Abstract
Introduction
External ano-genital warts (AGWs) due to human papilloma virus infection are the most common sexually transmitted ano-genital lesions of viral origin worldwide. Treatments include topical chemicals/drugs, excisional surgery, cryosurgery, electrosurgery and laser surgery. Nitric-zinc complex (NZC) is a new topically applied solution containing nitric acid, zinc, copper and organic acids that induces a caustic effect on condyloma. The objective of this study was to investigate the efficacy and tolerability of NZC in the treatment of AGWs.
Methods
Patients attending for AGWs between September 2016 and February 2018 were retrospectively studied. They received at least one NZC application for a maximum of four treatments (V0, V1, V2, V3) with average intervals of 25 days between sessions. Recurrences were evaluated at 3 and 6 months after clearance.
Results
One hundred patients (70 males, 30 females) with a mean age of 36.39 years were studied. The total number of AGWs diagnosed at the baseline visit (V0) in all patients was 418 with a mean of 4.18 AGWs per patient. A wart cure rate of 92% was observed in ≤ 4 treatment sessions (383 lesions cured at visit 4, V4, out of 418 lesions at baseline), with a cure rate of 49% with only one NZC application. Complete clearance was observed in 25, 52, 72 and 84% of patients at V1, V2, V3 and V4, respectively. Relapses were observed in 29% of patients at 3 months and in 5% at 6 months. Of note, patients with ≤ 5 AGWs at V0 showed better clearance results than patients with > 5 lesions (p < 0.05). The treatment was well tolerated by most patients.
Conclusion
NZC has been demonstrated to be effective for AGWs after 1–4 treatment sessions, obtaining a good response from the first application. The better response in patients with fewer warts suggests that the earlier diagnosis is made and treatment started, the better the expected results.
Funding
ISDIN.
Keywords: Anal warts, Genital warts, Nitric zinc complex, Sexually transmitted infections
Introduction
Human papillomavirus (HPV) infections are very common sexually transmitted infections worldwide with about 14 million new genital HPV infections occurring each year in the USA [1]. The Centers for Disease Control and Prevention (CDC) estimate that > 90% and 80%, respectively, of sexually active men and women will be infected with at least one type of HPV at some point in their lives [2]. Around one-half of these infections are with a high-risk HPV type, especially types 16 and 18, which are responsible for most HPV-caused cancers [3]; low-risk HPVs (mainly 6 and 11) usually do not cause cancer but cause skin warts on or around the genitals and anus [3, 4]. External ano-genital warts (AGWs) due to HPV infection are the most common sexually transmitted ano-genital lesions of viral origin worldwide [5–8]. AGWs are usually asymptomatic, but depending on the size and anatomic location, they can be painful or pruritic. Regardless of symptoms, warts can also cause significant psychosocial distress since they are perceived as disfiguring, impacting sexual lifestyle and causing concerns about future fertility and cancer risk [4, 5, 9]. Treatments for AGWs include topical chemicals or drugs, excisional surgery, cryosurgery, electrosurgery and laser surgery. There is no clear evidence that one recommended treatment is superior to another, and no single treatment is ideal for all patients or all warts. The choice of treatment for a single patient should be guided by several factors: wart size, number and anatomic site, patient preference, cost, convenience, adverse effects and operator’s experience [10]. Regrettably, the available treatments can be painful and can leave scars or hypopigmentation [11]; furthermore, recurrence rates after any treatment range from 6% to 100% [12]. Nitric-zinc complex solution (NZCS) is a new medical device: a topically applied solution containing nitric acid, zinc, copper and organic acids that has been recently demonstrated to induce a painless caustic effect on “difficult-to-treat warts,” including AGWs [13, 14]. The objective of the present study was to investigate retrospectively the efficacy and tolerability of NZC in the treatment of AGWs.
Methods
Patients attending for AGWs between September 2016 and February 2018 were retrospectively studied. Eligible patients were adult men and women with at least one anal or genital wart suitable for topical treatment. They received at least one NZCS application for a maximum of four treatment sessions (V0, V1, V2, V3) with average intervals of 25 days (ranging from 21 to 30 days) between sessions. NZCS was topically applied using a 30 μl capillary tube on each lesion until a whitening reaction appeared. The number and location of AGWs observed at each session in each patient were recorded as well as the demographic data (sex, age) and the clinical history of the patients, namely AGW location and duration (months), any previous treatment, cutaneous/systemic comorbidities and concomitant treatments, infectious comorbidities and especially human immunodeficiency virus (HIV) status. Data concerning sexual behaviour, when available, were also noted for each patient, namely sexual orientation, number of sexual partners during the last year and infectious comorbidities of the partners. AGW recurrences were evaluated at 3 and 6 months after clearance. Tolerability was assessed by asking patients to quantify the pain after each treatment session on a scale from 0 to 4: 0 meaning no pain, 1 mild pain, 2 mild/moderate pain, 3 moderate pain and 4 intense pain. The study was approved by the Independent Ethics Committee Institutional review board (IRB) SPRIM Advanced Life Sciences on 7 February 2018, and each patient signed written informed consent to participate in the study.
Statistical Analysis
A descriptive statistics analysis was carried out for all variables. Continuous variables were described as the number of valid cases, mean, standard deviation (SD), median, 25th and 50th percentile (P25–P75), minimum and maximum. Categorical variables were described as absolute and relative frequency over the total valid values (N). In cases of missing values, their number has been described per group.
Categorical variables were compared using the chi-square test to evaluate the homogeneity of the values between groups and between sessions. For all comparisons, statistical significance was set at 0.05. All analyses were performed on the data set using all available information on an intention-to-treat (ITT) basis. The statistical analysis was carried out using the SAS (Statistical Analysis System) program, version 9.2.
Results
One hundred patients were included in the study: 70 males and 30 females with a mean age of 36.39 years (ranging from 17 to 80 years). At the baseline visit (V0), the HPV lesions were genital in 59% of the patients, anal in 25%, inguinal in 10% and ano-genital in 6%. Regarding the history of AGWs, they had been present for an average of 10.96 months (ranging from 0.3 to 72 months) and previous treatments had already been performed in 54% of the patients, being partially effective in 88.9% and not effective in 11.1% of these subjects. The previously performed treatments were: liquid nitrogen cryotherapy, imiquimod 5% cream, diathermocoagulation, epigallocatechin gallate cream, tretinoin cream, laser therapy and photodynamic therapy. These treatments had been performed alone, in sequence or in combination. Cutaneous comorbidities (atopic dermatitis and psoriasis) were present in 18.2% of the patients; other comorbidities were HPV cervicitis (18.2%), hypertension (9.1%) and chronic renal failure (9.1%). Some patients, before participating in the study, had undergone surgery for other comorbidities as well as squamous carcinoma of the rectum and bone marrow, heart, kidney and liver transplantation. Oral therapies were taken by 19/100 (19%) patients: 7 of these 19 patients were taking long-term immunosuppressive drugs (cyclosporine, tacrolimus, prednisone, mycophenolate mofetil) following organ transplantation. Infectious comorbidities were recorded in 36% of the patients: bowenoid papulosis, HPV cervicitis, chronic cystitis, genital herpes, gonorrhoea, syphilis, hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, Molluscum contagiosum, urethritis and skin warts. Ninety-three of the 100 studied patients took an HIV test and had a negative result, while 7 patients refused to take the test and their HIV status remained unknown. Regarding sexual behaviour, 84% of the patients self-reported as heterosexual, 15% homosexual and 1% bisexual. When asked about the number of sexual partners during the last year, 74% of the patients reported having had sexual intercourse with one or two partners and 26% of the patients did not respond. Thirty-six patients reported information about the presence of sexually transmitted infections in their stable partners: of these partners, 21 had HPV cervicitis, 5 had skin warts, 4 had bacterial urethritis, 3 had Molluscum contagiosum and 3 had syphilis. At enrolment (V0), the total number of AGWs diagnosed in all patients was 418 with a mean of 4.18 ± 2.44 AGWs per patient (ranging from 1 to 12 AGWs per patient). Concerning tolerability, at V0, most of the patients quantified the pain from NZCS application as mild (45% of the subjects gave a score of 1 on a scale from 0 to 4) or mild/moderate (35% of the subjects gave a score of 2).
At V1, 25% of the treated patients were cured whereas 75% needed a second NZC application. The total number of AGWs present at V1 in all patients was 213 with a mean number of lesions per patient of 2.13 ± 2.18 (ranging from 0 to 10 AGWs per patient), reaching a wart cure rate of 49% of total warts diagnosed. Tolerability at V1 did not differ from V0.
At V2, 36% of the 75 patients treated at V1 were cured. The total number of AGWs present at V2 in all patients was 88 with a mean number of lesions per patient of 1.17 ± 1.35 (ranging from 0 to 4 AGWs per patient). Therefore, the AGW cure rate was of 58.7% versus V1, reaching a cumulative cure rate of 78.9% of the initial diagnosed AGWs and new warts that appeared during the study. Tolerability at V2 did not differ from V0 and V1.
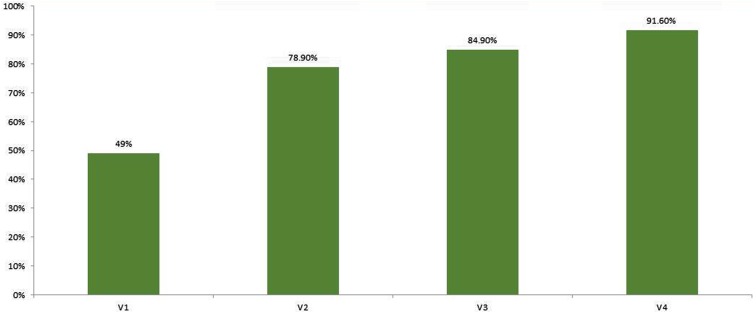
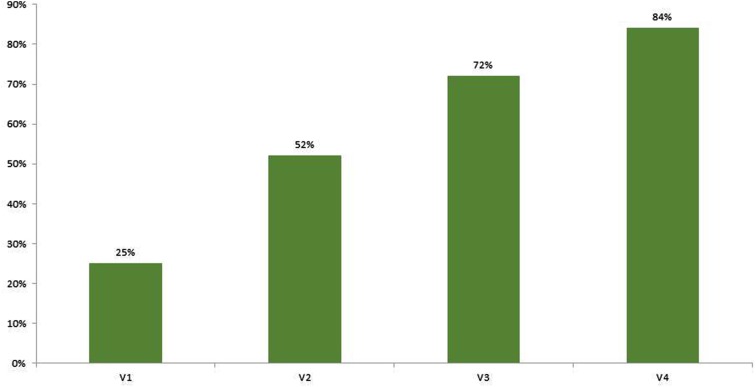
At V3, 41.7% of the 48 patients treated at V2 were cured. The total number of AGWs present at V3 in all patients was 63 with a mean number of lesions per patient of 1.31 ± 1.74 (ranging from 0 to 6 AGWs per patient), reaching a cumulative cure rate of 84.9%. Tolerability at V3 was slightly better than V0, V1 and V2: 56% of patients quantified the pain from NZC application as mild compared with 45% of patients at V0, 44.8% at V1 and 38.5% at V2. At V4, 42.9% of the 28 patients treated at V3 were cured. The total number of AGWs present at V3 in all patients was 35 with a mean number of lesions per patient of 1.25 (ranging from 0 to 6 AGWs per patient). Overall, considering the number of lesions cured, a cumulative cure rate of 92% was observed in ≤ 4 treatment sessions (383 lesions cured at visit 4 out of 418 lesions at baseline) (Table 1, Fig. 1). Complete clearance was observed in 25, 52, 72 and 84% of patients and the cumulative cure rate was 49, 78.9, 84.9 and 91.6% at V1, V2, V3 and V4, respectively (Table 2, Figs. 1, 2).
Table 1.
Cumulative number of cured warts at V1, V2, V3, V4
| V0 | V1 | V2 | V3 | V4 | |
|---|---|---|---|---|---|
| Number of total warts | 418 (100.0%) | 213 (100.0%) | 88 (100.0%) | 63 (100.0%) | 35 (100.0%) |
| Number of cured warts | 0 (0.0%) | 205 (49.0%) | 125 (58.7%) | 25 (28.4%) | 28 (44.4%) |
| Number of accumulated total warts | 0 (0.0%) | 205 (49.0%) | 330 (78.9%) | 355 (84.9%) | 383 (91.6%) |
Fig. 1.
Percentage of cured warts at visit 1 (V1), visit 2 (V2), visit 3 (V3) and visit 4 (V4)
Table 2.
Cured patients at V1, V2, V3, V4
| V0 | V1 | V2 | V3 | V4 | |
|---|---|---|---|---|---|
| Patients visited | 100 (100.0%) | 100 (100.0%) | 75 (100.0%) | 48 (100.0%) | 28 (100.0%) |
| Treated and cured | 25 (25.0%) | 27 (36.0%) | 20 (41.7%) | 12 (42.9%) | |
| Treated and not cured | 75 (75.0%) | 48 (64.0%) | 28 (58.3%) | 16 (57.1%) | |
| Cumulative cured patients | 25 (25.0%) | 52 (52.0%) | 72 (72.0%) | 84 (84.0%) | |
| Patients not cured | 75 (75.0%) | 48 (48.0%) | 28 (28.0%) | 16 (16.0%) | |
| Number of warts per subject, mean (SD) | 4.18 (2.44) | 2.13 (2.18) | 1.17 (1.35) | 1.31 (1.74) | 1.25 (1.65) |
Fig. 2.
Percentage of cured patients at visit 1 (V1), visit 2 (V2), visit 3 (V3) and visit 4 (V4)
New AGWs diagnosed during the study period (as recurrences or new lesions) were also treated and included in the efficacy analysis: relapses were observed in 29% of patients at 3 months and in 5% at 6 months.
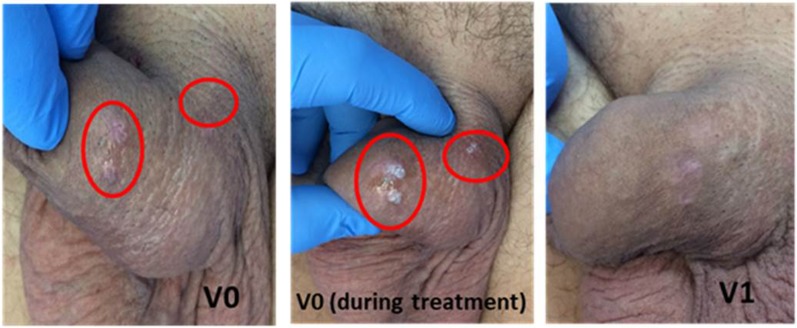
Of note, patients with ≤ 5 AGWs at V0 showed better clearance results than patients with > 5 lesions (p < 0.05) (Figs. 3, 4). Statistical analysis (chi-square test) did not find a significant difference in the cure rate between patients with infectious comorbidity and patients without (p = 0.6163 at V3, p = 0.6658 at V4). When evaluating the cure rate by type of infectious comorbidity present in partner(s), a statistically significant difference was found between patients whose partners had HPV infection (HPV cervicitis, urethral smear positive for HPV, genital condylomas) and patients whose partner had infections different from HPV or no infection. Indeed, at V4, 67.9% of patients whose partners had HPV infection were cured compared with 90.3% of patients whose partners did not have HPV infection (p = 0.0060). In addition, no statistically significant differences were found in the cure rate considering the following variables: lesion location (p = 0.0694 comparing at V4 the cured patients with anal versus genital, ano-genital, inguinal warts), sexual orientation (p = 0.3686 at V3 and p = 0.0568 at V4 comparing heterosexual versus homosexual patients) and the number of partners (p = 0.5957 at V3, p = 0.2603 at V4 comparing patients with 1 sexual partner versus patients with 2 sexual partners during the past year).
Fig. 3.
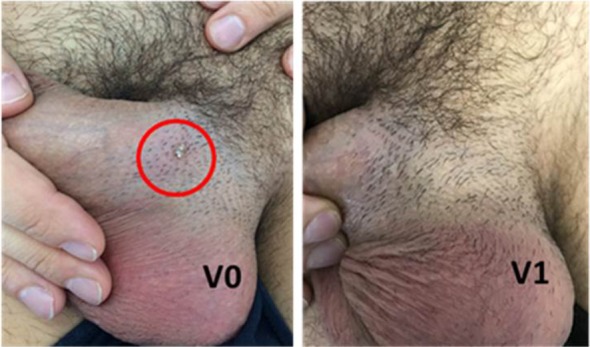
Warts of the shaft successfully treated with only one application of NZC
Fig. 4.
Warts of the shaft successfully treated with only one application of NZC
Discussion
NZC is an aqueous solution containing organic acids (lactic, oxalic and acetic acid at 8.6% each), small amounts of metallic ions (zinc and copper salts) and 65% nitric acid as the main compound. Application of NZCS alters the wart structure through acid hydrolysis of the peptide bonds between the cells and devitalization of the tissue proteins of the lesion (mummification). In addition, unlike liquid nitrogen cryotherapy, NZCS shows antiviral effects by reducing the amount of HPV DNA, as demonstrated by a novel ex vivo approach that studied epidermal histology and assessed the amount of HPV DNA in fragments of plantar warts before and after treatment [15]. The specific combination of ingredients of NZC preserves the active concentration of nitrites in the solution and the nitric acid produces a chemical fixation on the wart tissue with protein denaturation and mummification of the treated area [15]. Therefore, the antiviral effect of NZC can be attributed to the protein denaturation of HPV DNA by the nitric acid.
Ours is the largest series of patients with warts, especially AGWs, treated with NZC. Only three previous studies investigated the efficacy of NZC: Cusini et al. evaluated 37 patients with a total of 55 genital, plantar, hand and periungual warts finding an overall complete cure in 90% of the patients after 1–4 applications [13]. Rozas-Munoz et al. described 15 patients with a total of 128 genital, plantar and periungual warts showing a complete resolution in 88.3% of the warts and partial resolution in 11.7%. They also found that lesions with complete resolution had no recurrences within 3 months of follow-up [14].
Recently, Kelati et al. studied 11 patients with AGWs: although resistant to previous treatments with cryotherapy and/or imiquimod, all lesions were completely cured after a single application of NZC and only one case of recurrence at 6 months was observed [16].
Considering the lesions of the genital area alone, in the study by Cusini et al. 30/37 patients had genital warts and complete resolution was achieved in 87% of them [13], a patient cure rate very similar to ours (84%) with 1–4 applications. Rozas-Munoz et al. did not specify how many of their patients had genital lesions; they considered as a whole the 128 warts of their 15 patients and calculated the cure rate based on the number of cured warts. They observed complete resolution in all (100%) the treated genital warts after four treatment sessions [14], whereas we found complete resolution in 92% of the lesions after four sessions. The cure rate reported by Kelati et al. was also very satisfying with a complete resolution in 100% of the patients at 15 days [16]. The difference between our and other results is possibly due to the smaller sample sizes in the previous studies. Of note, seven of our patients were taking oral immunosuppressive therapy during NZC treatment (1 for psoriasis, 1 for atopic dermatitis and 5 because of a previous solid organ/bone marrow transplantation); nevertheless, one of them was completely cured with only two NZC applications and the other six with three NZC applications. Only two of the seven immunosuppressed patients relapsed at the 3-month follow-up and one at the 6-month follow-up. Despite the small number of immunosuppressed patients in our series, these data demonstrated that NZCS was also effective in a subgroup of difficult-to-treat patients known to be particularly at risk of severe and extensive infections. Indeed, previous studies on NZC efficacy have always dealt with immunocompetent patients [13, 14, 16], and ours was the first small series of immunosuppressed patients treated with NZC. In a previous study on the dermatological infections in organ transplant recipients (OTR), we found that viral infections, especially by HPV, were the most prevalent dermatological infections in these individuals [17]. Indeed, the prolonged duration of cellular-immunosuppressive therapies required to maintain graft function is responsible for the susceptibility to infection in OTRs, which may be more difficult to treat than in the general population. Furthermore, in OTR, the risk of developing non-melanoma skin cancers in chronic HPV infection is higher than in the general population [17]. In addition, our finding of a worse cure rate in patients whose partners had HPV infection compared with those whose partners did not have HPV infection confirms the high contagiousness of HPV [5, 6] and emphasizes the importance of assessing the HPV-infectious status of patients’ partners and, if necessary, the need to treat the infected couple in parallel.
A limitation of the current study is represented by the follow-up period that is limited to 6 months after clearance.
Conclusions
NZCS has been demonstrated to be effective for AGWs after 1–4 treatment sessions, obtaining a good response from the first application and maintaining remission over time. Indeed, the cure rate of NZCS in our study (complete resolution of 92% of warts in ≤ 4 treatment sessions) was one of the highest reported to date. The better response in patients with fewer warts suggests that the earlier diagnosis is made and treatment started, the better the expected results are.
Acknowledgements
We thank the participants of the study.
Funding
No funding or sponsorship was received for the publication of this article. Article processing charges were funded by ISDIN. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and/or Editorial Assistance
We thank Jane Marshall for her revision of the English language and Sonia Aladren for her global revision of the manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
GC and FD performed data collection, analysis and writing of the article; CG and AP provided editorial assistance and contributed to data management, analysis and review of the manuscript.
Disclosures
Giulia Ciccarese has collaborated with ISDIN conducting a didactival activity in the way of scientific advices. Francesco Drago has collaborated with ISDIN conducting a didactival activity in the way of scientific advices. Corinne Granger is an employee at ISDIN. Aurora Parodi has nothing to disclose.
Compliance with Ethics Guidelines
Authors received the approval of an Independent Ethics Committee on 7 February 2018. All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.8029265.
Giulia Ciccarese and Francesco Drago contributed equally.
References
- 1.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187–193. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 2.Chesson HW, Dunne EF, Hariri S, Markowitz LE. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41(11):660–664. doi: 10.1097/OLQ.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health and Nutrition Examination Survey, 2003–2006. J Infect Dis. 2011;204(4):566–573. doi: 10.1093/infdis/jir341. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Human papillomavirus-associated cancers—United States, 2004–2008. Morb Mortal Wkly Rep. 2012;61(15):258–261. [PubMed] [Google Scholar]
- 5.https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-fact-sheet#q. Accessed 22 Mar 2019.
- 6.Ciccarese G, Herzum A, Rebora A, Drago F. Prevalence of genital, oral, and anal HPV infection among STI patients in Italy. J Med Virol. 2017;89:1121–1124. doi: 10.1002/jmv.24746. [DOI] [PubMed] [Google Scholar]
- 7.Drago F, Herzum A, Ciccarese G, Bandelloni R. Prevalence of oral human papillomavirus in men attending an Italian sexual health clinic. Sex Health. 2016;13:597–598. doi: 10.1071/SH16155. [DOI] [PubMed] [Google Scholar]
- 8.Drago F, Herzum A, Ciccarese G, et al. Prevalence and persistence of oral HPV infection in Italy. J Eur Acad Dermatol Venereol. 2018 doi: 10.1111/jdv.15380. [DOI] [PubMed] [Google Scholar]
- 9.Broccolo F, Ciccarese G, Rossi A, et al. Human papillomavirus (HPV) and Epstein-Barr virus (EBV) in keratinizing versus non- keratinizing squamous cell carcinoma of the oropharynx. Infect Agent Cancer. 2018;9(13):32. doi: 10.1186/s13027-018-0205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Workowski KA, Bolan GA, Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 11.Burge SM, Bristol M, Millard PR, Dawber RP. Pigment changes in human skin after cryotherapy. Cryobiology. 1986;23:422–432. doi: 10.1016/0011-2240(86)90027-1. [DOI] [PubMed] [Google Scholar]
- 12.Lacey CJ, Woodhall SC, Wikstrom A, Ross J. 2012 European guideline for the management of anogenital warts. J Eur Acad Dermatol Venereol. 2013;27:e263–e270. doi: 10.1111/j.1468-3083.2012.04493.x. [DOI] [PubMed] [Google Scholar]
- 13.Cusini M, Micali G, Lacarrubba F, Puviani M, Barcella A, Milani M. Efficacy and tolerability of nitric-zinc complex in the treatment of external genital warts and “difficult-to-treat” warts: a “proof of concept”, prospective, multicentre, open study. G Ital Dermatol Venereol. 2015;150:643–648. [PubMed] [Google Scholar]
- 14.Rozas-Muñoz E, Mir-Bonafé J, Piquero-Casals J. Refractory warts successfully treated with a nitric-zinc complex solution. J Dermatol. 2019 doi: 10.1111/1346-8138.14795. [DOI] [PubMed] [Google Scholar]
- 15.Viennet C, Gheit T, Muret P, et al. Assessment of the efficacy of a new formulation for plantar wart mummification: new experimental design and human papillomavirus identification. Clin Exp Dermatol. 2013;38:85–88. doi: 10.1111/ced.12025. [DOI] [PubMed] [Google Scholar]
- 16.Kelati A, Khemis A, Montaudié H, Lacour J, Passeron T. Successful treatment of resistant condylomas with nitrizinc complex solution: a retrospective study in 11 patients. J Eur Acad Dermatol Venereol. 2019;33:e88–e89. doi: 10.1111/jdv.15241. [DOI] [PubMed] [Google Scholar]
- 17.Ciccarese G, Trave I, Herzum A, et al. Dermatological infections in organ transplant recipients: a retrospective study on 222 patients. J Eur Acad Dermatol Venereol. 2019;33:e36–e38. doi: 10.1111/jdv.15153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.