Abstract
Being more sensitive to salt stress among the cereals, growth of rice (Oryza sativa L.) has been habitually affected by salinity. Although, several practices have evolved to sustain the growth of rice under salinity, the enormous role of calcium (Ca2+) as a signalling molecule in salt stress mitigation is still arcane. Considering this fact, an experiment was performed aiming to explicate the mechanism of salt-induced growth inhibition in rice and its alleviation by exogenous Ca2+. At germination stage, 10 mM and 15 mM CaCl2 primed rice (cv. Binadhan-10 & Binadhan-7) seeds were grown in petri dishes for 9 days under 100 mM NaCl stress. At seedling stage, 9-day-old rice seedlings grown on sand were exposed to 100 mM NaCl alone and combined with 10 mM and 15 mM CaCl2 for 15 days. This research revealed that salinity radically slowed down growth of rice seedlings and Ca2+ treatment noticeably improved growth performances. At germination stage, 10 mM CaCl2 treatment significantly increased the final germination percentage, germination rate index (in Binadhan-7), shoot, root length (89.20, 67.58% in Bindhan-10 & 84.72, 31.15% in Bindhan-7) and biomass production under salinity. Similarly, at seedling stage, 10 mM CaCl2 supplementation in salt-stressed plants enhanced shoot length (42.17, 28.76%) and shoot dry weight (339.52, 396.20%) significantly in Binadhan-10 & Binadhan-7, respectively, but enhanced root dry weight (36.76%) only in Binadhan-10. In addition, 10 mM CaCl2 supplementation on salt-stressed seedlings increased the chlorophyll and proline content, and oppressed the accretion of reactive oxygen species thus protecting from oxidative damage more pronouncedly in Binadhan-10 than Binadhan-7 as reflected by the elevated levels of catalase and ascorbate peroxidase activity. The 15 mM CaCl2 somehow also enhanced some growth parameters but overall was less effective than 10 mM CaCl2 to alleviate salt stress, and sometimes showed negative effect. Therefore, supplementary application of calcium-rich fertilizers in saline prone soils can be an effective approach to acclimatize salt stress and cultivate rice successfully.
Keywords: NaCl stress, Sandponic culture, Calcium, Photosynthetic pigments, Antioxidant enzymatic activity
Introduction
Environmental adversity exerts substantial amount of stresses on plants’ existence (Mahmood-ur-Rahman et al. 2019), among which salinity is one of the dominant abiotic stresses that abruptly cease off the plant growth as well as developmental processes (Mbarki et al. 2018b; Safdar et al. 2019), resulting a huge annual yield loss all over the earth (Munns and Tester 2008). Next to drought, salinity is the prevalent soil problem in rice (Oryza sativa L.) and is contemplated as a powerful constraint in the way to amplify rice production worldwide (Ghosh et al. 2016). Currently, salinity affects nearly 6% of the entire world’s land (Munns and Tester 2008) and has become an escalating problem in shoreline areas. Globally, no less than 10 million hectares of agricultural land are abandoned annually because of salinity. Salt-induced land degradation is a very important issue affecting the status of food productivity worldwide (Geist 2017; FAO 2016).
Salinity impairs the plant growth more than any other noxious materials in the earth (Xiong and Zhu 2002) and modulates several physiological and biochemical processes in plants (Goyal et al. 2018). Surplus salts in growth media affects plant growth in two ways. Firstly, high concentration of salt in the soil solution results in stunted root growth and causes the root to uptake water (osmotic stress) that also impedes ion flux to the shoot and leaves, and stimulates stomatal closure (Yang and Guo 2018; Reddy et al. 2017). Secondly, high concentrations of salts, particularly Na+ in the transpiration stream of plants can be injurious to cells (salt-specific effect), resulting in an inhibition of several physiological and biochemical routes such as nutrient uptake (like potassium and calcium) and CO2 assimilation (Tang et al. 2015; Parihar et al. 2015; Safdar et al. 2019). Salinity negatively regulates stomatal conductance, plant water relations and degrades photosynthetic pigments which triggers lower transpiration rate, photosynthesis, growth and biomass production (Tang et al. 2015; Morton et al. 2019). In chloroplasts, inadequacy of CO2 assimilation due to excessive Na+ accumulation in plants resulting in hyper-reduction of electron transport complex (ETC) is the key reason of reactive oxygen species (ROS) generation. Hyper-reduction of mitochondrial ETC is also a vital means of ROS production under stress condition (Miller et al. 2010; Saini et al. 2018). The super-oxide radical, hydroxyl radical, singlet oxygen and hydrogen peroxide are the major ROS which causes oxidative stress to plants (Waszczak et al. 2018). The ROS can impair the function and structure of DNA, RNA and protein, thereby intensifying oxidative damage (Hossain et al. 2012; Kordrostami and Rabiei 2019). Moreover, higher Na+ concentration, impairs the function of the Calcium-Dependent Protein Kinase, OsCPK12 that leads to early senescence in plants (Asano et al. 2012; Campo et al. 2014). Therefore, exogenous calcium supplementation might help to overexpress the OsCPK12 gene and confer salt stress tolerance.
Among all cereals, rice is more vulnerable to salt stress and shows variability in sensitivity to salinity at different developmental phases throughout its lifespan. However, germination phase is considered fairly tolerant but the early seedling stage is the most salinity sensitive growth stage that directly affects the yield (Zeng et al. 2001; Mickky et al. 2019). Besides, salt stress can cause nutritional discrepancy, especially K+ insufficiency by impairing its uptake and initiating its leakage from the cell (Munns and Tester 2008; Parihar et al. 2015). Recently, several research works have revealed the salt stress response and tolerance mechanisms in crop plants (Gao et al. 2007; Mbarki et al. 2018b; Safdar et al. 2019) that includes a diversity of defense mechanisms such as osmotic, ionic and ROS homeostasis (Gupta and Huang 2014; Tang et al. 2015). The accumulation of high concentration of salt triggers the amplification of the cytosolic osmotic force. Under these circumstances, plants maintain cellular homeostasis by osmotic regulation processes, mostly by synthesizing osmolytes and depositing toxic ions in the cell-vacuoles (Reddy et al. 2017). In many plant species, production of different compatible osmoprotectants such as sucrose (Sami et al. 2016), glycine betaine (Kurepin et al. 2017), mannitol (Patel and Williamson 2016), trehalose (Kosar et al. 2018) and proline (Slama et al. 2015) helps to continue water relation, stabilize enzyme, protein complex and membrane under saline condition (Iqbal et al. 2015). Specifically, higher deposition of proline is considered to be interlinked with tolerance to osmotic stresses (Per et al. 2017). Plant defense against increased ROS under stressful condition is coupled with the maintenance of cellular redox equilibrium, which is mostly conferred by some non-enzymatic and enzymatic antioxidants such as ascorbate peroxidase (APX), catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), glutathione peroxidase (GPX), glutathione-S-transferase (GST), glutathione reductase (GR), monodehydroascorbate reductase (MDHAR) and dehydroascorbate reductase (DHAR) (Yan et al. 2013; Tang et al. 2015). The synchronized function of these enzymes strengthen a steadiness between the formation of and detoxification of ROS (Hanin et al. 2016).
Efforts are ongoing to improve plants ability to survive under diverse environmental stresses. In this prospect, the probable role of small biological molecules such as phyto-hormones and signalling molecules could be considered influential in amending plants adaptability against adverse environment conditions. Calcium (Ca2+) is an essential plant element and acts as signalling molecules associated with adaptive responses against environmental stresses (Mahmood-ur-Rahman et al. 2019). Scientist have shown that exogenous Ca2+ supplements to irrigation water has caused mitigation of salt stress (Sohan et al. 1999; Jaleel et al. 2008; Rahman et al. 2016). The affirmative effect of Ca2+ in stress alleviation has been associated with some defence mechanisms. Ca2+ is the crucial element that helps in keeping the structural and functional integrity of membranes with stabilization of cell wall as well as regulation of ion homeostasis (Arshi et al. 2010; Morgan et al. 2014) consequently, playing a significant role in plant growth and development. Besides, an exogenous addition of Ca2+ reduces the effects of NaCl toxicity by facilitating a high K+/Na+ selectivity. It also intensifies the antioxidant enzymatic system and ROS detoxification system (Rahman et al. 2016).
In Bangladesh context, research works are hardly found on the ameliorative roles of exogenous Ca2+ on salt-stressed rice. Therefore, in the current study, we aimed to examine the protective roles of exogenous Ca2+ in alleviating the harmful consequences of salinity on the salt-tolerant and susceptible rice cultivars both at germination and seedling stage. For this purpose, we have examined the germination indices, morpho-physiological and biochemical parameters to assess salt tolerance in rice under Ca2+ treatments with specific highlight on compatible solute production and ROS detoxification system. As far as we know, very few experiments were performed on Ca-mediated salinity stress tolerance in two contrasting rice cultivars both at germination and seedling stage.
Materials and methods
Germination stage experiment
Two rice cultivars viz. Binadhan-10 (high yielding, salt-tolerant) (Kibria et al. 2017) and Binadhan-7 (high yielding, salt-susceptible) (Tahjib-Ul-Arif et al. 2018a) were collected from Bangladesh Institute of Nuclear Agriculture (BINA), Mymensingh. The rice seeds were surface sterilized with 2.5% sodium hypochlorite for 15 min and washed thoroughly with distilled water several times. These seeds were soaked in 10 mM and 15 mM CaCl2 solution for 24 h. Followed by washing excess CaCl2, the 100 rice seeds were placed on each petri dishes with or without 100 mM NaCl. Therefore, the treatment combinations were: control (C), only tap water; 100 mM NaCl (S); 10 and 15 mM CaCl2 with control (C + Ca10 and C + Ca15); 10 and 15 mM CaCl2 with 100 mM NaCl (S + Ca10 and S + Ca15). All treatments were replicated for three times. The seeds were grown for 9 days under 100 mM NaCl stress condition and different morphological assessments were performed at germination stage.
Seedling stage experiment
For seedling stage experiment, twenty germinated rice seeds were sown in a 4.0 L plastic tray containing sterilized sands. The seedlings were grown for 3 days in tap water, following which 0.5 × Hoagland Nutrient solution (Hoagland and Arnon 1950) was added. The pH of the nutrient solution was maintained at 6.5. One group of 6-day old seedlings were treated with 10 and 15 mM CaCl2 for 72 h while another group was kept untreated. The both pretreated and non-pretreated seedlings were exposed to 100 mM NaCl stress with a nutrient solution in two steps by increasing the dosage by 50 mM NaCl per day to avoid osmotic shock. The treatment combinations were similar as that of germination stage experiments and also all the treatments replicated for three times. Seedlings were grown under 100 mM NaCl stress for 15 days and meanwhile, the nutrient solutions were renewed at the 4-day interval. Visual toxic symptoms of salt injury were evaluated by using modified standard evaluation score (SES) of IRRI (International Rice Research Institute (IRRI) 2002) at 10th day after salinization. Different morphological parameters were assessed at 15th day after salinization and the third leaf of each rice seedlings was taken for determining several physiological and biochemical parameters.
Assessment of germination indices
The number of germinated seeds were counted every day and final germination percentage (FGP) and germination rate index (GRI) on the 7th day of germination stage experiment were calculated by the following formulae (Afrin et al. 2019).
Here, n = 1, 2, 3, 4.
Assessment of growth parameters
At 9th day, different growth parameters were recorded at germination stage. The growth parameters were assessed by measuring shoot and root length, fresh weight (FW) and dry weight (DW). Shoot length was measured from shootlet base to the leaf tip and root length was measured from rootlet base to the root tip. Five seedlings from each treatment were weighed for FW determination and DW were determined by drying the sample in an oven at 70 °C for 4 days till it attained a constant weight. Also, the growth parameters (shoot and root length and fresh weight) at the seedling stage after 15 days salt exposure were recorded similarly as germination stage.
Measurement of relative water content (RWC)
RWC of rice seedlings was measured as described previously by Tahjib-Ul-Arif et al. 2018b using following the formula:
Proline content determination
Proline content was determined according to methods of Bates et al. 1973. 50 mg of fresh leaf sample was homogenized using 10 mL of 3.0% sulfosalicylic acid. After centrifuging at 11,500×g, the supernatant (2.0 mL) was mixed with 2 mL acid ninhydrin and 2 mL glacial acetic acid in a test tube. The test tubes were incubated for 1 h at 100 °C in a hot water bath and then transferred to an ice bath to terminate the reaction. 4.0 mL of toluene was added to each of the test tubes and then stirred vigorously for 15–20 s. The absorbance of the collected toluene was measured at 520 nm in a VIS/UV spectrophotometer (Shimadzu, UV-1201) against reagent blank. A standard curve was prepared with analytical grade proline for calculating proline content expressed as mg 100 g−1 fresh weight of leaves.
Determination of photosynthesis pigments
Chlorophyll (Chl) extraction was done by taking 0.05 g fresh leaf with 10 mL of 80% acetone. The absorbance of the supernatant was recorded at 645 nm and 663 nm wavelengths to determine the chlorophyll-a and chlorophyll-b according to the method developed by Roy et al. 2016 and results were expressed as mg g−1 FW. The formula for computing chlorophyll-a and chlorophyll-b were as follows:
where A663 = Absorbance at 663 nm wavelength and A645 = Absorbance at 645 nm wavelength.
Extraction and determination of antioxidant enzymatic activity
The 50 mg of the fresh leaf was used for extraction with 3 mL of 50 mM potassium phosphate buffer (pH 8.0). Catalase (CAT, EC: 1.11.1.6) activity was determined by following the method of Aebi 1984. The activity of catalase was calculated from the decrease in absorbance at 240 nm min−1 when the extinction coefficient of H2O2 was 40 M−1 cm−1. Peroxidase (POD, EC: 1.11.1.7) activity was determined by following the method of Nakano and Asada 1981. The activity of peroxidase was calculated from the increase in absorbance at 470 nm min−1 when the extinction coefficient was 26.6 mM−1 cm−1. Ascorbate peroxidase (APX, EC: 1.11.1.11) activity was determined by following the method of Nakano and Asada 1981. The activity of ascorbate peroxidase was calculated from the decrease in absorbance at 290 nm min−1 when the extinction coefficient was 2.8 mM−1 cm−1. The concentration of these antioxidant enzymes was expressed as mM min−1 g−1 FW.
Statistical analysis
Two-way analysis of variance (ANOVA) was performed following the General Linear Model (GLM) procedure of Minitab 17.0 software where the two factor were treatment and cultivars. Fisher’s least significant difference (LSD) method was employed to perform statistical comparisons among treatments considering 5% level of probability (P < 0.5).
Results
Exogenous Ca2+ enhances germination indices
Salt stress negatively affected the FGP of two tested rice cultivars. FGP of salt-stressed plants was reduced by 5.3% in Binadhan-10 while 6.6% in Binadhan-7 compared with that of control plants. Exogenous application of Ca2+ (10 and 15 mM CaCl2) significantly increased the FGP of salt-susceptible Binadhan-7 whereas non-significant change was observed in salt-tolerant Binadhan-10 over only salt-stressed plants. Moreover, GRI of salt-tolerant Binadhan-10 and salt-susceptible Binadhan-7 showed a significant reduction under salt stress condition in comparison with control condition. Exogenous application of 10 mM CaCl2 restored the GRI in both salt-stressed and non-stressed plants of both Binadhan-10 and Binadhan-7 (Table 1).
Table 1.
Effects of exogenous calcium on final germination percentage (FGP) and germination rate index (GRI) of two rice cultivars under 100 mM NaCl stress at germination stage
| Cultivars | Treatments | FGP (%) | GRI (day−1) |
|---|---|---|---|
| Binadhan-10 | Control | 100 ± 0.00a | 24.38 ± 0.256a |
| 10 mM CaCl2 | 100 ± 0.00a | 24.09 ± 0.344a | |
| 15 mM CaCl2 | 100 ± 0.00a | 23.92 ± 0.295a–c | |
| 100 mM NaCl (S) | 94.66 ± 1.33b | 22.87 ± 0.307cd | |
| S + 10 mM CaCl2 | 94.66 ± 1.33b | 23.01 ± 0.301b–d | |
| S + 15 mM CaCl2 | 92.00 ± 2.00b | 22.49 ± 1.124d | |
| Binadhan-7 | Control | 100 ± 0.00a | 24.12 ± 0.255ab |
| 10 mM CaCl2 | 100 ± 0.00a | 24.31 ± 0.155a | |
| 15 mM CaCl2 | 100 ± 0.00a | 24.06 ± 0.284ab | |
| 100 mM NaCl (S) | 93.33 ± 1.33b | 21.86 ± 0.577d | |
| S + 10 mM CaCl2 | 100 ± 0.00a | 24.02 ± 0.275a–c | |
| S + 15 mM CaCl2 | 100 ± 0.00a | 23.72 ± 0.124a–c | |
| Sig. level | * | * | |
Each value presents the means ± standard errors (n = 3) obtained from three independent replicates. Different letters in each column indicates statistically significant difference following Fisher’s least significant difference method (P < 0.05, *)
Exogenous Ca2+ synchronizes plantlet growth and biomass production at germination stage
Salt stress reduced the plantlet growth and biomass accumulation of both salt-tolerant Binadhan-10 and salt-susceptible Binadhan-7 rice cultivars. Shoot and root length, FW and DW of salt-stressed Binadhan-10 reduced by 57, 30, 47.4, 25.9, 48.6 and 27.3%, respectively, whilst by 61, 35.5, 46.1, 53.6, 62.5 and 37.5%, respectively, in Binadhan-7 over control plants. The reduction of these growth parameters due to salt stress was recovered after applying 10 mM exogenous CaCl2 by 89.20, 67.58, 65.09, 38.25, 26.98 and 25.35% respectively in Binadhan-10 where Binadhan-7 showed 84.72, 31.15, 33.73, 36.56, 66.67 and 80.45% accretion, respectively compared to salt-stressed without Ca2+ treated plants. On the other hand, application of 15 mM CaCl2 also enhanced shoot and root length, FW and DW more pronouncedly in salt-stressed Binadhan-10 than Binadhan-7, compared with only stressed plants, but it was less effective than 10 mM CaCl2. Under the non-stressed condition, Ca2+ application (both 10 mM and 15 mM CaCl2) increased the shootlet and rootlet growth and biomass production compared with that of control condition (Table 2).
Table 2.
Effects of exogenous calcium on shoot and root length, fresh weight (FW) and dry weight (DW), and relative water content (RWC) of two rice cultivars under 100 mM NaCl stress at germination stage
| Cultivars | Treatments | Shoot length (cm) | Root length (cm) | Shootlet FW (mg plant−1) | Shootlet DW (mg plant−1) | Rootlet FW (mg plant−1) | Rootlet DW (mg plant−1) | RWC (%) |
|---|---|---|---|---|---|---|---|---|
| Binadhan-10 | Control | 4.11 ± 0.02ab | 4.03 ± 0.19cd | 21.06 ± 0.13bc | 2.46 ± 0.35bc | 17.2 ± 1.14a–d | 2.93 ± 0.17a | 90.55 ± 1.74c |
| 10 mM CaCl2 | 4.36 ± 0.17a | 5.16 ± 0.52b | 26.33 ± 2.76a | 2.46 ± 0.73bc | 24.00 ± 4.98a | 2.93 ± 0.33a | 96.21 ± 0.83a | |
| 15 mM CaCl2 | 3.80 ± 0.07b | 6.32 ± 0.22a | 22.46 ± 0.71b | 2.13 ± 0.30bc | 17.53 ± 0.88a–d | 2.6 ± 0.11ab | 93.79 ± 0.93a–c | |
| 100 mM NaCl (S) | 1.76 ± 0.16gh | 2.82 ± 0.33e | 11.06 ± 0.86f–g | 1.26 ± 0.11e | 12.73 ± 1.33cd | 2.13 ± 0.13ab | 90.44 ± 1.86c–e | |
| S + 10 mM CaCl2 | 3.33 ± 0.21cd | 4.72 ± 0.73bc | 18.26 ± 0.43cd | 1.6 ± 0.41de | 17.6 ± 1.96a–d | 2.67 ± 0.24ab | 91.91 ± 0.81b–d | |
| S + 15 mM CaCl2 | 2.81 ± 0.18e | 4.98 ± 0.32b | 15.00 ± 0.91de | 1.26 ± 0.53e | 18.6 ± 1.25a–c | 2.53 ± 0.06ab | 95.50 ± 1.05a | |
| Binadhan-7 | Control | 3.70 ± 0.06bc | 4.04 ± 0.08 cd | 14.00 ± 1.27ef | 3.20 ± 0.11a | 24.00 ± 3.49a | 2.13 ± 0.26ab | 92.59 ± 0.22b–d |
| 10 mM CaCl2 | 4.10 ± 0.08ab | 4.85 ± 0.15bc | 12.07 ± .60e–g | 3.53 ± 0.06a | 19.20 ± 1.03a–c | 2.53 ± 0.17ab | 94.30 ± 0.44ab | |
| 15 mM CaCl2 | 2.98 ± 0.25de | 3.79 ± 0.15d | 9.53 ± 1.83gh | 2.60 ± 0.11b | 21.07 ± 0.76ab | 2.40 ± 0.06ab | 94.06 ± 1.01a–c | |
| 100 mM NaCl (S) | 1.44 ± 0.05h | 2.60 ± 0.03e | 7.53 ± 0.78 h | 1.20 ± 0.11e | 11.13 ± 0.06d | 1.33 ± 0.26c | 89.25 ± 2.01e | |
| S + 10 mM CaCl2 | 2.66 ± 0.02ef | 3.41 ± 0.05de | 10.07 ± 0.40gh | 2.00 ± 0.11cd | 15.20 ± 0.75b–d | 2.27 ± 0.11ab | 91.52 ± 1.43c–e | |
| S + 15 mM CaCl2 | 2.23 ± 0.03fg | 2.74 ± 0.04e | 7.60 ± 0.60h | 1.40 ± 0.11e | 14.40 ± 0.46b–d | 2.00 ± 0.20ab | 89.14 ± 0.54e | |
| Sig. level | * | * | * | * | * | * | * | |
Each value presents the means ± standard errors (n = 3) obtained from three independent replicates. Different letters in each column indicates statistically significant difference following Fisher’s least significant difference method (P < 0.05, *)
Exogenous Ca2+ amends the RWC
RWC of leaves showed an inverse trend with salinity. Under salt stress, salt-tolerant Binadhan-10 retained RWC but significant reduction of RWC (3.6%) was noticed in salt-susceptible Binadhan-7 in comparison with the control plants. A non-significant increase of RWC was observed both in Binadhan-10 (1.63%) and Binadhan-7 (2.54%) when the salt-stressed plants were treated with 10 mM CaCl2 and application of 15 mM CaCl2 in salt-stressed plants increased RWC significantly only in Binadhan-10, compared with only salt-stressed plants. On the other hand, under the non-stress condition, application of 10 mM CaCl2 only increased RWC in Binadhan-10 compared with the control condition. Overall, 10 mM CaCl2 performed better than the 15 mM CaCl2 (Table 2).
Exogenous Ca2+ improves the phenotypic response of rice seedlings
Several salt injuries including shoot growth reduction, root growth reduction, stunted growth and seedling drying were observed in the salinized conditions. In salt-stressed condition, Binadhan-10 showed moderate tolerance (SES was 5.0) while Binadhan-7 showed susceptible (SES was 7.0) characteristics based on SES score. But when the salt-stressed plants were treated with 10 mM CaCl2, Binadhan-10 showed tolerance (SES was 3.0) and Binadhan-7 showed moderate tolerance (SES was 5.0) characters. However, application of 15 mM CaCl2 in salt-stressed Binadhan-10 (SES was 5.0) and Binadhan-7 (SES was 7.0) did not enhance salt tolerance level compared with control (Table 3).
Table 3.
Effects of exogenous calcium on shoot length, root length, shoot dry weight, root dry weight and SES score of two rice cultivars under 100 mM NaCl stress at seedling stage
| Cultivars | Treatments | Shoot length (cm) | Root length (cm) | Shoot dry weight (mg plant−1) | Root dry weight (mg plant−1) | SES score |
|---|---|---|---|---|---|---|
| Binadhan-10 | Control | 20.4 ± 0.373b | 8.05 ± 0.167a | 188.00 ± 0.002b | 37.87 ± 0.001c | 1.0 |
| 10 mM CaCl2 | 21.89 ± 0.430a | 8.00 ± 0.352a | 198.00 ± 0.007a | 63.47 ± 0.003a | 1.0 | |
| 15 mM CaCl2 | 16.99 ± 0.137d | 7.30 ± 0.593a–c | 167.80 ± 0.002c | 38.40 ± 0.001c | 1.0 | |
| 100 mM NaCl (S) | 11.69 ± 0.784h | 6.30 ± 0.562c | 31.30 ± 0.001 h | 15.77 ± 0.0003f | 5.0 | |
| S + 10 mM CaCl2 | 16.62 ± 0.456de | 6.97 ± 0.218bc | 137.57 ± 0.002e | 25.83 ± 0.0006e | 3.0 | |
| S + 15 mM CaCl2 | 14.46 ± 0.289f | 6.95 ± 0.338c | 118.67 ± 0.002f | 17.50 ± 0.0004f | 5.0 | |
| Binadhan-7 | Control | 18.58 ± 0.435c | 8.03 ± 0.419a | 156.50 ± 0.003d | 37.50 ± 00c | 1.0 |
| 10 mM CaCl2 | 18.33 ± 0.214c | 8.02 ± 0.628a | 168.30 ± 0.002c | 48.77 ± 00b | 1.0 | |
| 15 mM CaCl2 | 15.81 ± 0.479e | 6.33 ± 0.251c | 152.33 ± 0.002d | 32.13 ± 00d | 1.0 | |
| 100 mM NaCl (S) | 10.08 ± 0.686i | 7.98 ± 0.577ab | 26.50 ± 0.0007h | 10.77 ± 0.09g | 7.0 | |
| S + 10 mM CaCl2 | 12.98 ± 0.275g | 7.99 ± 0.333a | 131.50 ± 0.0002e | 14.73 ± 00fg | 5.0 | |
| S + 15 mM CaCl2 | 11.09 ± 0.145hi | 6.76 ± 0.187c | 109.67 ± 0.001g | 10.97 ± 00g | 7.0 | |
| Sig. level | * | * | * | * | ||
Each value presents the means ± standard errors (n = 3) obtained from three independent replicates. Different letters in each column indicates statistically significant difference following Fisher’s least significant difference method (P < 0.05, *)
Retrieval of salinity induced growth retardation of rice seedlings by exogenous Ca2+
To appraise the effects of salinity on rice seedling growth, we determined the shoot and root length and DW. Shoot and root length, shoot and root DW of salt-stressed Binadhan-10 were significantly reduced by 42.69, 21.74, 83.35 and 58.36%, respectively, whereas Binadhan-7 showed a reduction by 45.75, 0.62, 83.07 and 71.28%, respectively, in relative to control plants. When salt-stressed plants were subjected to 10 mM CaCl2, shoot and root length, shoot and root DW of Binadhan-10 were increased by 42.17, 10.63, 339.52 and 63.79%, respectively, while Binadhan-7 showed accretion at 28.76, 0.13, 396.23 and 10.97%, respectively compared to only salt-stressed plants. On the other hand, application of 15 mM CaCl2 on salt-stressed plants did not recover growth and biomass retardation caused by salinity. Under the non-stressed condition, application of 10 mM CaCl2 did not affect plant growth and biomass production but 15 mM CaCl2 treatment negatively affected plant growth parameters. 10 mM CaCl2 performed better over 15 mM CaCl2 in both salt-tolerant Binadhan-10 and salt-susceptible Binadhan-7 (Table 3).
Effects of exogenous Ca2+ on photosynthetic pigments
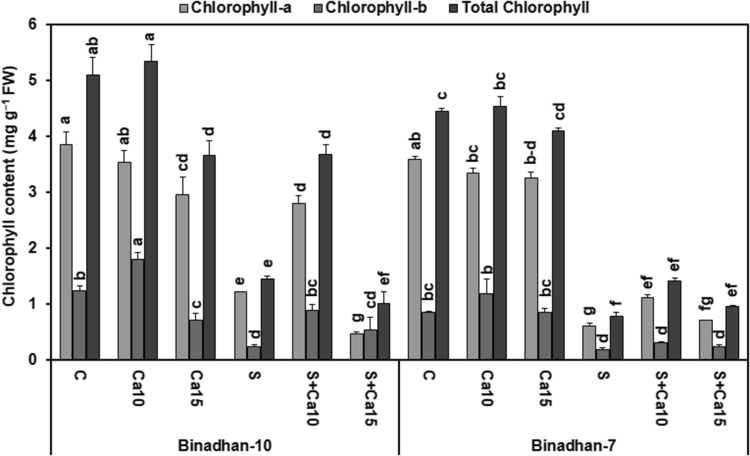
Salinity impaired the photosynthetic pigments of rice seedlings. Salt stress resulted in significant decrease of chl-a, chl-b and total chls by 68.62, 81.03 and 71.62%, respectively in Binadhan-10 and 83.4, 78.71 and 82.5%, respectively in Binadhan-7 compared with control seedlings. Exogenous Ca2+ (10 mM CaCl2) application to the salt-stressed plants improved chl-a, chl-b and total chls content by 56.6, 73.5 and 60.0%, respectively in Binadhan-10 and 46.58, 39.0 and 45.02%, respectively in Binadhan-7, compared with only salt-stressed seedlings. In contrast, 15 mM CaCl2 treatment on salt-stressed Binadhan-10 and Binadhan-7 seedlings did not improve chls content compared with only salt-stressed seedlings. Application of 10 mM CaCl2 on non-stressed rice seedlings did show any significant effects on chls content whereas 15 mM CaCl2 significantly decreased chls content compared with control seedlings. The increment of chls content due to 10 mM CaCl2 treatment was more prominent in salt-tolerant Binadhan-10 than salt-susceptible Binadhan-7 (Fig. 1).
Fig. 1.
Effects of exogenous calcium on chlorophyll content of two contrasting rice cultivars grown under 100 mM NaCl stress for 15 days at seedling stage. The vertical bar indicates means of three replicates (n = 3) and the error bar indicates standard errors. Statistically significant differences among the treatments and cultivars are indicated by different alphabetical letters at P < 0.05 according to Fisher’s least significant difference test. FW, fresh weight; C, control (nutrient solution only); Ca10, Nutrient solution + 10 mM CaCl2; Ca15, Nutrient solution + 15 mM CaCl2; S, salt (100 mM NaCl); S + Ca10, 100 mM NaCl + 10 mM CaCl2 and S + Ca15, 100 mM NaCl + 15 mM CaCl2
Effects of Ca2+ on proline content in rice seedlings
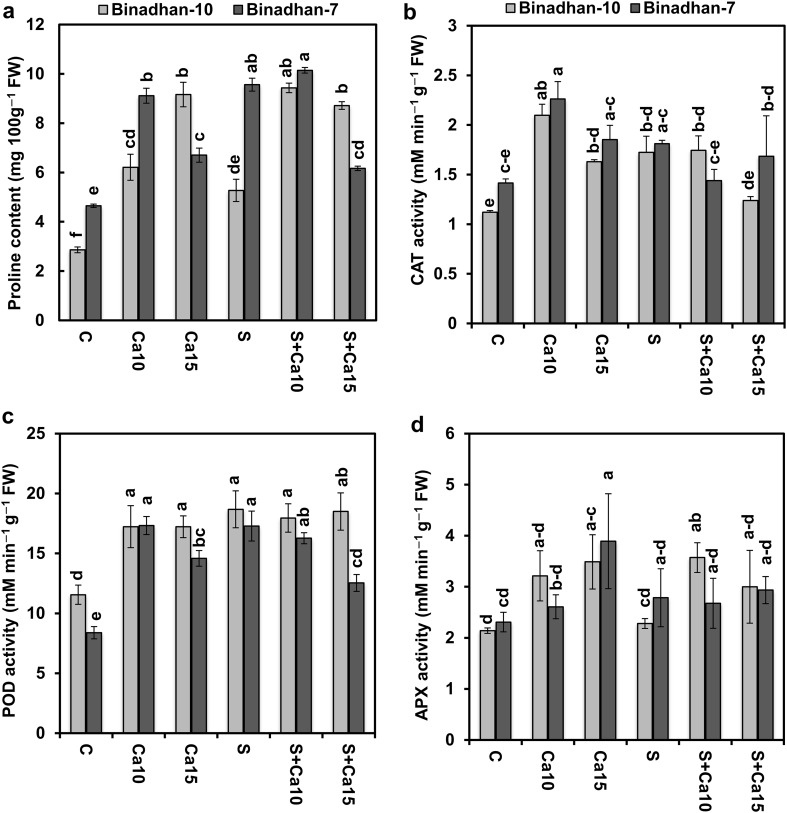
Salt stress significantly increased the proline content by 84.3 and 105.7%, respectively both in salt-tolerant Binadhan-10 and salt-susceptible Binadhan-7 cultivars compared with control seedlings. Application of Ca2+ accelerated the proline accumulation in both stressed and non-stressed seedlings. The proline content was increased in salt-stressed Binadhan-10 (78.83%) and Binadhan-7 (6.07%) when treated by 10 mM CaCl2 in comparison with only salt-stressed seedlings. On the other hand, 15 mM CaCl2 treatment on salt-stressed seedlings of Binadhan-10 showed an increase in proline content by 65.27% whereas Binadhan-7 showed a decrease by 35.52% over only salt-stressed seedlings (Fig. 2a).
Fig. 2.
Effect of exogenous calcium on a proline content, b catalase (CAT) activity, c peroxidase (POD) activity and d ascorbate peroxidase (APX) activity of two contrasting rice cultivars grown under 100 mM NaCl stress for 15 days. The vertical bar indicates means of three replicates (n = 3) and the error bar indicates standard errors. Statistically significant differences among the treatments and cultivars are indicated by different alphabetical letters at P < 0.05 according to Fisher’s least significant difference test. FW, fresh weight; C, control (nutrient solution only); Ca10, Nutrient solution + 10 mM CaCl2; Ca15, Nutrient solution + 15 mM CaCl2; S, salt (100 mM NaCl); S + Ca10, 100 mM NaCl + 10 mM CaCl2 and S + Ca15, 100 mM NaCl + 15 mM CaCl2
Exogenous Ca2+ ameliorates the antioxidant enzymatic activities
CAT activity in Binadhan-10 and Binadhan-7 increased by 53.9 and 27.8%, respectively compared with control seedlings. The 10 mM CaCl2 treatment on salt-stressed seedlings did not affect CAT activity in Binadhan-10 but decreased it in Binadhan-7 in relative to only salt-stressed seedlings. Compared with the control condition, application of either 10 mM or 15 mM CaCl2 on non-stressed seedlings enhanced CAT activity both in Binadhan-10 and Binadhan-7, but the increment was more prominent in 10 mM CaCl2 treatment than 15 mM CaCl2 (Fig. 2b).
The seedlings of Binadhan-10 and Binadhan-7 treated with NaCl increased POD activity by 61.6 and 106%, respectively compared with control seedlings. Moreover, 10 mM and 15 mM CaCl2 application in salt-stressed seedlings did not affect the POD activity in Binadhan-10 but decreased it in Binadhan-7 by 5.5% compared with salt-stressed control seedlings. Under the non-stressed condition, 10 mM and 15 mM CaCl2 also enhanced POD activity both in salt-tolerant Binadhan-10 and salt-susceptible Binadhan-7 but the increment was intensified in salt-tolerant one (Fig. 2c).
Rice seedlings of Binadhan-10 and Binadhan-7 exposed to salt stress had no significant effect on APX activity compared with control seedlings. 10 mM and 15 mM CaCl2 treatments on salt-stressed seedlings increased APX activity significantly in Binadhan-10 but did not show an effect in Binadhan-7. Application of 10 mM and 15 mM CaCl2 also in non-stressed seedlings enhanced APX activity significantly in Binadhan-10, but no change was found in Binadhan-7 compared to control seedlings (Fig. 2d).
Discussion
Salinity is one of the most severe environmental stresses that causes a decline in growth rate, along with a suite of metabolic alterations in plants. Plants tolerance to salinity is a genetic trait that varies among species, as mirrored in their growth response under certain conditions (Goyal et al. 2018; Safdar et al. 2019). So a trivial accumulation of salt in the cell may badly affect the growth and development by ruining the usual physiological and biochemical processes (Parihar et al. 2015; Goyal et al. 2018; Yang and Guo 2018). In this context, plants have developed some rapid mechanisms to overcome salinity induced damage and acclimate themselves under saline condition (Tang et al. 2015). In addition to their escaping mechanisms, small biological molecules such as phytohormones and signalling molecules amend plants adaptability against adverse environment factors. Our research showed that exogenous application of Ca2+ amends growth and development under salt stress by regulating osmo-protection and ROS scavenging capability of two contrasting rice cultivars.
As a first indication, reduction of FGP and GRI is a common phenomenon under salt stress condition (Safdar et al. 2019). In the present study, we found the reduction of FGR and GRI both in Binadhan-10 and Binadhan-7 rice cultivars (Table 1) due to induced salinity which was consistent with the previous results (Shereen et al. 2011; Bahrani and Hagh Joo 2012; Vibhuti et al. 2015), which reported that rice lines showed more than 80% germination up to 75 mM NaCl at 72 h, while at higher salinity levels, these lines germinated after a delay of 3–6 days in 100 and 200 mM NaCl, respectively. Germination requires imbibition, hydration of protoplasm and restoration of enzymatic activity (Bewley 1997; Soundararajan et al. 2017). All these steps require sufficient supply of water. Salinity inhibits the normal water uptake (Coskun et al. 2016) that delays the imbibition and other physiological processes, entail less or delayed germination but exogenous application of calcium ameliorated the FGP and GRI in the present study (Table 1) which is consistent with the findings in Phragmites karka seeds (Zehra et al. 2012) and six forest tree seeds (Liu et al. 2011). The present study also revealed that low concentration (10 mM CaCl2) of exogenous calcium increased FGP and GRI over high concentration (15 mM CaCl2), which is supported by previous reports in Cichorium intybus (Arshi et al. 2010), Festuca ovina (Salahshoor and Kazemi 2016) and Melilotus officinalis (Zhang et al. 2019).
Our present study showed the decline of RWC in induced saline condition (Table 2). It happened due to osmotic stress that makes the root rigid to uptake water (Goyal et al. 2018; Safdar et al. 2019). Exogenous supplementation of Ca2+ (10 mM CaCl2) improved RWC in both rice cultivars whereas 15 mM CaCl2 improved RWC only in salt-tolerant Binadhan-10 under salt stress (Table 2). It might be due to, Ca2+ positively affecting stomatal functioning by keeping the guard cells turgid (Agurla et al. 2018) or by regulating stomatal conductance or by ensuring CO2 availability (Sibole et al. 2003). Low RWC employed its adverse effect on all growth parameters measured in rice plants when exposed to 100 mM salt stress. In our study, we found salt stress significantly reduced the shoot and root length both at germination and seedling stage experiments (Tables 2, 3) which is consistent with the former findings on Brassica campestris (Keling and Zhujun 2010) and rice (Roy et al. 2016). Cells require turgor pressure for elongation (Guérin et al. 2016) but salinity abates the turgor pressure that might reduce the shoot and root length, thus leading to stunted growth. Exogenous application of Ca2+ ameliorated this detrimental effects of salinity, similar results were reported on Calligonum mongolicum (Xu et al. 2017), Glycine max (Yin et al. 2015) and rice (Tahjib-Ul-Arif et al. 2018c). Calcium helps in retaining RWC and turgor pressure and entail proper shoot and root growth (Tables 2, 3).
This mitigation of growth inhibition under NaCl-salt stress also might be due to the improved ion homeostasis with CaCl2 supplementation. As a primary and an immediate response of plants exposed to salt stress, stimulation of K+ leakage is occurred by Na+ (Bose et al. 2014). In plant growth medium, excess Na+ ion depolarizes root plasma membranes that activate guard cell outward rectifying potassium channels, decreasing cytosolic K+ (Demidchik et al. 2002), displacing Ca2+ from membranes, and consequently disrupting the ion homeostasis (Wu and Wang 2012; Gu et al. 2016). This excess of cell Na+ and shortage of Ca2+ and K+ might also be cause of ROS overproduction (Demidchik and Maathuis 2007). However, it was reported that exogenously-applied Ca2+ promotes membrane stability, ameliorates salt toxicity by decreasing Na+ content by reducing its uptake and transport and preventing its binding to cell wall, as well as increasing Ca2+ content in plants (Shabala and Pottosin 2014; Rahman et al. 2016). Therefore, treatment with Ca2+ might counter the adverse consequences by providing protection to the integrity and permeability of plasma membranes against Na+ toxicity (Zehra et al. 2012).
Plant growth is intimately related with photosynthetic pigments and rate of photosynthesis (Tang et al. 2015). The photosynthetic pigments such as chlorophyll ‘a’, ‘b’ and total chlorophyll content were significantly decreased in salt-stressed rice seedlings (Fig. 2) and similar results were reported in rice (Zhen-hua et al. 2012) and Pisum sativum (Ozturk et al. 2012). Under higher salt concentrations, the accumulation of Na+ and Cl− ions increased which hinder the process of chlorophyll synthesis by influencing the activity of some chlorophyll synthesizing enzymes containing Fe3+ (Silva et al. 2011) or by increasing the activity of chlorophyll depredating enzyme, chlorophyllase and excess accumulation of ROS (Hasanuzzaman et al. 2014). Exogenous application of Ca2+ (10 mM CaCl2) on salt-stressed rice cultivars increased the total chlorophyll content (Fig. 1). Previous study reported a similar trend of increment in total chlorophyll content by supplementary Ca2+ in Trigonella foenum-graecum (Oprica and Sandu 2014), Festuca arundinacea (Wang et al. 2017) and rice (Tahjib-Ul-Arif et al. 2018c). The addition of Ca2+ to the salt-stressed plants affects most of the physiological processes and our results showed that the exogenous addition of Ca2+ regulated the total content of photosynthetic pigments (Fig. 1). The increment of chlorophyll pigments under salt stress due to application of Ca2+ might be because of limited ROS production (Rahman et al. 2016).
Moreover, stunted shoot and root growth and physiological impairments (photosynthesis pigment degradation and impairment of electron flow) led to a reduction in shoot and root weight (biomass accumulation). In our study, we found a decreasing trend in fresh and dry weight of shoot and root under salt stress (Tables 2, 3). Salinity induces ABA-mediated stomatal closure that limits CO2 fixation and disturbs the normal electron flow for carbon reduction in Calvin cycle which is associated with low fresh as well as dry matter production (Mbarki et al. 2018a, b; Acosta-Motos et al. 2017). These deleterious effects were quelled by exogenous application of calcium (Tables 2, 3). The effect of Ca2+ on increasing growth characters might be due to several reasons including that Ca2+ contributes to the composition of the cell wall and the cell membrane, therefore maintaining the balance and stability of membranes through the contacting different protein and lipids on the surface of the membrane (Davis et al. 2003). Calcium helps plants to withstand saline stress through several mechanisms including the stabilization of cell membranes and walls (Tuna et al. 2007) and organization of Na+ and reduction of the toxic effects of NaCl through facilitating the transfer of Na+/K+ (Cuin et al. 2003). Furthermore, high rate of salinity causes an increase of Ca2+ in vacuole which is shifted from apoplast and the compartments within cells (Arshi et al. 2010). But it has been found that the lower concentration of Ca2+ improved the growth of rice plants and in the experiment, a lower concentration of Ca2+ performed better than higher concentration. Similar results were found in Dolichos lablab (D’Souza and Devaraj 2013) and Atriplex halimus (Soualem et al. 2014).
Production of osmolytes is a common phenomenon in stress condition to alleviate the physiological damage (Ghosh et al. 2016). In the present study, the proline content increased significantly both in salt-stress and combined treatment with salt and exogenous Ca2+ (Fig. 2a). Under salinity condition, the accumulation of proline showed increasing trend in rice (Polash et al. 2018), Hordeum vulgare (Reza et al. 2006), Pisum sativum (Ozturk et al. 2012) and Trifolium alexandrinum (Abdalla 2011). Proline may act as a non-toxic osmotic solute preferentially stabilizing the structure of macromolecules and organelles (Acosta-Motos et al. 2019). Increased proline in the stressed plants may be an adaptation to compensate the energy for growth and survival and thereby helps the plant to tolerate stress, as also observed in spinach leaves (Öztürk and Demir 2003) and in Phyllanthus amarus (Jaleel et al. 2008).
Salinity leads to other stresses. ROS production occurs when the plants are subjected to salt stress (Bose et al. 2014; Tang et al. 2015). In this circumstance, antioxidants are believed to play a very crucial role in the plant defence against ROS (Hanin et al. 2016). In the current study, we found increment in CAT activity with increasing the magnitude of NaCl in both tolerant and susceptible cultivars (Fig. 2b). Similar results are also found in rice (Nounjan et al. 2012) and Vigna radiata (Manivannan et al. 2007). CAT could convert hydrogen peroxide into oxygen and water to remove the peroxide in plants, and the higher action of CAT led to greater salt tolerance (Waszczak et al. 2018). The present study also revealed that the APX activity increased in all the rice cultivars with the increase of NaCl concentration (Fig. 2d) which was also supported by Weisany et al. (2012) on Glycine max, but also decrease in APX activity was also found in Pisum sativum (Ozturk et al. 2012). A major hydrogen peroxide detoxifying system in plant cells is the ascorbate–glutathione cycle, in which, APX enzymes play a key role catalyzing the conversion of H2O2 into H2O, using ascorbate as a specific electron donor (Hossain and Fujita 2013). APX activity was higher in salt-tolerant cultivars than salt-sensitive (Yasar et al. 2008) which is consistent with our results (Fig. 2d). Salinity stress has a significant effect on the activity of POD, which was revealed in our present study (Fig. 2c). Almost all the plants showed a similar increasing response in POD activity under salinity stress, which was reported in Glycine max (Weisany et al. 2012) and Solanum melongena (Shaheen et al. 2013). It is believed that POD in cytosol and peroxisomes efficiently remove any H2O2 found outside the chloroplast (Asada 1992). Application of Ca2+ under control condition enhanced POD activity, which might be due to its dependency on Ca2+ ions and in absence of Ca2+, POD is functionally inactive (Plieth and Vollbehr 2012). On the other hand, salt stress might have limited the Ca2+ uptake in combined treatment condition and somehow limited the further increment of POD activity.
Eventually, it can be concluded that Ca2+ participates in the regulatory mechanisms that activates the plants to adapt to adverse salt stress conditions by following reasons. First, exogenous Ca2+ improved the FGP and GRI as well as shoot length, root length, shoot weight, root weight, RWC and SES in both tolerant (Binadhan-10) and susceptible (Binadhan-7) rice plants. Second, Ca2+ augmented the photosynthetic capacity by restoring the photosynthetic pigments. Third, Ca2+ regulated the proline biosynthesis that compensates the energy for growth and survival and thereby helps the plant to tolerate stress. Fourth, Ca2+ reduced the oxidative damage through regulating the antioxidant defence and ROS detoxification system by boosting antioxidant enzymatic activity. Consequently, our outcomes offer a core foundation in salt stress mitigation by exogenous Ca2+ application and developing salt-tolerant rice genotypes by well tuning the level of endogenous Ca2+ through genetic manipulation of Ca2+ uptake mechanism. Finally, 10 mM CaCl2 showed better performance than that of 15 mM CaCl2 application to mitigate the adverse effect of salinity in rice.
Acknowledgments
The authors are also grateful to Promod Kumar Nagar for his valuable suggestions in manuscript preparation.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdalla MM. Impact of diatomite nutrition on two Trifolium alexandrinum cultivars differing in salinity tolerance. Int J Plant Physiol Biochem. 2011;3:233–246. [Google Scholar]
- Acosta-Motos J, Ortuño M, Bernal-Vicente A, Diaz-Vivancos P, Sanchez-Blanco M, Hernandez J. Plant responses to salt stress: adaptive mechanisms. Agronomy. 2017;7:18. [Google Scholar]
- Acosta-Motos JR, Diaz-Vivancos P, Acosta M, Hernandez JA, et al. Effect of biostimulants on plant responses to salt stress. In: Hasanuzzaman M, et al., editors. Plant tolerance to environmental stress: role of phytoprotectants. Boca Raton: CRC Press; 2019. pp. 363–380. [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Afrin S, Tahjib-Ul-Arif M, Sakil MA, Sohag AAM, Polash MAS, Hossain MA. Hydrogen peroxide priming alleviates chilling stress in rice (Oryza sativa L.) by enhancing oxidant scavenging capacity. Fundam Appl Agric. 2019;4:713–722. [Google Scholar]
- Agurla S, Gahir S, Munemasa S, Murata Y, Raghavendra AS. Mechanism of stomatal closure in plants exposed to drought and cold stress. In: Iwaya-Inoue M, editor. Survival strategies in extreme cold and desiccation. Singapore: Springer; 2018. pp. 215–232. [DOI] [PubMed] [Google Scholar]
- Arshi A, Ahmad A, Aref IM, Iqbal M. Effect of calcium against salinity-induced inhibition in growth, ion accumulation and proline contents in Cichorium intybus L. J Environ Biol. 2010;31:939–944. [PubMed] [Google Scholar]
- Asada K. Ascorbate peroxidase-a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant. 1992;85:235–241. [Google Scholar]
- Asano T, Hayashi N, Kobayashi M, Aoki N, Miyao A, Mitsuhara I, Ichikawa H, Komatsu S, Hirochika H, Kikuchi S, Ohsugi R. A rice calcium-dependent protein kinase OsCPK12 oppositely modulates salt-stress tolerance and blast disease resistance. Plant J. 2012;69:26–36. doi: 10.1111/j.1365-313X.2011.04766.x. [DOI] [PubMed] [Google Scholar]
- Bahrani A, Hagh Joo M. Response of some wheat (Triticum aestivum L.) genotypes to salinity at germination and early seedling growth stages. World Appl Sci J. 2012;16:599–609. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Bewley JD. Seed germination and dormancy. Plant Cell. 1997;9:1055. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose J, Rodrigo-Moreno A, Shabala S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot. 2014;65:1241–1257. doi: 10.1093/jxb/ert430. [DOI] [PubMed] [Google Scholar]
- Campo S, Baldrich P, Messeguer J, Lalanne E, Coca M, San Segundo B. Overexpression of a calcium-dependent protein kinase confers salt and drought tolerance in rice by preventing membrane lipid peroxidation. Plant Physiol. 2014;165:688–704. doi: 10.1104/pp.113.230268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun D, Britto DT, Huynh WQ, Kronzucker HJ. The role of silicon in higher plants under salinity and drought stress. Front Plant Sci. 2016;7:1072. doi: 10.3389/fpls.2016.01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuin TA, Miller AJ, Laurie SA, Leigh RA. Potassium activities in cell compartments of salt-grown barley leaves. J Exp Bot. 2003;54:657–661. doi: 10.1093/jxb/erg072. [DOI] [PubMed] [Google Scholar]
- D’Souza MR, Devaraj VR. Mercury-induced changes in growth and oxidative metabolism of field bean (Dolichos lablab) Res J Chem Environ. 2013;17:86–93. [Google Scholar]
- Davis TA, Volesky B, Mucci A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003;37:4311–4330. doi: 10.1016/S0043-1354(03)00293-8. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Maathuis FJ. Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytol. 2007;175:387–404. doi: 10.1111/j.1469-8137.2007.02128.x. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Davenport RJ, Tester M. Nonselective cation channels in plants. Annu Rev Plant Biol. 2002;53:67–107. doi: 10.1146/annurev.arplant.53.091901.161540. [DOI] [PubMed] [Google Scholar]
- FAO (2016) FAOSTAT: online statistical database. http://faostat.fao.org/. Accessed 30 July 2016
- Gao JP, Chao DY, Lin HX. Understanding abiotic stress tolerance mechanisms: recent studies on stress response in rice. J Integr Plant Biol. 2007;49(6):742–750. [Google Scholar]
- Geist H. The causes and progression of desertification. London: Routledge; 2017. [Google Scholar]
- Ghosh B, Md NA, Gantait S. Response of rice under salinity stress: a review update. Rice research: open access. 2016;26:1–8. [Google Scholar]
- Goyal MR, Gupta SK, Singh A, et al. Physiological and biochemical changes in plants under soil salinity stress: a review. In: Gupta SK, et al., editors. Engineering practices for management of soil salinity. 1. Palm Bay: Apple Academic Press; 2018. pp. 159–200. [Google Scholar]
- Gu MF, Li N, Long XH, et al. Accumulation capacity of ions in cabbage (Brassica oleracea L.) supplied with sea water. Plant Soil Environ. 2016;62:314–320. [Google Scholar]
- Guérin A, Gravelle S, Dumais J. Forces behind plant cell division. Proc Natl Acad Sci. 2016;113:8891–8893. doi: 10.1073/pnas.1609309113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta B, Huang B. Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genom. 2014 doi: 10.1155/2014/701596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanin M, Ebel C, Ngom M, Laplaze L, Masmoudi K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front Plant Sci. 2016;29:1787. doi: 10.3389/fpls.2016.01787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Alam MM, Rahman A, Hasanuzzaman M, Nahar K, Fujita M. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. Biomed Res Int. 2014 doi: 10.1155/2014/757219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. California agricultural experiment station, Circular 347, pp 1–32
- Hossain MA, Fujita M. Hydrogen peroxide priming stimulates drought tolerance in mustard (Brassica juncea L.) seedlings. Plant Gene Trait. 2013;4:109–123. [Google Scholar]
- Hossain MA, Piyatida P, da Silva JAT, Fujita M. Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot. 2012 [Google Scholar]
- International Rice Research Institute (IRRI) Standard evaluation system (SES) Manila: International Rice Research Institute; 2002. pp. 11–30. [Google Scholar]
- Iqbal N, Umar S, Khan NA. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea) J Plant Physiol. 2015;178:84–91. doi: 10.1016/j.jplph.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Jaleel CA, Kishorekumar A, Manivannan P, Saankar B, Gomathinayagam M, Panneerselvam R. Salt stress mitigation by calcium chloride in Phyllanthus amarus. Acta Bot Croat. 2008;67:53–62. [Google Scholar]
- Keling H, Zhujun Z. Effects of different concentrations of sodium chloride on plant growth and glucosinolate content and composition in Pakchoi. Afr J Biotechnol. 2010;9:4428–4433. [Google Scholar]
- Kibria MG, Hossain M, Murata Y, Hoque MA. Antioxidant defense mechanisms of salinity tolerance in rice genotypes. Rice Sci. 2017;24:155–162. [Google Scholar]
- Kordrostami M, Rabiei B, et al. Salinity stress tolerance in plants: physiological, molecular, and biotechnological approaches. In: Hasanuzzaman M, et al., editors. Plant abiotic stress tolerance. Switzerland: Springer; 2019. [Google Scholar]
- Kosar F, Akram NA, Sadiq M, Al-Qurainy F, Ashraf M. Trehalose: a key organic osmolyte effectively involved in plant abiotic stress tolerance. J Plant Growth Regul. 2018 [Google Scholar]
- Kurepin LV, Ivanov AG, Zaman M, Pharis RP, Hurry V, Hüner NP, et al. Interaction of glycine betaine and plant hormones: protection of the photosynthetic apparatus during abiotic stress. In: Hou HJM, et al., editors. Photosynthesis: structures, mechanisms, and applications. Cham: Springer; 2017. pp. 185–202. [Google Scholar]
- Liu TW, Wu FH, Wang WH, Chen J, Li ZJ, Dong XJ, Patton J, Pei ZM, Zheng HL. Effects of calcium on seed germination, seedling growth and photosynthesis of six forest tree species under simulated acid rain. Tree Physiol. 2011;31:402–413. doi: 10.1093/treephys/tpr019. [DOI] [PubMed] [Google Scholar]
- Mahmood-ur-Rahman, Ijaz M, Qamar S, Bukhari SA, Malik K. Abiotic stress signaling in rice crop. In: Hasanuzzaman M, editor. Advances in rice research for abiotic stress tolerance. Cambridge: Woodhead Publishing; 2019. pp. 551–569. [Google Scholar]
- Manivannan P, Jaleel CA, Kishorekumar A, Sankar B, Somasundaram R, Sridharan R, Panneerselvam R. Changes in antioxidant metabolism of Vigna unguiculata (L.) Walp. by propiconazole under water deficit stress. Colloids Surf B Biointerfaces. 2007;57:69–74. doi: 10.1016/j.colsurfb.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Mbarki S, Cerdà A, Zivcak M, Brestic M, Rabhi M, Mezni M, Jedidi N, Abdelly C, Pascual JA. Alfalfa crops amended with MSW compost can compensate the effect of salty water irrigation depending on the soil texture. Process Saf Environ. 2018;115:8–16. [Google Scholar]
- Mbarki S, Sytar O, Cerda A, Zivcak M, Rastogi A, He X, Zoghlami A, Abdelly C, Brestic M. Strategies to mitigate the salt stress effects on photosynthetic apparatus and productivity of crop plants. In: Kumar V, editor. Salinity responses and tolerance in plants. Cham: Springer; 2018. pp. 85–136. [Google Scholar]
- Mickky BM, Abbas MA, Sameh NM. Morpho-physiological status of fenugreek seedlings under NaCl stress. J King Saud Univ. 2019 [Google Scholar]
- Miller GAD, Suzuki N, Ciftci-Yilmaz S, Mittler RON. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- Morgan SH, Maity PJ, Geilfus CM, Lindberg S, Mühling KH. Leaf ion homeostasis and plasma membrane H+-ATPase activity in Vicia faba change after extra calcium and potassium supply under salinity. Plant Physiol Biochem. 2014;82:244–253. doi: 10.1016/j.plaphy.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Morton MJ, Awlia M, Al-Tamimi N, Saade S, Pailles Y, Negrão S, Tester M. Salt stress under the scalpel–dissecting the genetics of salt tolerance. Plant J. 2019;97:148–163. doi: 10.1111/tpj.14189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Nounjan N, Nghia PT, Theerakulpisut P. Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J Plant Physiol. 2012;169:596–604. doi: 10.1016/j.jplph.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Oprica L, Sandu L. Impact of inorganic salt solution on antioxidative enzyme activity and photosynthetic pigments content in Trogonella foenum-graecum seedlings. Ann “Alexandru Ioan Cuza Uni” Sec II Gen Mol Bio. 2014;15:31. [Google Scholar]
- Öztürk L, Demir Y. Effects of putrescine and ethephon on some oxidative stress enzyme activities and proline content in salt stressed spinach leaves. Plant Growth Regul. 2003;40:89–95. [Google Scholar]
- Ozturk L, Demir Y, Unlukara A, Karatas I, Kurunc A, Duzdemir O. Effects of long-term salt stress on antioxidant system, chlorophyll and proline contents in pea leaves. Rom Biotech Lett. 2012;17:7227–7236. [Google Scholar]
- Parihar P, Singh S, Singh R, Singh VP, Prasad SM. Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Res. 2015;22:4056–4075. doi: 10.1007/s11356-014-3739-1. [DOI] [PubMed] [Google Scholar]
- Patel TK, Williamson JD. Mannitol in plants, fungi, and plant–fungal interactions. Trends Plant Sci. 2016;21:486–497. doi: 10.1016/j.tplants.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Per TS, Khan NA, Reddy PS, Masood A, Hasanuzzaman M, Khan MI, Anjum NA. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: phytohormones, mineral nutrients and transgenics. Plant Physiol Biochem. 2017;115:126–140. doi: 10.1016/j.plaphy.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Plieth C, Vollbehr S. Calcium promotes activity and confers heat stability on plant peroxidases. Plant Signal Behav. 2012;7:650–660. doi: 10.4161/psb.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polash MAS, Sakil MA, Tahjib-Ul-Arif M, Hossain MA. Effect of salinity on osmolytes and relative water content of selected rice genotypes. Trop Plant Res. 2018;5:227–232. [Google Scholar]
- Rahman A, Nahar K, Hasanuzzaman M, Fujita M. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front Plant Sci. 2016;7:609. doi: 10.3389/fpls.2016.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy IN, Kim SM, Kim BK, Yoon IS, Kwon TR. Identification of rice accessions associated with K+/Na+ ratio and salt tolerance based on physiological and molecular responses. Rice Sci. 2017;24:360–364. [Google Scholar]
- Reza S, Heidari R, Zare S, Norastehnia A. Antioxidant response of two salt-stressed barley varieties in the presence or absence of exogenous proline. Gen Appl Plant Physiol. 2006;32:233–251. [Google Scholar]
- Roy PR, Tahjib-Ul-Arif M, Akter T, Ray SR, Sayed MA. Exogenous ascorbic acid and hydrogen peroxide alleviates salt-induced oxidative stress in rice (Oryza sativa L.) by enhancing antioxidant enzyme activities and proline content. Adv Environ Biol. 2016;10:148–155. [Google Scholar]
- Safdar H, Amin A, Shafiq Y, Ali A, Yasin R, Shoukat A, Hussan MU, Sarwar MI. A review: impact of salinity on plant growth. Nat Sci. 2019;17:34–40. [Google Scholar]
- Saini P, Gani M, Kaur JJ, Godara LC, Singh C, Chauhan SS, Francies RM, Bhardwaj A, Kumar NB, Ghosh MK. Reactive oxygen species (ROS): a way to stress survival in plants. In: Zargar SM, Zargar MY, editors. Abiotic stress-mediated sensing and signaling in plants: an omics perspective. Singapore: Springer; 2018. pp. 127–153. [Google Scholar]
- Salahshoor F, Kazemi F. Effect of calcium on reducing salt stress in seed germination and early growth stage of Festuca ovina L. Plant Soil Environ. 2016;62:460–466. [Google Scholar]
- Sami F, Yusuf M, Faizan M, Faraz A, Hayat S. Role of sugars under abiotic stress. Plant Physiol Biochem. 2016;109:54–61. doi: 10.1016/j.plaphy.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Shabala S, Pottosin I. Regulation of potassium transport in plants under hostile conditions: implications for abiotic and biotic stress tolerance. Physiol Plant. 2014;151:257–279. doi: 10.1111/ppl.12165. [DOI] [PubMed] [Google Scholar]
- Shaheen S, Naseer S, Ashraf M, Akram NA. Salt stress affects water relations, photosynthesis, and oxidative defense mechanisms in Solanum melongena L. J Plant Interact. 2013;8(1):85–96. [Google Scholar]
- Shereen A, Ansari R, Raza S, Mumtaz S, Khan MA, Khan MA. Salinity induced metabolic changes in rice (Oryza sativa L.) seeds during germination. Pak J Bot. 2011;43:1659–1661. [Google Scholar]
- Sibole JV, Cabot C, Poschenrieder C, Barceló J. Efficient leaf ion partitioning, an overriding condition for abscisic acid-controlled stomatal and leaf growth responses to NaCl salinization in two legumes. J Exp Bot. 2003;54:2111–2119. doi: 10.1093/jxb/erg231. [DOI] [PubMed] [Google Scholar]
- Silva EN, Ribeiro RV, Ferreira-Silva SL, Viégas RA, Silveira JA. Salt stress induced damages on the photosynthesis of physic nut young plants. Sci Agric. 2011;68:62–68. [Google Scholar]
- Slama I, Abdelly C, Bouchereau A, Flowers T, Savoure A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann Bot. 2015;115:433–447. doi: 10.1093/aob/mcu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohan D, Jasoni R, Zajicek J. Plant–water relations of NaCl and calcium-treated sunflower plants. Environ Exp Bot. 1999;42:105–111. [Google Scholar]
- Soualem S, Adda A, Belkhodja M, Merah O. Calcium supply reduced effect of salinity on growth in the Mediterranean shrub (Atriplex halimus L.) Life Sci J. 2014;11:278–284. [Google Scholar]
- Soundararajan P, Manivannan A, Jeong BR, et al. Reactive oxygen species signaling and seed germination: an overview. In: Singh VP, et al., editors. Reactive oxygen species in plants: boon or bane-revisiting the role of ROS. Hoboken: Wiley; 2017. pp. 291–306. [Google Scholar]
- Tahjib-Ul-Arif M, Roy PR, Sohag AAM, Afrin S, Rady MM, Hossain MA. Exogenous calcium supplementation improves salinity tolerance in BRRI dhan28; a salt-susceptible high-yielding Oryza sativa cultivar. J Crop Sci Biotechnol. 2018;21:383–394. [Google Scholar]
- Tahjib-Ul-Arif M, Sayed MA, Islam MM, Siddiqui MN, Begum SN, Hossain MA. Screening of rice landraces (Oryza sativa L.) for seedling stage salinity tolerance using morpho-physiological and molecular markers. Acta Physiol Plant. 2018;40:70. [Google Scholar]
- Tahjib-Ul-Arif M, Siddiqui MN, Sohag AAM, Sakil MA, Rahman MM, Polash MAS, Mostofa MG, Tran LSP. Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J Plant Growth Regul. 2018;37:1318–1330. [Google Scholar]
- Tang X, Mu X, Shao H, Wang H, Brestic M. Global plant-responding mechanisms to salt stress: physiological and molecular levels and implications in biotechnology. Crit Rev Biotechnol. 2015;35:425–437. doi: 10.3109/07388551.2014.889080. [DOI] [PubMed] [Google Scholar]
- Tuna AL, Kaya C, Ashraf M, Altunlu H, Yokas I, Yagmur B. The effects of calcium sulphate on growth, membrane stability and nutrient uptake of tomato plants grown under salt stress. Environ Exp Bot. 2007;59:173–178. [Google Scholar]
- Vibhuti CS, Bargali K, Bargali SS. Seed germination and seedling growth parameters of rice (Oryza sativa L.) varieties as affected by salt and water stress. Indian J Agric Sci. 2015;85:102–108. [Google Scholar]
- Wang G, Bi A, Amombo E, Li H, Zhang L, Cheng C, Hu T, Fu J. Exogenous calcium enhances the photosystem II photochemistry response in salt stressed tall fescue. Front Plant Sci. 2017;8:2032. doi: 10.3389/fpls.2017.02032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszczak C, Carmody M, Kangasjärvi J. Reactive oxygen species in plant signaling. Annu Rev Plant Biol. 2018;69:209–236. doi: 10.1146/annurev-arplant-042817-040322. [DOI] [PubMed] [Google Scholar]
- Weisany W, Sohrabi Y, Heidari G, Siosemardeh A, Ghassemi-Golezani K. Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycine max L.) Plant Omics. 2012;5:60. [Google Scholar]
- Wu GQ, Wang SM. Calcium regulates K+/Na+ homeostasis in rice (Oryza sativa L.) under saline conditions. Plant Soil Environ. 2012;58:121–127. [Google Scholar]
- Xiong L, Zhu J. Molecular and genetic aspects of plant responses to osmotic stress. Plant, Cell Environ. 2002;25:131–139. doi: 10.1046/j.1365-3040.2002.00782.x. [DOI] [PubMed] [Google Scholar]
- Xu D, Wang W, Gao T, Fang X, Gao X, Li J, Bu H, Mu J. Calcium alleviates decreases in photosynthesis under salt stress by enhancing antioxidant metabolism and adjusting solute accumulation in Calligonum mongolicum. Conserv Physiol. 2017 [Google Scholar]
- Yan K, Shao H, Shao C, Chen P, Zhao S, Brestic M, Chen X. Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol Plant. 2013;35:2867–2878. [Google Scholar]
- Yang Y, Guo Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018;217:523–539. doi: 10.1111/nph.14920. [DOI] [PubMed] [Google Scholar]
- Yasar F, Ellialtioglu S, Yildiz K. Effect of salt stress on antioxidant defense systems, lipid peroxidation, and chlorophyll content in green bean. Russ J Plant Physiol. 2008;55:782. [Google Scholar]
- Yin Y, Yang R, Han Y, Gu Z. Comparative proteomic and physiological analyses reveal the protective effect of exogenous calcium on the germinating soybean response to salt stress. J Proteom. 2015;113:110–126. doi: 10.1016/j.jprot.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Zehra A, Gul B, Ansari R, Khan MA. Role of calcium in alleviating effect of salinity on germination of Phragmites karka seeds. S Afr J Bot. 2012;78:122–128. [Google Scholar]
- Zeng L, Shannon MC, Lesch SM. Timing of salinity stress affects rice growth and yield components. Agric Water Manag. 2001;48:191–206. [Google Scholar]
- Zhang DW, Vu TS, Huang J, Chi CY, Xing Y, Fu DD, Yuan ZN. Effects of calcium on germination and seedling growth in melilotus officinalis L. (Fabaceae) under salt stress. Pak J Bot. 2019;51:1–9. [Google Scholar]
- Zhen-hua ZH, Qiang LI, Hai-xing SO, Xiang-min RO, Ismail AM. Responses of different rice (Oryza sativa L.) genotypes to salt stress and relation to carbohydrate metabolism and chlorophyll content. Afr J Agric Res. 2012;7:19–27. [Google Scholar]