Abstract
The culture of fungal species from agro-waste allows for the sustainable preparation of valuable biotechnological products and contributes to establish the Circular Economy concept. The Ganoderma lucidum species is well known as producer of laccases (EC 1.10.3.2), which serves as a tool to oxidize chemicals. When producing G. lucidum E47 basidiomes with edible purposes out of rice crop residues, its laccase remains as by-product. In this work, we report the biotechnological characterization and application of the laccase recovered from spent cultures of the G. lucidum E47 strain. We detected at least one polypeptide (ca. 59 kDa) which displays attractive activity and stability values when used in the range of 18–45 °C in mildly acidic environment (pH 4.8–5.8). These parameters can be enhanced in the presence of organic cosolvents such as butyl acetate and methyl iso-butyl ketone, but the opposite effect is observed with solvents of lower log P. The best activity–stability performance is reached when the biocatalyst is used in pH 4.8 buffer with 5% (v/v) butyl acetate at 37 °C. The laccase was capable of decolorizing xanthene, azo and triarylmethane dyes, exhibiting excellent selectivity on bromocresol green and bromocresol purple. Furthermore, the biocatalyst displayed an attractive activity when assessed for the decolorization of bromocresol green in a proof-of-concept effluent biotreatment.
Keywords: Agro-waste, Laccase, Biocatalysis, Ganoderma lucidum, Dyes, Decolorization
Introduction
Since the worldwide debate on climate change (Paris Agreement 2018), the notion of Circular Economy has regained momentum (Geissdoerfer et al. 2017). With the aim of diminishing the negative impact that the modern lifestyle cause to nature while maintaining the quality of currently available products and services, policymakers foster closed-loop “make-use-dispose” strategies. To boost a circular bio-based economy, the lignocellulosic residues generated as a consequence of agricultural activity must be harnessed, as addressed by the biorefinery concept (de Jong and Jungmeier 2015).
The Ganoderma genus is a white-rot fungi that is especially rich in terpenes and polysaccharides and it is of paramount importance in ancient Asiatic medicine (Wachtel-Galor et al. 2011). Furthermore, the Ganoderma species are well known as producers of laccases (EC 1.10.3.2). These enzymes are copper-containing oxidases performing a one-electron oxidation mechanism of phenols and related molecules. In vivo, these biocatalysts play a role in the formation of lignin by promoting the oxidative coupling of naturally occurring phenols (Loyd et al. 2018; Zhou et al. 2013). As biotechnological tools, laccases are the keystone in the process of breaking down lignocellulosic biomass—enabling the uptake of the polysaccharide fraction by oxidizing the lignin structure that act as a barrier. In addition, these enzymes are needed to treat biomass hydrolyzates containing lignin-derived phenols which hamper its fermentation (Roth and Spiess 2015). In the search of mild technologies to treat industrial effluents, laccases paved the way for the decolorization of those containing dyes (Sen et al. 2016; Singh et al. 2015; Upadhyay et al. 2016). The use of biocatalysts with laccase activity—either as whole cells or enzymatic extracts—with this purpose has attracted academic interest (Adak et al. 2016; Akpinar and Urek 2017; Shanmugam et al. 2017; Vats and Mishra 2018). Recently, Teerapatsakul et al. demonstrated the decolorization of commercial dyes by an immobilized enzymatic extract of Ganoderma sp. with laccase activity in a 5 L airlift bioreactor (Teerapatsakul et al. 2017). Torres-Farradá et al. described the use of crude laccase from Ganoderma sp. for the degradation of different anthraquinone and azo dyes (Torres-Farradá et al. 2017). Muguresan et al. employed laccase obtained from the solid-state fermentation (SSF) of G. lucidum to decolorize remazol brilliant Blue R and remazol black-5 (Murugesan et al. 2007).
With the intention to subscribe to a lignocellulosic biorefinery process, we previously started a biotechnological project dealing with the production of edible basidiomes from the G. lucidum E47 strain using rice crop residues as substrate—the staple food for over half the world’s population (Sumithra et al. 2014). The process leaves an undervalued protein extract with laccase activity that must be harnessed to achieve zero waste (Postemsky et al. 2014, 2017). In this study, we address the feasibility of closing the loop of the process by valorizing the laccase by-product. To gain knowledge on its biotechnological utility, we describe its primary characterization as biocatalyst and we further assess its application as a tool for the decolorization of dyes.
Experimental
Chemicals
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) was purchased from Sigma-Aldrich (Argentina). The organic dyes bengal rose (1), blue black naphtol (2), congo red (3), methyl orange (4), bromocresol green (5), bromocresol purple (6), bromophenol blue (7) and phenol red (8) were obtained from Mallinckrodt (USA). Analytical-grade solvents were acquired from Anedra (Argentina) and were used as received.
Laccase production
Cultures of the G. lucidum E47 strain (CERZOS, UNS-CONICET, Bahía Blanca, Argentina) were prepared as described in our previous report (Postemsky et al. 2017). In short, the inoculum was done in MYSA medium (20 g malt extract, 2 g yeast extract, 10 g sucrose, and 20 g agar per liter of distilled H2O, pH 5.5) and then kept in darkness for 7 days at 25 °C. A mixture of rice straw, husk and bran was used as the culture substrate for its SSF. The G. lucidum E47 laccase (GLL) was obtained from the spent culture substrate as follows (Postemsky et al. 2014). Briefly, 10 mL distilled water (pH 5.5) was added to a 30 mL glass flat bottom tube containing 3.0 g fresh residual mushroom used as culture substrate. The mixture was chopped with a scalpel and incubated at 4 °C for 16 h to allow extraction. Then, it was compressed to yield the enzymatic broth, which was divided into 1.5 mL aliquots. These were centrifuged (6000g, 3 min) and the supernatants were further lyophilized and stored at − 20 °C until use. The total protein content was determined by the Bradford assay using BSA as standard (Bradford 1976).
Laccase characterization
The protein extract was analyzed by SDS-PAGE using a 10% (w/v) polyacrylamide gel and coomassie blue staining according to the method of Laemmli (Laemmli 1970). A BRP-125 prestained broad-range protein marker (Genbiotech, Argentina) was used as reference.
Laccase activity was determined using the ABTS oxidation as a model reaction (Han et al. 2005; Kadnikova and Kostić 2002), which was adapted to run in 96-well microplates (360 µL well volume). The change of the absorbance at 420 nm (λmax for ABTS·+) was recorded every 15 s with an Infinite M200 PRO microplate reader (Tecan Trading AG, Switzerland). The standard reaction mixtures comprised 1.7 mM ABTS in 100 mM KOAc pH 5.8 buffer. They were initiated by adding an adequate volume of GLL stock (300 µL final reaction volume) and placed immediately in a Vortemp 56 shaking incubator (Labnet International, Slovenia) at 28 °C and 100 rpm (henceforth, standard conditions). One enzymatic unit (U) was defined as the amount of GLL in µg needed to oxidize 1 µmol of ABTS in 1 min under the standard conditions. Control reactions without ABTS and without GLL were run simultaneously. All reactions were run in triplicate.
To address the GLL activity at different temperatures, incomplete reaction mixtures were first incubated for 2 h at 18 °C, 28 °C, 37 °C, 45 °C, 60 °C and 75 °C, and then GLL was added to start them. These were allowed to proceed for 1 min at the corresponding temperatures before analysis. Results were relativized to the standard condition. Control reactions without ABTS and without GLL were run simultaneously. All reactions were carried out in triplicate. To assess the GLL stability at the aforementioned temperatures, incomplete reaction mixtures were prepared and incubated for 2 h at each temperature. Then, ABTS was added to start the reactions, which ran for 1 min at the corresponding temperatures before analysis. Results were relativized to the standard condition. The same protocols were followed to measure the GLL activity and stability at different pH values. The assayed buffer solutions (100 mM) were KOAc pH 3.8, pH 4.8 and pH 5.8, and KPi pH 6.7 and pH 7.8. To determine the effect of adding organic cosolvents on the GLL activity and stability, butyl acetate (BuOAc), methyl iso-butyl ketone (MIBK), tetrahydrofuran (THF), methyl ethyl ketone (MEK) and dimethylsulfoxide (DMSO) were added to the reaction mixtures under the standard condition [5% (v/v) final concentration]. These were treated and analyzed as described above.
Microscale screening for dyestuff decolorization
Microreactions were conducted in 96-well plates as described in section “Laccase characterization”. Each well contained 0.1 mM organic dye 1–8 instead of ABTS, 100 mM KOAc buffer pH 4.8 and 5% (v/v) BuOAc. Microplates were incubated at 37 °C and 100 rpm during 2 h. A total of four 10 µL enzyme additions were carried out every 30 min (340 µL final reaction volume). Control reactions without dye and without GLL were run simultaneously. All reactions were performed in triplicate. The GLL activity was measured every 30 min as the change of the absorbance at the maximum wavelength according to the one of the control without enzyme, as follows: bengal rose 550 nm; blue black naphtol, 610 nm; congo red, 530 nm; methyl orange, 470 nm; bromocresol green, 620 nm; bromocresol purple, 430 nm; bromophenol blue, 590 nm; and phenol red, 550 nm.
Decolorization of bromocresol green in a batch recirculation flow minireactor
The decolorization of 0.1 mM bromocresol green (5) was conducted in 100 mM KOAc buffer pH 5.0 with 5% (v/v) BuOAc (0.2 L final reaction volume). The minireactor system consisted of (i) a 0.5 L conical flask fitted with two connecting side arms, one at the neck (inlet) and the other at the very bottom (outlet); (ii) a Minipuls 3 peristaltic pump (Gilson, France) to allow the recirculation of the reaction mixture at 1.5 L h−1; and (iii) an diffuser providing a 125 L h−1 flow to the sine of the liquid. The system was settled in a thermostatic room at 25 °C. A total of six 1 mL enzyme aliquots were added. A twin system was assembled to run the blank reaction without GLL. Sampling was performed thoroughly until completion. To this end, 1 mL aliquots were taken and scanned in the 300–800 nm range with a Lambda 25 UV/VIS spectrophotometer (PerkinElmer, USA). The absorbance values at 404 nm (reduced form) and 617 nm (oxidized form) (Fassi et al. 2012) were used to analyze the reaction course by the A404/A617 ratio. Immediately, samples were measured for pH and returned to each reactor.
Results and discussion
Laccase biotechnological characterization
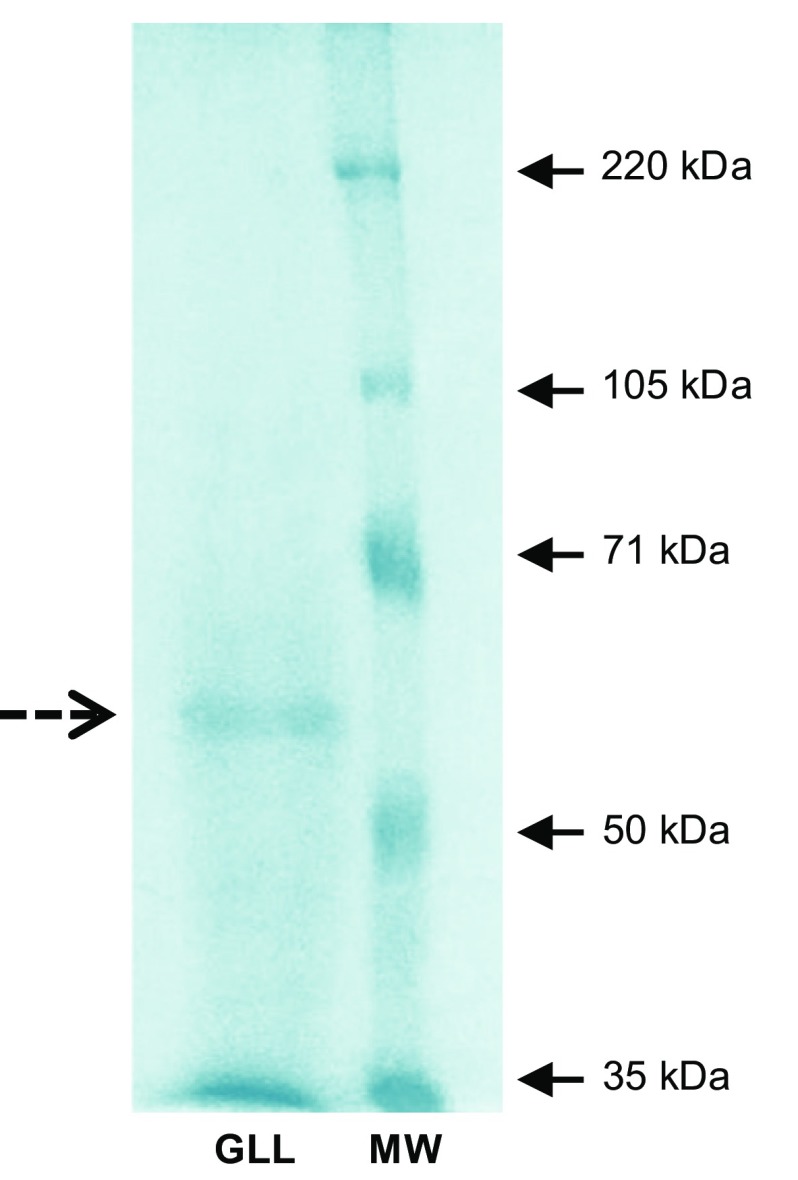
From SDS-PAGE analysis, it was determined that the G. lucidum E47 crude laccase (GLL) is composed of at least one polypeptide chain with an approximate molecular weight of 59 kDa (Fig. 1). This is in agreement with the observations made by several authors on crude preparations or pure laccases from other G. lucidum strains (Kumar et al. 2015; Manavalan et al. 2013; Sharma et al. 2013; Sitarz et al. 2013).
Fig. 1.
SDS-PAGE analysis of the Ganoderma lucidum E47 laccase. The band corresponding to the enzyme is signaled with a dashed arrow
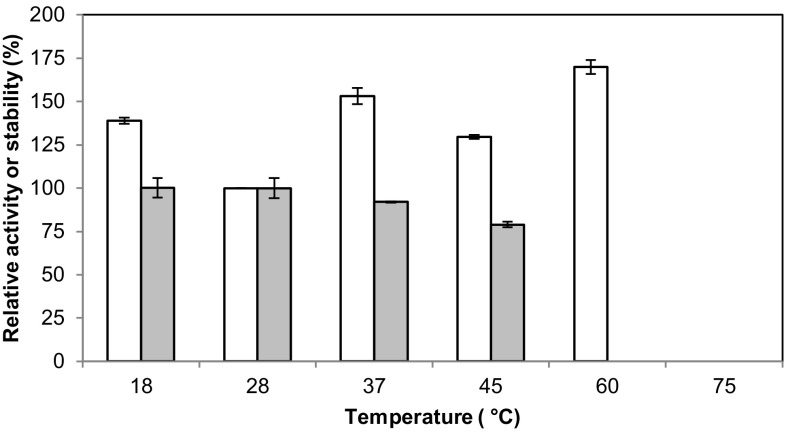
The GLL was characterized as biocatalyst using the well-known ABTS oxidation as a model reaction (Kadnikova and Kostić 2002). The enzymatic activity and stability were studied with different values of temperature and pH, as well as under the presence of several organic cosolvents, and the results were relativized to the standard conditions. When assaying the effect of temperature, it was observed that the GLL is more active both below and above 28 °C (Fig. 2). The protein conformation seems to be more stable at lower temperatures, thus favoring activity. This assumption relies on the fact that the protein stability after 2-h incubation at 18 °C was not reduced in comparison to the standard condition. Nevertheless, from a biotechnological perspective, this temperature might be inconvenient. On the other hand, higher activity rates were observed at 37 °C whereas the protein stability decreased slightly, thus showing a compromise between a favored enzymatic catalysis and the turnover. A further increment of the temperature up to 45 °C negatively affected both parameters. However, the reaction was found to proceed faster at 60 °C although no protein stability was recovered after a 2-h exposure at this temperature, as described by several authors (Ding et al. 2012; Huang et al. 2011; Sun et al. 2012). Furthermore, no laccase activity was detected at 75 °C. These observations may arise from the loss of the copper ions from the enzymes at these high temperatures, as reported by Palonen et al. (2003). Therefore, the optimal reaction temperature was considered to be 37 °C.
Fig. 2.
Effect of temperature on enzyme activity (white bars) and stability (gray bars)
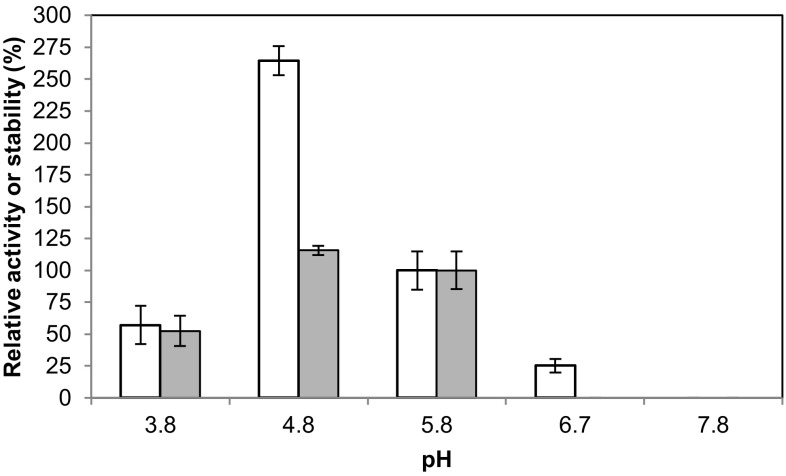
As expected from data in the literature, the GLL activity was shown to be strongly dependent on the pH of the medium (Baldrian 2006). More than a twofold increase in enzymatic activity was detected when assaying it at pH 4.8 in comparison with the reference value, pH 5.8 (Fig. 3). The protein stability also increased, but to a minor extent. At pH 3.8, both protein activity and stability were decreased to approximately a half. Very low activity was recorded at pH 6.7 and null activity was observed at basic pH. As addressed by Munoz and coworkers (Munoz et al. 1997), when pH > 7 the protein stability is dramatically decreased by the binding of a hydroxide anion to the T2/T3 coppers of laccase, thereby blocking the internal electron transfer from T1 to the T2/T3 centers. For these reasons, the optimal pH value was estimated to be 4.8, as typically seen for different G. lucidum laccases (Manavalan et al. 2013; Sharma et al. 2013) and a wide variety of other fungal laccases (Baldrian 2006; Heinzkill et al. 1998).
Fig. 3.
Effect of pH on enzyme activity (white bars) and stability (gray bars)
As mentioned, laccases offer a variety of biocatalytic applications. For this reason, a range of organic solvents were studied as additives [5% (v/v) final concentration] (Table 1). The selection criteria were established on the basis of their sustainable nature and toxicity (FDA 2018; Henderson et al. 2011) as well as for their commercial availability and their bulk price (data not shown). Their lipophilicity or hydrophobicity was addressed by their log P values. Only BuOAc enhanced both the enzymatic activity and stability (Table 1, entry 2). Interestingly, MIBK—which has a similar log P—did not affect the enzymatic activity but increased the protein stability (Table 1, entry 3). However, solvents with lower log P values caused reductions in the values of these parameters up to half of the standard values (Table 1, entries 4–6). When dealing with low water systems, it is generally accepted that for the sake of enzymatic catalysis, organic cosolvents with log P < 2 are a poor choice while those exhibiting log P > 4 are suitable. Solvents with log P values in between cause unpredictable effects on the enzyme microenvironment, therefore, they must be experimentally tested (Gupta 1992; Wan et al. 2010). Similar but milder effects might be assumed to occur in high water systems like ours. From our experiments, the threshold log P range for GLL is limited to 0.49 > log P > 1.31. The fact that BuOAc can be produced by biocatalytic procedures (Alves et al. 2014; Bubalo et al. 2015; Martins et al. 2011) from renewable sources (Wu et al. 2016) may encourage its use in biotechnological processes. Therefore, BuOAc was the cosolvent of choice to assess the performance of the GLL in the decolorization of dyes.
Table 1.
Effect of organic cosolvents on GLL activity and stability
| Entry | Organic solvent | Log P | FDA ICH classa | GSK guideb | Relative enzymatic activity (%) | Relative enzymatic stability (%) |
|---|---|---|---|---|---|---|
| 1 | None | – | – | – | 100 ± 6 | 100 ± 4 |
| 2 | BuOAc | 1.78 | 3 | Preferred | 115 ± 8 | 106 ± 10 |
| 3 | MIBK | 1.31 | 3 | Usable | 100 ± 8 | 147 ± 14 |
| 4 | THF | 0.49 | 2 | Usable | 60 ± 3 | 51 ± 2 |
| 5 | MEK | 0.29 | 3 | Preferred | 55 ± 8 | 136 ± 10 |
| 6 | DMSO | − 1.40 | 3 | Usable | 41 ± 3 | ND |
ND not determined
aAs indicated by the FDA, class 2 solvents should be limited because of their inherent toxicity while those in class 3 are less toxic and of lower risk to human health (FDA)
bAccording to scores in GSK Solvent Selection Guide (Henderson et al. 2011)
Dyestuff decolorization screening
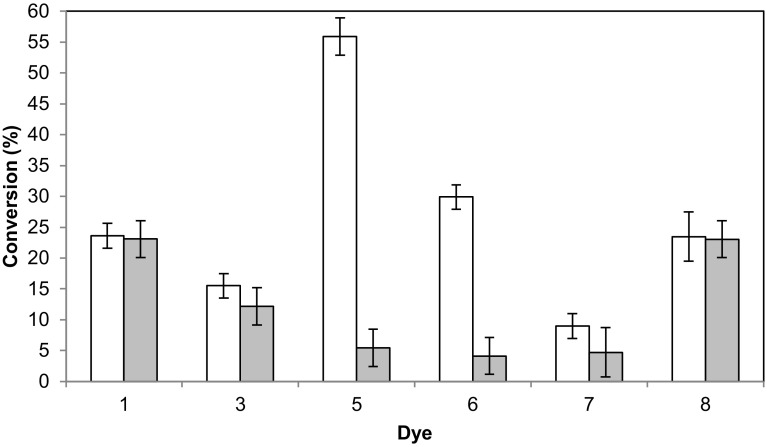
To easily gain an insight on the applicability of the GLL to decolorize dyestuff, eight xanthene, azo and triarylmethane dyes were screened as substrates in microassays, comprising bengal rose (1), blue black naphtol (2), congo red (3), methyl orange (4), bromocresol green (5), bromocresol purple (6), bromophenol blue (7) and phenol red (8). The enzyme exhibited no activity on compounds 2 and 4. On the contrary, it was capable of decolorizing the dyes 1, 3, 7 and 8, although with insignificant conversion. Interestingly, the GLL displayed an excellent selectivity when acting on compounds 5 and 6 (Fig. 4), exhibiting biocatalyzed reaction to blank reaction ratios of 10 and 7, respectively. No structure–activity relation was observed.
Fig. 4.
Dye decolorization screening: biocatalyzed reaction (white bars) versus blank reaction without enzyme (gray bars)
Bromocresol green decolorization in a batch recirculation flow minireactor
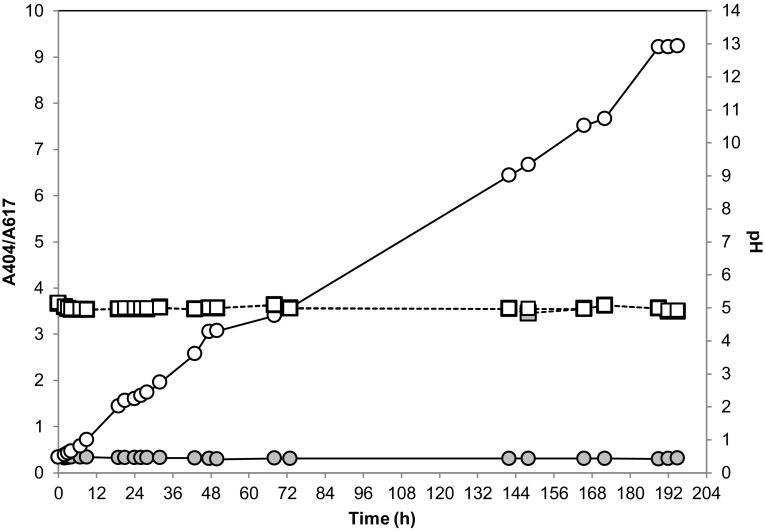
To further assess the performance of the GLL as a biotechnological tool, the decolorization of the compound 5 was conducted in a 0.5 L minireactor simulating a conventional effluent biotreatment process. For this reason, the reaction was run in a room at 25 °C and was allowed to mix by a constant in/out flow. The oxygenation needed for biocatalysis was supplied by an air diffuser—which also contributed to mixing—and pH was monitored in real time. As shown in Fig. 5, this proof-of-concept experiment demonstrated that the enzyme was capable of decolorizing bromocresol green 5 completely under these simple operative conditions. The rate of the process can be controlled at ease by adding a larger amount of GLL.
Fig. 5.
Reaction course plot for the decolorization of bromocresol green catalyzed by GLL (solid line, white circle markers) versus the blank reaction without enzyme (solid line, gray circle markers). Real-time pH plots are denoted with dashed lines with white or gray squares corresponding to the biocatalyzed reaction or the blank reaction without enzyme, respectively
Conclusions
By harnessing the laccase extract remaining as the by-product of the culture of G. lucidum E47, we demonstrated the feasibility to close the loop of our lignocellulosic biorefinery process. The enzyme was primary characterized and showed to be capable of decolorizing organic dyes efficiently. These results foster the use of the G. lucidum E47 laccase in biomass valorization processes.
Acknowledgements
This work was supported by grants from Universidad Nacional de San Luis (PROICO 2-1716), Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 2015 090, Proyecto Unidades Ejecutoras CERZOS e INTEQUI), Ministerio de Ciencia, Tecnología e Innovación Productiva de la República Argentina (PDTS 2014 0492) and Agencia Nacional de Promoción Científica y Tecnológica (PICT 2014 0654). MAP, PDP and MKS are members of the Research Career of CONICET, Argentina.
Abbreviations
- ABTS
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt
- BuOAc
Butyl acetate
- DMSO
Dimethyl sulfoxide
- GLL
Ganoderma lucidum laccase
- KOAc
Postassium acetate
- MEK
Methyl ethyl ketone
- MIBK
Methyl iso-butyl ketone
- THF
Tetrahydrofuran
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Martín A. Palazzolo, Phone: (+54)(9)(266)4520300 ext. 3153, Email: mapalazzolo@unsl.edu.ar
Pablo D. Postemsky, Email: pablop@criba.edu.ar
Marcela Kurina-Sanz, Email: marcelakurina@gmail.com.
References
- Adak A, Tiwari R, Singh S, Sharma S, Nain L. Laccase production by a novel white-rot fungus Pseudolagarobasidium acaciicola LA 1 through solid-state fermentation of Parthenium biomass and its application in dyes decolorization. Waste Biomass Valorization. 2016;7(6):1427–1435. doi: 10.1007/s12649-016-9550-0. [DOI] [Google Scholar]
- Akpinar M, Urek RO. Induction of fungal laccase production under solid state bioprocessing of new agroindustrial waste and its application on dye decolorization. 3 Biotech. 2017;7(2):98. doi: 10.1007/s13205-017-0742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves JS, Garcia-Galan C, Schein MF, Silva AM, Barbosa O, Ayub MA, Fernandez-Lafuente R, Rodrigues RC. Combined effects of ultrasound and immobilization protocol on butyl acetate synthesis catalyzed by CALB. Molecules. 2014;19(7):9562–9576. doi: 10.3390/molecules19079562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldrian P. Fungal laccases–occurrence and properties. FEMS Microbiol Rev. 2006;30(2):215–242. doi: 10.1111/j.1574-4976.2005.00010.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bubalo MC, Tušek AJ, VinkoviĿ M, RadoševiĿ K, SrĿek VG, RedovnikoviĿ IR. Cholinium-based deep eutectic solvents and ionic liquids for lipase-catalyzed synthesis of butyl acetate. J Mol Catal B Enzym. 2015;122:188–198. doi: 10.1016/j.molcatb.2015.09.005. [DOI] [Google Scholar]
- de Jong E, Jungmeier G. Chapter 1—biorefinery concepts in comparison to petrochemical refineries A2—Pandey, Ashok. In: Höfer R, Taherzadeh M, Nampoothiri KM, Larroche C, editors. Industrial biorefineries and white biotechnology. Amsterdam: Elsevier; 2015. pp. 3–33. [Google Scholar]
- Ding Z, Peng L, Chen Y, Zhang L, Shi G, Zhang K. Production and characterization of thermostable laccase from the mushroom, Ganoderma lucidum, using submerged fermentation. Afr J Microbiol Res. 2012;6(6):1147–1157. doi: 10.5897/AJMR11.1257. [DOI] [Google Scholar]
- Fassi S, Bousnoubra I, Sehili T, Djebbar K. Degradation of ‘’Bromocresol Green’’ by direct UV photolysis, Acetone/UV and advanced oxidation processes (AOP’s) in homogeneous solution (H2O2/UV, S2O8 2-/UV). Comparative study. J Mater Environ Sci. 2012;3(4):732–743. [Google Scholar]
- FDA Q3C—Tables and List Guidance for Industry. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073395.pdf. Accessed 21 June 2018
- Geissdoerfer M, Savaget P, Bocken NM, Hultink EJ. The circular economy–a new sustainability paradigm? J Clean Prod. 2017;143:757–768. doi: 10.1016/j.jclepro.2016.12.048. [DOI] [Google Scholar]
- Gupta MN. Enzyme function in organic solvents. Eur J Biochem. 1992;203(1–2):25–32. doi: 10.1111/j.1432-1033.1992.tb19823.x. [DOI] [PubMed] [Google Scholar]
- Han M-J, Choi H-T, Song H-G. Purification and characterization of laccase from the white rot fungus Trametes versicolor. J Microbiol. 2005;43(6):555–560. [PubMed] [Google Scholar]
- Heinzkill M, Bech L, Halkier T, Schneider P, Anke T. Characterization of laccases and peroxidases from wood-rotting fungi (family Coprinaceae) Appl Environ Microbiol. 1998;64(5):1601–1606. doi: 10.1128/aem.64.5.1601-1606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson RK, Jiménez-González C, Constable DJ, Alston SR, Inglis GG, Fisher G, Sherwood J, Binks SP, Curzons AD. Expanding GSK’s solvent selection guide–embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011;13(4):854–862. doi: 10.1039/c0gc00918k. [DOI] [Google Scholar]
- Huang W-T, Tai R, Hseu R-S, Huang C-T. Overexpression and characterization of a thermostable, pH-stable and organic solvent-tolerant Ganoderma fornicatum laccase in Pichia pastoris. Process Biochem. 2011;46(7):1469–1474. doi: 10.1016/j.procbio.2011.03.020. [DOI] [Google Scholar]
- Kadnikova EN, Kostić NM. Oxidation of ABTS by hydrogen peroxide catalyzed by horseradish peroxidase encapsulated into sol–gel glass.: effects of glass matrix on reactivity. J Mol Catal B Enzym. 2002;18(1–3):39–48. doi: 10.1016/S1381-1177(02)00057-7. [DOI] [Google Scholar]
- Kumar A, Sharma KK, Kumar P, Ramchiary N. Laccase isozymes from Ganoderma lucidum MDU-7: isolation, characterization, catalytic properties and differential role during oxidative stress. J Mol Catal B Enzym. 2015;113:68–75. doi: 10.1016/j.molcatb.2015.01.010. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loyd AL, Held BW, Linder ER, Smith JA, Blanchette RA. Elucidating wood decomposition by four species of Ganoderma from the United States. Fungal Biol. 2018;122(4):254–263. doi: 10.1016/j.funbio.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Manavalan T, Manavalan A, Thangavelu KP, Heese K. Characterization of optimized production, purification and application of laccase from Ganoderma lucidum. Biochem Eng J. 2013;70:106–114. doi: 10.1016/j.bej.2012.10.007. [DOI] [Google Scholar]
- Martins AB, Graebin NG, Lorenzoni AS, Fernandez-Lafuente R, Ayub MA, Rodrigues RC. Rapid and high yields of synthesis of butyl acetate catalyzed by Novozym 435: reaction optimization by response surface methodology. Process Biochem. 2011;46(12):2311–2316. doi: 10.1016/j.procbio.2011.09.011. [DOI] [Google Scholar]
- Munoz C, Guillén F, Martinez A, Martínez M. Laccase isoenzymes of Pleurotus eryngii: characterization, catalytic properties, and participation in activation of molecular oxygen and Mn2 + oxidation. Appl Environ Microbiol. 1997;63(6):2166–2174. doi: 10.1128/aem.63.6.2166-2174.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan K, Nam I-H, Kim Y-M, Chang Y-S. Decolorization of reactive dyes by a thermostable laccase produced by Ganoderma lucidum in solid state culture. Enzyme Microb Technol. 2007;40(7):1662–1672. doi: 10.1016/j.enzmictec.2006.08.028. [DOI] [Google Scholar]
- Muthayya S, Sugimoto JD, Montgomery S, Maberly GF. An overview of global rice production, supply, trade, and consumption. Ann N Y Acad Sci. 2014;1324(1):7–14. doi: 10.1111/nyas.12540. [DOI] [PubMed] [Google Scholar]
- Palonen H, Saloheimo M, Viikari L, Kruus K. Purification, characterization and sequence analysis of a laccase from the ascomycete Mauginiella sp. Enzyme Microb Technol. 2003;33(6):854–862. doi: 10.1016/S0141-0229(03)00247-3. [DOI] [Google Scholar]
- Paris Agreement: essential elements. UN Climate Change. https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement. Accessed 21 June 2018
- Postemsky PD, Delmastro SE, Curvetto NR. Effect of edible oils and Cu (II) on the biodegradation of rice by-products by Ganoderma lucidum mushroom. Int Biodeterior Biodegrad. 2014;93:25–32. doi: 10.1016/j.ibiod.2014.05.006. [DOI] [Google Scholar]
- Postemsky PD, Bidegain MA, González-Matute R, Figlas ND, Cubitto MA. Pilot-scale bioconversion of rice and sunflower agro-residues into medicinal mushrooms and laccase enzymes through solid-state fermentation with Ganoderma lucidum. Bioresour Technol. 2017;231:85–93. doi: 10.1016/j.biortech.2017.01.064. [DOI] [PubMed] [Google Scholar]
- Roth S, Spiess AC. Laccases for biorefinery applications: a critical review on challenges and perspectives. Bioprocess Biosyst Eng. 2015;38(12):2285–2313. doi: 10.1007/s00449-015-1475-7. [DOI] [PubMed] [Google Scholar]
- Sen SK, Raut S, Bandyopadhyay P, Raut S. Fungal decolouration and degradation of azo dyes: a review. Fungal Biol Rev. 2016;30(3):112–133. doi: 10.1016/j.fbr.2016.06.003. [DOI] [Google Scholar]
- Shanmugam S, Ulaganathan P, Swaminathan K, Sadhasivam S, Wu Y-R. Enhanced biodegradation and detoxification of malachite green by Trichoderma asperellum laccase: degradation pathway and product analysis. Int Biodeterior Biodegrad. 2017;125:258–268. doi: 10.1016/j.ibiod.2017.08.001. [DOI] [Google Scholar]
- Sharma KK, Shrivastava B, Sastry V, Sehgal N, Kuhad RC. Middle-redox potential laccase from Ganoderma sp.: its application in improvement of feed for monogastric animals. Sci Rep. 2013;3:1299. doi: 10.1038/srep01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RL, Singh PK, Singh RP. Enzymatic decolorization and degradation of azo dyes—a review. Int Biodeterior Biodegrad. 2015;104:21–31. doi: 10.1016/j.ibiod.2015.04.027. [DOI] [Google Scholar]
- Sitarz AK, Mikkelsen JD, Højrup P, Meyer AS. Identification of a laccase from Ganoderma lucidum CBS 229.93 having potential for enhancing cellulase catalyzed lignocellulose degradation. Enzyme Microb Technol. 2013;53(6–7):378–385. doi: 10.1016/j.enzmictec.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Sun J, Peng R-H, Xiong A-S, Tian Y, Zhao W, Xu H, Liu D-T, Chen J-M, Yao Q-H. Secretory expression and characterization of a soluble laccase from the Ganoderma lucidum strain 7071-9 in Pichia pastoris. Mol Biol Rep. 2012;39(4):3807–3814. doi: 10.1007/s11033-011-1158-7. [DOI] [PubMed] [Google Scholar]
- Teerapatsakul C, Parra R, Keshavarz T, Chitradon L. Repeated batch for dye degradation in an airlift bioreactor by laccase entrapped in copper alginate. Int Biodeterior Biodegradation. 2017;120:52–57. doi: 10.1016/j.ibiod.2017.02.001. [DOI] [Google Scholar]
- Torres-Farradá G, Manzano León AM, Rineau F, Ledo Alonso LL, Sánchez-López MI, Thijs S, Colpaert J, Ramos-Leal M, Guerra G, Vangronsveld J. Diversity of ligninolytic enzymes and their genes in strains of the genus Ganoderma: applicable for biodegradation of xenobiotic compounds? Front Microbiol. 2017;8:898. doi: 10.3389/fmicb.2017.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay P, Shrivastava R, Agrawal PK. Bioprospecting and biotechnological applications of fungal laccase. 3 Biotech. 2016;6(1):15. doi: 10.1007/s13205-015-0316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vats A, Mishra S. Identification and evaluation of bioremediation potential of laccase isoforms produced by Cyathus bulleri on wheat bran. J Hazard Mater. 2018;344:466–479. doi: 10.1016/j.jhazmat.2017.10.043. [DOI] [PubMed] [Google Scholar]
- Wachtel-Galor S, Yuen J, Buswell JA, Benzie IF. Ganoderma lucidum (Lingzhi or Reishi): a medicinal mushroom. In: Benzie IF, Wachtel-Galor S, editors. Herbal medicine: biomolecular and clinical aspects. Boca Raton: CRC Press; 2011. pp. 175–200. [Google Scholar]
- Wan Y-Y, Lu R, Xiao L, Du Y-M, Miyakoshi T, Chen C-L, Knill CJ, Kennedy JF. Effects of organic solvents on the activity of free and immobilised laccase from Rhus vernicifera. Int J Biol Macromol. 2010;47(4):488–495. doi: 10.1016/j.ijbiomac.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Wu L, Moteki T, Gokhale AA, Flaherty DW, Toste FD. Production of fuels and chemicals from biomass: condensation reactions and beyond. Chem. 2016;1(1):32–58. doi: 10.1016/j.chempr.2016.05.002. [DOI] [Google Scholar]
- Zhou X-W, Cong W-R, Su K-Q, Zhang Y-M. Ligninolytic enzymes from Ganoderma spp: current status and potential applications. Crit Rev Microbiol. 2013;39(4):416–426. doi: 10.3109/1040841X.2012.722606. [DOI] [PubMed] [Google Scholar]