Abstract
The emergence of multi-drug resistant (MDR) Gram-negative pathogens has become a serious worldwide health concern. Gram-negative bacteria such as Enterobacteriaceae (Klebsiella pneumoniae, Escherichia coli, Enterobacter spp.,) Acinetobacter spp., and Pseudomonas aeruginosa have rendered most antibiotics inactive, leaving aminoglycosides and polymyxins. Plazomicin (formerly ACHN-490), is a neoglycoside with unique structural modifications to the aminoglycoside pharmacophore that impart activity against many MDR Gram-negative organisms. ACHN-490 was recently approved by the US Food and Drug Administration for the treatment of complicated urinary tract infections caused by MDR Enterobacteriaceae. In this era of increasing Gram-negative resistance, it is imperative to critically evaluate new antibiotics so that we understand how to use them optimally. The objective of this article is to discuss available data detailing plazomicin’s biochemistry, pharmacokinetic/pharmacodynamic characteristics, in-vitro activity and current progress in clinical trials. In addition, plazomicin’s potential role in therapy for the treatment of MDR Gram-negative infections will be discussed.
Keywords: Aminoglycosides, Complicated urinary tract infection, Gram-negative, Multi-drug resistance, Plazomicin
Introduction
Multidrug-resistance (MDR) among Gram-negative pathogens is an urgent public health threat as bacteria continue to create new modes of resistance to evade the current mainstays of antimicrobial therapy [1, 2]. The evolution of β-lactam resistance among Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter spp., has forced clinicians to resort to antimicrobial agents that have poorly defined pharmacokinetic/pharmacodynamic (PK/PD) targets, as well as significant toxicity which limit their reliability in infection eradication [1, 3]. Plazomicin (formerly ACHN-490), a neoglycoside, has demonstrated activity against Gram-negative bacteria expressing a wide range of resistance mechanisms [4]. Aminoglycosides (AGs) are broad spectrum, bactericidal, antibiotics used for treating complicated urinary tract infections (cUTIs), nosocomial respiratory tract infections, and septicemia. Similar to other classes of antibiotics, resistance to AGs has emerged with up to 30% of Enterobacteriaceae demonstrating resistance to gentamicin, tobramycin, and/or amikacin [5, 6]. The limited availability of antibiotics to treat serious MDR infections attests to the need for novel agents such as plazomicin.
Data sources
Literature searches were conducted using the MEDLINE (1963–May 2018) and EMBASE (1988–May 2018) databases, searching using the terms “ACHN-490”, “aminoglycosides”, and “plazomicin”. English articles were used to obtain results, and additional citations were gained through information provided from literature specific to plazomicin. Current Trials, in addition to information outlined in FDA review documents, were also reviewed. Finally, data presented within this article was collected from conference proceedings; including published abstracts.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Chemistry and mechanism of action
AGs act primarily by impairing bacterial protein synthesis through binding to the A-site of the 16S ribosomal RNA (rRNA); functionally known as the decoding center of the 30s ribosome [7, 8]. The passage of these highly polar molecules across the outer membrane of Gram–negative bacteria is an energy-dependent process involving the drug-induced disruption of Mg2+ bridges between adjacent lipopolysaccharide molecules. The unique combination in the uptake of AGs, as well as the inhibition of bacterial protein synthesis, aid in producing the concentration-dependent killing exhibited by this class of antimicrobials [9–11].
AG resistance develops through several mechanisms that can coexist simultaneously within the same isolate. Mechanisms of resistance include modification of the AG target site through the mutation of the 16S rRNA or ribosomal proteins, methylation of 16S rRNA, reduced permeability by modification of the outer membrane, or the enzymatic inactivation of the antibiotic molecule through the development of AG-specific transferases. For Enterobacteriaceae, resistance to AGs is caused primarily by AG modifying enzymes (AMEs) [9]. AMEs are defined by various transferases including those that inactivate AGs by N-acetylation [AG acetyltransferase (AAC)], O-adenylation [AG-nucleotidyltransferase (ANT)], or O-phosphorylation [AG-phosphotransferases (APH)]. These enzymes can be present in the transferable elements or chromosomes of the bacteria. Transferases are diverse with respect to the positions of the AG scaffold that they attack, as well as the genes that encode them [5, 10].
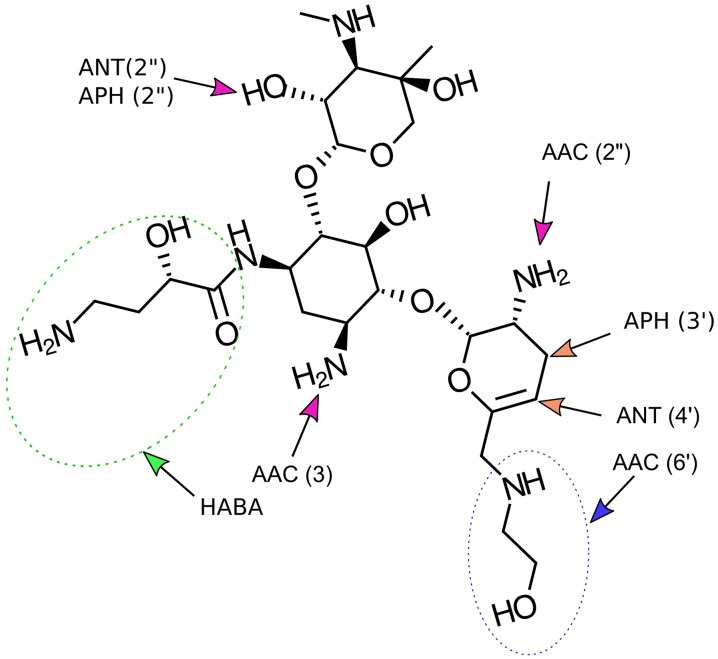
Synthesized through an eight-step process, plazomicin (C25H48N6O10) (Fig. 1), was derived utilizing the original chemistry of sisomicin (C19H37N5O7). The construction of plazomicin was consummated through the basic rendering of sis sulfate followed by a subsequent reaction with ethyl trifluorothioacetate to selectively form the 6′trifluoroacetamide. Comparable to sisomicin, which excludes the 3′-and 4′–OH groups characterized in the traditional AG pharmacophore, plazomicin is active against the APH (3′) and ANT (4′) enzymes. Contrary to its parent compound, the extension in plazomicin’s spectrum of activity is exhibited through the institution of a hydroxyamino butyric acid (HABA) substituent at position 1 and a hydroxyethyl substituent at position 6′. The aforementioned modification at the 1′ position provides additional protection from the AAC (3), ANT (2″), and APH (2″) AMEs, while the addition of the hydroxyethyl group at the 6′ position supplements further protection from the AAC (6′) AME, present in various Enterobacteriaceae (see Fig. 1) [11].
Fig. 1.
Structure of plazomicin and modification sites. The hydroxyamino butyric acid (HABA) substituent (green dotted line) at position 1 and the hydroxyethyl compound addition at the 6′-N position, enhancing plazomicin’s activity against various aminoglycoside-modifying enzymes. The 2′-N position remains unblocked to AACs
Represented in Fig. 1 is plazomicin derived from its parent molecule, sisomicin. Distinct differences between the two molecules are represented through the presence of the two side chains located on plazomicin’s C1 and C6′ nitrogen atoms. Plazomicin is protected from the APH (3′) and ANT(2″) AMEs through the lack in hydroxyl groups at positions C3′ and C4′, and consequently inactivity to the AAC (2′) AME is preserved [7–11].
Microbiology
The in vitro activity of plazomicin and comparator AGs against various Gram-negative and Gram-positive pathogens, several of which are characterized by AMEs and extended spectrum beta-lactamases (ESBLs), are presented in Tables 1 and 2. The minimum inhibitory concentration (MIC50 and MIC90, range) values are representative of pooled data from studies conducted with plazomicin. Preference was given to those studies conducted using isolates collected within the last 3 years when summarizing these data. Standard MIC determination methods were conducted under neutral pH conditions in accordance with Clinical and Laboratory Standard Institute procedures [11–16].
Table 1.
| Species | AG | MIC50 (mg/L) | MIC90 (mg/L) | MIC range |
|---|---|---|---|---|
| All Enterobacteriaceae | Plazomicin | 0.5 | 1 | 0.25 to 8 |
| AMKa | 8 | 64 | 1 to > 64 | |
| GENb | 32 | > 64 | 0.5 to > 64 | |
| TOBc | 32 | > 32 | 0.5 to > 32 | |
| Escherichia coli | Plazomicin | 0.5 | 1 | 0.5 to 4 |
| AMK | < 8 | > 32 | < 8 to 32 | |
| GEN | 8 | > 8 | < 2 to 8 | |
| TOB | 32 | > 32 | 1 to 32 | |
| Enterobacter spp. | Plazomicin | 0.5 | 1 | < 0.5 to 16 |
| AMK | 8 | 64 | < 1 to > 64 | |
| GEN | 32 | > 64 | < 1 to > 64 | |
| TOB | 0.5 | 64 | 0.5 to 64 | |
| Klebsiella pneumoniae | Plazomicin | 0.5 | 1 | 0.25 to 8 |
| AMK | 32 | > 32 | < 8 to 32 | |
| GEN | 4 | 8 | < 2 to 8 | |
| TOB | 32 | > 32 | 0.5 to 64 | |
| Pseudomonas aeruginosa | Plazomicin | 8 | 64 | 0.5 to > 64 |
| AMK | 16 | > 64 | 1 to > 64 | |
| GEN | > 64 | > 64 | 8 to > 64 | |
| TOB | 2 | 16 | 0.5 to > 64 | |
| Acinetobacter spp. | Plazomicin | 16 | 32 | 1 to > 64 |
| AMK | > 64 | > 64 | 4 to > 64 | |
| GEN | > 64 | > 64 | 2 to > 64 | |
| TOB | > 64 | > 64 | 2 to 64 | |
| Methicillin-resistant Staphylococcus aureus | Plazomicin | 1 | 2 | 0.25 to 4 |
| AMK | 16 | > 64 | 0.5 to > 64 | |
| GEN | 32 | > 64 | 0.25 to > 64 | |
| TOB | – | – | – |
aAMK amikacin
bGEN gentamicin
cTOB tobramycin
Table 2.
| Organism group | Phenotype | MIC (mg/L) | ||
|---|---|---|---|---|
| AMK | GEN | Plazomicin | ||
| Escherichia coli | ATCC 25,992a | 2 | 1 | 1 |
| ANT(2″)-Ib | 4 | > 64 | 1 | |
| AAC(6′)-Ic | 32 | 4 | 0.25 | |
| AAC(3)-II | 4 | > 64 | 2 | |
| APH(3′)-Ibd | 0.5 | 0.25 | 0.25 | |
| AAC(3)-Iva | 4 | 32 | 1 | |
| Klebsiella pneumoniae | ATCC 10,031 | 0.5 | 0.12 | 0.25 |
| Providencia stuartii | AAC (2′) | > 64 | > 64 | 8 |
| Pseudomonas aeruginosa | ATCC 27,853 | 2 | 1 | 2 |
| Wild-type pump | 4 | 1 | 4 | |
| ΔEfflux pumps | 0.5 | 0.125 | 0.125 | |
| MexXY up | 4 | 2 | 8 | |
| ANT (4′)-II | 32 | 4 | 4 | |
| AAC(3)-I | 4 | 64 | 8 | |
| AAC(6′)-II | 4 | 32 | 2 | |
| Acinetobacter spp. | APH(3′)-VI | > 64 | 1 | 1 |
| ATCC 19,606 | 16 | 16 | 8 | |
| AAC(6′)-I | 32 | 8 | 2 | |
| Staphylococcus aureus | ATCC 29,213 | 4 | 0.5 | 1 |
| ANT(4′)-I | > 64 | 0.5 | 1 | |
| APH(3′)-III | 2 | 0.5 | 0.5 | |
| APH(2″) + AAC(6′) | 64 | > 64 | 4 | |
| ESBLes | CTX-Mf, AmpCg, SHVh, TEMi | 16 | 8 | 1 |
| KPCsj | 16 | 64 | 1 | |
aATCC American Type Culture Collection quality control strain
bAAC N-Acetyltransferase catalyzes acetyl CoA-dependent acetylation of amino group
cANT O-Adenyltransferase catalyzes ATP-dependent adenylation of hydroxyl group
dAPHO-phosphotransferase catalyzes ATP-dependent phosphorylation of hydroxyl group
eESBLs Extended-spectrum beta lactamases produced by E. coli, K. pneumoniae, and Enterobacter spp.
fCTX-M Class A ESBL
gAmpC Class C ESBL
hSHV Class B ESBL
iTEM Class A ESBL
jKPC Klebsiella pneumoniae carbapenamase Class A beta-lactamase
The observed in-vitro activity of plazomicin encompasses a variety of AG-resistant organisms, such as Staphylococcus aureus, members of the Enterobacteriaceae family, P. aeruginosa and Acinetobacter spp., as well as AMEs and ESBLs expressed within the aforementioned organisms [11–15].
When tested against 493 methicillin-resistant S. aureus isolates, plazomicin exhibited superior activity against these isolates in comparison to amikacin and gentamicin [11]. The MIC50 and MIC90 values for plazomicin were 1 and 2 mg/L respectively, in comparison to 32 and > 64 mg/L for gentamicin, and 16 and > 64 mg/L for amikacin [11–15].
Of 407 A. baumanni isolates gathered through a surveillance study, high rates of non-susceptibility to the three traditional AGs was observed [11]. Among the isolates studied, 80%, exhibited a plazomicin MIC50 of ≤ 8 mg/L, while the remaining 20% displayed an MIC50 of ≥ 16 mg/L. High -level AG resistance (HLAR), defined as an MIC50 of > 64 mg/L, was documented in 21 strains. The molecular analysis of 20 of the 21 strains with HLAR revealed AAC (3)-Ib as the most prevalent AME responsible for the observed resistance. A MIC50 of 8 mg/L for plazomicin was documented for 4 of the 20 strains presenting with HLAR predominately attributed to the AAC (3)-Ib AME; for the remaining 16 strains, an MIC50 of > 16 mg/L was observed, similar to that of the traditional AGs [11, 16, 17].
In P. aeruginosa, the activity of plazomicin was comparable to amikacin. A plazomicin MIC of 16 mg/L was found in 62% of P. aeruginosa isolates, while 38% or P. aeruginosa positive isolates displayed MICs ≥ 32 mg/L. Two isolates presented with AAC (3)-Ib, an AME possibly attributable to the documented resistance. The plazomicin MIC for these two strains harboring the AAC (3)-Ib AME was found to be > 64 mg/L [11, 16]. Upregulation of efflux pumps, most commonly orchestrated through the MexXy efflux pump, compromises the antimicrobial effect of AGs. The presence of the MexXy efflux pump in P. aeruginosa and Acinetobacter spp. correlates to plazomicin’s decline in activity among these organisms [16–18]. The most prevalent AG-modifying enzymes that contribute to AG resistance in Enterobacteriaceae are AAC (3)-II, AAC (6′)-I, and ANT (2″)-I [2, 3]. As represented in Table 2, plazomicin remains highly potent against each of these AMEs [11–19].
Also represented in Table 2 is plazomicin’s activity against common ESBLs. While ESBLs do not directly inhibit AG activity, they are frequently plasmid-encoded [20]. The encoded plasmids are responsible for carrying AME genes, which prohibit AG activity [21]. Nevertheless, plazomicin in vitro activity against ESBL-producing strains was retained when tested against 209 isolates in which these enzymes were present. Gentamicin’s and amikacin’s MICs were documented as 8 mg/L and 16 mg/L, respectively; plazomicin maintained MICs at 1 mg/L for all ESBL-producing strains [12–15]. These results indicate the improved activity of plazomicin against AMEs as well as ESBLs harboring strains that are typically resistant to AGs [12–21].
Although the data is limited, there are several in-vitro studies that have assessed the potential for combination therapy with plazomicin. Utilizing the checkerboard assay method, Thwaites et al. assessed various MDR Enterobacteriaceae with MIC values ranging from 0.5 to 8 mg/L The assay demonstrated synergy when plazomicin was used in combination with piperacillin/tazobactam, meropenem, and ceftazidime. However, for the combination of levofloxacin, tigecycline and colistin, neither synergy nor antagonism was observed with plazomicin. Time-kill analysis was performed in this study to further confirm the initial observation of synergy. The results from the time-kill analysis further demonstrated plazomicin’s synergistic capabilities with a > 3-log reduction in organisms from baseline exhibited when used in combination with both ceftazidime and piperacillin/tazobactam [22].
Rodriguez-Avial et al., using checkerboard techniques, also observed synergistic activity when plazomicin was combined with meropenem, colistin, and fosfomycin against carbapenemase-producing Enterobacteriaceae. Similar to the study conducted by Thwaites et al., antagonism was not observed in any of the combinations [23].
The outcomes of these studies demonstrate the potential use of plazomicin in combination with other antimicrobials in the treatment of MDR Gram-negative infections [22, 23].
Pharmacokinetics/Pharmacodynamics
The pharmacokinetic (PK) properties of plazomicin have been evaluated through two phase 1, randomized, double-blind, placebo-controlled studies conducted in healthy human subjects.
Study 1 was a parallel-group design, with escalating single (SD) and multiple doses (MD), while the second study included patients receiving a longer duration of the highest tolerated dose from the first study. The dosing of traditional AGs (5–7 mg/kg daily) served as a reference for the dosing of plazomicin utilized in the first study. The patient cohorts in study 1 were dosed ascendingly (cohorts 1a–4),beginning with 1 mg/kg, 4 mg/kg, 7 mg/kg, 11 mg/kg, and concluding with 15 mg/kg. Patients received either a SD or MD of plazomicin, for a descending number of days (cohort 1b for 10 days, cohort 2 for 10 days, cohort 3 for 5 days, and cohort 4 for 3 days). The subjects that received plazomicin in study 2 received 15 mg/kg once daily for 5 days [24].
The 28 subjects of study 1 who received the plazomicin injection were included in the SD and MD PK analyzes. The mean peak plasma plazomicin concentration was attained either at or shortly following initiation. Following the single dose administration, the mean ± standard deviation Cmax ranged from 8 ± 0.8 mg/L in the 1-mg/kg group to 144 ± 45 mg/L in the 15-mg/kg group. Following the conclusion of the infusion, the decline in plazomicin concentrations appeared to be multiphasic, with a decline immediately following initiation, and then a biphasic elimination in the terminal phase; beginning at 16–24 h. The elimination half-life was measured at 12 h following the initial dosing. The mean area under the curve (AUC) ranged from 15 ± 1 h × mg h/L in the 1 mg/kg group to 246 ± 39h × mg h/L in the 15 mg/kg group. Study 1 results for AUC versus dose (mg) and Cmax versus dose (mg), following the initial once-daily 10-min intravenous infusion of the plazomicin injection, were linear and dose-proportional [24].
The mean plasma PK parameters for the patients that received multiple doses in cohorts 1b–4 were generally similar to that of the patients who received a single-dose for each respective cohort. Elimination half-lives were also were similar in both groups across each study, ranging from 3 to 4 h. Steady-state volumes were attained following the second dose of plazomicin, with the volume of distribution having limited variability across both studies. Similar PK parameters were observed in the patients who received 15 mg/kg MD daily versus those that received a SD daily of 15 mg/kg for both studies 1 and 2. The Cmax from those patients in study 1 who received multiple doses of plazomicin was documented to be 117 ± 28 mg/L, while the Cmax was 113 ± 17 mg/L for those patients who had received 15 mg/kg once-daily in study 2. The AUC0–24 was 224 ± 37 mg h/L, and 235 ± 44 mg h/L for the 15-mg/kg allocated group in study 1 versus study 2, respectively. In addition, these studies demonstrated that plazomicin’s 20% protein binding was concentration-independent and that, following excretion, 97.5% of the drug was recovered in the urine. Although the AUC has been shown to be an important indicator in aminoglycoside efficacy, a higher Cmax has been correlated with better tissue penetration which is imperative in terms of preventing adaptive antimicrobial resistance [5, 24].
To further confirm the targeted dose exposure PK/PD relationships, a plazomicin murine septicemia study was conducted using a dose of 28 mg/kg to simulate the 15-mg/kg once-daily dosing in humans. Using this dosing strategy an AUC of 269 mg h/L was obtained. The mice studied were infected with a variety of Enterobacteriaceae spp. exhibiting MIC values ranging from 2 to 16 mg/L. Overall, plazomicin therapy increased survival for 86% of the mice with organism MIC values of ≤ 4 mg/L and 54% for those with organisms exhibiting MICs of ≥ 8 mg/L [25].
The efficacy in plazomicin’s dosing strategy was additionally evaluated through a small randomized, double-blind, phase two study, comparing plazomicin 10 mg/kg, 15 mg/kg, and levofloxacin 750 mg (each administered daily). Plazomicin dosed at 15 mg/kg once-daily was shown to be an effective treatment in the adult patients diagnosed with a cUTI. The microbiological infection was eradicated in over 85% of patients who received plazomicin, with 80% of patients who received the 15 mg/kg daily dose of plazomicin being pronounced clinically cured, per the complete resolution of baseline signs and symptoms of infection. In addition, a lower rate of disease recurrence was observed in the 15-mg/kg once-daily plazomicin group in comparison to the levofloxacin group [26].
These various studies demonstrate that the recommended 15-mg/kg once-daily dosing of plazomicin provides the most optimal pharmacokinetics and pharmacodynamics outcomes required to eradicate resistant organisms [24–26].
Within the phase 3 trial, “Combating Antibiotic-Resistant Enterobacteriaceae (CARE)”; the management of plazomicin through therapeutic drug monitoring (TDM) for 22 patients, noted as being critically ill, was evaluated. Renal function varied in this patient population, ranging from a creatinine clearance (CrCl) of < 36 mL/min to 224 mL/min. Patient’s received 4–14 days of plazomicin therapy, with therapeutic drug monitoring utilized in each patient to attain an AUC of 262 mg*h/L for plazomicin. At the conclusion of therapy, two patients had CrCl that rose above baseline; however, the elevated serum creatinine did resolve within 48 h post-treatment. While the third patient experienced renal decline, the investigators were able to attribute this matter to septic shock unrelated to the plazomicin therapy [27]. In an additional study evaluating plazomicin, Komirenko et al. compared groups with varying renal function, normal to severe (CrCl > 90 ml/min, CrCl < 15 ml/min r,espectively). Plazomicin was administered as a single dose of 7.5 mg/kg to six individuals of four renal function groups (normal, mild, moderate, and severe) and the collection of PK samples took place on days 1–5. The variation of Cmax across each group was not severely impacted by a decline in renal function (37.9 ± 5.01, 32.8 ± 4.30, 39.2 ± 6.43, and 41.4 ± 7.83, respectively to each group); however, the AUC0–24, as well as the total clearance, did show an inversely proportional relationship to the decline in renal function. An approximate fivefold increase in plazomicin AUC levels was exhibited in the severe renal impairment group in relation to the normal renal function group (647 ± 259 mg h/L and 136 ± 17.2 mg h/L, respectively); while the total clearance time, indicated in L/h, decreased nearly fivefold in the severely renally impaired group when compared to the individuals with a normal Crcl (0.96 ± 0.379 L/h and 4.64 ± 1.17 L/h, respectively) [28].
These results demonstrate that for patients with comprised renal function the modification of plazomicin dosing, including the application of therapeutic drug monitoring, would be recommended in providing optimal bacterial eradication while protecting the patient from kidney injury via elevated drug exposure [27, 28].
Resistance
In-vitro studies indicate that the development of resistance to plazomicin is similar to that of the traditional aminoglycosides. The development of plazomicin resistance was evaluated in an in vitro chemostat model, in which exposure to plazomicin was applied to 10 isolates (equal numbers in the E. coli and K. pneumoniae groups) in increasing concentrations. Resistant isolates in this study were defined as those that had with a fourfold increase in MIC readings post-baseline, following a baseline MIC reading of 4. In the strains that developed resistance, per the study definition, an AUC of 66 mg h/L was identified. This AUC is far below the AUC achieved with the recommended 15-mg/kg once-daily dosing; increasing the risk of the development in common AG resistance mechanisms. Resistance was not detected in those isolates for which an AUC of > 66 mg h/L was observed. Despite plazomicin’s success in evading most mechanisms of aminoglycoside resistance, the presence of the New Delhi metallo-β-lactamases (NDM), in strains of E. coli and K. pneumoniae did render the drug ineffective. Due to the encoded plasmids of NDM being avid producers of 16S rRNA methylases, the plazomicin-associated MICs were > 8 mg/L; indicating resistance to this metallo-β-lactamase [29].
Drug–drug interactions
The likelihood of drug–drug interactions with plazomicin was determined following the in vitro testing of plazomicin against a panel of drug-metabolizing enzymes as well as drug transporters. Due to plazomicin being 97.5% excreted, unchanged, through the urine, the interaction with drug enzymes is unlikely. Interactions with major drug transporters, such as the p-glycoprotein, was also not observed in plazomicin trials [24–28].
Clinical efficacy
At this time, plazomicin has been evaluated for the treatment of cUTI including acute pyelonephritis (AP) in one Phase II and one Phase III clinical study [30]. A small, pathogen-specific Phase III trial in patients with carbapenem-resistant Enterobacteriaceae (CRE) bacteremia or hospital-acquired/ventilator-associated bacterial pneumonia (HABP/VABP) was also recently completed (Table 3) [30, 31].
Table 3.
Clinical studies
| Connolly et al. [26, 30] | EPIC [32, 33] | CARE [27, 31] | ||
|---|---|---|---|---|
| Design | Phase II, randomized, double-blind | Phase III, randomized, multi-centered, double-blind |
Cohort 1 Phase III, pathogen-specific, randomized, multi-centered, open-label |
Cohort 2 Phase III, pathogen-specific, single arm |
| Patients |
Adults with cUTI/AP n = 92a |
Adults with cUTI/AP n = 388b |
Adults with CRE bacteremia or HAPBP/VAPBP and APACHE II ≥ 15 n = 37b |
Adults with CRE bacteremia, HAPBP/VAPBP or cUTI not eligible for cohort 1 n = 27 |
| Intervention | Plazomicin 10 mg/kg/day vs. plazomicin 15 mg/kg/day vs. levofloxacin 750 mg/day × 5 days |
Plazomicin 15 mg/kg/day vs. meropenem 1 g q8h Optional switch to oral therapy after 4 days study drug |
Plazomicin 15mg/kg/day vs. colistin | Plazomicin 1 5mg/kg/day |
| Primary outcome(s) |
Microbiological eradication at TOC:c Treatment difference plazomicin 15 mg/kg/day – levofloxacin MITT 2.2% (95% CI − 22.9%, 27.2%) ME 7.6% (95% CI − 16%, 31.3%) |
Clinical + microbiological cure at day 5: treatment difference plazomicin – meropenem, 3.4% (95% CI −10.0%, 3.1%) TOC: 11.6% (95% CI 2.7%, 20.3%)d | 28-day all-cause mortality or significant infection-related complications: plazomicin 4 (23%) vs. colistin 10 (50%) (one-sided P = 0.058) | 28-day all-cause mortality 6 (22.2%) |
APACHE Acute Physiology and Chronic Health Evaluation, CI confidence interval, CRE carbapenem-resistant Enterobacteriaceae, cUTI complicated urinary tract infection, HABP hospital-acquired bacterial pneumonia, ME microbiologically evaluable, MITT modified intention-to-treat, mMITT microbiologic modified intention-to-treatm, TOC test of cure, VABP ventilator-associated bacterial pneumonia
aModified intention-to-treat-population
bMicrobiologic modified intention-to-treat-population
c5–12 days after last treatment
dDays 15–19
The Phase III cUTI/AP study (EPIC) was a randomized, multicenter, multinational, double-blind trial evaluating the safety and efficacy of once-daily plazomicin (15 mg/kg) versus meropenem (1 g, every 8 h) in adults with cUTI/AP [32, 33]. Patients could be switched to oral levofloxacin (or alternative in the case of resistance) following 4 days of IV therapy for a total treatment duration of 7–10 days. Key exclusion criteria included severe renal impairment (CrCl ≤ 30 mL/min), body weight > 150 kg, and urine pathogen resistant to meropenem. A total of 609 patients were randomized, of whom 388 received at least one dose of any study drug and had at least one qualifying pathogen on the baseline urine culture (microbiological modified intention-to-treat population, mMITT). All Enterobacteriaceae were considered to be qualifying pathogens, while P. aeruginosa and Acinetobacter spp. were not [32, 33].
Baseline demographic and clinical characteristics were generally well balanced between study arms. The mean age was 59.4 years, and slightly over half (52.8%) were female. Most patients (98.5%) were enrolled from Eastern Europe and were predominantly Caucasian (99.5%). The mean CrCl was approximately 75 mL/min, with 34.0% of patients having a CrCl of 30–60 mL/min. Overall, 27.5% and 26.0% of patients were infected with ESBL-producing and aminoglycoside-resistant pathogens, respectively. CRE was isolated from the baseline urine culture in 15 (3.9%) patients [29, 30]. The MIC50/90 for plazomicin against Enterobacteriaceae were 0.5/1 mg/L. Among ESBL-producing and aminoglycoside-resistant Enterobacteriaceae, the plazomicin MIC50/90 were 0.25/0.5 mg/L and 0.25/2 mg/L, respectively. Blood cultures were positive in 48 (12.4%) patients [32].
Plazomicin demonstrated non-inferiority to meropenem for the co-primary efficacy endpoints of composite clinical and microbiological cure at day 5 (treatment difference plazomicin–meropenem − 3.4%, 95% CI − 10.0, 3.1) and the test-of–cure time point (TOC, day 17 ± 2) (difference 11.6–15%, 95% CI 2.7, 20.3) [31, 32]. The difference favoring the plazomicin arm at the TOC time point was driven by a fairly large decrease in microbiological eradication in the meropenem arm (microbiological eradication day 5, 98.4% vs. 98.0%, TOC 89.0% vs. 74.6%, plazomicin vs. meropenem, respectively). Microbiological eradication favoring the plazomicin arm was sustained through to the late follow-up visit (LFU, day 24–32) (84.3% vs. 65.0%) [31, 32]. The reason behind this precipitous decline in the meropenem microbiological eradication rates is unclear, although this pattern was also recently observed in the meropenem–vaborbactam cUTI trial where the composite cure rate dropped over 20% from the end-of-therapy to the TOC visit in both β-lactam arms, again driven by microbiological failure [32, 33]. It is possible that plazomicin may have a benefit for sustained microbiological eradication due to PK properties such as the post-antibiotic effect and achievement of high urine concentrations. Although the clinical relevance of these findings is not clear, it is notable that, at the LFU visit (days 24–32), clinical relapse was also higher in the meropenem group (1.6% vs. 7.1%) [33].
Efficacy outcomes in subgroups were generally consistent with the main analysis. Microbiological eradication rates tended to favor plazomicin among all Enterobacteriaceae and specifically in ESBL-producing and aminoglycoside-resistant strains [33].
Although the results of this study support the use of plazomicin as an effective carbapenem-sparing alternative for cUTI, several limitations affect the generalizability of the findings. First, the vast majority of the patients were Caucasian and therefore not representative of countries such as the US which have a more diverse racial makeup. Next, due to concerns from non-clinical studies about plazomicin’s activity against P. aeruginosa and Acinetobacter spp., these pathogens were excluded from the mMITT analysis. Although not common causes of cUTI, in clinical scenarios where they are likely pathogens, an alternative agent may be preferred until more clinical data are available. Finally, patients with severe renal impairment were excluded. As elderly patients with age-related declines in renal function make up a large proportion of patients with cUTI, more data about the safety and efficacy of plazomicin in these patients are needed.
In addition to the EPIC study, an earlier Phase II, multicenter, randomized, double-blind study evaluated the efficacy, safety, and PK of two doses of plazomicin (10 mg/kg and 15 mg/kg) compared to levofloxacin for the treatment of cUTI/AP [26, 30]. As a Phase II study, no formal hypothesis testing was conducted and the overall sample size was relatively small (n = 145). However, this study does provide important data pertaining to a possible plazomicin dose–response relationship as well as on the use of plazomicin in a cohort of patients who differed considerably from the Phase III study by demographic characteristics. In this study, the majority of patients (53.8%) were enrolled from North American sites, the racial distribution was more diverse (37.2% American Indian or Alaska Native, 29.7% Asian and 15.2% African American), the mean age was only 42.6 years and 80.0% were female (ITT population) [31, 33]. There did appear to be a trend toward higher microbiological eradication at the TOC time point in the 15 mg/kg arm (plazomicin 10 mg/kg: 6/12 50% vs. 15 mg/kg: 31/51 60.8% vs. levofloxacin: 17/29 58.6%), although the number of indeterminate results (5, 15, and 8, respectively) and the small, imbalanced sample sizes do make the results difficult from which to draw conclusions [31, 33].
Finally, the CARE study was a Phase III pathogen-specific, multicenter, randomized, open-label trial evaluating plazomicin versus colistin (plus background therapy with meropenem or tigecycline) for CRE bacteremia or HABP/VABP. A parallel single arm cohort (cohort 2) included patients treated with plazomicin for bacteremia, HABP/VABP who were not eligible for primary analysis and patients treated with plazomicin monotherapy for cUTI. Key exclusion criteria for the main analysis (cohort 1) included polymicrobial infection, APACHE II score < 15 and known colistin-resistant infection. The original planned sample size was for over 350 patients including 286 in the mMITT population. However, due to enrollment difficulties (2100 patients screened but less than 2% met inclusion criteria and were included in the trial) the study was stopped after 37 patients had been enrolled in the mMITT population (17 plazomicin and 20 colistin). With regards to demographic characteristics, most patients (59.5%) were at least 65 years of age and 67.6% were enrolled from Greece. Nearly half (45.9%) of patients had an APACHE II score above 20. Although balance was achieved between groups with regards to pre-specified stratification criteria (i.e., infection type, APACHE II score, and time from initial empiric therapy to randomization), due to the small sample size, there were disparities in important demographic characteristics such as age, gender, study site and background therapy. The majority of patients (78.4%) were in the bacteremia stratum. More than half of bacteremic patients had an intravascular central catheter at the time of diagnosis. All patients in the HABP/VABP stratum had VABP and none had positive blood cultures. Carbapenem-resistant K. pneumoniae was the qualifying pathogen in 36/37 patients, while carbapenem-resistant Enterobacter aerogenes was isolated from the blood culture of a single patient in the plazomicin group. Prior antibiotics were used for up to 72 h before enrollment in almost all of the patients, most commonly colistin or meropenem [27, 31].
The primary endpoint was a composite of 28-day all-cause mortality or significant disease-related complications [new/worsening acute respiratory distress syndrome, new lung abscess within 7 days, worsening septic shock within 7 days, new CRE bacteremia within 7 days or persistent ( ≥ 7 days) CRE bacteremia]. A total of 10 (50%) and 4 (23.4%) of patients in the colistin and plazomicin arms experienced the primary outcome, respectively (one-sided P = 0.094). Eight (40%) and two (11.8%) experienced 28-day mortality, respectively (one-sided P = 0.058). Numerical trends favored plazomicin for the primary outcome in all subgroups, particularly in the analysis restricted to bacteremic patients (8/15, 53.3% vs. 2/14, 14.3%; one-sided P = 0.033). Only one patient with bacteremia assigned to plazomicin experienced 28-day mortality. It should be noted, however, that 6 and 5 patients in the colistin and plazomicin arms, respectively, had negative blood cultures within 24 h prior to initiation of the study drug.
A total of 27 patients were enrolled in cohort 2: 14 patients with bacteremia, 9 with HABP/VABP and 4 with cUTI. All patients were enrolled in Greece and all had carbapenem-resistant K. pneumoniae as the qualifying pathogen. There was a total of six (22.2%) deaths in the cohort and all occurred in patients with either bacteremia (2/14) or HABP/VABP (4/9) [31, 32].
Although the results from the CARE study were numerically favorable for plazomicin, the efficacy analysis was limited by several factors. Most significantly, the sample size of cohort 1 consisted of only 37 patients. As such, the results were presented descriptively without the use of inferential statistics. Furthermore, the small sample size led to substantial uncertainty regarding the precise values of the treatment effects. Despite randomization, important differences in baseline characteristics were present. Finally, cohort 2 lacked a control group and patients were not comparable enough to the plazomicin group of cohort 1 to allow a synthesized analysis [31].
On May 3, 2018, the US FDA Antimicrobial Drugs Advisory Committee voted unanimously that the evidence supported the cUTI/AP indication but voted 4–11 (yes–no) that results from the CARE study did not provide “substantial evidence of safety and effectiveness of plazomicin for the treatment of bloodstream infection in patients with limited or no treatment options.” The US FDA subsequently follow suit by approving the cUTI/AP indication but rejecting the bacteremia indication [33].
Safety
To date, the safety of plazomicin has been evaluated in 612 subjects who received varying doses in six phase 1, one phase 2, and two phase 3 studies. This safety database identifies signals for anticipated risks of an aminoglycoside, including nephrotoxicity and ototoxicity, and more general side effects such as headache, nausea and vomiting.
In the phase 2 and phase 3 cUTI/AP studies, nephrotoxicity (defined as an increase in serum creatinine ≥ 0.5 mg/dL) were identified more frequently in the plazomicin arms than in the comparator arms (5.6% vs. 2.4% and 7.0% vs. 4.0%, respectively). Serum creatinine increases ≥ 1 mg/dL were infrequent: 9 (3%) plazomicin treated patients and 3 (1.0%) of meropenem patients in the phase 3 cUTI/AP study. Two patients in each treatment arm of the phase 3 cUTI/AP study experienced acute kidney failure.
As with other AGs, plazomicin-associated nephrotoxicity is significantly associated with increased trough levels (Cmin). Pooled PK data from the two cUTI/AP studies also revealed that the incidence of plazomicin-induced nephrotoxicity was higher in patients with baseline CrCl 30–60 mL/min versus > 60 mL/min, even at comparable Cmin. The incidence of nephrotoxicity at different Cmin thresholds stratified by CrCl is outlined in Table 4.
Table 4.
Incidence of nephrotoxicity by baseline creatinine clearance and plazomicin trough level
| Plazomicin trougha (mg/L) | Nephrotoxicitybn/N (%) | |
|---|---|---|
| CrCl > 60 mL/min.c | CrCl 30–60 mL/min.c | |
| ≥ 0.5 | 9/148 (6) | 13/100 (13) |
| ≥ 1.0 | 6/88 (7) | 12/78 (15) |
| ≥ 1.5 | 4/53 (8) | 10/56 (18) |
| ≥ 2.0 | 4/30 (13) | 10/46 (22) |
| ≥ 2.5 | 3/18 (17) | 8/31 (26) |
| ≥ 3.0 | 3/13 (23) | 7/19 (37) |
CrCl creatinine clearance
aDay 1 trough level; data pooled from PK population of phase 1 and 2 cUTI/AP studies
bSerum creatinine increase ≥ 0.5 mg/L while on plazomicin or during follow-up
cEstimated using the Cockcroft–Gault Equation
Classification and regression tree analysis of these data identified Cmin of 3 mg/L and CrCl around 60 mL/min as critical thresholds associated with a higher incidence of nephrotoxicity. On the other hand, nephrotoxicity did not appear to be associated with duration of treatment (up to 7 days) or concomitant nephrotoxic medications.
In the CARE study, plazomicin doses were adjusted to target an AUC0–24h range of 200–400 mg h/L, corresponding to 75% and 150% of the mean AUC0–24h (262 mg h/L) in cUTI patients with normal renal function. Overall, 86.2% of patients in the study required dosage adjustment to stay within range and a mean of 1.9 adjustments per patient. Among patients in the CARE study with baseline and post-baseline serum creatinine measurements, there were fewer patients with serum creatinine increases ≥ 0.5 mg/L in the plazomicin compared to the colistin arm (2/12, 16.7% vs. 8/16, 50.0%). No patients in the plazomicin arm experienced a serum creatinine increase ≥ 1 mg/L versus 7/16 (43.8%) in the colistin arm. Based on data from this study, the t arget AUC0–24h range was subsequently modified to 210–315 mg h/L.
Assuming an approximately linear relationship between Cmin and AUC0–24h and the exposure–response relationship for nephrotoxicity observed in the cUTI/AP studies, the FDA projected the upper bound of the target range corresponds to Cmin values of 5.7 mg/L and 8.7 mg/L (AUC0–24h 315 and 400 mg h/L, respectively), which would be expected to be associated with high rates of nephrotoxicity. Additionally, PK/PD targets for net bacterial stasis derived from a neutropenic thigh model and plazomicin MIC values for Enterobacteriaceae suggests that the lower bound exceeds the AUC0–24h required to attain PK/PD values several-fold. As a result, therapeutic drug monitoring to maintain Cmin < 3 mg/L is recommended for all patients with a CrCl 15–90 mL/min, rather than dosing guided by AUC0–24h.
Plazomicin’s ototoxic potential was evaluated using pure tone audiometry in Phase 1 and 2 studies. An independent expert review of these data concluded there was no widespread signal for drug-induced ototoxicity. Similarly, no clear signal of vestibulotoxicity was found following review of electronystagmography, modified Romberg testing and dynamic visual acuity testing results performed in Phase 1 and 2 studies. Due to the practical impediments limiting the use of typical measures such as audiometric tests or Romberg testing in hospitalized patients, evaluation of plazomicin’s ototoxic potential in the cUTI/AP studies was primarily based on the use of several validated inventories related to patient-perceived changes in hearing, tinnitus, and dizziness as well as patient-reported adverse events. Hyperacusis, tinnitus, and vertigo were reported by three patients. Whereas aminoglycoside-related ototoxicity is typically non-reversible and bilateral in nature, these events were somewhat atypical in that the hyperacusis and vertigo resolved and the tinnitus was unilateral. The clinical safety data parallel the results of animal models suggesting that at equivalent doses plazomicin had a lower ototoxic potential than both gentamicin and amikacin [34].
Conclusion
With the increasing prevalence of MDR Gram–negative organisms, there has been a resurgence in the use AGs, particularly in combination regimens [5–9]. Plazomicin retains AG concentration-dependent bactericidal activity, while evading common AMEs [6]. The prolonged antibiotic effect and structural modifications enhance plazomicin’s action against Gram–negative pathogens that render broad-spectrum antibiotics defenseless [5–11].
The variety of data presented supports the observed clinical cure and microbiological eradication demonstrated in plazomicin trials, attesting to its recent FDA approval in the treatment of MDR Enterobacteriaceae cUTIs, including acute pyelonephritis [30]. Despite the limited population of patients included in the CARE study evaluating the utility of plazomicin against CRE, a potential therapeutic role for plazomicin was suggested through the decline of mortality in those patients treated with plazomicin as salvage therapy [27]. In-vitro testing has shown that plazomicin is active against the most prevalent of AMEs, including those carried by ESBL-producing pathogens [11–20]. Plazomicin could have the potential to be used a narrower-spectrum alternative to broad-spectrum beta-lactams, specifically carbapenems. Use of plazomicin in these settings may reduce selective pressure attributing to the development of beta-lactam resistance [21]. It would also be interesting to learn whether plazomicin’s narrower spectrum translates into a lower risk of Clostridium difficile infections. The promising results of synergy experiments suggest a potential role for plazomicin in combination regimens. The combinations exhibited synergy and were successful in overcoming the heteroresistance to broad-spectrum beta-lactams traditionally observed in the Enterobacteriaceae species [22, 23].
The recommended dosing profile, 15 mg/kg once-daily, attributes to plazomicin’s possible role in therapy, as it may be a convenient option for outpatient therapy in patients with Enterobacteriaceae infections requiring an extended duration of antimicrobial treatment [24].
Nevertheless, the safety profile of plazomicin is consistent with that of the traditional aminoglycosides; indicating the need for plazomicin dose adjustment through using a lower targeted dose of 10 mg/kg once- daily or the integration of TDM in individuals with comprised renal function [27, 28]. The observed toxicities associated with plazomicin include nephrotoxicity and ototoxicity, both documented in lesser amounts than that of the other systemically available AGs, although relatively few patients have been exposed to plazomicin at this point [34].
Based upon the attested spectrum of activity profile, as well as an established PK/PD profile validated by clinical studies, plazomicin has the ability to serve as an efficacious therapy for treating MDR Enterobacteriaceae infections.
Acknowledgments
Michael J. Rybak is Editor in Chief of Infectious Diseases and Therapy.
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Michael J. Rybak has received grant support, consulted or spoken on behalf of Accelerate, Allergan, Achaogen, Bayer, Merck, Melinta, and Theravance. Jacinda C. Abdul-Mutakabbir, Razieh Kebriaei, and Sarah C. J. Jorgensen have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced digital features
To view enhanced digital features for this article go to 10.6084/m9.figshare.7738892.
References
- 1.Wenzler E, Goff DA, Humphries R, Goldstein EJ. Anticipating the unpredictable: a review of antimicrobial stewardship and Acinetobacter infections. Infect Dis Ther. 2017;6(2):149–172. doi: 10.1007/s40121-017-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doi Y, Bonomo RA, Hooper DC, Kaye KS, Johnson JR, Clancy CJ, et al. Gram-negative bacterial infections: research priorities, accomplishments, and future directions of the Antibacterial Resistance Leadership Group. Clin Infect Dis. 2017;64(suppl_1):S30–S35. doi: 10.1093/cid/ciw829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eliopoulos GM, Drusano GL, Ambrose PG, Bhavnani SM, Bertino JS, Nafziger AN, Louie A. Back to the future: using aminoglycosides again and how to dose them optimally. Clin Infect Dis. 2007;45(6):753–760. doi: 10.1086/520991. [DOI] [PubMed] [Google Scholar]
- 4.Shaeer KM, Zmarlicka MT, Chahine EB, Piccicacco N, Cho JC. Plazomicin: a next-generation aminoglycoside. Pharmacotherapy. 2019;39(1):77–93. doi: 10.1002/phar.2203. [DOI] [PubMed] [Google Scholar]
- 5.Mingeot-Leclercq MP, Glupczynski Y, Tulkens PM. Aminoglycosides: activity and resistance. Antimicrob Agents Chemother. 1999;43(4):727–737. doi: 10.1128/AAC.43.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawser SP, Bouchillon SK, Hoban DJ, Badal RE. In vitro susceptibilities of aerobic and facultative anaerobic Gram-negative bacilli from patients with intra-abdominal infections worldwide from 2005–2007: results from the SMART study. Int J Antimicrob Agents. 2009;34(6):585–588. doi: 10.1016/j.ijantimicag.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Cox G, Ejim L, Stogios PJ, Koteva K, Bordeleau E, Evdokimova E, Sieron AO, Savchenko A, Serio AW, Krause KM, Wright GD. Plazomicin retains antibiotic activity against most aminoglycoside modifying enzymes. ACS Infect Dis. 2018;4(6):980–7. doi: 10.1021/acsinfecdis.8b00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura Y, Matsunaga H, Vaara M. Polymyxin B octapeptide and polymyxin B heptapeptide are potent outer membrane permeability-increasing agents. J Antibiot (Tokyo) 1992;45(5):742–749. doi: 10.7164/antibiotics.45.742. [DOI] [PubMed] [Google Scholar]
- 9.Livermore DM, Mushtaq S, Warner M, Zhang JC, Maharjan S, Doumith M, Woodford N. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J Antimicrob Chemother. 2010;66(1):48–53. doi: 10.1093/jac/dkq408. [DOI] [PubMed] [Google Scholar]
- 10.Busse HJ, Wöstmann C, Barker EP. The bactericidal action of streptomycin: membrane permeabilization caused by the insertion of mistranslated proteins into the cytoplasmic membrane of Escherichia coli and subsequent caging of the antibiotic inside the cells due to degradation of these proteins. J Gen Microbiol. 1992;138(3):551–561. doi: 10.1099/00221287-138-3-551. [DOI] [PubMed] [Google Scholar]
- 11.Aggen JB, Armstrong ES, Goldblum AA, Dozzo P, Linsell MS, Gliedt MJ, Hildebrandt DJ, Feeney LA, Kubo A, Matias RD, Lopez S. Synthesis and spectrum of the neoglycoside ACHN-490. Antimicrob Agents Chemother. 2010;54(11):4636–4642. doi: 10.1128/AAC.00572-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almaghrabi Reem, Clancy Cornelius J., Doi Yohei, Hao Binghua, Chen Liang, Shields Ryan K., Press Ellen G., Iovine Nicole M., Townsend Bethany M., Wagener Marilyn M., Kreiswirth Barry, Nguyen M. Hong. Carbapenem-Resistant Klebsiella pneumoniae Strains Exhibit Diversity in Aminoglycoside-Modifying Enzymes, Which Exert Differing Effects on Plazomicin and Other Agents. Antimicrobial Agents and Chemotherapy. 2014;58(8):4443–4451. doi: 10.1128/AAC.00099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castanheira M, Davis AP, Mendes RE, Serio AW, Krause KM, Flamm RK. In vitro activity of plazomicin against Gram-negative and Gram-positive isolates collected from US hospitals and comparative activities of aminoglycosides against carbapenem-resistant Enterobacteriaceae and isolates carrying carbapenemase genes. Antimicrob Agents Chemother. 2018;62(8):e00313–e00318. doi: 10.1128/AAC.00313-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castanheira M, Deshpande LM, Woosley LN, Serio AW, Krause KM, Flamm RK. Activity of plazomicin compared with other aminoglycosides against isolates from European and adjacent countries, including Enterobacteriaceae molecularly characterized for aminoglycoside-modifying enzymes and other resistance mechanisms. J Antimicrob Chemother. 2018;73(12):3346–3354. doi: 10.1093/jac/dky344. [DOI] [PubMed] [Google Scholar]
- 15.Galani I, Souli M, Daikos GL, Chrysouli Z, Poulakou G, Psichogiou M, et al. Activity of plazomicin (ACHN-490) against MDR clinical isolates of Klebsiella pneumoniae, Escherichia coli, and Enterobacter spp. from Athens, Greece. J Chemother. 2012;24(4):191–194. doi: 10.1179/1973947812Y.0000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landman D, Kelly P, Bäcker M, Babu E, Shah N, Bratu S, Quale J. Antimicrobial activity of a novel aminoglycoside, ACHN-490, against Acinetobacter baumannii and Pseudomonas aeruginosa from New York City. J Antimicrob Chemother. 2010;66(2):332–334. doi: 10.1093/jac/dkq459. [DOI] [PubMed] [Google Scholar]
- 17.Hocquet D, Vogne C, El Garch F, Vejux A, Gotoh N, Lee A, et al. MexXY-OprM efflux pump is necessary for a adaptive resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother. 2003;47(4):1371–1375. doi: 10.1128/AAC.47.4.1371-1375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobel ML, McKay GA, Poole K. Contribution of the MexXY multidrug transporter to aminoglycoside resistance in Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother. 2003;47(10):3202–3207. doi: 10.1128/AAC.47.10.3202-3207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walkty A, Karlowsky JA, Baxter MR, Adam HJ, Boyd D, Bharat A, Mulvey MR, Charles M, Bergevin M, Zhanel GG. Frequency of 16S ribosomal RNA methyltransferase detection among Escherichia coli and Klebsiella pneumoniae clinical isolates obtained from patients in Canadian hospitals (CANWARD, 2013–2017) Diagn Microbiol Infect Dis. 2018 doi: 10.1016/j.diagmicrobio.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Sirot D. Extended-spectrum plasmid-mediated β-lactamases. J Antimicrob Chemother. 1995;36(suppl A):19–34. doi: 10.1093/jac/36.suppl_A.19. [DOI] [PubMed] [Google Scholar]
- 21.Dhillon RH, Clark J. ESBLs: a clear and present danger? Critical care research and practice. 2012 doi: 10.1155/2012/625170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thwaites M, Hall D, Stoneburner A, Shinabarger D, Serio AW, Krause KM, et al. Activity of plazomicin in combination with other antibiotics against multidrug-resistant Enterobacteriaceae. Diagn Microbiol Infect Dis. 2018;92(4):338–345. doi: 10.1016/j.diagmicrobio.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Salguero C, Rodriguez-Avial I, Picazo JJ, Culebras E. Can plazomicin alone or in combination be a therapeutic option against carbapenem-resistant Acinetobacter baumannii? Antimicrob Agents Chemother. 2015;59(10):5959–5966. doi: 10.1128/AAC.00873-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cass RT, Brooks CD, Havrilla NA, Tack KJ, Borin MT, Young D, et al. Pharmacokinetics and safety of single and multiple doses of ACHN-490 injection administered intravenously in healthy subjects. Antimicrob Agents Chemother. 2011;55(12):5874–5880. doi: 10.1128/AAC.00624-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdelraouf Kamilia, Kim Aryun, Krause Kevin M, Nicolau David P. Assessment of the In Vivo Efficacy of Plazomicin (PLZ) Alone or in Combination with Meropenem (MEM) or Tigecycline (TGC) against Enterobacteriaceae (EB) Isolates Exhibiting Various Resistance Mechanisms in an Immunocompetent (I+) Murine Septicemia Model. Open Forum Infectious Diseases. 2017;4(suppl_1):S470–S470. doi: 10.1093/ofid/ofx163.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connolly LE, Riddle V, Cebrik D, Armstrong ES, Miller LG. A multicenter, randomized, double-blind, phase 2 study of the efficacy and safety of plazomicin compared with levofloxacin in the treatment of complicated urinary tract infection and acute pyelonephritis. Antimicrob Agents Chemother. 2018;62(4):e01989-17. doi: 10.1128/AAC.01989-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKinnell JA CL, Pushkin R, Jubb AM, O’Keeffe B, Serio AW, Smith A, Gall J, Riddle V, Krause KM, Pogue JM. Improved outcomes with plazomicin (PLZ) compared with colistin (CST) in patients with bloodstream infections (BSI) caused by carbapenem-resistant Enterobacteriaceae (CRE): results from the CARE study. In: Open Forum Infectious Diseases 2017 Oct 4;Vol. 4, No. suppl_1, pp. S531–S531. New York: Oxford University Press.
- 28.Komirenko AS, Riddle V, Gibbons JA, Van Wart S, Seroogy JD. A phase 1 study to assess the pharmacokinetics of intravenous plazomicin in adult subjects with varying degrees of renal function. Antimicrob Agents Chemother. 2018;62(12):e01128-18. doi: 10.1128/AAC.01128-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thwaites M, Hall D, Shinabarger D, Serio AW, Krause KM, Marra A, Marra A, Pillar C. Evaluation of the bactericidal activity of plazomicin and comparators against multidrug-resistant enterobacteriaceae. Antimicrob Agents Chemother. 2018;62(8):e00236-18. doi: 10.1128/AAC.00236-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Food and Drug Administration briefing document. Plazomicin sulfate injection. Document prepared for the meeting of the Antimicrobial Drugs Advisory Committee (AMDAC). 2 May 2018. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM606039.pdf. Accessed 15 Oct 2018.
- 31.Shrimant M. NDA 210303 Clinical review plazomicin injection (Zemdri™). Center for Drug Evaluation and Research. US Food and Drug Administration. 13 June 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210303Orig1s000MedR.pdf Accessed 15 Oct 2018.
- 32.Shurland SM. NDA 210303 Clinical microbiology review plazomicin injection (Zemdri™). Center for Drug Evaluation and Research. US Food and Drug Administration. 17 May 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210303Orig1s000MicroR.pdf Accessed 15 Oct 2018.
- 33.Nambiar S, Cox E. NDA 210303 Summary review plazomicin injection (Zemdri™) . Center for Drug Evaluation and Research. US Food and Drug Administration. 25 June 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210303Orig1s000SumR.pdf. Accessed15 Oct 2018.
- 34.Kostrub CF, Dolan DF, Altschuler RA, Baird TJ, Tapp RL, Boggs JA, Bruss JB. Ototoxic potential of ACHN-490 compared to gentamicin and amikacin in the guinea pig. In: presented at: 20th European congress of clinical microbiology and infectious diseases. Vienna, AT, 10-13 April 2010. Abstract P1249.