Abstract
The rising population entails enhancement in wheat productivity to ensure substantial food supply which often get hindered by various biotic and abiotic stresses. Lodging, due to rain and high velocity wind causes significant economic and yield losses in cereals. Hence, lodging is emerging as a major hurdle to achieve the required yield targets. Various morphological, biochemical, anatomical and genetic traits contribute to produce a plant competent enough to bear lodging stress. Hence, in this review, we intend to elaborate the cause and impact relationship of lodging and tried to link lodging tolerance traits to field practices to minimize the losses. Because of the complex nature of lodging phenomenon, it is still obscure to identify best correlated traits to screen genotype in breeding programmes. However, the genotypes with best correlated traits like plant height, culm wall thickness should be introduced/selected in breeding programmes to inculcate lodging tolerance in a high yielding variety as in recent era lodging tolerance is a key factor to enhance productivity and farmer’s income as well.
Keywords: Lodging, Wheat, Productivity, Plant traits
Introduction
Lodging refers to the permanent displacement of the plant shoot from its normal upright position. This usually occurs as a combined effect of inadequate anchorage strength of the crop and impact of adverse weather conditions like rain, wind and hailstorm (Mulsanti et al. 2018). Wheat (Triticum aestivum L.) is a staple food crop in India which is cultivated on about 30 mha land (DAC&FW 2017) but still there is need to increase wheat productivity to feed ever growing population of the country. It is predicted that, up to 60–110 percent increase is required in the overall agri-production to attain food security by the mid of this century (Alexandratos and Bruinsma 2012). Besides the adoption of new technologies/approaches for meeting the production targets, challenges in the pathway are also increasing (Sharma et al. 2015). Although, with the combined efforts of multidisciplinary research, we have become the second largest wheat producer in the world, during independence Indian agriculture scenario was very awful, as only 6.46 million tonnes of wheat was produced, which was insufficient to fulfill the food requirements for the existing population (Dronamraju 2008). Due to lodging prone, low yielding (< 1 tonne/ha) nature of Indian wheat varieties under high fertility conditions, there remained a continuous need for a breakthrough in wheat production. So, the only option was to modify plant architecture to reduce plant height well adapted to intensive agriculture and input applied. Then, breeding of non lodging, high yielding wheat varieties was accorded as the major priority area.
Lodging has led to upto 80% harvestable yield loss and is therefore, one of the major limitation to wheat production (Foulkes et al. 2011). Hence, lodging tolerance has been a driver of plant breeding to minimize these losses (Berry and Spink 2012). Changing rainfall patterns makes the situation more intricate when we target for lodging tolerant genotype. Under field conditions, a lodged plant community widen the yield gaps firstly, by reducing the photosynthetic capacity via changing plant architecture (Berry and Spink 2012; Xiao et al. 2015) and secondly, by disturbing the assimilate partitioning to produce more dry matter instead of spike growth and yield (Berry et al. 2007). Lodging interferes with conducting tissue and thus impedes the storage of photo-assimilates in the developing kernel. Reduced light interception creates conducive environment for foliar diseases which affect plant development and hence decreases production. However, according to Curtis and Halford (2014) in wheat, grain development/production is governed by the metabolic activities of both source and sink and would increase if sink is more efficient to transport assimilates even under lodged conditions. So, the higher vegetative growth favors better assimilate partitioning if it re-translocate the photosynthates from its site of synthesis to the desired sink. But, sometimes the robust plant architecture exhibiting lodging tolerant traits only for aboveground portion is not sufficient to overcome the lodging impact as poorly developed shallow dysplastic root, failure of anchorage system, bending/buckling at the root cone also makes a crop to fall. Generally, high velocity winds with/after rain are the main culprit of lodging, but the complex phenomenon of lodging is also influenced by soil type, topography and crop management practices (Berry et al. 2004; Tams et al. 2004).
Severe yield losses has been reported in different parts of world due to lodging (Table 1). A recent example of lodging losses was recorded 2 years back in the rabi season 2014–2015, when wheat production had fallen to 86.53 mt because of unseasonal weather vagaries. Large scale lodging was reported due to unexpected hailstorms and untimely heavy rains in major wheat growing areas of India like Punjab, Haryana, Uttar Pradesh and Madhya Pradesh. Delayed harvesting because of severe lodging and pre-harvest sprouting, negatively affected both yields and quality of wheat (USDA 2014). This unseasonal precipitation pattern completely destroyed millions of acres of cultivated land and flattens the wheat crop in North West Plain zone of India.
Table 1.
Yield losses due to wheat lodging in different parts of world
| S. no. | Region/area | Yield potential losses (t ha−1 or percentage basis) | Reference |
|---|---|---|---|
| 1 | UK | 0.73–8.3 t ha−1 | Pinera-Chavez et al. (2016) |
| 2 | Australia | 1.7 t ha−1 | Peake et al. (2014) |
| 3 | North West Mexico (Yaqui Valley) | 0.63–7.2 t ha−1 | Pinera-Chavez et al. (2016) |
| 4 | Canada | 16% | Vera et al. (2014) |
| 5 | Southern Queensland and northern NSW | 20,000 ha area or > $20 million | https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2009/11/increasing-yield-of-irrigated-wheat-in-queensland-and-northern-nsw?refresh=024430491 |
Scientific studies to understand the multifaceted interplay of plant architecture, agronomic practices and weather conditions have recently begun, and consequently guidelines for lodging control are often based on perceived wisdom rather than practical comprehension. The hostile weather and unpredictability of its occurrence is the major hindrance to understand lodging. Hence, most of the lodging studies do not reflect the field reality. Thus, exhaustive study is necessary to obtain comprehensive measurements of plant, soil and weather conditions during the natural course of lodging itself to understand the complexity of lodging. This review not only includes the impact of lodging on wheat crop, but also highlighted the traits responsible and the unexplored areas to improve lodging tolerance.
Economic fallouts of lodging
In general, lodging effects are directly correlated to plant growth stage and lodging angle and losses are much more if crop lodged horizontally on the ground at anthesis or early grain filling. A yield reduction of about 0.5% per day of lodging was recorded during grain filling period by Stapper and Fischer (1990). They also reported a large reduction in bread making quality of wheat grains harvested from lodged plant population. Multiple studies on cereals concluded that lodging caused myriad of changes to quality of produce (Rajkumara 2008; Ashraf 2014; Shah et al. 2016). Quality deterioration is not acceptable because of the specific properties of wheat varieties for making bread, biscuit and other industrial products. Crop fall during early grain filling affect the grain quality by decreasing the grain size, Hagberg falling number, thousand grain weight and specific weight (Lang et al. 2012). Reduction in grain size and thousand grain weight highlights the disturbed supply of assimilates to spike which is the most common consequence of crop lodging. Furthermore, high percentage of small and poor quality grain in the produce limit its use and likelihood of achieving a premium price. In later stage, lodging causes less reduction in grain quality but pre-harvest sprouting deteriorate milling and baking quality of the cultivars with weak seed dormancy (Kono 1995).
Types of lodging
Lodging tolerance is a heritable varietal character that is determined and reflected by various traits of plant’s architecture but type of lodging is defined by the plant part bended/broken during the lodging event. In general, two type of lodging have been identified, (1) stem lodging and (2) root lodging. Stem lodging can further be categorized on the basis of stem responses i.e. bending and breaking. The bending type, represented by internode bending at the middle of the internode and the other one is characterized by breaking of stem generally below the third internode. Bending type of stem lodging is not much deterimental because bended culm can even transport photosynthates to kernel for grain filling and further, can also recover to upright position with time and improvement of climatic conditions. Weight differences between consecutive internodes are the main reason for buckling of lower internode (Packa et al. 2015).
Root lodging occurs due to failure of efficient root anchorage resulting in straight, unbroken culms leaning from the crown. A wider root spread angle was directly correlated to root lodging tolerance (Pinthus 1967). Practically, root and shoot lodging could occur simultaneously/separately but root lodging is more common than stem lodging (Berry et al. 2003b). Under long term adverse climatic conditions, permanent loss of the original vertical position of the plant causes deformation of the ears and shriveling of the kernels. High intensity of rain and wind significantly deteriorate soil strength and increasing the load which a plant must bear.
Plant traits associated with lodging
Plant height
Height of a wheat plant is comprised of total above ground internodal length along with length of peduncle and spike which determine the resistibility against lodging. Height is the most practical and easily selectable plant trait to determine lodging behavior of a cultivar. It was hypothesized that shorter height may limit various physico-chemical processes which ultimately reduces yield. But, besides owing good yield potential heavier canopy of tall varieties generally reduce the ability to resist lateral forces which enhance lodging index. Plant height is directly correlated to center of gravity of culm, as lesser plant height lowers the value of centre of gravity, self weight movement of shoot and thus provides resistance to short stature varieties (Okuno et al. 2014). It is well documented that short height provides advantage over taller cultivars as theses are less susceptible to lodging (Curtis and Halford 2014). A strong positive correlation between plant height and lodging was identified among a diverse population of 140 spring and spelt wheat cultivars under artificially created lodging conditions at early and late milk stage and variability was higher among cultivars of intermediate height rather than shorter and taller plant types (Navabi et al. 2006; Longin and Würschum, 2014). Optimization of plant height for maximum photosynthesis could be a fruitful strategy to increase productivity under lodging.
-
(b)
Culm traits
The two major contributing traits for stem strength are plant’s standing power and straw shortness. Internodal length, strength of the lower internodes and weight distribution generally define the type of lodging. But, stem traits like solidness, diameter and wall thickness of basal internodes are the best reported indicators of culm strength against lodging (Hasnath Karim and Jahan 2013; Longin and Würschum 2014; Xiao et al. 2015). However, wider culm diameter with thicker basal internode and fewer tiller per unit area with heavy spikes are some characteristics suggested to be chosen for lodging tolerance (Tripathi et al. 2003) (Fig. 2). Among height related traits, third and fourth internodes had the maximum correlation with stem lodging (Kashiwagi et al. 2005) but, resistance to breaking and lodging index in internodes are important at inflection (Kono 1995). Thicker stem wall and solid culm increase the value of per unit plant biomass and demand more photosynthetic investment for strengthening its supporting components. During an experimental study of understanding lodging characteristics, a strong positive correlation between stem wall thickness and lodging resistance in the four wheat genotypes was reported (r = 0.972, P < 0.05) by Kong et al. (2013). About 49.7% of the variations related to lodging in wheat were explained by variation in stem weight (Zuber et al. 1999). A significant correlation between weight of the three lower internodes (WOL) and lodging resistance (r = 0.986, P < 0.05) suggested WOL as an important factor affecting the rigidity of wheat stem (Kong et al. 2013). Influence of spike weight is stage specific and lodging caused by heavier spikes could be compensated by heavier stem (Zuber et al. 1999) hence, thicker and heavier stems (mg per cm) are the indicators of better lodging tolerance in tall genotypes. But in plants shorter than 90 cm height, stem weight per cm is of less importance for lodging resistance.
-
(c)
Stem anatomical traits
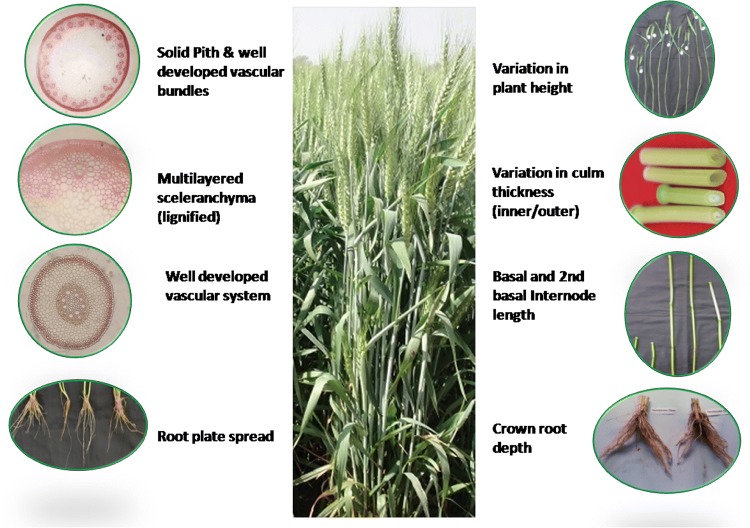
Anatomical characters are believed to be a good indicator of plant adaptation to abiotic stress. Wheat culm is mainly built up of ground tissue (parenchyma) and mechanical tissue (sclerenchyma). The pattern of organization of these cells, their relative proportion and biochemical composition determine the behavior of a genotype under weather uncertainties (Figs. 1 and 2). Depending on genotypic variability, wheat culm may be solid, partially solid or hollow type. Completely or partially filled stems have comparatively thicker stem wall and higher proportion of sclerenchymatous tissues which make it relatively more tolerant to lodging than hollow stemmed wheat (Kong et al. 2013). For a lodging ideotype, a wide genetic range for wall width from 0.732 to 1.11 mm was suggested by Berry et al. (2007) with a maximum material strength of 54.6 Mpa for wheat. A significant positive correlation has been found between stem wall density and stem material strength. Stem stability increases with the increasing proportion of pith parenchyma as parenchyma cells have a good shock absorbing capacity and can withstand against the harmful effects of environmental (wind, rain) forces without any mechanical damage to the culm (Kokubo et al. 1989). The potential to tolerate bending forces against lodging is also directly linked with the ratio of outer and internal culm diameter. Higher percentage of sclerenchymatous layers is equally correlated to lodging tolerance as plant height (Hasnath Karim and Jahan 2013). As more sclerification will provide a mechanical strength to soft tissues and can prove to be of high significance, as it can prevent collapse and bulking of stem. Multi-layered sclerenchyma not only provide better tolerance against lodging, but also decrease the respiratory losses.
Fig. 2.
Plant traits associated with lodging tolerance
Fig. 1.
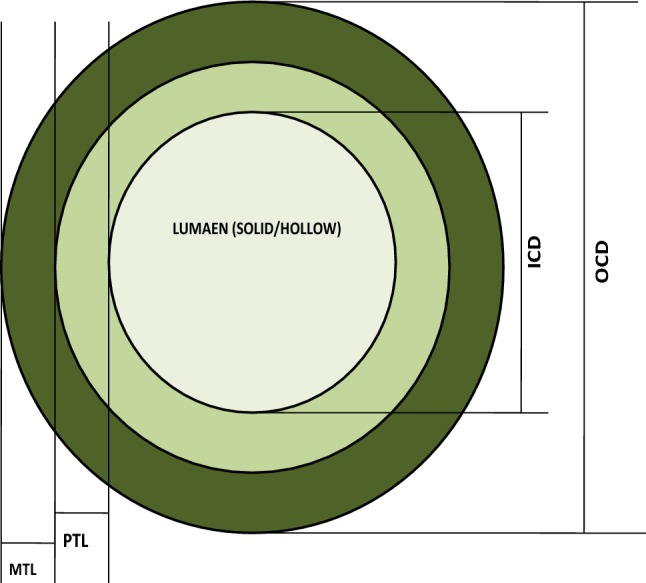
Transverse section of wheat culm internode, OCD outer culm diameter (culm thickness), ICD internal culm diameter, MTL mechanical tissue layer, PTL parenchymatous tissue layer
A well-built translocation system is the marker of strong stem. A significant positive correlation between the number of vascular bundles per mm2 and bending resistance was reported by some researchers (Wang et al. 2006) while no correlation was observed by others (Kelbert et al. 2004; Kong et al. 2013). Two rows of vascular bundles are dispersed in wheat culm, with smaller vascular bundles embedded in the outer sclerenchyma tissues while the larger vascular bundles are embedded in the inner parenchyma tissues. The sheath around vascular bundles and sclerenchyma tissue were composed of fiber cells. Nevertheless, most of the favorable trait combinations of wheat culm are genotype specific and required associations were not recorded for most of the traits, but still, a solid stemmed cultivar with thicker sclerenchyma layer and parenchymatous pith is relatively tolerant to lodging.
-
(d)
Root traits
The measurement of underground traits is a complicated task due to unavailability of standardized methodology and root traits are highly influenced by rhizospheric fluctuation and above ground agro ecological conditions (N fertilizer, temperature). But, it is well documented that wider and deeper roots improve lodging tolerance (Hasnath Karim and Jahan 2013). Hence, a separate system of classification was developed for stem and root lodging, as the basis of lodging tolerance was different for different winter wheat cultivars (Berry et al. 2003a, b). To avoid root lodging and increasing anchorage strength, coronal root should be targeted as desirable trait as the spreading angle and strength of coronal roots has been proven significant in many research experiments. Wider root spreading angle helps in the formation of larger root-soil cone having more tolerance for bending moments. Although certain studies have shown that root-clump weight, tensile strength and root pulling strength also enhance lodging tolerance.
-
(e)
Biochemical and physiological traits
Development of a lodging tolerant high yielding variety is a cumbersome endeavor which needs thorough understanding of the background biochemical and physiological pathways behind the development of a stronger plant phenotype (Table 2). For instance, plant pushing resistance is an important trait and easy to record merely by hand scoring and can be used by breeders as indirect selection parameter for lodging tolerance. Biochemical molecules like lignin, silica, cellulose and hemicellulose contribute for strengthening plant culm. Culm wall thicknesses also represent a positive correlation with lignin, cellulose, water soluble carbohydrates along with other structural carbohydrates. Percentage of cellulose and lignin is closely associated with plant strength (Ma 2009) while silica deposition in epidermal cells is a good indicator of lodging tolerant wheat genotypes. Zheng et al. (2017) also reported improved hardness, toughness and stem elasticity due to silica accumulation. Some researchers reported that excess carbohydrates normally stored in the basal part of culm/stem provide rigidity and breaking resistance to plant (Wang et al. 2012; Kong et al. 2013; Peng et al. 2014). The most common form of water soluble carbohydrates are fructans and variability in these sugars could be used to screen large number of genotypes. WSC estimation includes complex inter-relationship pathway of source sink and also includes both its concentration and content of sugars (fructans, glucose, fructose and sucrose). High sugar content in culm and its active remobilization during stress conditions may keep the plant alive for longer duration and contributes to the functional stay green trait of delay senescence (Campbell 1963; Thomas and Howarth 2000). Lignin is a complex phenolic polymer that forms an important structural component (20% of the secondary cell wall and filling pores between the polysaccharides) while cellulose constitute about one-third of primary cell wall and more than half of the secondary wall (Taylor et al. 1999). However, the complete biosynthetic pathway of these bimolecules is not fully undersood but their biosynthesis and accumulation has been affected by mechanical injury and environmental stresses (Moura et al. 2010; Zheng et al. 2017). A significant positive correlation was recorded between cell wall cellulose content and stem bending strength of barley cultivars by Kokubo et al. (1989). Some researchers also recorded impact of wall cellulose on the total sugar release during different enzymatic reactions (Fan et al. 2012; Lindedam et al. 2012).The altered ratio of different biochemical components is the result of disturbed physiological processes, hence; emphasis should be given to understand the impaired fundamental physiological processes like photosynthesis, respiration, nutrient uptake and their translocation. For instance disturbed photosynthetic machinery always produce lesser amount of soluble carbohydrate content in stem basal internodes which is significantly associated with lodging tolerance. More accumulation of starch contributes higher bending strength to the stem. So, non-constituent components of wheat culm viz. soluble sugars and starch play important role to enhance physical strength of culm (Arai-Sanoh et al. 2011). Conductive tissue may get damaged due to bending/breakage of culm which block the supply of xylem and phloem ultimately disturbing the grain development.
Table 2.
Correlation of plant traits with lodging tolerance
| Plant trait | Correlation with lodging tolerance | Reference |
|---|---|---|
| Plant height | − | Okuno et al. (2014) |
| Internode length | − | Xiao et al. (2015) |
| Stem solidness | + | Xiang et al. (2016) |
| Stem/Culm diameter | + | Xiao et al. (2015) |
| Culm wall thickness | + | Xiang et al. (2016) |
| Number of vascular bundles | + | Hasnath Karim and Jahan (2013) |
| Crop canopy | − | Wang et al. (2006) |
| Stem material strength | + | Pinera-Chavez et al. (2016) |
| Tiller number | − | Tripathi et al. (2003) |
| Lignin content | + | Okuno et al. (2014) |
| Cellulose content | + | Kong et al. (2013) |
| Silicon content | + | Zhang et al. (2010) |
| Root plate spread | + | Pinthus (1967) |
Lodging characterization: natural vs artificial conditions
Measurement of lodging in field is a cumbersome practice due to complexities associated with data recording especially when crop is lying on the ground. Though under controlled/glasshouse conditions lodging characterization is not an easy task. Literature suggested different means and methods to study lodging under natural/field or glasshouse or in field with movable wind tunnels. Various theoretical models have been developed by researchers to standardize a universal method to quantify lodging. To revive the field conditions under artificial setup, portable wind tunnel has also been used (Sterling et al. 2003) and high intensity winds were blown over the plants to measure the degree of lodging tolerance. Various instruments were used/self designed by the researchers to identify the basis of stem bending moments and stem strength (Kono and Takahashi 1964).
Varietal strength to lodging could be calculated by measuring the breaking strength of culm (1/4 × Breaking load × Distance between fulcra) or by lodging index (Bending moment by whole plant/Breaking strength) or by measuring bending stress (Breaking strength/Cross section modulus) (Quang Duy et al. 2004) etc. But none of the parameters are satisfactory to define lodging in true sense as they are influenced by various environmental vagaries. Lodging could either be determined on a scale of 1–9, where 1 represents no lodging and 9 represent severe lodging. Lodging score has two components, one is the percentage of the area lodged and another is the angle at which crop get lodged, hence, lodging can be estimated by putting the values in the formula to calculate the score i.e.
In a lodging prone year, these methods are used to calculate the severity of lodging to analyze the impact of lodging on total harvest and economy. Otherwise, the screening/scaling is also used to test any research/breeding material in wheat improvement programme. Plant genotype recovery percentage also plays an important role to identify a tolerant variety and the retrieval of original upright position after lodging event is a desirable trait. However, the recovery percentage decreases with increasing plant age.
Strategies to mitigate lodging
Lodging is a persistent but discontinuous problem, because the lodging incidences primarily depend upon weather uncertainties rather than plant variety. Secondly, identification of lodging associated traits is complicated due to the challenge of screening under natural field conditions. But, we can reduce the lodging events by following some precised agricultural practices.
Agronomic practices
Cultural practices
Earlier, identification of morphological traits was the focused area for lodging tolerance but later on research became more concentrated on establishment of mechanical models for lodging resistance (Crook and Ennos 1993; Berry et al. 2006) and exploring the physiological and biochemical components of the culms and their histological distribution (Zhu et al. 2004). Lodging risk can efficiently be managed by calculating the correlation between agronomic practices as husbandry decisions including varietal choice (tall/semi dwarf/dwarf), sowing date (early/timely/late), seed rate (high/low or as per recommendations), drilling depth (deep/shallow), soil fertility (high/low) and the application of plant growth regulators (Berry et al. 2000; Pham et al. 2004; Shah et al. 2016) which strongly influenced crop lodging. A reduction of 30% in lodging risk with a delayed sowing of 15 days was recorded (Spink et al. 2000b). Early sowing increases the duration of crop cycle and hence the lodging risk because of higher grain filling duration, enhanced tillering potential and also increased the number of base bending moments significantly along with higher respiratory losses in later stages. Due to lodging, yield losses of 30% and 40% has been reported by Pinthus (1973) and Easson et al. (1993) respectively, but a crop lodging just after head emergence may cause up to 61% of yield reduction (Berry and Spink 2012; Acreche and Slafer 2011).
A higher plant density is more likely to increase lodging incidences (Easson et al. 1993) because more tillers per unit area compete hard for space, light and nutrients and ultimately produce plants having longer, thinner and weaker stems with less dry matter in the lower internode (Berry et al. 2000) and poor root anchorage.
High soil fertility levels also favor lodging. Application of nitrogenous fertilizers drives the crop to produce a dense canopy owing to higher photosynthetic potential but with shading effect because of low red: far red ratio. It is well documented that higher N application improves plant’s growth and development but reduces culm diameter and culm wall thickness along with cellulose and lignin contents (Wang et al. 2012; Zhang et al. 2015). Recent studies reported that agronomic practices also alter the expression of lignin synthesizing genes. Higher application of N fertilizer down regulates the expression of PAL (Phenylalanine ammonia-lyase), CoMT (Caffeic acid O-methyltransferase), 4CL3 (4-Coumarate:coenzyme A ligase)3, CCR (Cinnamoyl CoA reductase), CAD2 (Cinnamyl alcohol dehydrogenase 2), CAD7 (Cinnamyl alcohol dehydrogenase7) which ultimately results in lignin deficiency in the secondary cell walls (Zhang et al. 2017). The reduced mechanical strength of stem subsequently causes poor lodging tolerance in the crop. High planting density is an effective and approachable methodology to enhance productivity but also make the crop prone to lodging (Shah et al. 2016). High seed rate also resulted in higher lodging index ultimately because of denser canopy. Deeper sowing facilitates development of good crown growth which provides stronger anchorage with better absorption and assimilates partitioning to the aerial parts. Integrated agronomic practices including proper irrigation and balanced fertilizer use influence soil water-nutrient uptake and agro–ecosystem conditions, which finally influence the phenology, rhizospheric architecture and stage specific sensitivity to crop lodging (Wu and Ma 2015a, b). Hence, agricultural practices related to sowing and fertilizer application may play a significant role for lodging tolerance.
-
(b)
Application of plant growth regulators
Plant growth regulators are synthetic chemicals which play important roles against lodging by interfering plant growth and development processes. Generally, PGR’s reduce cell division and elongation to reduce plant height, improve stem mechanical strength and hence seemed to be an effective and promising intervention to reduce lodging (Bridgemohan and Bridgemohan 2014). They contribute indirectly to improve quantity and quality of stem strength (Rajala and Peltonen-Sainio 2002; Stachecki et al. 2004). PGR’s like Trinexapac-ethyl, Chlormequat chloride, mepiquat chloride etc. control stem elongation by hampering the normal Gibberellic acid (GA) biosynthesis pathway. Trinexapac-ethyl and paclobutrizol were reported to cause a linear decline in plant height but no affect on the productive plant tillers. GA inhibitors play key role in developing semi-dwarf and sturdier plants (Rademacher 2000; Berry et al. 2004). Hence, being antagonistic to GA, these PGR’s produce stouter stem with profuse belowground growth and better grain filling. Paclobutrizol application increases culm diameter, wall thickness and lignin concentration along with reduction in length of second basal internode (Peng et al. 2014). In countries like France, UK, Germany, which have the largest cereal production, plant growth regulators are applied in 70% of wheat grown area (Berry et al. 2004). Resistance of dwarfs to lodging can help to maximize harvestable yield by reducing harvest losses. In paddy, the application of paclobutrizol gives 15% higher yield by reducing height and lodging 60–90% respectively (French et al. 1990). With a concentration of 400 and 600 mg/L of PBZ, culm bending resistance can be increased significantly as the application of this concentration can reshape parenchyma cell from rounded to hexagonal and intercellular space get reduced (Syahputra 2012).
GA also seems to positively regulate lignin content. The bending stress (r = 0.81) in the rice cultivars show most significant correlation with the lignin content of the culm. GA plays a role in lodging resistance by either resulting in increase in culm diameter or by increasing lignin content. Consequently, the GA-deficient and insensitive mutants show decrease in the bending moment at breaking in contrast to GA-overproducing mutants which show increase in this parameter (Okuno et al. 2014). The bending moment at breaking is considered as an effcient parameter for measuring the strength of the culm (Okuno et al. 2014).
Besides GA, other plant hormones like auxin and cytokinins also play a role for culm strength by inducing the expression of lignin biosynthetic gene. Hyper-accumulation of auxin leads to enhanced expression of selected lignin biosynthetic genes. It has been shown by researchers that the level of salicylic acid (SA) is inversely related to lignin content in plants like Arabidopsis thaliana and down-regulation of the HCT enzyme leads to severe stunting of the plants with significant reduction in lignin levels.
-
2.
Genetics of lodging and lodging tolerance mechanism
Indian wheat scenario has completely changed after the discovery of “Norin 10” dwarfing genes and we became self sufficient in wheat production as these varieties have high yielding traits along with semi dwarf non-lodging plant habit. The genes conferring semi-dwarfing traits are for culm length shortening with a semi-dominant gain-of-function mutation in the reduced height (Rht) homologous genes in wheat (Gale and Marshall 1973). The wheat varieties of modern world are having at least one of these genes. These wheats were relatively shorter, photo insensitive, having good degree of lodging resistance and highly responsive to input doses. Hence, introduction of Rht genes have played a remarkable role in reduction of plant height associated with significant yield enhancement in Indian wheat varieties (Marshall et al. 2013). Development of varieties like, Kalyan Sona, Sonalika, Choti Lerma and Safed Lerma made the wheat revolution to happen in India by occupying 10 million hectare area because of their wide agronomic and climatic adaptability.
The carbon partitioned to grain and thereby the harvest index (HI) is increased in proportion to reduction in height and thus the risk of yield penalties caused by lodging is reduced. But some researchers have pointed that since 2000, the lodging risk has been increased in wheat varieties due to high yield and no further shortening of plant height (Berry et al. 2014). However, further reduction in height will also affect overall wheat production.
Hence, to enhance lodging tolerance in the breeding population, combination of multiple traits associated with lodging is required because of their high heritability values. Lodging characters are highly heritable with a range from 0.73 to 0.93 (Berry et al. 2007). The stem related traits like culm diameter, culm wall width, material strength and plant height have significant heritability values of 0.70 while, a range between 0.51 and 0.93 for heritability for traits such as stem diameter, stem wall width and stem dry weight per length was reported (Keller et al. 1999). But, among all traits, plant height is the best reported heritable morphological trait. Genetic linkage between various lodging related traits has been identified by Berry et al. (2003b) but the most detrimental correlation has been found between stem diameter to stem wall width (positive correlation), stem diameter to material strength (negative correlation) and stem strength to anchorage strength (zero/no correlation).
Recent advances in molecular biology and biotechnological techniques enhanced understanding of tolerance against lodging in many crops viz. rice, maize, sorghum etc. The alteration of lignin quantity/quality is a viable option to counter the problem of lodging, as lignin, a complex phenolic polymer play critical role to provide structural support, rigidity and solute transport. However, molecular mechanisms underlying lignin formation in wheat are still not clear and hence could be an important step towards the understanding and development of molecular tools for breeding of wheat cultivars with optimal level of lignin and improved feedstock quality without negatively affecting lignin-related agronomic benefits. The type and concentration of lignin units vary among different plant species and cell types and the synthesis may affect environmental stresses at various developmental stages (Li et al. 2009; Moura et al. 2010).
Lignin biosynthesis pathway has been explored extensively in many plants (rice, sorghum tobacco and Arabidopsis), and these studies have led to the identification of genes encoding the enzymes catalyzing many of the steps in the pathway. Suppression of some identified genes early in the pathway such as phenylalanine ammonia-lyase (PAL), Cinnamate 4-Hydroxylase (C4H), p-HydroxycinnamoylCoa:Quinate/ShikimateP-Hydroxycinnamoyltransferas (HCT), P-Coumarate 3-Hydroxylase1 (C3H) and Caffeoyl-CoaO-Methyltransferase (CCoAOMT), Ferulate5-Hydroxylase (F5H) and caffeic acid O-methyltransferase2 (COMT2), results in altered total lignin content. Genetic repression of Cinnamyl Alcohol Dehydrogenase (CAD), encoding an enzyme that catalyzes the last step in the biosynthesis of monolignols, also causes reduction in total lignin content. Total lignin cannot be used as a direct indicator of stem strength as its biochemical distribution in wheat culm/straw is not consistent throughout the plant histology (Ghaffar and Fan 2015). During a comparative study of wheat cultivars, it was found that occurrence of CCR1, COMT1 and CAD1 genes along with their corresponding biochemical components contributes for better lodging tolerance (Ma 2007). Some workers concluded that deficiency of cellulose may cause brittle stem hence cellulose is more crucial to maintain bending strength instead of lignin (Wang et al. 2012).
Molecular marker studies in lodging tolerance
Lodging related traits has been directly or indirectly affected by the pleiotropic effects of height related genes. Allele for reduced height is associated with lodging tolerance and out of nine quantitative trait loci (QTL) identified for lodging tolerance, five correspond well to height related QTLs (Keller et al. 1999). Along with height related QTL’s, some other QTL has also been identified responsible for wheat lodging (Table 3). It was also reported that QTLs on chromosome 2D and 6BL were responsible for culm stiffness. QTLs have also been identified for lodging tolerance and stem wall width on chromosome 1B, 4B, 4D, 6D and 7D (Verma et al. 2005), and for stem carbohydrate content on chromosome 6B, 7A and 7D (Rebetzke et al. 2008; McIntyre et al. 2011). Some QTLs related to root traits has also been reported (Verma et al. 2005, Hamada et al. 2012) on chromosome 2D, 5A, 5D and 7D. In a study conducted with a doubled-haploid (DH) population, two QTL for stem strength (on chromosome 3A and 3B), two for pith diameter (on chromosome 1A and 2D) and two for culm wall thickness and stem diameter (on chromosome 2D and 3B) were identified (Hai et al. 2005). Recently, six QTL linked for plant height has been identified, among which three are associated with enhanced yield and three with anchorage strength, however individual QTL for plant height, culm diameter, stem material strength, stem failure moment, root plate depth and root plate spread were also identified in two DH winter wheat population (Berry and Berry 2015). A significant positive correlation was found between culm diameter and pith diameter but no single identical QTL has been found for these two traits. Stem strength can be improved by selecting culm thickness and culm diameter/pith diameter ratio with marker-assisted selection (MAS) in wheat improvement breeding programmes. However, stem solidness can be manipulated by a single chromosome region on chromosome 3BL in wheat population derived from a cross of hollow and solid stemmed parents and the trait showed 99% of variation in lodging tolerance (Kong et al. 2013).
Table 3.
Quantitative trait loci identified for lodging and lodging related traits in the different wheat population
| Number of QTLs | Wheat material | Chromosome number | Related traits | Reference |
|---|---|---|---|---|
| 3 | Doubled haploid population derived from two Canadian wheat cultivars | 4D, 5A and 6D | Plant height and yield | Ma (2009) |
| – | Population of 96 doubled haploid lines from the cross ‘Milan’ × ‘Catbird’ wheat | IB, ID, 2B, 2D, 4B, 4D, 6D and 7D | Plant height, Internode characters, root sheath depth, tensile breakage force, ear length | Verma et al. (2005) |
| 3 | A doubled-haploid population generated from the cross RL4452 × ‘AC Domain’ spring wheat | 4B, 4D | Plant height and other agronomic traits | McCartney et al. (2005) |
| 3 | 132 F12 RILs derived by single-seed descent from a cross between the Chinese facultative wheat Ning7840 and the US soft red winter wheat Clark | 1B, 4AL and 5A | Lodging score, stem solidness | Marza et al. (2006 |
| 6 | A doubled-haploid population derived from anther culture of the wheat cross CA9613 and H1488 | 1A, 2D, 3A and 3B | Stem strength, culm wall thickness, pith diameter and stem diameter | Hai et al. (2005) |
Future prospects
Recent studies suggest that reduced height of dwarfs and semi-dwarfs may limit several metabolic processes and therefore, results in yield stagnation. So, it is unlikely to improve wheat yield with further reduction in plant height which is probably close to optimal value. Hence, genetic improvement of culm strength is required to tackle lodging. But here also direct selection of a trait is inadequate as specific selection of allelic combinations is essential for directed manipulation. Traits other than height and basal internode length, like culm diameter and solidness should be focused. A group of UK researchers favors the hypothesis that improvement in total plant biomass could enhance lodging tolerance in wheat and presented this as a practically feasible option against lodging tolerance. Some wheat genotypes has already been reported with targeted values for certain plant traits but still there is a scope to improve in other germplasms to develop a ideotype for lodging tolerance (Berry et al. 2007). In an experimental study conducted by our group, it was observed that these plant traits works in complement, wider culm diameter along with a partially solid/solid stem and thicker culm wall with high lignin are effective combinations for lodging tolerance. Higher activities of lignin synthesizing enzymes (PAL, TAL) facilitate formation of strong stem even in a semi solid culm type with increased capacity to tolerate wind gusts. It is better to conduct lodging related stress studies under natural condition as compare to control/glasshouse, as under control various other stresses can effect plant performance and interfere with lodging complexity and plant behavior. Nature is the best room to screen and identify true lodging tolerance.
Acknowledgements
Funding was provide by Indian Council of Agricultural Research (Incentivizing Agriculture).
Contributor Information
Rinki Khobra, Phone: 0184-2209231, Email: rinkikhobra@gmail.com.
Sindhu Sareen, Email: sareen9@hotmail.com.
Braj Kishor Meena, Email: pbkmeena@yahoo.com.
Arvind Kumar, Email: arvind.duhan27@gmail.com.
Vinod Tiwari, Email: pici.dwr@gmail.com.
G. P. Singh, Email: GP.Singh@icar.gov.in
References
- Acreche MM, Slafer GA. Lodging yield penalties as affected by breeding in Mediterranean wheats. Field Crops Res. 2011;122(1):40–48. doi: 10.1016/j.fcr.2011.02.004. [DOI] [Google Scholar]
- Alexandratos N, Bruinsma J (2012) World agriculture towards 2030/2050: the 2012 revision. ESA Working paper No. 12–03. Rome, FAO
- Arai-Sanoh Y, Ida M, Zhao R, Yoshinaga S, Takai T, Ishimaru T, Kato N. Genotypic variations in non-structural carbohydrate and cell wall components of the stem in rice, sorghum, and sugarcane. Biosci Biotechnol Biochem. 2011;75:1104–1112. doi: 10.1271/bbb.110009. [DOI] [PubMed] [Google Scholar]
- Ashraf M. Stress-induced changes in wheat grain composition and quality. Crit Rev Food Sci Nutr. 2014;54(12):1576–1583. doi: 10.1080/10408398.2011.644354. [DOI] [PubMed] [Google Scholar]
- Berry PM, Berry ST. Understanding the genetic control of lodging-associated plant characters in winter wheat (Triticum aestivum L.) Euphytica. 2015;205(3):671–689. doi: 10.1007/s10681-015-1387-2. [DOI] [Google Scholar]
- Berry PM, Spink J. Predicting yield losses caused by lodging in wheat. Field Crops Res. 2012;137:19–26. doi: 10.1016/j.fcr.2012.07.019. [DOI] [Google Scholar]
- Berry PM, Griffin JM, Sylvester-Bradley R, Scott RK, Spink JH, Baker CJ, Clare RW. Controlling plant form through husbandry to minimise lodging in wheat. Field Crops Res. 2000;67:59–81. doi: 10.1016/S0378-4290(00)00084-8. [DOI] [Google Scholar]
- Berry P, Spink J, Gay A, Craigon J. A comparison of root and stem lodging risks among winter wheat cultivars. J Agric Sci. 2003;141(2):191–202. doi: 10.1017/S002185960300354X. [DOI] [Google Scholar]
- Berry PM, Spink J, Sterling M, Pickett AA. Methods for rapidly measuring the lodging resistance of wheat cultivars. J Agron Crop Sci. 2003;189:390–401. doi: 10.1046/j.0931-2250.2003.00062.x. [DOI] [Google Scholar]
- Berry PM, Sterling M, Spink JH, Baker CJ, Sylvester-Bradley R, Mooney SJ, Tams AR, Ennos AR. Understanding and reducing lodging in cereals. Adv Agron. 2004;84:217–271. doi: 10.1016/S0065-2113(04)84005-7. [DOI] [Google Scholar]
- Berry PM, Sterling M, Mooney SJ. Development of a model of lodging for barley. J Agron Crop Sci. 2006;192:151–158. doi: 10.1111/j.1439-037X.2006.00194.x. [DOI] [Google Scholar]
- Berry PM, Sylvester- Bradley R, Berry S. Ideotype design for lodging-resistant wheat. Euphytica. 2007;154:165–179. doi: 10.1007/s10681-006-9284-3. [DOI] [Google Scholar]
- Berry PM, Kendall S, Rutterford Z, Orford S, Griffiths S. Historical analysis of the effects of breeding on the height of winter wheat (Triticum aestivum) and consequences for lodging. Euphytica. 2014;203(2):375–383. doi: 10.1007/s10681-014-1286-y. [DOI] [Google Scholar]
- Bridgemohan P, Bridgemohan RSH. Evaluation of anti-lodging plant growth regulators on the growth and development of rice (Oryza sativa) J Cereals Oilseed. 2014;5(3):12–16. [Google Scholar]
- Campbell CM. Influence of seed formation of corn on accumulation of vegetative dry matter and stalk strength. Crop Sci. 1963;4:31–34. doi: 10.2135/cropsci1964.0011183X000400010011x. [DOI] [Google Scholar]
- Crook MJ, Ennos AR. The mechanics of root lodging in winter wheat (Triticum aestivum L.) J Exp Bot. 1993;44:1219–1224. doi: 10.1093/jxb/44.7.1219. [DOI] [Google Scholar]
- Curtis T, Halford NG. Food security: the challenge of increasing wheat yield and the importance of not compromising food safety. Ann Appl Biol. 2014;164(3):354–372. doi: 10.1111/aab.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Agriculture, Cooperation & Farmers Welfare, Ministry of Agriculture & Farmers Welfare, Annual report (2017–2018), Government of India, Krishi Bhawan, New Delhi
- Dronamraju KR (2008) Emerging consequences of biotechnology: biodiversity loss and IPR issues. World Scientific, Singapore, Science – 460 pages. ISBN: 978-981-277-501-6
- Easson DL, White EM, Pickles SL. The effects of weather, seed rate and genotype on lodging and yield in winter wheat. J Agric Sci. 1993;121:145–156. doi: 10.1017/S0021859600077005. [DOI] [Google Scholar]
- Fan WX, Hou YX, Feng SW, Zhu FK, Ru ZG (2012) Study on cellulose and lodging resistance of wheat straw. J Henan Agric Sci 9
- Foulkes MJ, Slafer GA, Davies WJ, Berry PM, et al. Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. J Exp Bot. 2011;62:469–486. doi: 10.1093/jxb/erq300. [DOI] [PubMed] [Google Scholar]
- French P, Matsuyuki H, Ueno H. Paclobutrazol: control of lodging in Japanese paddy Rice. In: Grayson BT, Green MB, Copping LG, editors. Pest management in rice. Dordrecht: Springer; 1990. pp. 474–485. [Google Scholar]
- Gale MD, Marshall GA. The chromosomal location of Gai 1 and Rht 1, genes for Gibberellin insensitivity and semi-dwarfism, in a derivative of Norin 10 wheat. Heredity. 1973;37:283–289. doi: 10.1038/hdy.1976.88. [DOI] [Google Scholar]
- Ghaffar SH, Fan M. Revealing the morphology and chemical distribution of nodes in wheat straw. Biomass Bioenergy. 2015;77:123–134. doi: 10.1016/j.biombioe.2015.03.032. [DOI] [Google Scholar]
- Hai L, Guo H, Xiao S, Jiang G, Zhang X, Yan C, Xin Z, Jia J. Quantitative trait loci (QTL) of stem strength and related traits in a doubled-haploid population of wheat (Triticum aestivum L.) Euphytica. 2005;141:1–9. doi: 10.1007/s10681-005-4713-2. [DOI] [Google Scholar]
- Hamada A, Nitta M, Nasuda S, Kato K, Fujita M, Matsunaka H, Okumoto Y. Novel QTLs for growth angle of seminal roots in wheat (Triticum aestivum L.) Plant Soil. 2012;354(1–2):395–405. doi: 10.1007/s11104-011-1075-5. [DOI] [Google Scholar]
- Hasnath Karim MD, Jahan MA. study of lodging resistance and its associated traits in bread wheat. ARPN J Agric Biol Sci. 2013;8:10. [Google Scholar]
- Kashiwagi T, Sasaki H, Ishimaru K. Factors responsible for decreasing sturdiness of the lower part in lodging of rice (Oryza sativa L.) Plant Prod Sci. 2005;2:166–172. doi: 10.1626/pps.8.166. [DOI] [Google Scholar]
- Kelbert AJ, Spaner D, Briggs KG, King JR. The association of culm anatomy with lodging susceptibility in modern spring wheat genotypes. Euphytica. 2004;136:211–221. doi: 10.1023/B:EUPH.0000030668.62653.0d. [DOI] [Google Scholar]
- Keller M, Karutz C, Schmid JE, Stamp P, Winzeler M, Keller B, Messmer MM. Quantitative trait loci for lodging resistance in a segregating wheat × spelt population. Theor Appl Genet. 1999;98:1171–1182. doi: 10.1007/s001220051182. [DOI] [Google Scholar]
- Kokubo A, Kuraishi S, Sakurai N. Culm strength of barley: correlation among maximum bending stress, cell wall dimensions, and cellulose content. Plant Physiol. 1989;91:876–882. doi: 10.1104/pp.91.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong E, Liu D, Guo X, Yang W, Sun J, Li X, Zhan K, Cui D, Lin J, Zhang A. Anatomical and chemical characteristics associated with lodging resistance in wheat. Crop J. 2013;1:43–49. doi: 10.1016/j.cj.2013.07.012. [DOI] [Google Scholar]
- Kono M. Physiological aspects of lodging. In: Matsuo T, Kumazawa K, Ishii R, Ishihrar K, Hirata H, editors. Science of the rice plant: physiology. Tokyo: Food and Agriculture Policy Research Center; 1995. pp. 971–982. [Google Scholar]
- Kono M, Takahashi J. The effect of wind force with reference to lodging of paddy rice. J Soil Sci Plant Nutr. 1964;10(6):20–27. doi: 10.1080/00380768.1964.10431141. [DOI] [Google Scholar]
- Lang YZ, Yang XD, Wang ME, Zhu QS. Effects of lodging at different filling stages on rice yield and grain quality. Rice Sci. 2012;19:315–319. doi: 10.1016/S1672-6308(12)60056-0. [DOI] [Google Scholar]
- Li X, Yang Y, Yao J, Chen G, Li X, Zhang Q, Wu C. FLEXIBLE CULM 1 encoding a cinnamyl-alcohol dehydrogenase controls culm mechanical strength in rice. Plant Mol Biol. 2009;69:685–697. doi: 10.1007/s11103-008-9448-8. [DOI] [PubMed] [Google Scholar]
- Lindedam J, Andersen SB, DeMartini J, Bruun S, Jørgensen H, Felby C, et al. Cultivar variation and selection potential relevant to the production of cellulosic ethanol from wheat straw. Biomass Bioenergy. 2012;37:221–228. doi: 10.1016/j.biombioe.2011.12.009. [DOI] [Google Scholar]
- Longin CFH, Würschum T. Genetic variability, heritability and correlation among agronomic and disease resistance traits in a diversity panel and elite breeding material of spelt wheat. Plant Breeding. 2014;133(4):459–464. doi: 10.1111/pbr.12182. [DOI] [Google Scholar]
- Ma QH. Characterization of a cinnamoyl-CoA reductase that is associated with stem development in wheat. J Exp Bot. 2007;58:2011–2021. doi: 10.1093/jxb/erm064. [DOI] [PubMed] [Google Scholar]
- Ma QH. The expression of caffeic acid 3-O-methyltransferase in two wheat genotypes differing in lodging resistance. J Exp Bot. 2009;60:2763–2771. doi: 10.1093/jxb/erp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A, Cowan S, Edwards S, Griffiths I, Howarth C, Landon T, White E. Crops that feed the world 9. Oats-a cereal crop for human and livestock feed with industrial applications. Food Secu. 2013;5(1):13–33. doi: 10.1007/s12571-012-0232-x. [DOI] [Google Scholar]
- Marza F, Bai GH, Carver BF, Zhou WC. Quantitative trait loci for yield and related traits in the wheat population Ning7840 × Clark. Theor Appl Genet. 2006;112(4):688–698. doi: 10.1007/s00122-005-0172-3. [DOI] [PubMed] [Google Scholar]
- McCartney CA, Somers DJ, Humphreys DG, Lukow O, Ames N, Noll J, Cloutier S, McCallum BD. Mapping quantitative trait loci controlling agronomic traits in the spring wheat cross RL4452 × ‘AC domain’. Genome. 2005;48(5):870–883. doi: 10.1139/g05-055. [DOI] [PubMed] [Google Scholar]
- McIntyre L, Casu RE, Rattey A, Dreccer MF, Kam JW, van Herwaarden AF, Shorter R, Xue GP. Linked gene networks involved in nitrogen and carbon metabolism and levels of water soluble carbohydrate accumulation in wheat stems. Funct Integr Genomics. 2011;11(4):585–597. doi: 10.1007/s10142-011-0232-5. [DOI] [PubMed] [Google Scholar]
- Moura JCMS, Bonine CAV, Viana JOF, Dornelas MC, Mazzafera P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J Integr Plant Biol. 2010;52:360–376. doi: 10.1111/j.1744-7909.2010.00892.x. [DOI] [PubMed] [Google Scholar]
- Mulsanti IW, Yamamoto T, Ueda T, et al. Finding the superior allele of japonica-type for increasing stem lodging resistance in indica rice varieties using chromosome segment substitution lines. Rice. 2018;11:25. doi: 10.1186/s12284-018-0216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navabi A, Iqbal M, Strenzke K, Spaner D. The relationship between lodging and plant height in a diverse wheat population. Can J Plant Sci. 2006;86:723–726. doi: 10.4141/P05-144. [DOI] [Google Scholar]
- Okuno A, Hirano K, Asano K, Takase W, Masuda R, Morinaka Y, et al. New approach to increasing rice lodging resistance and biomass yield through the use of high gibberellin producing varieties. PLoS ONE. 2014;9(2):e86870. doi: 10.1371/journal.pone.0086870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packa D, Wiwart M, Suchowilska E, Bieńkowska T. Morpho-anatomical traits of two lowest internodes related to lodging resistance in selected genotypes of Triticum. Int Agrophys. 2015;29:475–483. doi: 10.1515/intag-2015-0053. [DOI] [Google Scholar]
- Peake AS, Huth NI, Carberry PS, Raine SR, Smith RJ. Quantifying potential yield and lodging-related yield gaps for irrigated spring wheat in sub-tropical Australia. Field Crops Res. 2014;158:1–14. doi: 10.1016/j.fcr.2013.12.001. [DOI] [Google Scholar]
- Peng D, Chen X, Yin Y, Lu K, Yang W, Tang Y, Wang Z. Lodging resistance of winter wheat (Triticum aestivum L.): lignin accumulation and its related enzymes activities due to the application of paclobutrazol or gibberellin acid. Field Crops Res. 2014;157:1–7. doi: 10.1016/j.fcr.2013.11.015. [DOI] [Google Scholar]
- Pham QD, Akira A, Hirano M, Sagawa S, Kuroda E. Analysis of lodging resistant characteristic of different rice genotypes grown under the standard and nitrogen–free basal dressing accompanied with sparse planting density practices. Plant Prod Sci. 2004;7:243–251. doi: 10.1626/pps.7.22. [DOI] [Google Scholar]
- Pinera-Chavez FJ, Berry PM, Foulkes MJ, Jesson MA, Reynolds MP. Avoiding lodging in irrigated spring wheat. I. Stem and root structural requirements. Field Crops Res. 2016;196:325–336. doi: 10.1016/j.fcr.2016.06.009. [DOI] [Google Scholar]
- Pinthus MJ. Spread of the root system as an indicator for evaluating lodging resistance of wheat. Crop Sci. 1967;7:107–110. doi: 10.2135/cropsci1967.0011183X000700020005x. [DOI] [Google Scholar]
- Pinthus ML. Lodging in wheat, barley, and oats: the phenomenon, its causes, and preventative measures. Adv Agron. 1973;25:210–256. [Google Scholar]
- Rademacher W. Growth retardants: effects on gibberellin biosynthesis and other metabolic pathways. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:501–531. doi: 10.1146/annurev.arplant.51.1.501. [DOI] [PubMed] [Google Scholar]
- Rajala A, Peltonen-Sainio P. Timing applications of growth regulators to alter spring cereal development at high latitudes. Agric Food Sci Finl Agric. 2002;11:233–244. doi: 10.23986/afsci.5721. [DOI] [Google Scholar]
- Rajkumara S. Lodging in cereals—a review. Agric Rev. 2008;29(1):55–60. [Google Scholar]
- Rebetzke GJ, Van Herwaarden AF, Jenkins C, Weiss M, Lewis D, Ruuska S, Tabe L, Fettell NA, Richards RA. Quantitative trait loci for water-soluble carbohydrates and associations with agronomic traits in wheat. Aust J Agric Res. 2008;59(10):891–905. doi: 10.1071/AR08067. [DOI] [Google Scholar]
- Shah AN, Tanveer M, Rehman AU, Anjum SA, Iqbal J, Ahmad R. Lodging stress in cereal—effects and management: an overview. Environ Sci Pollut Res. 2016;24(6):5222–5237. doi: 10.1007/s11356-016-8237-1. [DOI] [PubMed] [Google Scholar]
- Sharma I, Tyagi BS, Singh G, Venkatesh K, Gupta OP. Enhancing wheat production—a global perspective. Indian J Agric Sci. 2015;85(1):3–13. [Google Scholar]
- Spink JH, Whaley JM, Semere T, Wade AP, Sparkes DL, Foulkes MJ (2000b) Prediction of optimum plant population in winter wheat Project Report No 234 Home-Grown Cereals Authority, London
- Stachecki S, Praczyk T, Adamczewski K. Adjuvant effects on plant growth regulators in winter wheat. J Plant Prot Res. 2004;44(4):365–371. [Google Scholar]
- Stapper M, Fischer RA. Genotype, sowing date and plant spacing influence on high yielding irrigated wheat in Southern New South Wales II Growth, yield and nitrogen use. Aust J Agric Res. 1990;41:1021–1041. doi: 10.1071/AR9901021. [DOI] [Google Scholar]
- Sterling M, Baker CJ, Berry PM, Wade A. An experimental investigation of the lodging of wheat. Agric For Meteorol. 2003;119:149–165. doi: 10.1016/S0168-1923(03)00140-0. [DOI] [Google Scholar]
- Syahputra BSA (2012) Effect of paclobutrazol on lodging resistance, growth and yield of direct seeded rice. Dissertation, University Putra Malaysia
- Tams AR, Mooney SJ, Berry PM (2004) The effect of lodging in cereals on morphological properties of the root-soil complex. In: 3rd Australian New Zealand soils conference, 5–9 December 2004, University of Sydney, Australia
- Taylor NG, Scheible WR, Cutler S, Somerville CR, Turner SR. The irregular xylem3 locus of Arabidopsis encodes a cellulose synthase required for secondary cell wall synthesis. Plant Cell. 1999;11:769–780. doi: 10.1105/tpc.11.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H, Howarth CJ. Five ways to stay green. J Exp Bot. 2000;51:329–337. doi: 10.1093/jexbot/51.suppl_1.329. [DOI] [PubMed] [Google Scholar]
- Tripathi SC, Sayre KD, Kaul JN, Narang RS. Growth and morphology of spring wheat (Triticum aestivum L.) culms and their association with lodging: effects of genotypes, N levels and ethephon. Field Crops Res. 2003;84:271–290. doi: 10.1016/S0378-4290(03)00095-9. [DOI] [Google Scholar]
- USDA (2014) Wheat Outlook/WHS-14e/May 13, 2014 Economic Research Service. http://usda.mannlib.cornell.edu/usda/ers/WHS//2010s/2014/WHS-05-13-2014.pdf
- Vera CL, Irvine RB, Duguid SD, Rashid KY, Clarke FR, Slaski JJ. Short communication: comparative effect of lodging on seed yield of flax and wheat. Can J Plant Sci. 2014;94(1):119–126. doi: 10.4141/cjps2013-046. [DOI] [Google Scholar]
- Verma V, Worland AJ, Sayers EJ, Fish L, Caligari PDS, Snape JW. Identification and characterization of quantitative trait loci related to lodging resistance and associated traits in bread wheat. Plant Breeding. 2005;124(3):234–241. doi: 10.1111/j.1439-0523.2005.01070.x. [DOI] [Google Scholar]
- Wang J, Zhu J, Qinqin L, Li X, Nianjun T, Li Z, Li B, Zhang A. The effect of the anatomical structure and chemical components of the culm on lodging resistance in wheat. Sci Bull. 2006;51(5):1–7. [Google Scholar]
- Wang J, Zhu J, Huang RZ, Yang YS. Investigation of cell wall composition related to stem lodging resistance in wheat (Triticum aestivum L.) by FTIR spectroscopy. Plant Signal Behav. 2012;7(7):856–863. doi: 10.4161/psb.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Ma BL. Integrated nutrient management (INM) for sustaining crop productivity and reducing environmental impact: a review. Sci Total Environ. 2015;512:415–427. doi: 10.1016/j.scitotenv.2014.12.101. [DOI] [PubMed] [Google Scholar]
- Xiang DB, Zhao G, Wan Y, Tan ML, Song C, Song Y. Effect of planting density on lodging-related morphology, lodging rate, and yield of tartary buckwheat (Fagopyrum tataricum) Plant Prod Sci. 2016;19(4):479–488. doi: 10.1080/1343943X.2016.1188320. [DOI] [Google Scholar]
- Xiao Y, Liu J, Li H, Cao X, Xia X, He Z. Lodging resistance and yield potential of winter wheat: effect of planting density and genotype. Front Agric Sci Eng. 2015;2(2):168–178. doi: 10.15302/J-FASE-2015061. [DOI] [Google Scholar]
- Zhang FZ, Jin Z, Guo-hui MA, Shang W, Liu H, Mei-lan X, Liu Y. Relationship between lodging resistance and chemical contents in culms and sheaths of Japonica rice during grain filling. Rice Sci. 2010;17:311–318. doi: 10.1016/S1672-6308(09)60032-9. [DOI] [Google Scholar]
- Zhang WJ, Wu LM, Ding YF, Weng F, Wu XR, Li GH, Liu ZH, Tang S, Ding CQ, Wang SH. Top-dressing nitrogen fertilizer rate contributes to reduce culm physical strength through decreasing in structural carbohydrates contents in japonica rice. J Integr Agric. 2015;15(5):992–1004. doi: 10.1016/S2095-3119(15)61166-2. [DOI] [Google Scholar]
- Zhang W, Wu L, Ding Y, Yao X, Wu X, Weng F, Li G, Liu Z, Tang S, Ding G, Wang S. Nitrogen fertilizer application affects lodging resistance by altering secondary cell wall synthesis in japonica rice (Oryza sativa) J Plant Res. 2017;130(5):859–871. doi: 10.1007/s10265-017-0943-3. [DOI] [PubMed] [Google Scholar]
- Zheng M, Chen J, Shi Y, Li Y, Yin Y, Yang D, et al. Manipulation of lignin metabolism by plant densities and its relationship with lodging resistance in wheat. Sci Rep. 2017;7:41805. doi: 10.1038/srep41805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Shi GX, Li ZS, Kuang TY, Li B, Wei QK, Bai KZ, Hu YX, Lin JX. Anatomical and chemical features of high-yield wheat cultivar with reference to its parents. J Integr Plant Biol. 2004;46(5):565–572. [Google Scholar]
- Zuber U, Winzeler H, Messmer MM, Keller M, Keller B, Schmid JE, Stamp P. Morphological traits associated with lodging resistance of spring wheat (Triticum aestivum L.) J Agron Crop Sci. 1999;182:17–24. doi: 10.1046/j.1439-037x.1999.00251.x. [DOI] [Google Scholar]