or recruitment of new individuals in the population. The smaller population size (only 5 individuals recorded), degraded habitat and higher geographic isolation (154 km from MAN) of BKN population may be an explanation for the low level of genetic diversity. Comparatively, MAN population was much larger and intact due to being part of KAZRI field station, Jodhpur; and more number of sampled individuals may have contributed to higher levels of genetic diversity. The high level of genetic diversity in E. foliata is consistent with the findings of Hamrick and Godt (1996), according to which long life span, fecundity and high out-crossing rates in gymnosperms contribute to maintainance of high intra-population genetic diversity. Therefore, high genetic diversity was found in E. foliata at the species level (HT = 0.23 and 0.25) than at population level (HS = 0.16 and 0.23) in both the estimates based on 11 natural and two genetic populations and these results are corroborating with the estimates of Ephedra gerardiana (Meena et al. 2016) and other gymnosperms (Jianfeng et al. 2013; Wang et al. 2016; Chen et al. 2017) analysed using ISSR markers.
In addition, the traits such as out-crossing, wind pollination, seed dispersal associated with the life history of Ephedra species may have promoted high genetic diversity (Hamrick et al. 1992; Nag et al. 2015). E. foliata exhibits a high ratio of male plants over female plants. It is conventionally propagated through seeds, however, the absence of proper seed setting is a major problem. Furthermore, the damage of seeds by pests, significantly affects the propagation by seeds (Singh 2004). Inbreeding depression in conifer species is a common phenomenon which often results in empty seeds formation due to the early abortion of the embryo (Kormutak and Lindgren 1996; Williams and Savolainen 1996). Therefore, more field studies and genetic investigations are required to better understand the mating system of E. foliata.
The over harvesting of Leptadenia pyrotechnica for thatching (Jhumpa, the traditional circular hut) and medicine, and Calligonum polygonoides for food, fodder, and medicine has adversely affected the desert ecosystem (Verma et al. 2014; Kumar et al. 2015). These species are keystone species of the region and considered as the best sand dune stabilizers and provide initial protection to E. foliata from grazing animals and other anthropogenic threats. When these associates are affected, newly recruited individuals of E. foliata automatically get affected and perish. Natural habitats of E. foliata have also been adversely affected by anthropogenic factors like intensive grazing, expansion of cultivation, collections of plants for fuel-wood, medicine, trade and commerce. Besides, the developmental activities like infrastructure, highways, industries, and urbanization has also contributed towards population’s fragmentation in E. foliata (Silori et al. 2005; Shekhawat et al. 2014).
A relatively high level of genetic differentiation (GST = 0.31; FST = 0.26) was estimated among the 11 natural populations of E. foliata (Zhao et al. 2016). These values are comparatively higher than the levels found in Ephedra gerardiana (GST = 0.27), an endemic Himalayan plant (Meena et al. 2016). Though, this estimate of GST computed for 11 natural populations was also higher than the average values estimated for gymnosperms (Nybom and Bartish 2000), long lived perennial herbaceous (Nybom 2004) and widely distributed out crossing species (Hamrick and Godt 1996), however significantly lower levels of differentiation were observed when populations were analyzed based on Bayesian clustering (GST = 0.08, FST = 0.15) and these levels were congruent with levels estimated for gymnosperms. Moreover, some other endangered and endemic gymnosperms like Pinus nigra (Rubio-Moraga et al. 2012) and Podocarpus sellowii (Dantas et al. 2015), Cephalotaxus oliveri (Wang et al. 2016) and Chinese fir (Chen et al. 2017) also have revealed higher genetic differentiation. Therefore, the results of the present investigation are corroborating with the findings of other gymnosperm species.
Geographical isolation limits the gene flow and thus is one major factor that influence genetic differentiation (Pfeifer and Jetschke 2006). The moderate gene flow (Nm = 1.11) among populations of E. foliata also revealed that genetic differentiation among populations may be due to the problems in seed setting, along with ongoing habitat fragmentation and habitat loss (Singh 2004). Other native species of the Indian Desert i.e. P. cineraria and C. wightii also revealed moderate and low levels of gene flow, respectively, thus corroborating with the present findings (Sharma et al. 2011; Harish et al. 2013). The transition of climate from a humid tropical during Neogene to the present day arid climate may also have contributed to the limited gene flow and elevated genetic differentiation among the species growing in Indian Thar Desert (Dhir and Singhvi 2012). A weak but positive correlation (r = 0.375, p = 0.062) between genetic and geographic distances of populations indicated that the isolation of populations is contributing towards shaping the present population structure in E. foliata. Moreover, the genetic drift alone did not contribute to population differentiation as evident by the lack of significant correlation; however, in case of isolation of populations from each other, the genetic drift is an important factor influencing the genetic structure, leading to genetic variations among populations (Fischer et al. 2000), therefore E. foliata populations revealed higher genetic diversity at species level than at populations. However, very low level of differentiation (GST = 0.08, FST = 0.15), and high gene flow (Nm = 5.46) among two natural populations/gene pools inferred from STRUCTURE analysis revealed that E. foliata occurring naturally in arid and semi-arid parts of India is not facing any genetic bottle-necks in its natural habitats.
High genetic differentiation revealed by E. foliata is in congruence with the level of differentiation observed in P. cineraria, (a keystone tree species), and C. wightii (an endangered medicinal plant) growing in arid and semi-arid regions of north western parts of India (Sharma et al. 2011; Harish et al. 2013). A weak but positive correlation between genetic and geographical distances among populations revealed that microhabitats have played an important role in constituting the present genetic structure in E. foliata. Although, the values of Pearson correlation coefficient (r) calculated among genetic diversity, rainfall, temperature, and altitude of populations did not reveal a significant correlation, but positive for rainfall, and negative correlation for temperature and altitude supports the pattern that populations growing in drier and high altitude regions possess less genetic variability. The results of UPGMA, PCoA, and Bayesian clustering showed clear geographic trends among natural populations of E. foliata and its comparison with the ex situ population revealed that effect of climate change during the past may have played an important role in shaping the present genetic diversity and population structure in E. foliata growing in wild in north western parts of India (Dhir and Singhvi 2012). The high genetic differentiation among populations of E. foliata could be due to the geographic isolation, regional climatic conditions, habitat fragmentation and degradation, and over-exploitation in its natural habitats. The present endeavour is the maiden attempt to analyze the genetic diversity and population genetic structure in E. foliata using DAMD and ISSR markers.
Implications for conservation
The assessment of genetic diversity and population structure in threatened and endangered species provide important information for development of conservation strategies and implementation of management programs. The extant of genetic diversity and scale of population differentiation estimated in the present study investigating 11 natural populations of E. foliata have confirmed parallel decrease of genetic diversity in the analyzed populations and that small populations harbour low genetic diversity than the large ones. The maximum genetic diversity was found in ex situ CLT population followed by naturally growing MAN population and the lowest in BKN population of E. foliata. Intra-population diversity was significantly higher than the inter-population diversity revealing that majority of diversity is preserved within populations. High level of genetic differentiation and moderate gene flow among the analyzed 11 natural populations suggest that population isolation is probably long term. These results also indicated that E. foliata does not face any threat of genetic drift or population extinction in immediate future. However, highly fragmented state of its natural habitats warrants speedy measures for maintenance of the available genetic diversity (Kharin 2002; Silori et al. 2005; Quattrocchi 2012; Joshi et al. 2013; Mathur and Sundaramoorthy 2013). It is also equally important to understand the adaptation biology and evolutionary process of the species. The major goal of conservation is the maintenance of genetic diversity in order to prevent the potential extinction of a species (Frankham 2005; Zhang et al. 2012) and in situ conservation is a preferred choice because it allows the ongoing evolutionary mechanism (Liu et al. 2006). The present study detected two genetic clusters in E. foliata growing naturally in north western parts of India and these two genetic clusters could be managed as two evolutionary units and should be given highest priority for conservation. The populations that harbour high genetic diversity and high allelic diversity may be considered suitable candidates for high adaptive variation and these populations are pointed to conservation (Vinceti et al. 2013). Out of the 11 natural populations analyzed, MAN from Rajasthan and DDH from Gujarat possessing high genetic diversity and highest private alleles, should get prioritized for conservation as these populations have the high adaptive capability in changing environments (Harish et al. 2013). Furthermore, seedlings or micro-propagated plants from healthy populations may also be introduced to genetically penurious populations (BKN, JSM and BPR) to increase the levels of heterozygosity in these populations and to conserve diverse gene pools. Populations from Gujarat (NNV and DDH) are genetically more differentiated than Rajasthan populations and should get special management priorities. In addition, more efforts should be made to raise conservation awareness among the local inhabitants, farmers and prohibition of deforestation in E. foliata distribution areas should be strictly implemented. Further study to explain the barriers or bottlenecks that inhibit the proper seed setting in E. foliata will improve our understanding towards conservation of this highly valuable and sole representative of gymnosperms occurring naturally in arid and semi-arid regions of north western parts of India.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The financial support from Council of Scientific and Industrial Research (CSIR), New Delhi, India (OLP-101) is duly acknowledged.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alqasoumi S, Abdel-Kader MS. Screening of some traditionally used plants for their hepatoprotective effect. In: Rao V, editor. Phytochemicals as nutraceuticals—global approaches to their role in nutrition and health. Rijeka: InTech; 2012. [Google Scholar]
- Bhandari MM. Flora of the Indian desert. Jodhpur: Scientific; 1978. p. 439. [Google Scholar]
- Bissa S. Evaluation of antibacterial potential of Ephedra foliata Boiss. ex CA Mey. The Bioscan. 2015;10:1169–1172. [Google Scholar]
- Botstein D, White RL, Skolnick M, Davis RW. Construction of a genetic linkage map in man using restriction fragment length polymorphism. Am J Hum Genet. 1980;32:314–331. [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Peng Z, Wu C, Ma Z, Ding G, Cao G, Ruan S, Lin S. Genetic diversity and variation of Chinese fir from Fujian province and Taiwan, China, based on ISSR markers. PLoS ONE. 2017;12(4):e0175571. doi: 10.1371/journal.pone.0175571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R, Pereira G, Garrido I, Tavares-de-Sousa MM, Espinosa F. Comparison of RAPD, ISSR, and AFLP molecular markers to reveal and classify Orchardgrass (Dactylis glomerata L.) germplasm variations. PloS ONE. 2016;11(4):e0152972. doi: 10.1371/journal.pone.0152972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas LG, Esposito T, de Sousa AC, Félix L, Amorim LL, Benko-Iseppon AM, Batalha-Filho H, Pedrosa-Harand A. Low genetic diversity and high differentiation among relict populations of the neotropical gymnosperm Podocarpus sellowii (Klotz.) in the Atlantic forest. Genetica. 2015;143:21–30. doi: 10.1007/s10709-014-9809-y. [DOI] [PubMed] [Google Scholar]
- Dhir RP, Singhvi AK. The Thar desert and its antiquity. Curr Sci. 2012;102:10. [Google Scholar]
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using software STRUCTURE: a simulation study. Mol Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Lischer HEL. Arlequin suite version 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Res. 2010;10:564e567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Husi R, Prati D, Peintinger M, van Kleunen M, Schmid B. RAPD variation among and within small and large populations of the rare clonal plant Ranunculus reptans (Ranunculaceae) Am J Bot. 2000;87:1128–1137. doi: 10.2307/2656649. [DOI] [PubMed] [Google Scholar]
- Frankham R. Genetics and extinction. Biol Conserv. 2005;126:131–140. doi: 10.1016/j.biocon.2005.05.002. [DOI] [Google Scholar]
- Freitag H, Maier-Stolte M. The genus Ephedra in NE tropical Africa. Kew Bull. 2003;58:415–426. doi: 10.2307/4120624. [DOI] [Google Scholar]
- Gavali D, Sharma D. Traditional knowledge and biodiversity conservation in Gujarat. Indian J Tradit Knowl. 2004;3:51–58. [Google Scholar]
- Ghafoor S, Shah MM, Ahmad H, Swati ZA, Shah SH, Pervez A, Farooq U. Molecular characterization of Ephedra species found in Pakistan. Genet Mol Res. 2007;6:1123–1130. [PubMed] [Google Scholar]
- Hamrick JL, Godt MJW. Effects of life history traits on genetic diversity in plant species. Philos Trans R Soc Lond B Biol Sci. 1996;351:1291–1298. doi: 10.1098/rstb.1996.0112. [DOI] [Google Scholar]
- Hamrick JL, Godt MJW, Sherman-Broyles SL. Factors influencing levels of genetic diversity in woody plant species. New For. 1992;6:95–124. doi: 10.1007/BF00120641. [DOI] [Google Scholar]
- Harish GA, Phulwaria M, Rai MK, Shekhawat NS. Conservation genetics of endangered medicinal plant Commiphora wightii in Indian Thar Desert. Gene. 2013;535:266–272. doi: 10.1016/j.gene.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Heath DD, Iwama GK, Devlin RH. PCR primed with VNTR core sequences yields species specific patterns and hypervariable probes. Nucleic Acids Res. 1993;21:5782–5785. doi: 10.1093/nar/21.24.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JB, Li JW, Wang LJ, Liu LJ, Si SW. Utilization of a set of high-polymorphism DAMD markers for genetic analysis of a cucumber germplasm collection. Acta Physiol Plant. 2011;33:227–231. doi: 10.1007/s11738-010-0525-7. [DOI] [Google Scholar]
- Ickert-Bond SM, Renner SS. The gnetales: recent insights on their morphology, reproductive biology, chromosome numbers, biogeography and divergence times. J Syst Evol. 2016;54:1–16. doi: 10.1111/jse.12190. [DOI] [Google Scholar]
- IUCN (2017) The IUCN Red list of threatened species, version 2017-1. www.iucnredlist.org. Accessed 06 June 2017
- Jaccard P. Nouvelles recherches sur la distribution florale. Bull Soc Vaud Sci Nat. 1908;44:223–270. [Google Scholar]
- Jianfeng L, Shengquing S, Ermei C, Wenjuan Y, Zeping J. Genetic diversity of the critically endangered Thuja sutchuenensis revealed by ISSR markers and the implications for conservation. Int J Mol Sci. 2013;14:14860–14871. doi: 10.3390/ijms140714860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PN, Soni HB, Sunderraj SFW, Joshua J. Conservation and management strategies for threatened plant species of Kachchh Desert Island, Gujarat, India. IJE. 2013;2:45–59. [Google Scholar]
- Kamboj VP. Herbal medicine. Curr Sci. 2000;78:35–39. [Google Scholar]
- Kharin N. Vegetation degradation in Central Asia under the impact of human activities. New York: Springer; 2002. [Google Scholar]
- Kormutak A, Lindgren D. Mating system and empty seeds in Silver fir (Abies alba Mill.) For Genet. 1996;3:231–235. [Google Scholar]
- Kotia A. Threatened plants and their habitats in Indian Thar Desert. In: Rawat GS, editor. Special habitats and threatened plants of India. Dehradun: ENVIS Bulletin: Wildlife and Protected Areas Wildlife Institute of India; 2008. pp. 115–121. [Google Scholar]
- Kumar M, Tiwari M, Mohil P, Bharti V, Jain U. Calligonum polygonoides Linn: an important rare shrub species in Thar Desert of India. Indian J Plant Sci. 2015;4(2):63–66. [Google Scholar]
- Liu J, Wang L, Geng Y, Wang Q, Luo L, Zhong Y. Genetic diversity and population structure of Lamiophlomis rotata (Lamiaceae), an endemic species of Qinghai-Tibet Plateau. Genetica. 2006;128:385–394. doi: 10.1007/s10709-006-7517-y. [DOI] [PubMed] [Google Scholar]
- Lodha D, Rathore N, Kataria V, Shekhawat NS. In-vitro propagation of female Ephedra foliata Boiss. & Kotschy ex Boiss.: an endemic and threatened gymnosperm of the Thar Desert. Physiol Mol Biol Plants. 2014;20(3):375–383. doi: 10.1007/s12298-014-0232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel N. The detection of disease clustering and generalized regression approach. Cancer Res. 1967;27:209–220. [PubMed] [Google Scholar]
- Mathur M, Sundaramoorthy S. Economic assessment and conservation priorities of the Indian Thar Desert medicinal plants. Indian J Nat Prod Resour. 2013;4:283–294. [Google Scholar]
- Meena B, Tiwari V, Singh N, Mahar KS, Sharma YK, Rana TS. Estimation of genetic variability and population structure in Ephedra gerardiana (Ephedraceae): an endangered and endemic high altitude medicinal plant. Agri Gene. 2016;1:116–125. doi: 10.1016/j.aggene.2016.08.002. [DOI] [Google Scholar]
- Nag A, Ahuja PS, Sharma RK. Genetic diversity of high elevation populations of an endangered medicinal plant. AoB Plants. 2015;7:plu076. doi: 10.1093/aobpla/plu076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics. 1978;89:583–590. doi: 10.1093/genetics/89.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybom H. Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol Ecol. 2004;13:1143–1155. doi: 10.1111/j.1365-294X.2004.02141.x. [DOI] [PubMed] [Google Scholar]
- Nybom H, Bartish IV. Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Perspect Plant Ecol Evol Syst. 2000;3:93–114. doi: 10.1078/1433-8319-00006. [DOI] [Google Scholar]
- Pavlicek A, Hrda S, Flegr J. FreeTree—freeware program for construction of phylogenetic trees on the basis of distance data and bootstrapping/jackknife analysis of the tree robustness. Application in the RAPD analysis of the genus Frenkelia. Folia Biol (Praha) 1999;45:97–99. [PubMed] [Google Scholar]
- Peakall R, Smouse PE. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics. 2012;28:2537–2539. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer M, Jetschke G. Influence of geographical isolation on genetic diversity of Himantoglossum hircinum (Orchidaceae) Folia Geobot. 2006;41:3–20. doi: 10.1007/BF02805258. [DOI] [Google Scholar]
- Powell W, Morgante M, Andre C, Hanafey M, Vogel J, Tingey S, Rafalski A. The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol Breed. 1996;2:225–238. doi: 10.1007/BF00564200. [DOI] [Google Scholar]
- Prevost A, Wilkinson MJ. A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor Appl Genet. 1999;98:107–112. doi: 10.1007/s001220051046. [DOI] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin AL, Wang MM, Cun YZ, Yang FS, Wang SS, Ran JH, Wang XQ. Phylogeographic evidence for a link of species divergence of Ephedra in the Qinghai-Tibetan plateau and adjacent regions to the Miocene Asian aridification. PLoS ONE. 2013;8:e56243. doi: 10.1371/journal.pone.0056243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocchi U. CRC world dictionary of medicinal and poisonous plants: common names, scientific names, eponyms, synonyms and etymology. London: Taylor & Francis; 2012. p. 1578. [Google Scholar]
- Rubio-Moraga A, Candel-Perez D, Lucas-Borja ME, Tiscar PA, Viñegla B, Linares JC, Gómez-Gómez L, Ahrazem O. Genetic diversity of Pinus nigra Arn. populations in southern Spain and northern Morocco revealed by inter simple sequence repeat profiles. Int J Mol Sci. 2012;13:5645–5658. doi: 10.3390/ijms13055645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed S, Barozai YK, Ahmed A, Tareen RB, Ali GM, Shehzad A, Begum S. Genetic diversity of Ephedra procera from high altitudes of Quetta valley, Balochistan, using RAPD and ISSR. Pak J Weed Sci Res. 2015;21:163–172. [Google Scholar]
- Sahni KC. Gymnosperms of India and adjacent countries. Dehradun: Bishen Singh Mahendra Pal Singh; 1990. pp. 139–140. [Google Scholar]
- Sharma SK, Kumar S, Rawat D, Kumaria S, Kumar A, Rao SR. Genetic diversity and gene flow estimation in Prosopis cineraria (L.) Druce: a key stone tree species of Indian Thar Desert. Biochem Syst Ecol. 2011;39:9–13. doi: 10.1016/j.bse.2010.12.018. [DOI] [Google Scholar]
- Shekhawat NS, Rai MK, Phulwaria M, Rathore JS, Gupta AK, Purohit M, Patel AK, Kataria V, Shekhawat S. Tree biotechnology with special reference to species of fragile ecosystems and arid environments. In: Ramawat KG, Mérillon JM, Ahuja MR, editors. Tree biotechnology. London: CRC Press; 2014. p. 197. [Google Scholar]
- Silori CS, Dixit AM, Gupta L, Mistry N. Observation on medicinal plant richness and associated conservation issues in district Kachchh, Gujarat. In: Trivedi PC, editor. Medicinal plants: utilization and conservation. Jaipur: Avishkar Publishers; 2005. p. 154. [Google Scholar]
- Singh AK. Endangered economic species of Indian Desert. Genet Res Crop Evol. 2004;51:371–380. doi: 10.1023/B:GRES.0000023452.91250.52. [DOI] [Google Scholar]
- Singh N, Pal AK, Roy RK, Tewari SK, Tamta S, Rana TS. Assessment of genetic variation and population structure in indigenous Gladiolus cultivars inferred from molecular markers. Nucleus. 2016;59:235–244. doi: 10.1007/s13237-016-0181-4. [DOI] [Google Scholar]
- SPSS Inc (2007). IBM SPSS for Windows, version 16.0. Chicago, SPSS Inc
- Takeuchi M, Nakashima A, Mizukami H, Hiraoka N, Kohda H. RAPD analysis of Ephedra plants. Nat Med. 2003;57:50–54. [Google Scholar]
- Verma N, Jha KK, Chaudhary S, Singh O, Kumar A. Phytochemistry, pharmacology and traditional uses of Leptadenia pyrotechnica—an important medicinal plant. Indian J Pharm Biol Res. 2014;2(1):128–134. doi: 10.30750/ijpbr.2.1.20. [DOI] [Google Scholar]
- Vinceti B, Loo J, Gaisberger H, van Zonneveld MJ, Schueler S, Konrad H, Kadu CA, Geburek T. Conservation priorities for Prunus Africana defined with the aid of spatial analysis of genetic data and climatic variables. PLoS ONE. 2013;8(3):e59987. doi: 10.1371/journal.pone.0059987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wang Z, Xia F, Su Y. Local adaptation to temperature and precipitation in naturally fragmented populations of Cephalotaxus oliveri, an endangered conifer endemic to China. Sci Rep. 2016;6:25031. doi: 10.1038/srep25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CG, Savolainen O. Inbreeding depression in conifers: implications for breeding strategy. For Sci. 1996;42:102–117. [Google Scholar]
- Wu H, Ma Z, Wang MM, Qin AL, Ran JH, Wang XQ. A high frequency of allopolyploid speciation in the gymnospermous genus Ephedra and its possible association with some biological and ecological features. Mol Eco. 2016;25(5):1192–1210. doi: 10.1111/mec.13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh FC, Yang RC, Boyle T. Microsoft windows-based freeware for population genetic analysis, quick user guide. Edmonton: Center for International Forestry Research, University of Alberta; 1999. [Google Scholar]
- Zhang R, Zhou Z, Du K. Genetic diversity of natural populations of endangered Ormosia hosiei, endemic to China. Biochem Syst Ecol. 2012;40:13–18. doi: 10.1016/j.bse.2011.09.005. [DOI] [Google Scholar]
- Zhao H, Wang Y, Yang D, Zhao X, Li N, Zhou Y. An analysis of genetic diversity in Marphysa sanguinea from different geographic populations using ISSR polymorphisms. Biochem Syst Ecol. 2016;64:65–69. doi: 10.1016/j.bse.2015.11.002. [DOI] [Google Scholar]
- Zhu TT, Jin L, Du T, Cui ZJ, Zhang XF, Wu D. Genetic relationship analysis of Ephedra intermedia from different habitat in Gansu by ISSR analysis. Zhonq Yao Cai. 2013;36(9):1397–1401. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.