Abstract
Transformed hairy root culture in common buckwheat (Fagopyrum esculentum Moench Rubra cultivar) was investigated for accumulation of amino acids and specific flavonoids. Leaves and stems of F. esculentum were used a starting material for induction of hairy roots via the Agrobacterium rhizogenes A4 strain. The transformed lines were confirmed by PCR detection of rol B gene, and their capability to continuously form hairy roots. Three lines from each explant types depending upon growth kinetics were observed. The hairy root lines were used to measure the contents of 17 amino acids and 3 flavonoids. Overall, the hairy root lines exhibited elevated accumulation of semi-essential amino acids such as lysine, isoleucine, valine, histidine and phenylalanine. Content of proline was increased 3–5 times, likely due to the biotic stress reaction induced with A. rhizogenes. Determination of flavonoids by high-performance liquid chromatography, hesperidine and kaempferol-3-rutinoside, were accumulated in hairy root cultures and didn’t detected in non-transformed root. The increase in flavonoids positively correlated with the antioxidant capacity of the hairy root cultures.
Keywords: Common Buckwheat, Agrobacterium rhizogenes, Semi-essential amino acids, Flavonoids
Introduction
Agrobacterium rhizogenes-mediate transformation is instrumental for induction of hairy roots (Georgiev et al. 2013). The ability of the A. rhizogenes to promote hairy root is determined by a part of the Ri (root inducing) plasmid, the so-called T-DNA that is transferred and inhered into the plant’s genomic DNA. The T-DNA encodes auxin biosynthesis genes as well as genes that are required for the formation of opines, which may be classified chemically as non-protein amino acids (Petit et al. 1983; Jung and Tepfer 1987). The hairy roots revealed fast growth. They are singular due their genetic and biosynthetic stability which can improve growth regulators activities (Giri and Narasu 2000; Arafa et al. 2015). The hairy roots cultures of different plants were reported to enhance the production of diverse metabolites (Srivastava and Srivastava 2007; Goel et al. 2011; Mehrotra et al. 2013; Gabr et al. 2016a; Alok et al. 2016). Furthermore, hairy roots cultures are advised as a promising source of biological active compounds that are not detectable even in parental plants (Berkov et al. 2003; Weathers et al. 2005; Gyurkovska et al. 2011; Pollier et al. 2011).
Common buckwheat (Fagopyrum esculentum Moench) in comparison to other cereals is characterized by high composition of the amino acid’s lysine, arginine, aspartic acid and less proline and glutamic acid. Thus, common buckwheat proteins typically have higher biological value than other cereals because of their high lysine levels (Ikeda 2002). The absence of gluten in buckwheat flour place it as a potential source for gluten-free diets (Alvarez-Jubete et al. 2010). Furthermore, common buckwheat is known as a medicinal food source due to its valuable content of flavonoids that have protective or curative properties, such as cholesterol reduction (Kayashita et al. 1997), tumor inhibition (Chan 2003), carcinogenesis and inflammation control (Ishii et al. 2008), and diabetes (Kawa et al. 2003). Additionally, common buckwheat (F. esculentum Moench) is rich of natural antioxidants, such as tocopherols, rutin, and phenolic acids (Ghimeray et al. 2010; Sedej et al. 2010; Dziadek et al. 2016; Verardo et al. 2010). Buckwheat plants accumulate high amount of various phenolic compounds and antioxidants as compared to cereals plants such as amaranth, wheat and quinoa (Alvarez-Jubete et al. 2010; Gallardo et al. 2006; Gorinstein et al. 2008; Kim et al. 2011) The buckwheat is particularly rich in flavonoid rutin and phenolics such as chlorogenic, ferulic, vanillic, p-anisic and p-coumaric acids (Sytar et al. 2014a, b; Sytar et al. 2016).
The development of an efficient protocol for successful hairy root induction by Agrobacterium rhizogenes is the main step at in vitro culturing method for the greater production of biological active compounds. To choose an effective Agrobacterium line and growth conditions to produce hairy roots culture is highly plant species dependent and must be resolved empirically. Therefore, our goal was to investigate the efficiency of use different hairy root transformed lines from leaf and stem of Fagopyrum esculentum; to determine the changes in protein, amino acids composition, flavonoids and flavons levels, as well as the antioxidant activity.
Materials and methods
Plant materials and culture conditions
Seedlings of F. esculentum Rubra cultivar were grown on hormone-free medium of Murashige-Skoog (MS) (Murashige and Skoog 1962) sucrose complemented (30 g/L). Farther culture medium was hardening with agar (8 g/L). Cultures were incubated at 25 ± 1 °C under cool white fluorescent light at 40 μmol/m2s under 16 h photoperiod for 3 weeks.
Preparation of Agrobacterium rhizogenes
A. rhizogenes A4 strain was originated from glycerol stock. It was grown in YEBS liquid culture. The composition of YEBS culture is next: 1 g/L yeast extract, 5 g/L beef extract, 5 g/L sucrose, 5 g/L bacto-peptone and 0.5 g/L magnesium sulphate. The pH 7 of current liquid culture was adjusted at 28 °C via shaking at 150 rpm. Appropriate antibiotic (Rifampici) was added to the respective cultures.
Establishment of hairy root cultures
Establishment of hairy root cultures was performed via method described by Gabr et al. (2012). Briefly, leaf and stem explants were used for inoculation with A. rhizogenes A4 strain (OD600 nm = 1.0). Each explant was fixed separately during 5 min in bacterial suspension. Thereafter, to remove excess of bacteria the infected explants were blotted on sterile filter paper. Next step of sterilization was 2 days incubation in liquid hormone-free MS medium with 30 g sucrose/L on a rotary shaker at 100 rpm under complete dark conditions. After co-cultivation, the infected explants were transferred to a fresh hormone-free MS liquid medium containing 30 g sucrose/L and 500 mg/L of antibiotic cefotaxime (cefotaxime sodium salt, Sigma-Aldrich) and incubated under the same conditions for 14 days to eliminate the Agrobacterium. After 3 weeks of subculture, the hairy root cultures (from both explants) were harvested and their amino acids, flavonoids, flavons, phenolic acids contents and antioxidant capacity were determined. Roots from uninfected seedlings were used as controls (non-transformed cultures).
Growth kinetics of transformed root cultures
Hairy roots from transformed leaves and stem were classified into three lines from each explant. This grouping was optimized through evaluating growth kinetics, for number of roots at various time intervals (7, 14, and 21 days), fresh weight (FW) and dry weight (DW) at the 21 days.
PCR detection
Extraction of genomic DNA from investigated cultures was done by using DNA-Kit (purification kit Wizard R genomic DNA, A1120, Promega, USA). PCR analyses of transformed hairy root cultures was done by use the A. rhizogenes rolb gene as target to validate the transformation event. To confirm the absence of Agrobacteria contamination in the tissue was used gene virD2. T100 thermal cycler (Bio-Rad, USA) was used to carry the polymerase chain reaction amplification. Primers of rol b gene were used as follow, 5′-ATGCGCTTTCGCGAAATCCAA-3′, and 5′-TTCAGGTTTACTGCAGCAGGC amplifying a 564 bp fragment (Bonhomme et al. 2000). Further, gene virD2 was identified using 5′-CCTGACCCAAACATCTCGGCT-3′ and 5′-ATGCCCGATCGAGCTCAAGT-3′ primers, amplifying a 338 bp fragment (Gabr et al. 2016a). The following conditions for PCR analysis were exposed. The initial denaturation was done during 4 min at 95 °C, followed by 30 cycles of amplification (1 min 94 °C, 1 min 55 °C and 1 min 72 °C) and last 5 min at 72 °C. The initial denaturation for virD2 gene was 3 min at 95 °C, followed by 30 cycles of amplification (30 s 95 °C, 30 s 56 °C and 45 s 72 °C) and last 10 min at 72 °C. Amplified products were evaluated on agarose gel (1.5%).
Sample preparation
Both transformed and non-transformed root cultures were harvested and quickly frozen and powdered in liquid nitrogen, then lyophilized in freeze dryer. 0.05 g of lyophilized sample were distilled with 1 mL of 70% aqueous methanol (v/v) in an ultrasonic bath under ice cooling condition for 15 min. Farther was done centrifugation of samples at 6000 rpm for 5 min. The supernatants were collected into the pellets and twice re-extracted with 0.5 mL 70% methanol.
Determination of total flavonoids
The content of total flavonoids was estimated by the method of Ordon et al. (2006). Briefly, a 0.5 mL of ethanolic solution AlCl3 (20 g/L) was combined to 0.5 mL of extraction solution. The mixture was kept for 1 h at room temperature. The absorbance was measured after 1 h of extraction at 240 nm. Extracted samples were assessed at a final concentration of 0.1 mg/mL. The content of total flavonoids was expressed as quercetin (QE) [mg QE/g dry weight (DW)].
Determination of total flavonols
The content of total flavonols was estimated by the method of Kumaran and Joel (2007). First, 2 mL of extraction solution, 2 mL of ethanolic solution AlCl3 (20 g/L) and 3 mL of sodium acetate solution (50 g/L) were mixed. The mixture was kept for 2.5 h at 20 °C. The absorbance was measured at 440 nm. Extracted samples were assessed at a final concentration of 0.1 mg/mL, and content of total flavonols was expressed as QE [mg QE/g dry weight (DW)].
2,2-diphenyl- 1-picryl-hydrazyl (DPPH) radical scavenging activity
The antioxidant activity of both non-transformed and transformed hairy root cultures was estimated using the DPPH assay by Lee et al. (2007) with some modifications. For sample preparation was taken 0.1 mL from extracts and vortexed with 3.9 mL of DPPH working solution for 30 s. Farther, the mixture was incubated at room temperature in the dark for 30 min. The absorbance of mixture was evaluated at 515 nm. As control sample was used the DPPH solution without extract. The percentage of DPPH radical-scavenging activity is determined as follows: [(Acontrol − Asample)/Acontrol] × 100, where Acontrol is the control reaction absorbance (presenting all reagents except the test compound), and Asample is the same absorbance.
Determination of flavonoids content by high performance liquid chromatography (HPLC)
HPLC analysis was based on method described in research work of Gabr et al. (2016b). The flavonoids content of transformed and non-transformed root cultures was performed with a Crystal 200 series HPLC pump (ATI Unicam, Cambridge, UK). The mobile phase flow rate was 1.4 mL min−1 and consists with methanol and water (both acidified with 0.3% orthophosphoric acid p.a. w/v). The dead volume was determined by injecting water. Flavonoids were eluted from water with linear gradient to 50% methanol for 5 min, following by isocratic elution with 50% methanol for 20 min. The absorption at λ = 288 nm was used for substances detection. Substances identification were carried out by the comparison of retention times and absorption spectra with standards of rutin (quercetin-3-rutinosid), hesperidin (hesperetin 7-rutinoside) and kaempferol (kaempferol-3-rutinoside). The content of flavonoids was calibrated to a known concentration of standard and sample peak areas and calculated in mg/g dry weight (DW).
Determination of total protein
Determination of total protein was based on the Smith method in some modifications (Smith et al. 1985). The bicinchoninic acid assay, also known as the Smith assay is based on the modification of the biuret test which are used in modern spectrophotometric analysis of peptides. In the presence of peptides Cu+ forms a deep purple complex with bicinchoninic acid, which absorbs around 550–562 nm, producing the signature mauve color (Smith et al. 1985). 0.2 g of the lyophilized plant material were mixed with 4 mL 2.5% trichloroacetic acid. The mixture was kept for 30 min at 20 °C and after was done centrifugation of samples at 6000 rpm for 5 min. Supernatant was transferred in another tube. Farther to the sample was added 2 mL 2.5% trichloroacetic acid and same extraction procedure with centrifugation was repeated. Last step of sample extraction was with 4 mL H2Odist and centrifugation with supernatant removing. After all steps of extraction to the tube sample was added 5 mL 0.05 N NaOH. This mixture was centrifuged at 6000 rpm for 10 min and used for the protein estimation. To the 3 mL of mixture was added 0.5 mL of biuret reagent and absorbance was evaluated at 515 nm with a Jenway UV/Vis 6405 spectrophotometer (Jenway, Chelmsford, UK). As control was used mixture of 3 mL of 0.05 N NaOH and biuret reagent.
Determination of amino acids composition
Acid hydrolysis of amino acids
To 0.5 g of the lyophilized plant material were mixed with 30 mL of 6 N HCl. Samples were directly hydrolyzed with 6 N HCl to obtain hydrolysates suitable for analysis of all amino acids except for sulfur amino acids. Then the tube is frozen and sealed under the vacuum and placed in a heating block for 20–24 h at 110 °C. 2-mercaptoethanol was added to the hydrochloric acid to minimize degradation of specific amino acids. After finishing hydroxylation procedure, the mixture was neutralized by adding 30 mL of neutralizing solution (120 g NaOH/L dilution buffer pH 2.2). Farther, the mixture was diluted with a 100 mL of dilution buffer (pH 2.2). The content of amino acids after acid hydrolysis with 6 M HCl was determined using an automatic AA analyzer (AAA 400; Ingos, Prague, Czech Republic).
Sulfur amino acids (Cys, Met) oxidative hydrolysis
Sulfur amino acids (Cys, Met) are oxidized with a mixture of hydrogen peroxide and formic acid 1:9 (1-part 33% hydrogen peroxide and 9 parts of 85% formic acid). For this purpose, 0.5 g of the lyophilized sample were mixed with 5 mL of oxidation mixture, and then the mixture was oxidized in a refrigerator at 0–4 °C for 16 h. After oxidation 1 mL of 6 N HCl was added to the samples, which could stand for 15 min. After 15 min, 80 mL of 6 N HCl was added to the samples for hydrolyzation process at a temperature of 105 °C for 23 h. After cooling hydrolysates, the samples were evaporated to 80 mL in a vacuum evaporator at 50 °C. The residue after evaporation to honey consistency is washed 2 times with 5 mL of deionized water and again evaporated till dryness. Dried contents were diluted by 50 mL of dilution buffer pH 2.2 and stored in a refrigerator. Content of oxidative products (methionine sulfoxide and cysteic acid) were determined by using an automatic amino acid analyzer (AAA 400; Ingos, Prague, Czech Republic).
Statistical analysis
Results are expressed as mean ± SD. Statistical analyses were conducted with SPSS 14.0 (SPSS, Inc, Chicago, IL, USA). The one-way analysis of variance (ANOVA) followed with Duncan’s test was used. Differences were considered significant at P ≤ 0.05.
Results and discussions
Growth kinetics of transformed root cultures
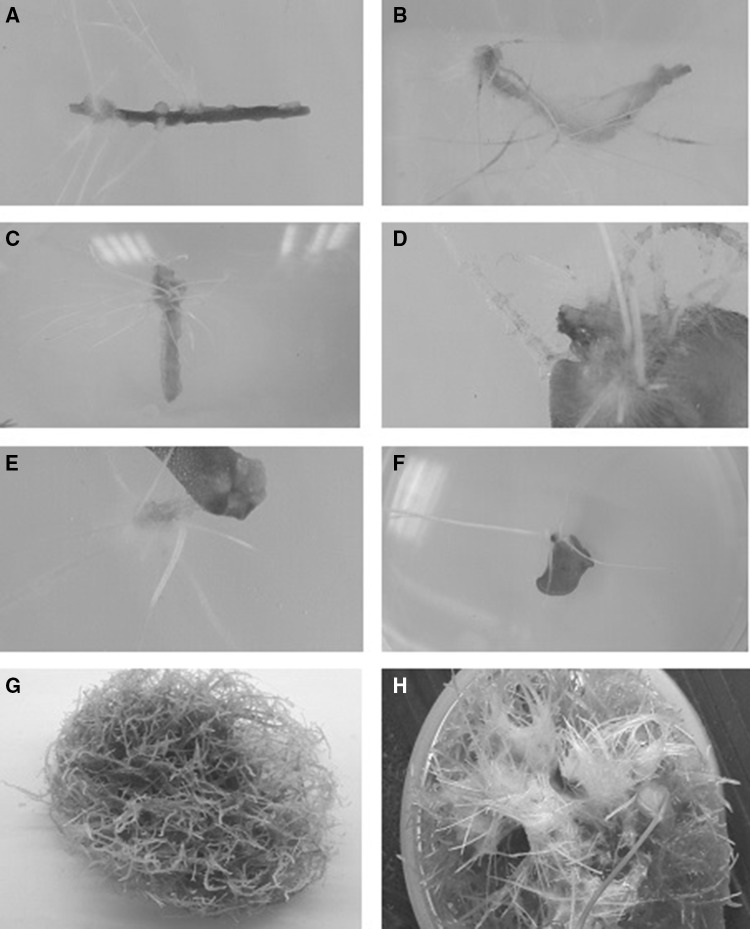
Leaf and stem explants from in vitro seedling of F. esculentum Rubra cultivar were subjected to hairy root transformation using the A. rhizogenes strain A4. The cultivation of each explant (leaf and stem) hairy root cultures was done on free MS medium for 21 days and their growth parameters (root number, FW and DW) were investigated. From each explant, three independent hairy root lines were used for further experimentation. These lines were classified according to their growth kinetics: The first hairy root line had low branching, second line had high branching and high growth rate and the third line had much thicker roots than those of both lines (Fig. 1). Growth kinetics, for number of roots at various time intervals (7, 14, and 21 days), fresh weight (FW) and dry weight (DW) at the 21 days was measured for all lines (Table 1). During the culture period, root numbers were investigated in each line from both explants leaf and stem. The root numbers dramatically increased from day 7 to day 21 with all explant lines. Line 2 from stem explant showed the highest root numbers (25 ± 2.00/explant) compared to other lines from both explants. FW and DW of the different hairy root lines at the end of 21 days showed that line 1 from leaf explant produced the highest FW and DW 25.679 ± 1.43 and 1.740 ± 0.089 g, respectively. In this respect, Huang et al. (2016) observed that biomass of Fagopyrum tataricum hairy root cultures were declined rapidly after 20 days. And they found that the maximal FW and DW were 13.5 and 1.78 g, respectively at the 24 days.
Fig. 1.
Hairy root lines from both leaf and stem explants were classified to three groups a stem line 1, b stem line 2, c stem line 3, d leaf line 1, e leaf line 2, f leaf line, g stem hairy root proliferation after 5 weeks and h leaf hairy root proliferation after 5 weeks
Table 1.
Root number, fresh weight (FW) and dry weight (DW) in the different hairy root transformed lines from leaf and stem
| Explant | Root numbers/explant | FW | DW | |||
|---|---|---|---|---|---|---|
| 7 days | 14 days | 21 days | ||||
| Leaf | Line 1 | 3.67 ± 0.58 | 10.67 ± 1.53 | 18.67 ± 2.08 | 25.679 ± 1.43 | 1.740 ± 0.089 |
| Line 2 | 3.00 ± 0.58 | 9.00 ± 2.00 | 23.00 ± 1.73 | 24.243 ± 1.28 | 1.273 ± 0.054 | |
| Line 3 | 3.00 ± 1.00 | 8.00 ± 1.00 | 17.67 ± 1.15 | 16.383 ± 4.43 | 1.050 ± 0.080 | |
| Stem | Line 1 | 3.00 ± 0.58 | 9.00 ± 0.58 | 21.00 ± 2.00 | 24.854 ± 1.52 | 1.463 ± 0.124 |
| Line 2 | 7.33 ± 0.58 | 14.67 ± 3.51 | 25.00 ± 2.00 | 21.122 ± 1.17 | 1.278 ± 0.075 | |
| Line 3 | 3.00 ± 0.58 | 7.33 ± 1.53 | 15.33 ± 2.52 | 18.746 ± 0.27 | 1.144 ± 0.103 | |
Data represented mean ± SD, n = 5
PCR detection of A. rhizogenes rolb gene
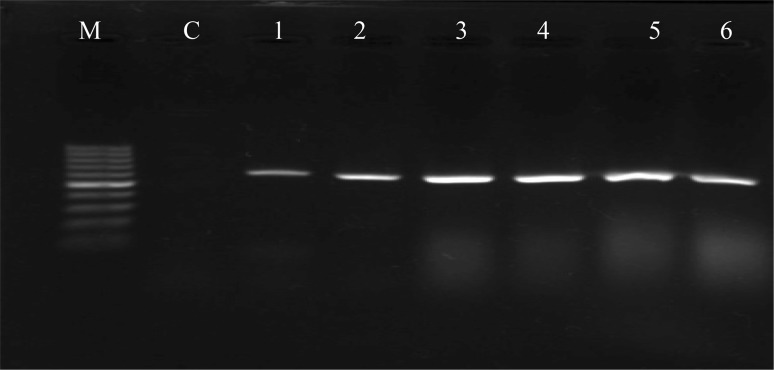
Confirmation of the successful transformation system of the Ri-plasmid, responsible for the induction of hairy root, was confirmed at the molecular level (Lucho et al. 2018). To confirm the insertion of rol genes, PCR-based analysis of rolB gene was used to evaluate the genetic transformation of hairy root transformed lines. Furthermore, the non-transferred virD2 gene was used to approve the absence of A. rhizogenes in hairy root lines. Figure 2 shows the results of the PCR analysis of the respective genes. All the hairy root lines showed presence of rolB gene in its genome, confirming transformation with the pRi plasmid, whereas none of the lines showed amplicon for virD2, excluding contamination with A. rhizogenes. Previous studies regarding literature data analysis found that metabolites production of hairy root cultures also depends on expression of rol gene (Tiwari et al. 2008; Bulgakov 2008; Gabr et al. 2016b).
Fig. 2.
PCR amplification of the rolB gene from genomic DNA isolated from different hairy root lines, and non-transformed roots (C) of F. esculentum Rubra cultivar. M: DNA ladder 100 kp, C: non-transformed roots, Hairy root lines developed from leaves (Lanes 1–3) and stem (Lanes 4–6)
Amino acids composition and total protein in the hairy root cultures from leaf and stems
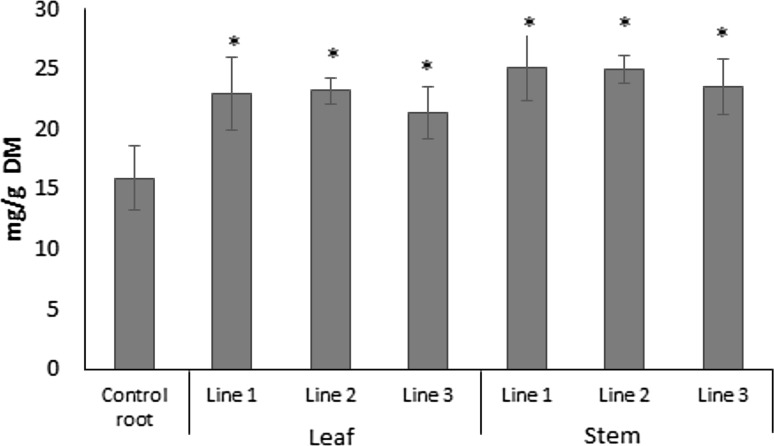
The sprouts of buckwheat are important nutrition source regarding a sulfur-containing amino acid methionine and amino acid leucine (Sytar et al. 2018). So far there are no reports about changes in total protein content and amino acid composition in hairy root cultures of buckwheat. Therefore, we aimed to address this gap of knowledge. It was shown greater level of total protein in the all hairy root transformed lines from leaf and stem compared to the root control (Fig. 3). We measured amino acid composition of amino acids in the different hairy root transformed lines from leaf and stem of common buckwheat. The results showed significant increase of semi-essential amino acids (lysine, isoleucine, valine, histidine and phenylalanine) and non-essential amino acids (serine, tyrosine, glycine, proline and asparagic acid) in the different hairy root transformed lines from leaf and stem (Table 2).
Fig. 3.
Total protein content of the different hairy root transformed lines from leaf and stem. Data are presented as mean ± SD, n = 3.).* indicate significant differences to control at P < 0·05, P < 0·01 or P < 0·001
Table 2.
Comparison of amino acids content in the different hairy root transformed lines from leaf and stem
| Compounds | Control root | Leaf | Stem | ||||
|---|---|---|---|---|---|---|---|
| (Line 1) | (Line 2) | (Line 1) | (Line 2) | (Line 3) | |||
| Asparagic acid | 7.1 ± 0.33 | 10.9 ± 0.31a | 12.6 ± 0.43a | 10.3 ± 0.30a | 16.1 ± 0.51a | 14.7 ± 0.44a | 12.5 ± 0.35a |
| Threonine | 4.0 ± 0.37 | 6.3 ± 0.76a | 6.8 ± 0.67a | 5.4 ± 0.56a | 8.4 ± 1.11a | 7.8 ± 0.87a | 6.9 ± 0.57a |
| Serine | 4.6 ± 0.31 | 6.0 ± 0.80a | 7.6 ± 0.69a | 6.2 ± 0.67a | 8.6 ± 0.80a | 7.8 ± 0.78a | 6.8 ± 0.62a |
| Glutamic acid | 31.8 ± 2.42 | 47.6 ± 3.86a | 25.5 ± 2.34a | 19.4 ± 2.97a | 38.4 ± 2.22a | 32.0 ± 2.51b | 45.1 ± 3.94a |
| Proline | 0.8 ± 0.12 | 3.0 ± 0.14a | 3.9 ± 0.21a | 2.3 ±0.19a | 5.1 ± 0.29a | 9.9 ±0.21a | 3.9 ± 0.28a |
| Glycine | 4.4 ± 0.27 | 6.0 ± 0.39a | 7.1 ± 0.44a | 5.5 ± 0.48a | 8.2 ± 0.56a | 7.8 ± 0.59a | 6.5 ± 0.79a |
| Alanine | 5.1 ± 0.44 | 7.7 ± 0.64a | 11.7 ± 0.54a | 9.5 ± 0.57a | 14.2 ± 0.47a | 11.4 ± 0.48a | 8.8 ± 0.38a |
| Valine | 4.5 ± 0.28 | 6.5 ± 0.48a | 7.8 ± 0.53a | 6.4 ± 0.47a | 8.6 ± 0.36a | 8.0 ± 0.41a | 7.1 ± 0.51a |
| Isoleucine | 3.2 ± 0.27 | 4.6 ± 0.36a | 5.5 ± 0.45a | 4.7 ± 0.73a | 6.4 ± 0.83a | 5.9 ± 0.79a | 5.2 ± 0.48a |
| Leucine | 5.7 ± 0.56 | 8.6 ± 0.54a | 10.5 ± 0.67a | 8.8 ± 0.76a | 12.1 ±0.79a | 11.3 ± 0.67a | 9.8 ± 0.57a |
| Tyrosine | 2.4 ± 0.26 | 2.2 ± 0.35b | 2.5 ± 0.47b | 1.4 ± 0.56a | 2.4 ±0.29b | 2.7 ± 0.32b | 2.3 ± 0.38b |
| Phenylalanine | 3.1 ± 0.36 | 4.4 ± 0.37a | 5.6 ± 0.41a | 4.5 ± 0.37a | 6.4 ± 0.35a | 6.1 ± 0.29a | 5.0 ± 0.34a |
| Histidine | 4.6 ± 0.46 | 5.9 ± 0.34a | 6.4 ± 0.35a | 5.3 ± 0.43a | 7.5 ± 0.45a | 7.0 ± 0.48a | 6.2 ± 0.39a |
| Lysine | 5.4 ± 0.35 | 8.4 ± 0.28a | 10.0 ± 0.86a | 8.6 ± 0.53a | 11.8 ± 0.34a | 10.9 ± 0.37a | 9.6 ± 0.43a |
| Arginine | 3.3 ± 0.39 | 5.3 ± 0.42a | 6.8 ± 0.43a | 5.4 ± 0.28a | 7.8 ± 0.34a | 7.1 ± 0.37a | 6.5 ± 0.32a |
| Cystine | ND | 0.8 ± 0.13 | 1.3 ± 0.09a | 1.1 ± 0.12a | 1.3 ± 0.23 | 1.7 ± 0.21a | 0.8 ± 0.08a |
| Methionine | ND | 0.9 ± 0.21 | 1.6 ± 0.11a | 1.6 ± 0.11a | 1.7 ± 0.33 | 2.1 ± 0.20a | 1.1 ± 0.09a |
| Total amino acids | 89.9 ± 6.83 | 135.2 ± 10.37a | 133.1 ± 9.69a | 106.3 ± 10.1a | 165.0 ± 10.27a | 154.2 ± 9.99a | 144.0 ± 10.52a |
Data represented mean ± SD, n = 5
ND not detected. The measurement unit used is g per 1000 g analytical DM
a letter in column are significant at the P ≤ 0.05
b letter in column are not significant at the P ≤ 0.05
Different changes in glutamic acid levels in the hairy root transformed lines from leaf have been observed. Glutamic acid levels were increased only in Line 1, but not in Line 2 and 3. At the same time glutamic acid a level of the hairy root transformed lines 1 and 3, which were obtained from the stem, were significantly increased but Line 2 was at the control level. The glutamine or glutamic acids are involved in combination with other compounds in the mannitol opine biosynthesis (Hong et al. 2006).
Content of proline was increased 3–5 times probably due to the biotic stress reaction occurred by interaction with A. rhizogenes. It was also suggested that proline accumulated in many plant species in response to different stresses and may also play a role as a signaling molecule and metabolite in flowering and development processes (Mattioli et al. 2009). Additionally, proline as a signaling molecule able to affect mitochondrial functions, cell proliferation process and cause specific gene expression, which can be important for plant recovery upon stress (Szabados and Savouré 2010).
Sulfur-containing amino acids, such as cysteine and methionine were not detectable in control root but were detectable in the different hairy root transformed lines from leaf and stem. The highest content of methionine and cysteine has been estimated in the hairy root transformed line from stem (Line 2). These results suggest that the hairy roots have enhanced sulfur metabolism.
Total flavonoids and flavons contents in the hairy root cultures from leaf and stems
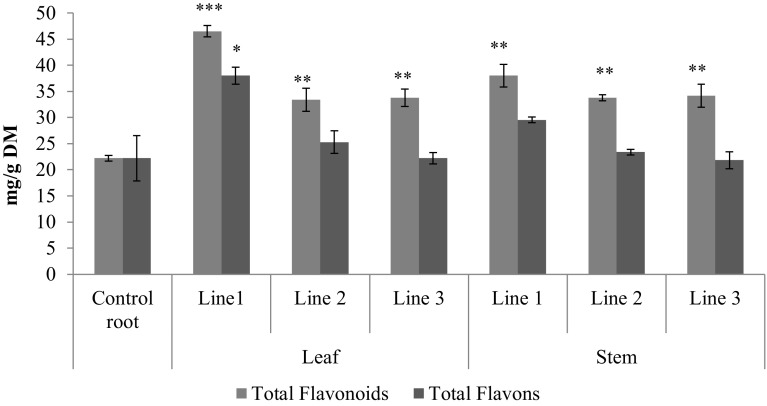
Buckwheat seed and hull an average may contain from 3.87 to 13.14 mg per g of flavonoids (Oomah and Mazza 1996). Cultures extracts of hairy roots induced by A. rhizogenes-mediated transformation of some model plants showed efficient production of flavonoids and enhanced antioxidant activities (Kim et al. 2010; Thwe et al. 2016). In our results, we found significant increase of total flavonoids and flavons contents in all experimental hairy root transformed lines from leaf and stems (Fig. 4). An average total flavonoids content between 32 and 45 mg per g DM in all experimental hairy root transformed lines from leaf and stems what is significantly higher compared to the total flavonoids content from literature data. Total flavons showed no significant differences between hairy root lines from the stem. However, only line 1 in leaf showed significant increase (P < 0.05) in its total flavons as compared to control. In contrast, total flavonoids showed significant increase in all lines from stem (P < 0.01) and leaf (P < 0.001, P < 0.01, P < 0.05) as compared to control. Generally, hairy root transformed line 1 from leaf was characterized by the highest flavonoids and flavons levels. Therefore, the hairy root transformed line 1 from leaf was used for production of total flavonoids and flavons.
Fig. 4.
Total flavonoids and total flavons contents in the different hairy root transformed lines from leaf and stem. Data are presented as mean ± SD, n = 3,).*,** or *** indicate significant differences to control at P < 0·05, P < 0·01 or P < 0·001
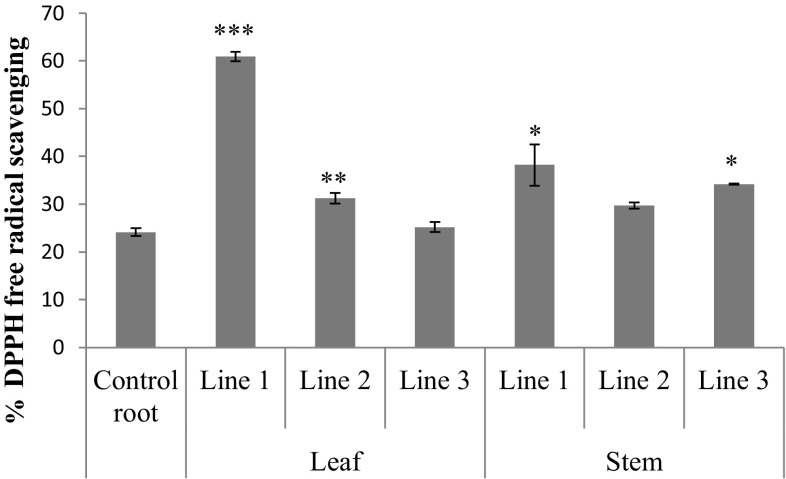
We also studied the antioxidant activities of the hairy root culture extracts. The extracts of transformed hairy root lines (leaf line 1 and 2; steam line 1 and 3) showed significant increases in antioxidant activity compared to the control root extract (Fig. 5, Table 2).
Fig. 5.
Free radical scavenging activity (DPPH) of the different hairy root transformed lines from leaf and stem. Data are presented as mean ± SD, n = 3,). *,** or *** indicate significant differences to control at P < 0·05, P < 0·01 or P < 0·001
Flavonoids composition in the hairy root cultures from leaf and stems
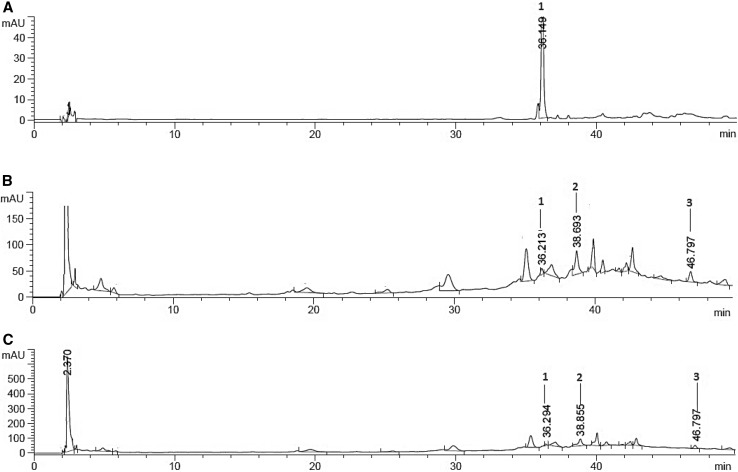
By HPLC we identified the three flavonoids compounds rutin, hesperidin, rosmarinic acid and kaempferol-3-rutinoside (Table 3, Fig. 6). Rutin was detected in control root at higher levels compared to the different hairy root transformed lines from leaf and stem. Rutin is biological active compound, which can increase resistances to plant disease occurred by bacterial pathogens via rutin-priming defense signal regulated by the salicylic acid-dependent pathway (Wei et al. 2016). Thus, it is possible that decreasing rutin content in the experimental hairy root transformed lines can be a novel strategy mediated by the pRi plasmid for suppressing the plant defense system to A. rhizogenes. Hesperidin is known bioflavonoid with high antioxidant properties which also plays a role in plant defense reactions. Hesperidin was identified in the different hairy root transformed lines from leaf and stem compared to the root control where hesperidin was not detected. The rosmarinic acid and kaempferol-3-rutinoside were detected in the different hairy root transformed lines from leaf and stem. Similar results with higher levels of rutin and kaempferol-3-rutinoside from transgenic calli compared to the wild-type tissues were obtained by Gális et al. (2004), where transformation of the AK-6b gene by A. tumefaciens modulated caffeic acid derivatives and flavonoids metabolism.
Table 3.
Comparison of flavonoid compounds in the different hairy root transformed lines from leaf and stem
| Compounds | Control root | Leaf | Stem | ||||
|---|---|---|---|---|---|---|---|
| (Line 1) | (Line 2) | (Line 1) | (Line 2) | (Line 3) | |||
| Rutin | 9.29 ± 0.286 | 5.8 ± 0.141*** | 2.84 ± 0.212*** | 7.07 ± 0.353** | 2.61 ± 0.212*** | 6.60 ± 0.282** | 8.63 ± 0.282 |
| Hesperidine | ND | 10.65 ± 0.353 | 10.77 ± 0.304 | 9.64 ± 0.339 | 13.57 ± 0.282 | 12.65 ± 0.353 | 10.63 ± 0.353 |
| Kaempferol-3-rutinoside | ND | 6.95 ± 0.212 | 6.02 ± 0.212 | 2.89 ± 0.141 | 5.75 ± 0.141 | 4.40 ± 0.282 | ND |
ND not detected
The measurement unit used is µg mg−1 DM. The data is presented as mean ± SD (n = 5)
*,** or *** indicate significant differences to control at P < 0·05, P < 0·01 or P < 0·001 respectively
Fig. 6.
HPLC chromatograph of flavonoid compounds in the hairy root transformed culture. a control; b: leaf; c: stem. 1: Rutin (RT 36. 2), 2: Hesperidin (RT 38.7), 3: Kaempferol-3-rutinoside (RT 46.8)
The capability of hairy roots of F. esculentum to produce the flavonoid compound rutin was previously investigated (Kim et al. 2010; Thwe et al. 2016).
Conclusion
The different growth kinetics and metabolite content of the transformed hairy root lines pinpoints a possible correlation between the accumulation of different secondary metabolites and their hairy root development. Based on the results of our investigation it seems that rutin production in transgenic root cultures of F. esculentum may depend on the explant and lines. The suppression of rutin accumulation in the hairy root culture is probably a plant defense avoidance strategy. Furthermore, hairy root cultures (line 1) of common buckwheat showed a significant increase in semi-essential amino acids such as lysine, isoleucine, valine, histidine and phenylalanine, as well as total flavonoids contents and antioxidant activity. The greatest content of methionine and cysteine found in the hairy root transformed line from stem (line 2). Jointly, presented data indicate that buckwheat hairy root culture can be a reasonable alternative source for flavonoids and semi-essential amino acids.
Acknowledgements
Funding was provided by Agentúra na Podporu Výskumu a Vývoja (Grant No. APVV-15-0721).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ahmed M.M. Gabr and Oksana Sytar have contributed equally to this article.
Contributor Information
Ahmed M. M. Gabr, Email: a_m_gabr2@yahoo.com
Oksana Sytar, Email: oksana.sytar@gmail.com.
References
- Alok A, Shukla V, Pala Z, Kumar J, Kudale S, Desai N. In vitro regeneration and optimization of factors affecting Agrobacterium mediated transformation in Artemisia Pallens, an important medicinal plant. Physiol Mol Biol Plants. 2016;22:261. doi: 10.1007/s12298-016-0353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Jubete L, Wijngaard H, Arendt EK, Gallagher E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010;119:770–778. doi: 10.1016/j.foodchem.2009.07.032. [DOI] [Google Scholar]
- Arafa NM, Gabr AMM, Ibrahim MM, Shevchenko Y, Smetanska I. Study the effect of hairy root transformation on rapid growth (growth morphology) of Nepeta cataria in vitro cultures. J Innov Pharm Biol Sci. 2015;2:439–450. [Google Scholar]
- Berkov S, Pavlov A, Kovacheva P, Stanimirova P, Philipov S. Alkaloid spectrum in diploid and tetraploid hairy root cultures of Datura stramonium. Z Naturforsch. 2003;58:42–46. doi: 10.1515/znc-2003-1-207. [DOI] [PubMed] [Google Scholar]
- Bonhomme V, Laurain-Mattar D, Fliniaux MA. Effects of the rolC gene on hairy root: induction development and tropane alkaloid production by Atropa belladonna. J Nat Prod. 2000;63:1249–1252. doi: 10.1021/np990614l. [DOI] [PubMed] [Google Scholar]
- Bulgakov VP. Functions of rol genes in plant secondary metabolism. Biotechnol Adv. 2008;26:318–324. doi: 10.1016/j.biotechadv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Chan PK. Inhibition of tumor growth in vitro by the extract of Fagopyrum cymosum. Life Sci. 2003;72:1851–1858. doi: 10.1016/S0024-3205(03)00013-4. [DOI] [PubMed] [Google Scholar]
- Dziadek K, Aneta Kopec A, Pastucha E, Pia-tkowska E, Leszczynska T, Pisulewska E, Witkowicz R, Francik R. Basic chemical composition and bioactive compounds content in selected cultivars of buckwheat whole seeds, dehulled seeds and hulls. J Cereal Sci. 2016;69:1–8. doi: 10.1016/j.jcs.2016.02.004. [DOI] [Google Scholar]
- Gabr AMM, Ghareeb H, El Shabrawi HM, Smetanska I, Bekheet SA. Enhancement of silymarin and phenolic compound accumulation in tissue culture of Milk thistle using elicitor feeding and hairy root cultures. J Genet Eng Biotechnol. 2016;14:327–333. doi: 10.1016/j.jgeb.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabr AMM, Mabrok HB, Ghanem KZ, Blaut M, Smetanska I. Lignan accumulation in callus and Agrobacterium rhizogenes-mediated hairy root cultures of flax (Linum usitatissimum) Plant Cell, Tissue Organ Cult. 2016;126:255–267. doi: 10.1007/s11240-016-0995-4. [DOI] [Google Scholar]
- Gabr AMM, Sytar O, Ahmed AR, Smetanska I. Production of phenolic acid and antioxidant activity in transformed hairy root cultures of common Buckwheat (Fagopyrum esculentum M.) Aust J Basic Appl Sci. 2012;6:577–586. [Google Scholar]
- Gális I, Kakiuchi Y, Simek P, Wabiko H. Agrobacterium tumefaciens AK-6b gene modulates phenolic compound metabolism in tobacco. Phytochemistry. 2004;65(2):169–179. doi: 10.1016/j.phytochem.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Gallardo C, Jimenez L, Garcia-Conesa MT. Hydroxycinnamic acid composition and in vitro antioxidant activity of selected grain fractions. Food Chem. 2006;99:455–463. doi: 10.1016/j.foodchem.2005.07.053. [DOI] [Google Scholar]
- Georgiev MI, Eibl R, Zhong JJ. Hosting the plant cells in vitro: recent trends in bioreactors. Appl Microbiol Bio-technol. 2013;97(9):3787–3800. doi: 10.1007/s00253-013-4817-x. [DOI] [PubMed] [Google Scholar]
- Ghimeray AK, Sharma P, Briatia X. The proceeding of the Intl. Korea: Symp. On Buckwheat Sprouts. Bongpyoung; 2010. Phenolic compounds and free radical scavenging activity of seed, seedling and sprout of buckwheat; pp. 41–45. [Google Scholar]
- Giri A, Narasu ML. Transgenic hairy roots: recent trends and applications. Biotechnol Adv. 2000;18:1–22. doi: 10.1016/S0734-9750(99)00016-6. [DOI] [PubMed] [Google Scholar]
- Goel MK, Mehrotra S, Kukreja AK. Elicitor-induced cellular and molecular events are responsible for productivity enhancement in hairy root cultures: an insight study. Appl Biochem Biotechnol. 2011;165(5–6):1342–1355. doi: 10.1007/s12010-011-9351-7. [DOI] [PubMed] [Google Scholar]
- Gorinstein S, Lojek A, Ciz M, Pawelzik E, Delgado-Licon E, Medina OJ, Moreno M, Salas IA, Goshev I. Comparison of composition and antioxidant capacity of some cereals and pseudocereals. Int J Food Sci Technol. 2008;43:629–637. doi: 10.1111/j.1365-2621.2007.01498.x. [DOI] [Google Scholar]
- Gyurkovska V, Alipieva K, Maciuk A, Dimitrova P, Ivanovska N, Haas CH, Bley T, Georgiev M. Anti-inflammatory activity of devil’s claw in vitro systems and their active constituents. Food Chem. 2011;125:171–178. doi: 10.1016/j.foodchem.2010.08.056. [DOI] [Google Scholar]
- Hong SB, Peebles CAM, Shanks JV, San KY, Gibson SI. Terpenoid indole alkaloid production by Catharanthus roseus hairy roots induced by Agrobacterium tumefaciens harboring rol ABC genes. Biotechnol Bioeng. 2006;93:386–390. doi: 10.1002/bit.20699. [DOI] [PubMed] [Google Scholar]
- Huang X, Yao J, Zhao Y, Xie D, Jiang X, Xu Z (2016) Efficient Rutin and Quercetin Biosynthesis through Flavonoids-Related Gene Expression in Fagopyrum tataricum Gaertn. Hairy Root Cultures with UV-B Irradiation. Front Plant Sci 7:63. 10.3389/fpls.2016.00063 [DOI] [PMC free article] [PubMed]
- Ikeda K. Buckwheat: composition, chemistry and processing. Adv Food Nutr Res. 2002;44:395–434. doi: 10.1016/S1043-4526(02)44008-9. [DOI] [PubMed] [Google Scholar]
- Ishii S, Katsumura T, Shiozuka C, Ooyauchi K, Kawasaki K, Takigawa S, Fukushima T, Tokuji Y, Kinoshita M, Ohnishi M, Kawahara M, Ohba K. Anti-inflammatory effect of buckwheat sprouts in lipopolysaccharide-activated human colon cancer cells and mice. Biosci Biotechnol Biochem. 2008;72:3148–3157. doi: 10.1271/bbb.80324. [DOI] [PubMed] [Google Scholar]
- Jung G, Tepfer D. Use of genetic transformation by the Ri T-DNA of Agrobacterium rhizogenes to stimulate biomass and tropane alkaloid production in Atropa belladonna and Calystegia septum roots grown in vitro. Plant Sci. 1987;50:145–151. doi: 10.1016/0168-9452(87)90151-8. [DOI] [Google Scholar]
- Kawa JM, Taylor CG, Przybylski R. Buckwheat concentrate reduces serum glucose in streptozotocin diabetic rats. J Agric Food Chem. 2003;51:7287–7291. doi: 10.1021/jf0302153. [DOI] [PubMed] [Google Scholar]
- Kayashita J, Shimaoka I, Nakajoh M, Yamazaki M, Norihisa K. Consumption of buckwheat protein lowers plasma cholesterol and raises fecal neutral sterols in cholesterol-fed rats because of its low digestibility. J Nutr. 1997;127:1395–1400. doi: 10.1093/jn/127.7.1395. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Park KJ, Lim JH (2011) Metabolomic analysis of phenolic compounds in buckwheat (Fagopyrum esculentum M.) sprouts treated with methyl jasmonate. J Agric Food Chem 59(10):5707–5713. 10.1021/jf200396k [DOI] [PubMed]
- Kim YK, Hui X, Park WT, Park N, Lee SY, Park SU (2010) Genetic transformation of buckwheat (‘Fagopyrum esculentum’ M.) with ‘Agrobacterium rhizogenes’ and production of rutin in transformed root cultures. Austr J Crop Sci 4 (7):485-490
- Kumaran A, Joel Karunakaran R. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT - Food Sci Technol. 2007;40(2):344–352. doi: 10.1016/j.lwt.2005.09.011. [DOI] [Google Scholar]
- Lee SY, Cho S, Park M, Kim YK, Choi J, Park SU. Growth and rutin production in hairy root cultures of Buckwheat) Prep Biochem Biotechnol. 2007;37(3):239–246. doi: 10.1080/10826060701386729. [DOI] [PubMed] [Google Scholar]
- Lucho SR, do Amaral MN, Benitez LC, Milech C, Kleinowski AM, Bianchi VJ, Braga EJB. Validation of reference genes for RT-qPCR studies in Stevia rebaudiana in response to elicitor agents. Physiol Mol Biol Plant. 2018;24:767. doi: 10.1007/s12298-018-0583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli R, Costantino P, Trovato M. Proline accumulation in plants: Not only stress. Plant Signal Behav. 2009;4(11):1016–1018. doi: 10.4161/psb.4.11.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra S, Goel MK, Rahman LU, Kukreja A. Molecular and chemical characterization of plants regenerated from Ri-mediated hairy root cultures of Rauwolfia serpentina. Plant Cell, Tissue Organ Cult. 2013;114:31–38. doi: 10.1007/s11240-013-0302-6. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Oomah BD, Mazza G. Flavonoids and antioxidative activities in buckwheat. J Agric Food Chem. 1996;44(7):1746–1750. doi: 10.1021/jf9508357. [DOI] [Google Scholar]
- Ordon Ez AAL, Gomez JD, Vattuone MA, Isla MI. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006;97:452–458. doi: 10.1016/j.foodchem.2005.05.024. [DOI] [Google Scholar]
- Petit A, David C, Dahl GA, Ellis JG, Guyon P, Casse-Delbart F, Tempe J. Further extension of the opine concept: Plasmids in Agrobacterium rhizogenes cooperate for opine degradation. Mol Gen Genet. 1983;190:204–214. doi: 10.1007/BF00330641. [DOI] [Google Scholar]
- Pollier J, Morreel K, Geelen D, Goossens A. Metabolite profiling of triterpene saponins of Medicago truncatula hairy roots by liquid chromatography Fourier transform ion cyclotron resonance mass spectrometry. J Nat Product. 2011;74:1462–1476. doi: 10.1021/np200218r. [DOI] [PubMed] [Google Scholar]
- Sedej I, Mandić A, Sakač M, Mišan A, Tumbas V. Comparison of antioxidant components and activity of buckwheat and wheat flours. Cereal Chem J. 2010;87:387–392. doi: 10.1094/CCHEM-02-10-0018. [DOI] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Srivastava AK. hairy root culture for mass-production of high-value secondary metabolites. Critical Rev Biotechnol. 2007;27:29–43. doi: 10.1080/07388550601173918. [DOI] [PubMed] [Google Scholar]
- Sytar O, Brestic M, Zivcak M, Tran LS. The contribution of buckwheat genetic resources to health and dietary diversity. Curr Genomics. 2016;17(3):193–206. doi: 10.2174/1389202917666160202215425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytar O, Chrenková M, Ferencová J, Polačiková M, Rajský M, Brestič M. Nutrient capacity of amino acids from buckwheat seeds and sprouts. J Food Nutr Res J Food Nutr Res. 2018;57:38–47. [Google Scholar]
- Sytar O, Borankulova A, Hemmerich I, Rauh C, Smetanska I. Effect of chlorocholine chlorid on phenolic acids accumulation and polyphenols formation of buckwheat plants. Biological Res. 2014;47:19. doi: 10.1186/0717-6287-47-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytar O, Kosyan A, Taran N, Smetanska I. Antocyanins as marker for selection of buckwheat plants with high rutin content. Gesunde Pflanzen. 2014;66:165–169. doi: 10.1007/s10343-014-0331-z. [DOI] [Google Scholar]
- Szabados L, Savouré A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15(2):89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Thwe A, Arasu MV, Li X, Park CH, Kim SJ, Al-Dhabi NA, Park SU. Effect of different Agrobacterium rhizogenes strains on hairy root induction and phenylpropanoid biosynthesis in Tartary Buckwheat (Fagopyrum tataricum Gaertn) Front Microbiol. 2016;7:318. doi: 10.3389/fmicb.2016.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari RK, Trivedi M, Guang ZC, Guo GQ, Zheng GC. Agrobacterium rhizogenes mediated transformation of Scutellaria baicalensis and production of flavonoids in hairy roots. Biol Plant. 2008;52:26. doi: 10.1007/s10535-008-0004-9. [DOI] [Google Scholar]
- Verardo V, Arráez-Román D, Segura-Carretero A, Marconi E, Fernández-Gutiérrez A, Caboni MF. Identification of buckwheat phenolic compounds by reverse phase high performance liquid chromatographyeelectrospray ionization-time of flight-mass spectrometry (RP-HPLCeESI -TOF-MS) J Cereal Sci. 2010;52:170–176. doi: 10.1016/j.jcs.2010.04.009. [DOI] [Google Scholar]
- Weathers PJ, Bunk G, McCoy MC. The effect of phytohormones on growth and artemisinin production in Artemisia annua hairy roots. Vitro Cell Dev Biol Plant. 2005;41:47–53. doi: 10.1079/IVP2004604. [DOI] [Google Scholar]
- Wei Y, Xiaonan X, Yang L, Yingzi W, Ming L, Yong W, Xinhua D, Zhaohui C. Rutin-mediated priming of plant resistance to three bacterial pathogens initiating the early SA signal pathway. PLoS One. 2016;11(1):e0146910. doi: 10.1371/journal.pone.0146910. [DOI] [PMC free article] [PubMed] [Google Scholar]