Abstract
Introduction
This study assesses insulin-degrading enzyme (IDE) and regulator of calcineurin 1 (RCAN1) as potential mediators of brain insulin deficiency and neurodegeneration in experimental and human Alzheimer's disease (AD).
Methods
Temporal lobes from Long Evans rats treated with intracerebral streptozotocin or vehicle and postmortem frontal lobes from humans with normal aging AD (Braak 0-2), moderate (Braak 3-4) AD, or advanced (Braak 5-6) AD were used to measure IDE and RCAN mRNA and protein.
Results
Intracerebral streptozotocin significantly increased IDE and RCAN mRNA and protein. In humans with apolipoprotein E (ApoE) ε3/ε4 or ε4/ε4 and AD, IDE was elevated at Braak 3-4, but at Braak 5-6, IDE expression was significantly reduced. RCAN1 mRNA was similarly reduced in ApoE ε4+ patients with moderate or severe AD, whereas RCAN1 protein declined with the severity of AD and ApoE ε4 dose.
Discussion
The findings suggest that IDE and RCAN1 differentially modulate brain insulin signaling in relation to AD severity and ApoE genotype.
Keywords: Alzheimer's disease, Insulin deficiency, Insulin resistance, Streptozotocin, Insulin-degrading enzyme, RCAN1, Neurodegeneration
1. Introduction
Alzheimer's disease (AD) is the most common form of dementia-associated neurodegeneration [1], [2], yet despite decades of intensive research based on the β-amyloid (Aβ) and pTau hypotheses, no effective treatments have emerged. On the other hand, growing evidence supports an alternative concept that cognitive impairment and neurodegeneration in AD are mediated by deficits in insulin and insulin-like growth factor (IGF-1) signaling and metabolic functions in the brain [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. The fact that progressive brain insulin and IGF-1 resistances accompany declines in ligand expression in the brain suggests that metabolic abnormalities in AD mimic combined effects of types 1 and 2 diabetes [16], [17], [18], [19], [20]. The term “Type 3 diabetes” was coined [6], [7] to reflect this fundamental concept, as well as emphasize the need for both trophic factor supplementation and insulin sensitizer treatment interventions. Further research in this field would improve understanding of how therapeutic strategies already developed for diabetes mellitus and other insulin resistance diseases could be repurposed for selectively targeting neurodegeneration. In addition, we must determine the underlying causes of insulin/IGF-linked brain metabolic dysfunction to develop rational strategies for prevention and treatment.
Two molecules (enzymes) that could have major roles in mediating brain insulin deficiency in AD are insulin-degrading enzyme (IDE) [21], [22] and regulator of calcineurin 1 (RCAN1) [23], [24], [25], [26]. IDE is a 110-kDa thiol zinc-metalloendopeptidase [27], [28] encoded by a gene located on chromosome 10q23.33 [29], [30] and expressed in many organs and tissues including the brain, liver, testis, kidney, heart, and skeletal muscle [31]. The surface membrane and the cytosolic, peroxisomal, and mitochondrial subcellular localizations of IDE [32], [33], [34], [35] suggest its functions are diverse. Initially, IDE was described as an insulinase [36], but later, it was demonstrated to degrade other small polypeptides including atrial natriuretic peptide, transforming growth factor-α, amylin, bradykinin, kallidin, and Aβ [37], [38], [39].
The fact that IDE can degrade insulin, amylin, and Aβ suggests that AD may be linked to type 2 diabetes mellitus via chromosome 10 [40], [41], [42]. Correspondingly, the finding that Aβ plaque size inversely correlates with IDE expression and activity [35], [43] suggests that IDE deficiency could mediate plaque buildup and possibly cognitive impairment in AD. On the other hand, the paradoxical findings of progressive brain insulin deficiency and insulin resistance in AD [19], [44] suggest that increased rather than decreased IDE activity is the culprit. A third possible scenario is that increased IDE activity preferentially targets and degrades insulin rather than Aβ, driving brain insulin deficiency vis-à-vis Aβ accumulation, as it occurs in the intracerebral streptozotocin (i.c. STZ) model [45], [46], [47], [48], [49].
The RCAN1 gene is located within the Down locus on chromosome 21 [50], [51]. Alternative splicing of RCAN1 produces three protein isoforms, RCAN1.1 L, RCAN1.1S, and RCAN1.4 [51], [52]. RCAN1 is expressed at high levels in the brain, spinal cord, kidney, liver, mammary gland, placenta, skeletal muscle, and heart [51], [52], and its principal function is to inhibit calcineurin, a serine-threonine phosphatase [53], [54]. RCAN1's potential role in neurodegeneration is through inhibition of calcineurin [55], [56] and attendant-increased glycogen synthase kinase 3β (GSK-3β) activity [55], [56], leading to hyperphosphorylation of tau and neurofibrillary tangle formation [57], activation of stress pathways, and increased neuronal apoptosis [51], [57], [58], [59]. In Down syndrome and AD, hyperphosphorylated tau immunoreactive neurofibrillary tangles, dystrophic neurites, and cortical neuritic plaques correlate with severity of cognitive impairment and neurodegeneration [60], [61], [62].
Importantly, calcineurin may have an important role in mediating AD neurodegeneration via dysregulation of Ca2+ [63] and Ca2+/calmodulin-dependent signaling [64] in relation to cognition and neuronal plasticity [65], [66], [67], Aβ synaptotoxicity and dendritic spine pathology [68], [69], driver of neuroinflammation [70], [71], excitotoxicity [72], and suppression of synaptic mRNA transcripts [73]. Apart from its roles in protecting against the development and progression of various structural and functional AD-associated pathologies that correlate with cognitive impairment, RCAN1 causes hypoinsulinemia and has been linked to diabetes mellitus, pancreatic β cell dysfunction, and altered mitochondrial function [25], [74], [75]. Therefore, either increased or reduced levels of RCAN1 expression in the brain could have profound effects on AD pathogenesis and progression. In particular, similar to IDE, upregulated expression of RCAN1 could promote brain insulin deficiency in AD.
The present work further evaluates the potential roles of IDE and RCAN1 as mediators of neurodegeneration in an established experimental rat model of i.c. STZ and in human brains with moderate or severe AD pathology. Intracerebral STZ has been widely used as a model of sporadic AD-type neurodegeneration because cognitive impairment is associated with Aβ and pTau accumulations, mitochondrial dysfunction, neuroinflammation, insulin and IGF deficiency and resistance, and impaired insulin/IGF-1 signaling through pathways that regulate energy metabolism, neuronal plasticity, cellular homeostasis, and neuronal survival [45], [46], [48], [76], [77], [78], [79], [80]. STZ functions in part by killing insulin-producing cells, particularly β cells in pancreatic islets. However, i.c. STZ mainly has neurotoxic effects, reducing insulin gene expression [46] and insulin polypeptide levels [79] in the brain, although with prolonged survival and after development of neurodegeneration with cognitive impairment, peripheral insulin resistance, and diabetes eventually emerge [48]. The relevance of this model to AD is further exemplified by reversal of cognitive deficits and neurodegeneration by treatment with insulin sensitizers [45], [47], [48], [81] or insulin analogs [79].
In human brains, greater severities, that is, higher histopathologic Braak stage scores of AD, are associated with decreased signaling through insulin and IGF-1 receptors, insulin receptor substrate, and downstream Akt pathways that mediate neuronal survival, plasticity, growth, metabolism, and cholinergic function and inhibit neuroinflammation, oxidative stress, and apoptosis [6], [7], [82]. Furthermore, the fact that apolipoprotein E (ApoE) ε4 allele, the strongest dose-dependent risk factor for late-onset sporadic AD [83], [84], [85], [86], also promotes insulin resistance supports the concept that impairments in brain insulin signaling mediate AD-type neurodegeneration. Correspondingly, brain insulin resistance and its associated deficits in cognition can be partially reversed by consistent implementation of healthy lifestyle choices [87], [88], [89], [90] or treatment with insulin sensitizers [91], that is, approaches that are currently used to treat peripheral insulin resistance disease states. In addition, intranasal insulin has been used to normalize brain insulin levels and improve cognition [92], [93], [94], [95], [96], [97] and glucose metabolism [98] in AD. Similarly, treatment with incretins could be used to stimulate endogenous production and release of insulin in the brain [19], [99].
The concept that brain insulin/IGF resistance and deficiency are at the core of AD neurodegeneration and link multiple facets of disease in both the i.c. STZ model and human cases of AD highlights the need to determine the underlying mechanisms of dysregulated signaling and metabolism in the brain. Understanding the dynamic alterations in brain insulin/IGF-1 signaling that mediate AD progression [6,19] is critical because abnormalities present in the early stages, for example, insulin deficiency, may be superseded by additional alterations in signaling due to insulin resistance [6], [7], [82], [100], [101], [102] and impairments in central nervous system expression of related gut hormones [103], [104], [105] that required different treatment strategies. Our overarching hypothesis is that alterations in RCAN1 and IDE expression are integrally related to the stages and mediators of AD neurodegeneration. In these regards, we hypothesize that ApoE genotype has modifying effects on both IDE and RCAN1 expressions and thereby drive brain insulin deficiency and resistance with AD progression.
2. Methods
2.1. Experimental model
A model of sporadic AD was produced in 8-week-old male and female Long Evans rats (6/group) by intracerebral (i.c.) administration of 0.9 mg/kg STZ (0.2 mg/rat) under ketamine/xylocaine anesthesia [5], [46]. Controls were administered as i.c. saline. The rats were sacrificed 6 weeks later, and the temporal lobes were snap frozen and stored at −80°C for molecular and biochemical studies. The use of experimental animals was approved by the Institutional Animal Care and Use Committee at the Lifespan Rhode Island Hospital and in accordance with guidelines established by the National Institutes of Health.
2.2. Human subjects
Postmortem fresh frozen human frontal lobe tissue samples from Brodmann Area 8/9 were obtained from the Duke Kathleen Price Bryan Brain Bank and Biorepository (Durham, NC). The banked brains are processed according to a standardized protocol that ensures storage of high-quality fresh frozen tissue for molecular and biochemical analyses and rendering of accurate histopathological diagnoses. In addition, the brain banking protocol includes genotyping for ApoE [ε3/ε3, ε3/ε4, or ε4/ε4] (https://neurology.duke.edu/research/research-centers/joseph-and-kathleen-bryan-alzheimers-disease-research-center/brain-bank). However, because the banked brains are deidentified, clinical information other than standard demographics is not available. For this study, we obtained 72 brain samples from men and women. The cases were grouped for analysis based on their Braak stages of AD and Apo E genotypes as follows: Braak 0-2 = normal aging, Braak 3-4 = moderate AD, Braak 5-6 = severe or advanced AD. Permission to use deidentified postmortem human brain tissue for this research was granted by the Lifespan Hospitals Institutional Review Board.
2.3. Quantitative reverse transcriptase polymerase chain reaction assays
Total RNA was extracted from 100-mg samples of fresh frozen temporal lobe using the RNeasy Mini Kit (Qiagen, Hilden, Germany). RNA was reverse transcribed using the AMV First Strand cDNA Synthesis Kit (Roche, Indianapolis, IL). The cDNA templates were used to measure RCAN1 and IDE by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) in a Roche LightCycler 480 System [106]. Primer pairs were designed using MacVector 12 software (Cary, NC) (Table 1). Relative mRNA abundance was calculated using the 2ˆΔCt method, with results normalized to actin or hypoxanthine phosphoribosyltransferase 1.
Table 1.
Primer pairs used for qRT-PCR analysis of human and rat IDE and RCAN genes
| Primers | Direction | Sequence | Position | Amplicon |
|---|---|---|---|---|
| Human | ||||
| IDE | Forward | TACCTCCGCTTGCTGATGAC | 2110 | 106 |
| IDE | Reverse | GGAGCTGAGGTATGAAGGCC | 2215 | |
| RCAN1 | Forward | AGGCTCCAGCTGCATAAGAC | 258 | 111 |
| RCAN1 | Reverse | CTGCTTGTCTGGATTTGGCG | 368 | |
| Rat | ||||
| IDE | Forward | CTGTGCCCCTTGTTTGATGC | 523 | 139 |
| IDE | Reverse | TGAAGGGGTGCTTGGGATTC | 661 | |
| RCAN1-2-3 | Forward | AACTTCAGCAACCCCCTGTC | 231 | 80 |
| RCAN1-2-3 | Reverse | AGTTTCATCTCCTTCCCCAGG | 310 |
Abbreviations: IDE, Insulin-degrading enzyme; RCAN1, regulator of calcineurin 1; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction.
2.4. Preparation of protein homogenates
Brain tissue samples were homogenized in buffer containing protease and phosphatase inhibitors using a TissueLyser II (Qiagen, Germantown, MD). Supernatants obtained after centrifugation (14000 × g for 10 min at 4°C) were used in enzyme-linked immunosorbent assays (ELISAs).
2.5. Direct binding duplex ELISAs
Direct binding duplex ELISAs measured immunoreactivity to RCAN1 and IDE, followed by large acidic ribosomal protein (RPLPO) [107] to normalize expression of target proteins. RCAN1 and IDE were detected with horseradish peroxidase–conjugated secondary antibody and Amplex UltraRed (Invitrogen, Carlsbad, CA), and RPLPO was subsequently detected with biotinylated anti-RPLPO (Proteintech Group Inc, Chicago, IL) and streptavidin-conjugated alkaline phosphatase with 4-methylumbelliferyl phosphate (4-MUP). Fluorescence intensities (Amplex Red: Ex565/Em595; 4-MUP: Ex360/Em450) were measured in a SpectraMax M5 (Molecular Devices, Sunnyvale, CA). The calculated specific protein-to-RPLPO ratios were used for intergroup statistical comparisons.
2.6. Statistics
Data corresponding to group means ± standard deviation are tabulated. Intergroup comparisons were made using Student t tests or analysis of variance with post hoc Tukey tests using GraphPad Prism 7 software (San Diego, CA).
2.7. Sources of reagents
Mouse or rabbit polyclonal antibodies to RCAN1 and IDE were purchased from Abcam (Cambridge, MA). Rabbit polyclonal antibody to RPLPO (RPL23 16086-1-AP) was from Proteintech Inc (Chicago, IL). The Amplex UltraRed fluorophore horseradish peroxidase and 4-MUP alkaline phosphatase substrates were obtained from Invitrogen (Carlsbad, CA).
3. Results
3.1. Effects of i.c. STZ on IDE and RCAN1 expression
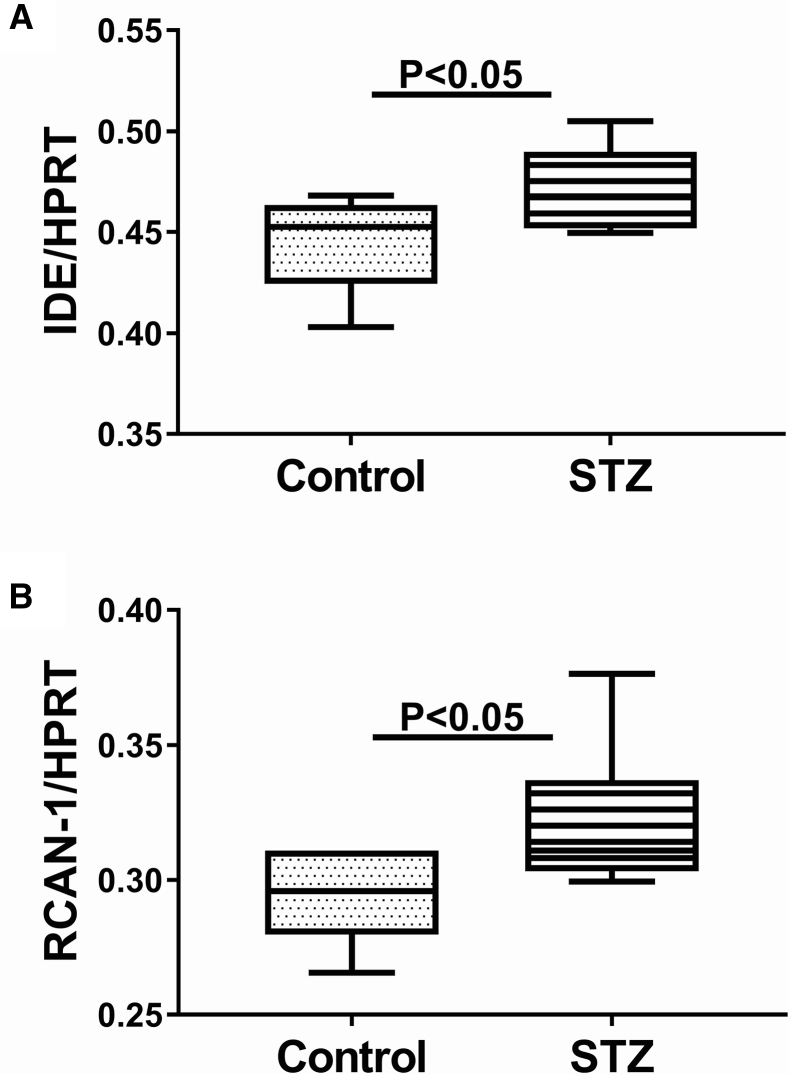
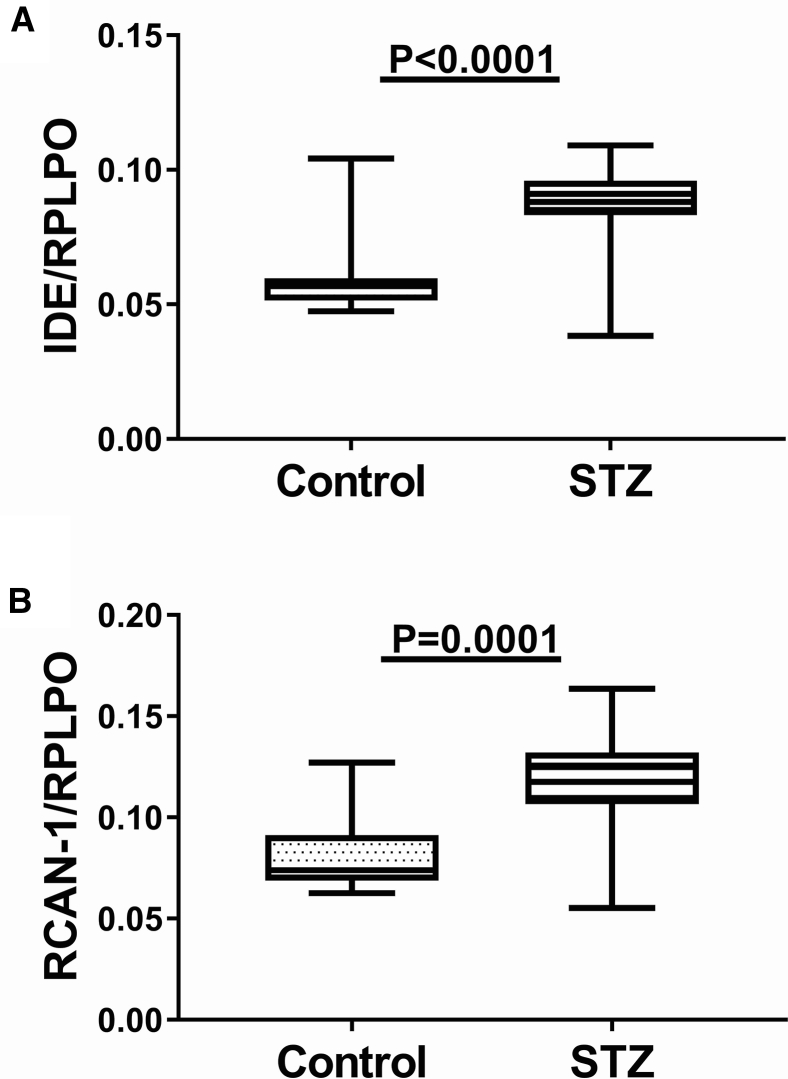
The mean mRNA level of IDE measured by qRT-PCR analysis and normalized to hypoxanthine phosphoribosyltransferase was significantly higher in i.c. STZ temporal lobe samples relative to control (Fig. 1A). Duplex ELISA also demonstrated upregulation of IDE in i.c. STZ brains with a more striking difference from control compared with the effects on mRNA (Fig. 2A). In addition, RCAN1 mRNA (Fig. 1B) and protein (Fig. 2B) expression were also significantly elevated in the i.c. STZ temporal lobe samples relative to control.
Fig. 1.
Increased temporal lobe mRNA expression of IDE and RCAN1 in i.c. STZ-treated rats. Long Evans rats were administered single i.c. injections of STZ or vehicle at 4 weeks of age (N = 6/group). The rats were sacrificed 4 weeks later to measure temporal lobe mRNA expression of (A) IDE and (B) RCAN1 by qRT-PCR analysis, with results normalized to HPRT measured simultaneously in the same samples. Graphs depict box plots with mean levels of expression (horizontal bars), 95% confidence interval limits (upper and lower boundaries of the boxes) and ranges (upper and lower stems). Student t tests were used for intergroup comparisons. Abbreviations: IDE, Insulin-degrading enzyme; RCAN1, regulator of calcineurin 1; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction; HPRT, hypoxanthine phosphoribosyltransferase; STZ, streptozotocin.
Fig. 2.
Increased temporal lobe protein expression of IDE and RCAN1 in i.c. STZ-treated rats. Long Evans rats were given single i.c. injections of STZ or vehicle at 4 weeks of age (N = 6/group). Four weeks later, the rats were sacrificed to measure temporal lobe protein expression of (A) IDE and (B) RCAN1 by duplex direct-binding ELISAs with results normalized to RPLPO measured sequentially in the same samples. Graphs depict box plots with mean levels of expression (horizontal bars), 95% confidence interval limits (upper and lower boundaries of the boxes), and ranges (upper and lower stems). Student t tests were used for intergroup comparisons. Abbreviations: IDE, Insulin-degrading enzyme; RCAN1, regulator of calcineurin 1; STZ, streptozotocin; ELISA, enzyme-linked immunosorbent assays; RPLPO, ribosomal protein.
3.2. Subject population
All brains included in this study had been banked using an established protocol including dissections and snap freezing of fresh frozen pieces from specific regions. Formalin-fixed paraffin-embedded histological sections were used to render diagnoses and stage severity of AD. Among the 72 cases studied, 38 were genotyped as ApoE ε3/ε3, 25 as ApoE ε3/ε4, and 9 as ApoE ε4/ε4 (Table 2). Analysis of variance with the Tukey post hoc test (repeated measures with 5% false discovery correction) demonstrated no significant differences in mean age among the subgroups, except for ApoE ε3/ε4, Braak 0-2 which were significantly younger than the AD group but not the ApoE ε3/ε3 controls. Owing to the small number of Braak 0-2, ApoE ε3/ε4 cases, control data from the ApoE ε3/ε3 and ApoE ε3/ε4 cases were combined for statistical comparisons with those of the AD groups.
Table 2.
Human subjects
| Stage | Apolipoprotein E ε3/ε3 |
Apolipoprotein E ε3/ε4 |
Apolipoprotein E ε4/ε4 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| # | Age | M/F | # | Age | M/F | # | Age | M/F | |
| Braak 0-2 | 18 | 78.33 ± 11.16 | 9 M; 9 F | 3 | 72.33 ± 14.15 | 2 M; 1 F | 0 | ||
| Braak 3-4 | 14 | 81.57 ± 6.81 | 6 M; 8 F | 15 | 84.13 ± 4.02 | 9 M; 6 F | 3 | 84.03 ± 3.60 | 2 M; 1 F |
| Braak 5-6 | 6 | 84.01 ± 2.45 | 3 M; 3 F | 7 | 83.71 ± 5.47 | 2 M 5 F | 6 | 81.11 ± 4.47 | 3 M; 3 F |
NOTE. Postmortem banked fresh frozen frontal lobe brain samples were used for qRT-PCR analysis and ELISA measurements of IDE and RCAN1. ANOVA with post hoc Tukey tests (multiple comparisons with 5% false discovery correction) revealed that the Braak 0-2, Apo E ε3/ε4 group was significantly younger than the AD groups (P < .05).
Abbreviations: AD, Alzheimer's disease; M, male; F, female; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction; ELISA, enzyme-linked immunosorbent assays; IDE, insulin-degrading enzyme; ANOVA, analysis of variance; RCAN1, regulator of calcineurin 1.
3.3. AD Braak Stage and ApoE genotype effects on IDE expression
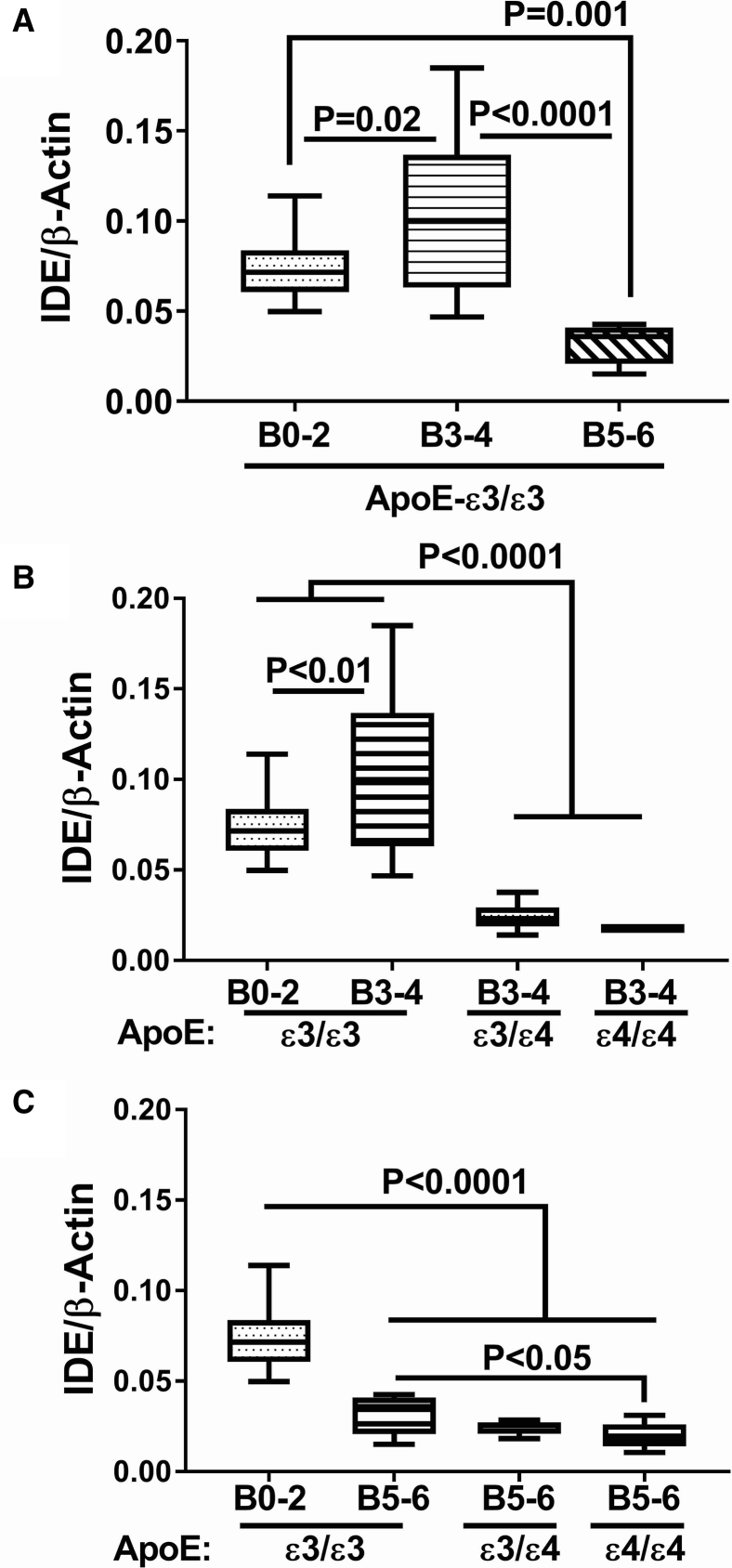
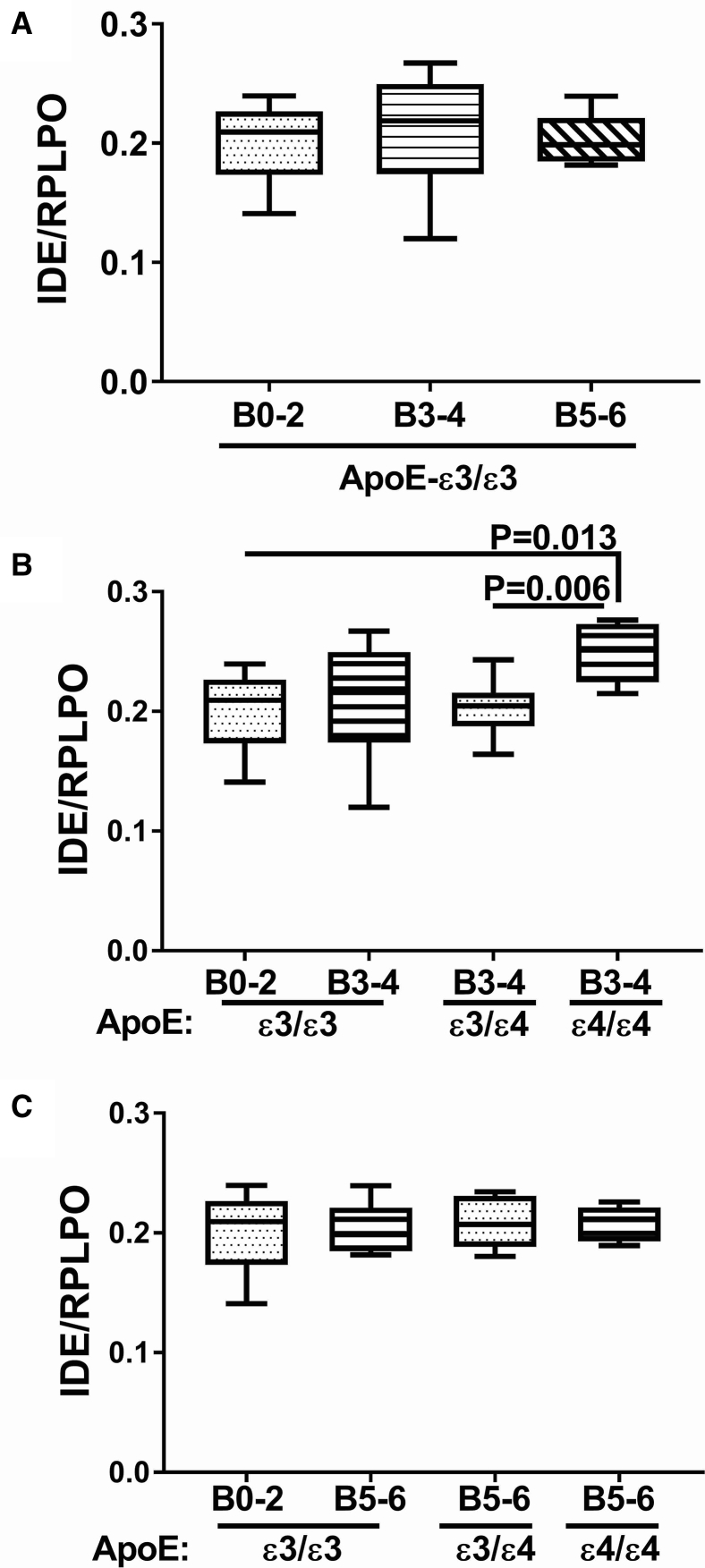
Among cases with an ApoE ε3/ε3 genotype, the mean level of IDE mRNA was highest at Braak stage 3-4, whereas with advanced AD pathology (Braak 5-6), the mean level of IDE was significantly reduced relative to control (Braak stage 0-2) and AD (Braak stage 3-4) (Fig. 3A). Focusing on the effects of ApoE ε4 dose, at Braak Stages 3-4, IDE mRNA levels were significantly and similarly reduced in brains from patients with an ApoE ε3/ε4 or ε4/ε4 genotype (Fig. 3B). IDE mRNA levels were significantly reduced in brains with Braak 5-6 stage AD, irrespective of ApoE genotype (Fig. 3C). However, a modest but significant ApoE ε4 dose effect further reduced IDE expression in brains from subjects with ApoE ε4/ε4 compared with ApoE ε3/ε3 (Fig. 3C). In contrast to the mRNA levels, IDE protein expression was almost invariant with respect to AD Braak stage and ApoE genotype (Fig. 4). The only exception was that IDE protein expression was significantly elevated at AD Braak stage 3-4 from patients with ApoE ε4/ε4 compared with the other ApoE genotypes (Fig. 4B).
Fig. 3.
Effects of AD Braak stage and ApoE genotype on IDE gene expression in human postmortem brains. Fresh frozen postmortem human frontal lobe tissue samples from cases with Braak stage [B] 0-2 (normal aging), B3-4 (moderate AD), or B5-6 (severe AD) were used to measure IDE mRNA expression by qRT-PCR analysis with results normalized to β-actin mRNA measured simultaneously in the same samples. Statistical comparisons assessed changes in IDE mRNA expression in relation to: (A) AD severity in cases with ApoE ε3/ε3 genotype, (B) ApoE genotype in cases with moderate AD (B3-4), and (C) ApoE genotype in cases with severe AD. Graphs depict box plots with mean levels of expression (horizontal bars), 95% confidence interval limits (upper and lower boundaries of the boxes), and ranges (upper and lower stems). One-way ANOVA with post hoc repeated measures Tukey tests were used for intergroup comparisons. Abbreviations: AD, Alzheimer's disease; ANOVA, analysis of variance; ApoE, apolipoprotein E; IDE, insulin-degrading enzyme; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction.
Fig. 4.
Effects of AD Braak stage and ApoE genotype on IDE protein expression in human postmortem brains. Fresh frozen postmortem human frontal lobe tissue samples from cases with Braak stage (B) 0-2 (normal aging), B3-4 (moderate AD), or B5-6 (severe AD) were used to measure IDE protein expression by duplex ELISA with results normalized to RPLPO measured simultaneously in the same samples. Graphs depict box plots with mean levels of expression (horizontal bars), 95% confidence interval limits (upper and lower boundaries of the boxes), and ranges (upper and lower stems). Statistical comparisons assessed changes in IDE protein expression in relation to: (A) AD severity in cases with ApoE ε3/ε3 genotype, (B) ApoE genotype in cases with moderate AD (B3-4), and (C) ApoE genotype in cases with severe AD. One-way ANOVA with post hoc repeated measures Tukey tests were used for intergroup comparisons. Abbreviations: AD, Alzheimer's disease; ANOVA, analysis of variance; ApoE, apolipoprotein E; IDE, Insulin-degrading enzyme; RPLPO, ribosomal protein; ELISA, enzyme-linked immunosorbent assays; RPLPO, ribosomal protein.
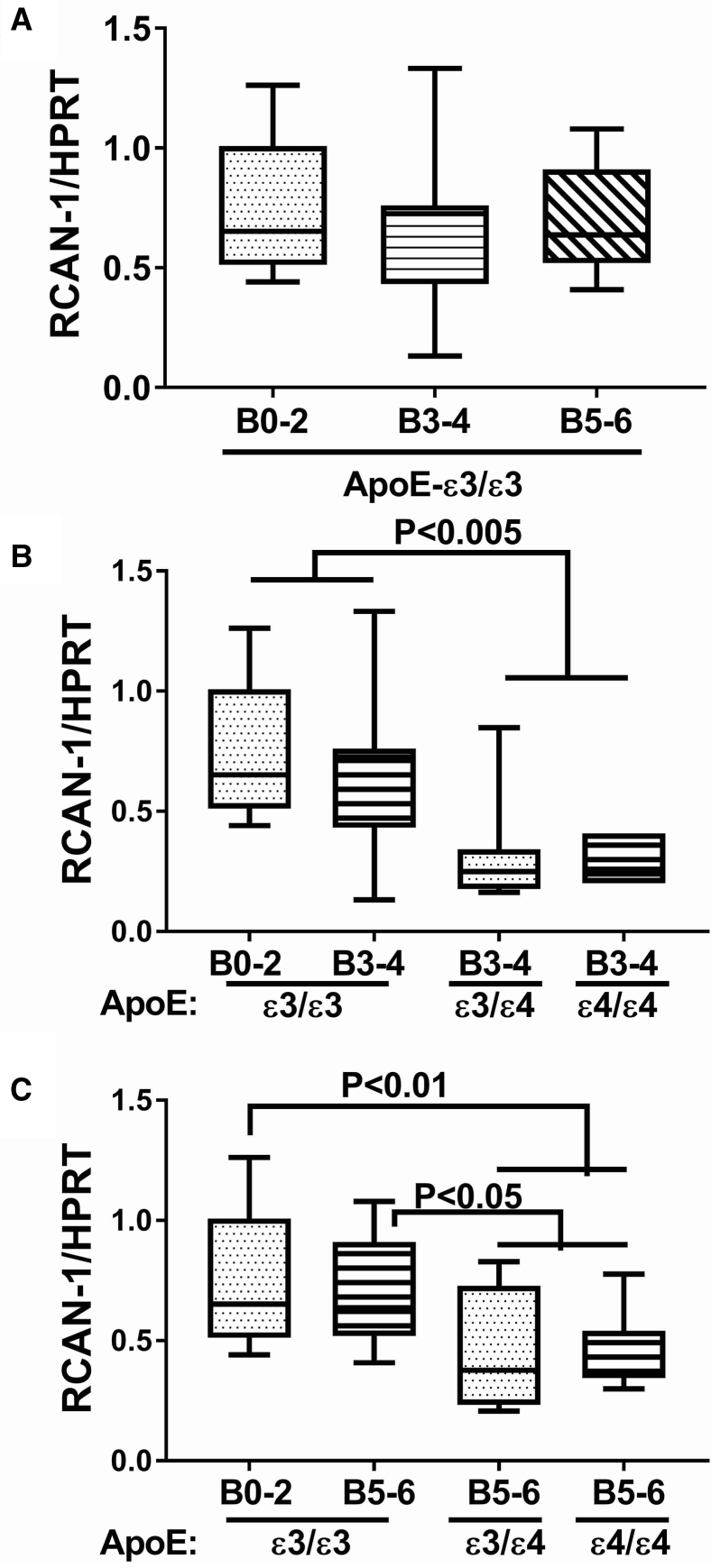
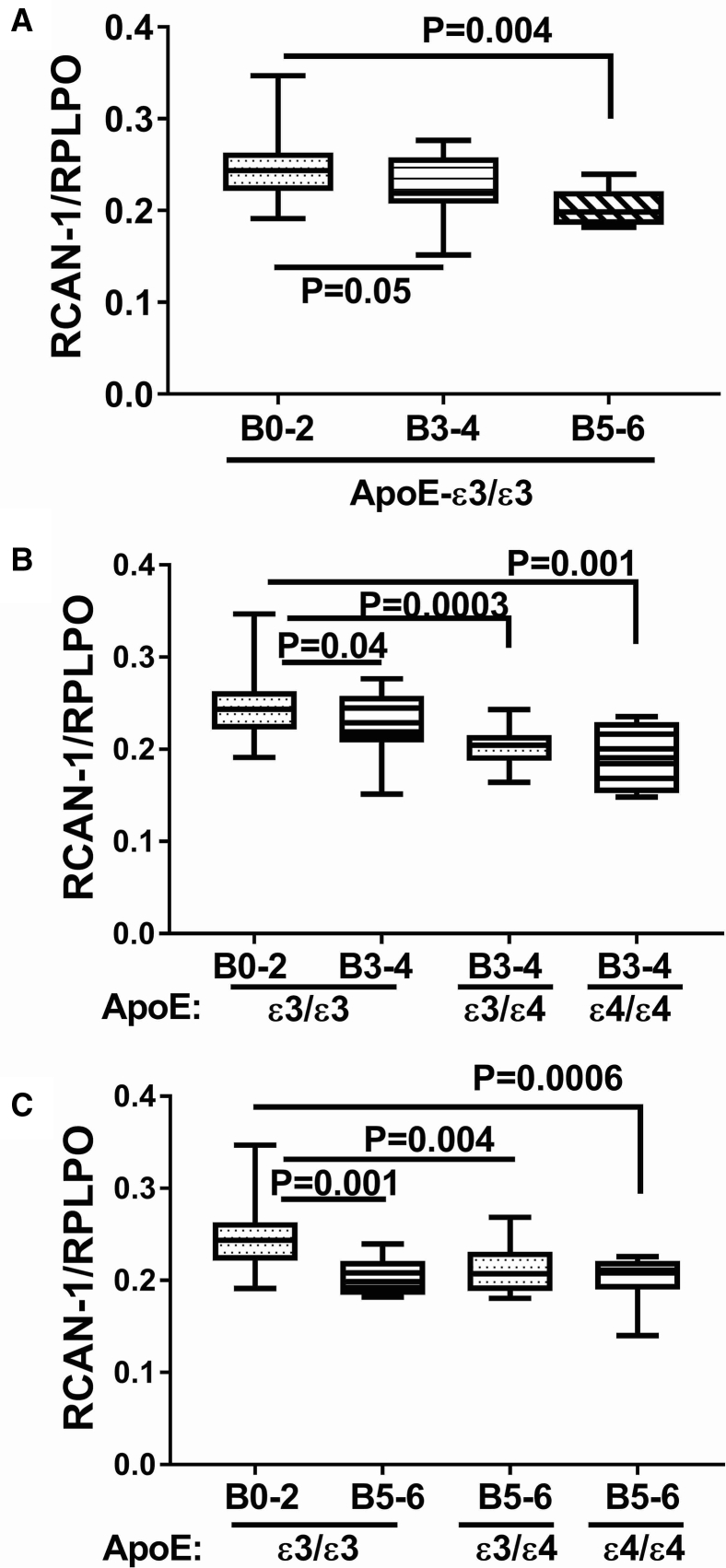
3.4. Effects of AD Braak stage and ApoE genotype on RCAN expression
Among the ApoE ε3/ε3 cases, the mean levels of RCAN1 mRNA were similar, that is, invariant with respect to AD Braak stage (Fig. 5A). In contrast, ApoE ε4 genotype had significant inhibitory effects on RCAN1 expression both at AD Braak 3-4 (Fig. 5B) and Braak 5-6 (Fig. 5C). It is noteworthy that significant reductions RCAN1 mRNA were observed for ApoE ε3/ε4 and ε4/ε4 relative to ApoE ε3/ε3 in brains with either moderate or severe AD; however, an ApoE ε4 dose effect was not detected. In general, the effects of AD grade and ApoE genotype on RCAN1 protein expression were concordant with their effects on the mRNA. In cases with an ApoE ε3/ε3 genotype, RCAN1 protein expression progressively and significantly declined with AD Braak stage severity (Fig. 6A). In cases with an ApoE ε3/ε4 genotype, frontal lobe RCAN1 protein expression also progressively and significantly declined with AD Braak stage severity, and the levels in all AD subgroups were significantly lower than those in control (Fig. 6B). At AD Braak stage 5-6, the mean levels of RCAN1 protein were significantly and similarly reduced relative to control for all ApoE genotypes (Fig. 6C).
Fig. 5.
Effects of AD Braak stage and ApoE genotype on RCAN1 gene expression in human postmortem brains. Fresh frozen postmortem human frontal lobe tissue samples from cases with Braak stage (B) 0-2 (normal aging), B3-4 (moderate AD), or B5-6 (severe AD) were used to measure RCAN1 mRNA expression by qRT-PCR analysis with results normalized to HPRT mRNA measured simultaneously in the same samples. Graphs depict box plots with mean levels of expression (horizontal bars), 95% confidence interval limits (upper and lower boundaries of the boxes), and ranges (upper and lower stems). Statistical comparisons assessed changes in RCAN-1 mRNA expression in relation to: (A) AD severity in cases with ApoE ε3/ε3 genotype, (B) ApoE genotype in cases with moderate AD (B3-4), and (C) ApoE genotype in cases with severe AD. One-way ANOVA with post hoc repeated measures Tukey tests were used for intergroup comparisons. Abbreviations: AD, Alzheimer's disease; ANOVA, analysis of variance; ApoE, apolipoprotein E; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction; RCAN1, regulator of calcineurin 1; HPRT, hypoxanthine phosphoribosyltransferase.
Fig. 6.
Effects of AD Braak stage and ApoE genotype on RCAN1 protein expression in human postmortem brains. Fresh frozen postmortem human frontal lobe tissue samples from cases with Braak stage (B) 0-2 (normal aging), B3-4 (moderate AD), or B5-6 (severe AD) were used to measure RCAN-1 protein expression by duplex ELISA with results normalized to RPLPO measured simultaneously in the same samples. Statistical comparisons assessed changes in RCAN-1 protein expression in relation to: (A) AD severity in cases with ApoE ε3/ε3 genotype, (B) ApoE genotype in cases with moderate AD (B3-4), and (C) ApoE genotype in cases with severe AD. Graphs depict box plots with mean levels of expression (horizontal bars), 95% confidence interval limits (upper and lower boundaries of the boxes), and ranges (upper and lower stems). One-way ANOVA with post hoc repeated measures Tukey tests were used for intergroup comparisons. Abbreviations: AD, Alzheimer's disease; ANOVA, analysis of variance; ApoE, apolipoprotein E; ELISA, enzyme-linked immunosorbent assays; IDE, insulin-degrading enzyme; RCAN1, regulator of calcineurin 1; HPRT, hypoxanthine phosphoribosyltransferase; RPLPO, ribosomal protein.
4. Discussion
The progressive declines in brain expression of genes and proteins required for insulin signaling as well as evidence for increased insulin resistance with severity of AD led to the concept that AD is a form of brain diabetes mellitus, that is, type 3 diabetes [6], [7]. Deficiencies in insulin supply could be mediated by reduced expression of mRNA transcripts in the brain [6], [7], [46], [48], [104] or impaired transport of insulin from the peripheral circulation [108], [109], [110]; the main source of insulin in the brain is thought to be from the peripheral circulation [97]. In addition, insulin resistance marked by reduced receptor responsiveness also contributes to neurodegeneration due to the associated impairments in downstream signaling through multifaceted PI3K-Akt pathways. Insulin resistance can be driven by insulin deficiency due to trophic factor withdrawal–mediated injury or killing of insulin responsive (receptor-bearing) cells or failure of systems that mediate insulin uptake. Regardless of the mechanism, insufficient supply of insulin and impaired insulin receptor responsiveness compromise glucose utilization, which is needed for energy metabolism, neuronal plasticity, cognition, cell survival, mitochondrial function [16], [19], and inhibition of neuroinflammation and oxidative stress, all of which are linked to AD neurodegeneration, that is, aberrant phosphorylation of tau and Aβ accumulation in the brain [16], [19]. These concepts are supported by findings in AD that working memory and cognition are improved by the administration of intranasal insulin [92], [93], [95], [111], [112], [113] and suggest that one of the core avenues for preventing or reducing severity of neurodegeneration is to increase understanding of the causes of brain insulin deficiency.
Potential roles for aberrant IDE and RCAN1 expression as mediators of brain insulin deficiency and ultimately insulin resistance in AD have been suggested due to their known roles in Down syndrome–associated neurodegeneration [114], [115] and diabetes mellitus [116]. However, studies in humans and experimental animals have led to inconsistent and inconclusive findings. Herein, we have readdressed this question by simultaneously examining cerebral mRNA and protein levels of IDE and RCAN1 in an established i.c. STZ rat model of sporadic AD [46], [48], [76], [81], [117] and in histopathologically well-characterized human postmortem brains with normal aging, moderately severe AD (Braak 3-4), or advanced AD (Braak 5-6) pathology from patients with an ApoE ε3/ε3, ε3/ε4, or ε4/ε4 genotype.
In a previous study, we confirmed that the i.c. STZ model was associated with increased temporal lobe levels of pTau, ubiquitin, Aβ, and SNAP-25 and reduced expression of synaptophysin as in AD [6], [7], [82], [104]. In addition, i.c. STZ significantly reduced the mean levels of Akt, p70S6K, mechanistic target of rapamycin, and phosphorylated glycogen synthase kinase 3β [11], [17], [18], [48], [118], which would compromise both insulin and IGF-1 signaling through neuronal and glial metabolic pathways, as well as account for the observed increases in brain levels of pTau and Aβ in the i.c. STZ model.
Our studies demonstrated moderately but significantly higher levels of IDE and RCAN1 mRNA and more pronounced increases in the corresponding proteins in temporal lobe tissue from the i.c. STZ-treated relative to control rats. The finding of increased RCAN1 expression is consistent with previous observations in human AD brain neurons and Down syndrome brains [23], [25], [26], [119], [120] and supports the concept that upregulated RCAN1 expression can have a role in neurodegeneration. Mechanistically, increased RCAN1 promotes GSK-3β activity [55], [56], which increases tau phosphorylation [57]. Mitochondrial dysfunction, oxidative stress, and neuroinflammation promoted by i.c. STZ [25], [74], [75] could also have increased RCAN1 expression and thereby further activate GSK-3β–driven pathogenic processes, including tau phosphorylation.
The significantly elevated temporal lobe expression levels of IDE mRNA and protein observed in our i.c. STZ rat model discordant with previous findings in closely related in vivo models [79], [116], STZ-treated astrocyte cultures [121], transgenic AD mouse models [122], [123], [124], and human AD gene linkage studies [125]. On the other hand, the responses were concordant with findings in human AD brains [126], [127], genetic models of AD [128], and experimental i.c. STZ [129] or toxin [130] exposure models, and they were partially concordant with experimental models of insulin resistance and diabetes mellitus [131]. Variability in IDE responses could be linked to the genotypic or phenotypic features of the models or the specific experimental conditions used to generate the models. For example, genetic models that caused high levels of Aβ or that used high doses of i.c. STZ (4 mg/rat) with shorter intervals to study (2 weeks) than in the present study (0.3-0.4 mg/rat and 6 weeks) reported inhibition of IDE. Based on the experimental conditions used herein, we conclude that brain insulin deficiency and resistance in the i.c. STZ could be mediated by upregulated expression of RCAN1 and IDE.
Besides neurons, microglia and astrocytes are potential sources of RCAN1 and IDE [126], [132], [133] expression. IDE and RCAN1 expression have been detected in activated microglia and astrocytes by immunohistochemical staining and analysis of cultured cells [49], [132], [133], [134]. In human AD and the i.c. STZ model, increased activation of microglia and astrocytes is at the core of the neuroinflammation, which is linked to many aspects of neurodegeneration [19]. In this study, the finding that the temporal lobes of i.c. STZ-treated rats had constitutively increased levels of RCAN1 and IDE expression may suggest that these responses could serve as etiopathic cofactors of brain insulin deficiency. We propose that oxidative stress driven by progressive neuroinflammatory and gliotic pathologies may have a role in activating RCAN1 and IDE, thereby causing brain insulin deficiency in the setting of progressive insulin resistance in AD, Down syndrome, and i.c. STZ models, that is, type 3 diabetes.
In contrast to the i.c. STZ model, the human brain studies demonstrated reduced RCAN1 expression in AD at both mRNA and protein levels, with the major inhibitory effects associated with ApoE-ε4 dose rather than severity of AD. These findings contrast with the i.c. STZ results and with earlier studies demonstrating higher levels of RCAN1 in AD, Down syndrome, and Down syndrome + AD relative to normal control brains [25], [119], [120]. However, the outcome differences could be attributed in part to the facts that in one study, the investigators used in situ approaches to assess neuronal labeling [119], yet RCAN1 is expressed in various cell types including activated microglia and astrocytes [119], [135], [136], and in another article, the analysis was focused on Down syndrome and Down syndrome + AD [120] rather than sporadic AD or AD associated with the ApoE ε4 allele. The reductions in RCAN1 expression demonstrated by both qRT-PCR analysis and ELISAs strengthen the conclusion that RCAN1 expression declines with severity of AD but more strikingly with ApoE ε4 dose. Conceivably, the disparate observations that the i.c. STZ model and human AD could reflect could be explained by differences in disease durations and the greater complexity of AD versus i.c. STZ-mediated neurodegeneration. To address the question of timing, that is, disease duration, future studies should assess RCAN1 expression in very early stages of AD to determine if the levels initially increase but subsequently decline, perhaps as a compensatory mechanism to preserve brain metabolic homeostasis. Correspondingly, elevated RCAN1 expression has been detected in peripheral blood lymphocytes of asymptomatic healthy young carriers of ApoE-ε4 [137].
In the present study of human brains, IDE mRNA was upregulated in moderate AD (Braak 3-4) among patients with ApoE ε3/ε3 genotype, whereas IDE protein expression was significantly elevated only in patients with Braak 3-4 AD and an ApoE ε4/ε4 genotype. Importantly, the findings suggest that IDE upregulated expression in AD occurs at intermediate stages of neurodegeneration and at least partly linked to ApoE ε4. IDE's upregulation in the earlier rather than late stages of AD could account for insulin depletion in the brain. On the other hand, its downregulation in the later stages of AD could be tied to accumulations of Aβ with disease progression, particularly in ApoE ε4 carriers or homozygotes. In previous studies, variability in findings pertaining to alterations in IDE expression [127], with some studies reporting increased IDE in AD brains [128], [138] and others showing reduced [122], [123], [139] or selective cellular increases [126] in IDE, could have been due to heterogeneity in AD stage and ApoE ε4 genotypes. Although genetic linkage studies failed to demonstrate significant or generalizable associations between IDE and AD [138], several polymorphisms have been shown to either increase or decrease AD risk [139], [140], [141].
5. Conclusions
The findings in this study illustrate that in RCAN1 and IDE, mRNA and protein expressions are both altered in the i.c. STZ model of sporadic AD and in humans with AD, particularly when associated with ApoE ε4. In the i.c. STZ model, which exhibits brain pathology and neurobehavioral deficits that correspond to the early or intermediate stages of AD, both RCAN and IDE expression were upregulated at mRNA and protein levels. These results suggest that several key molecular, biochemical, and structural abnormalities including tau phosphorylation, reduced brain insulin levels, and oxidative stress/neuroinflammation in the early stages of AD could be mediated in part by increased expression of RCAN and/or IDE, similar to the findings in Down syndrome. Corresponding with results obtained with the i.c. STZ model, increased IDE expression was detected in brains with moderate (Braak 3-4) but not advanced (Braak 5-6) AD, suggesting that IDE plays a role in insulin deficiency and attendant insulin resistance in the early and intermediate stages of AD. However, in contrast, human brains with moderate or advanced AD mainly exhibited reductions in RCAN and IDE expression, which may represent compensatory homeostatic responses mobilized to maintain brain function.
Research in context.
-
1.
Systematic review: The authors searched national and international biomedical literature databases, including PubMed and Google Scholar to perform a systematic review of the current state of knowledge related to the main scientific questions. The database searches were from 1990 to 2019.
-
2.Interpretation: The authors have objectively interpreted their results and declare that their findings contribute to the accumulated knowledge base because:
-
a.The work covers new territory about potential mechanisms of brain insulin deficiency in Alzheimer’s disease (AD).
-
b.In an established rat model of sporadic AD in which intracerebral streptozotocin produces all typical features of AD, including brain insulin deficiency and insulin resistance, temporal lobe levels of both insulin degrading enzyme (IDE) and regulator of calcineurin-1 (RCAN1) were significantly elevated with neurodegeneration.
-
c.In humans, altered expression of IDE and RCAN1 shifted with apolipoprotein E (ApoE-ε4) genotype dose and severity of neurodegeneration, with higher levels of IDE correlating with earlier stages of disease when brain insulin levels decline.
-
a.
-
3.
Future directions: Future research directions should mechanistically assess the roles IDE and RCAN1 play in neurodegeneration by determining if their inhibition favorably alters the course of neurodegeneration.
Acknowledgments
The authors thank Dr Emine B. Yalcin for assisting with technical aspects of the qRT-PCR studies. This research was supported by grants AA11431, AA024092, and NS096525 from the National Institutes of Health.
Footnotes
Conflict of Interest: The authors declare that there is no conflict of interest.
References
- 1.Reitz C., Brayne C., Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7:137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayeux R., Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harbor Perspect Med. 2012;2 doi: 10.1101/cshperspect.a006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao W.Q., Lacor P.N., Chen H., Lambert M.P., Quon M.J., Krafft G.A. Insulin receptor dysfunction impairs cellular clearance of neurotoxic oligomeric a{beta} J Biol Chem. 2009;284:18742–18753. doi: 10.1074/jbc.M109.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correia S.C., Santos R.X., Perry G., Zhu X., Moreira P.I., Smith M.A. Insulin-resistant brain state: The culprit in sporadic Alzheimer's disease? Ageing Res Rev. 2011;10:264–273. doi: 10.1016/j.arr.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoyer S. Is sporadic Alzheimer disease the brain type of non-insulin dependent diabetes mellitus? A challenging hypothesis. J Neural Transm. 1998;105:415–422. doi: 10.1007/s007020050067. [DOI] [PubMed] [Google Scholar]
- 6.Rivera E.J., Goldin A., Fulmer N., Tavares R., Wands J.R., de la Monte S.M. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer's disease: Link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 7.Steen E., Terry B.M., Rivera E.J., Cannon J.L., Neely T.R., Tavares R. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease–is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 8.Talbot K. O3-02-02: Expression of pIRS-1 (S312 and S616) is elevated in MCI and AD and correlates with cognitive impairment and neurofibrillary pathology. Alzheimer Dement. 2006;2:S54. [Google Scholar]
- 9.Salkovic-Petrisic M., Hoyer S. Central insulin resistance as a trigger for sporadic Alzheimer-like pathology: An experimental approach. J Neural Transm Suppl. 2007:217–233. doi: 10.1007/978-3-211-73574-9_28. [DOI] [PubMed] [Google Scholar]
- 10.Zhao W.Q., De Felice F.G., Fernandez S., Chen H., Lambert M.P., Quon M.J. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008;22:246–260. doi: 10.1096/fj.06-7703com. [DOI] [PubMed] [Google Scholar]
- 11.de la Monte S.M. Insulin resistance and Alzheimer's disease. BMB Rep. 2009;42:475–481. doi: 10.5483/bmbrep.2009.42.8.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong M., Neusner A., Longato L., Lawton M., Wands J.R., de la Monte S.M. Nitrosamine exposure causes insulin resistance diseases: Relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and Alzheimer's disease. J Alzheimers Dis. 2009;17:827–844. [PMC free article] [PubMed] [Google Scholar]
- 13.Moloney A.M., Griffin R.J., Timmons S., O'Connor R., Ravid R., O'Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer's disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2010;31:224–243. doi: 10.1016/j.neurobiolaging.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Kuljis R.O., Salkovic-Petrisic M. Dementia, diabetes, Alzheimer's disease, and insulin resistance in the brain: progress, dilemmas, new opportunities, and a hypothesis to tackle intersecting epidemics. J Alzheimers Dis. 2011;25:29–41. doi: 10.3233/JAD-2011-101392. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y., Liu F., Grundke-Iqbal I., Iqbal K., Gong C.X. Deficient brain insulin signalling pathway in Alzheimer's disease and diabetes. J Pathol. 2011;225:54–62. doi: 10.1002/path.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de la Monte S.M. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer's disease. Curr Alzheimer Res. 2012;9:35–66. doi: 10.2174/156720512799015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de la Monte S.M. Contributions of brain insulin resistance and deficiency in amyloid-related neurodegeneration in Alzheimer's disease. Drugs. 2012;72:49–66. doi: 10.2165/11597760-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de la Monte S.M. Therapeutic targets of brain insulin resistance in sporadic Alzheimer's disease. Front Biosci (elite Ed) 2012;4:1582–1605. doi: 10.2741/482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de la Monte S.M. Insulin resistance and neurodegeneration: Progress towards the development of new therapeutics for Alzheimer's disease. Drugs. 2017;77:47–65. doi: 10.1007/s40265-016-0674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de la Monte S.M. Relationships between diabetes and cognitive impairment. Endocrinol Metab Clin North Am. 2014;43:245–267. doi: 10.1016/j.ecl.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jha N.K., Jha S.K., Kumar D., Kejriwal N., Sharma R., Ambasta R.K. Impact of insulin degrading enzyme and neprilysin in Alzheimer's disease biology: Characterization of putative cognates for therapeutic applications. J Alzheimers Dis. 2015;48:891–917. doi: 10.3233/JAD-150379. [DOI] [PubMed] [Google Scholar]
- 22.Tang W.J. Targeting insulin-degrading enzyme to treat type 2 diabetes mellitus. Trends Endocrinol Metab. 2016;27:24–34. doi: 10.1016/j.tem.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ermak G., Davies K.J. Chronic high levels of the RCAN1-1 protein may promote neurodegeneration and Alzheimer disease. Free Radic Biol Med. 2013;62:47–51. doi: 10.1016/j.freeradbiomed.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lloret A., Fuchsberger T., Giraldo E., Vina J. Molecular mechanisms linking amyloid beta toxicity and Tau hyperphosphorylation in Alzheimers disease. Free Radic Biol Med. 2015;83:186–191. doi: 10.1016/j.freeradbiomed.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 25.Peiris H., Keating D.J. The neuronal and endocrine roles of RCAN1 in health and disease. Clin Exp Pharmacol Physiol. 2018;45:377–383. doi: 10.1111/1440-1681.12884. [DOI] [PubMed] [Google Scholar]
- 26.Wu Y., Ly P.T., Song W. Aberrant expression of RCAN1 in Alzheimer's pathogenesis: A new molecular mechanism and a novel drug target. Mol Neurobiol. 2014;50:1085–1097. doi: 10.1007/s12035-014-8704-y. [DOI] [PubMed] [Google Scholar]
- 27.Authier F., Posner B.I., Bergeron J.J. Insulin-degrading enzyme. Clin Invest Med Medecine clinique experimentale. 1996;19:149–160. [PubMed] [Google Scholar]
- 28.Farris W., Mansourian S., Chang Y., Lindsley L., Eckman E.A., Frosch M.P. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim M., Hersh L.B., Leissring M.A., Ingelsson M., Matsui T., Farris W. Decreased catalytic activity of the insulin-degrading enzyme in chromosome 10-linked Alzheimer disease families. J Biol Chem. 2007;282:7825–7832. doi: 10.1074/jbc.M609168200. [DOI] [PubMed] [Google Scholar]
- 30.Reitz C., Cheng R., Schupf N., Lee J.H., Mehta P.D., Rogaeva E. Association between variants in IDE-KIF11-HHEX and plasma amyloid beta levels. Neurobiol Aging. 2012;33:199.e13–199.e17. doi: 10.1016/j.neurobiolaging.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo W.L., Montag A.G., Rosner M.R. Insulin-degrading enzyme is differentially expressed and developmentally regulated in various rat tissues. Endocrinology. 1993;132:604–611. doi: 10.1210/endo.132.2.7678795. [DOI] [PubMed] [Google Scholar]
- 32.Authier F., Bergeron J.J., Ou W.J., Rachubinski R.A., Posner B.I., Walton P.A. Degradation of the cleaved leader peptide of thiolase by a peroxisomal proteinase. Proc Natl Acad Sci U S A. 1995;92:3859–3863. doi: 10.1073/pnas.92.9.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leissring M.A., Farris W., Wu X., Christodoulou D.C., Haigis M.C., Guarente L. Alternative translation initiation generates a novel isoform of insulin-degrading enzyme targeted to mitochondria. Biochem J. 2004;383:439–446. doi: 10.1042/BJ20041081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu W.Q., Walsh D.M., Ye Z., Vekrellis K., Zhang J., Podlisny M.B. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J Biol Chem. 1998;273:32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- 35.Vekrellis K., Ye Z., Qiu W.Q., Walsh D., Hartley D., Chesneau V. Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J Neurosci. 2000;20:1657–1665. doi: 10.1523/JNEUROSCI.20-05-01657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirsky I.A., Broh-Kahn R.H. The inactivation of insulin by tissue extracts; the distribution and properties of insulin inactivating extracts. Arch Biochem. 1949;20:1–9. [PubMed] [Google Scholar]
- 37.Duckworth W.C., Bennett R.G., Hamel F.G. Insulin degradation: Progress and potential. Endocr Rev. 1998;19:608–624. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- 38.Kurochkin I.V., Goto S. Alzheimer's beta-amyloid peptide specifically interacts with and is degraded by insulin degrading enzyme. FEBS Lett. 1994;345:33–37. doi: 10.1016/0014-5793(94)00387-4. [DOI] [PubMed] [Google Scholar]
- 39.Tundo G.R., Sbardella D., Ciaccio C., Grasso G., Gioia M., Coletta A. Multiple functions of insulin-degrading enzyme: A metabolic crosslight? Crit Rev Biochem Mol Biol. 2017;52:554–582. doi: 10.1080/10409238.2017.1337707. [DOI] [PubMed] [Google Scholar]
- 40.Qiu W.Q., Folstein M.F. Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer's disease: Review and hypothesis. Neurobiol Aging. 2006;27:190–198. doi: 10.1016/j.neurobiolaging.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Li H., Cao L., Ren Y., Jiang Y., Xie W., Li D. GLP-1 receptor regulates cell growth through regulating IDE expression level in Abeta1-42-treated PC12 cells. Biosci Rep. 2017 doi: 10.1042/BSR20171284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haque R., Nazir A. Insulin-degrading enzyme: a link between Alzheimer's and type 2 diabetes mellitus. CNS Neurol Disord Drug Targets. 2014;13:259–264. doi: 10.2174/18715273113126660139. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J., Guo Y., Wang Y., Song L., Zhang R., Du Y. Long-term treadmill exercise attenuates Abeta burdens and astrocyte activation in APP/PS1 mouse model of Alzheimer's disease. Neurosci Lett. 2018;666:70–77. doi: 10.1016/j.neulet.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto N., Ishikuro R., Tanida M., Suzuki K., Ikeda-Matsuo Y., Sobue K. Insulin-signaling pathway regulates the degradation of amyloid beta-protein via astrocytes. Neuroscience. 2018;385:227–236. doi: 10.1016/j.neuroscience.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 45.de la Monte S.M., Tong M., Lester-Coll N., Plater M., Jr., Wands J.R. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: Relevance to Alzheimer's disease. J Alzheimers Dis. 2006;10:89–109. doi: 10.3233/jad-2006-10113. [DOI] [PubMed] [Google Scholar]
- 46.Lester-Coll N., Rivera E.J., Soscia S.J., Doiron K., Wands J.R., de la Monte S.M. Intracerebral streptozotocin model of type 3 diabetes: Relevance to sporadic Alzheimer's disease. J Alzheimers Dis. 2006;9:13–33. doi: 10.3233/jad-2006-9102. [DOI] [PubMed] [Google Scholar]
- 47.Tong M., Dominguez C., Didsbury J., de la Monte S.M. Targeting Alzheimer's disease neuro-metabolic dysfunction with a small molecule nuclear receptor agonist (T3D-959) reverses disease pathologies. J Alzheimers Dis Parkinsonism. 2016;6:238–244. doi: 10.4172/2161-0460.1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de la Monte S.M., Tong M., Schiano I., Didsbury J. Improved brain insulin/IGF signaling and reduced neuroinflammation with T3D-959 in an experimental model of sporadic Alzheimer's disease. J Alzheimer Dis. 2017;55:849–864. doi: 10.3233/JAD-160656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Angelova D.M., Brown D.R. Altered processing of beta-amyloid in SH-SY5Y cells induced by model senescent Microglia. ACS Chem Neurosci. 2018;9:3137–3152. doi: 10.1021/acschemneuro.8b00334. [DOI] [PubMed] [Google Scholar]
- 50.Hattori M., Fujiyama A., Taylor T.D., Watanabe H., Yada T., Park H.S. The DNA sequence of human chromosome 21. Nature. 2000;405:311–319. doi: 10.1038/35012518. [DOI] [PubMed] [Google Scholar]
- 51.Ermak G., Morgan T.E., Davies K.J. Chronic overexpression of the calcineurin inhibitory gene DSCR1 (Adapt78) is associated with Alzheimer's disease. J Biol Chem. 2001;276:38787–38794. doi: 10.1074/jbc.M102829200. [DOI] [PubMed] [Google Scholar]
- 52.Fuentes J.J., Pritchard M.A., Estivill X. Genomic organization, alternative splicing, and expression patterns of the DSCR1 (Down syndrome candidate region 1) gene. Genomics. 1997;44:358–361. doi: 10.1006/geno.1997.4866. [DOI] [PubMed] [Google Scholar]
- 53.Davies K.J., Ermak G., Rothermel B.A., Pritchard M., Heitman J., Ahnn J. Renaming the DSCR1/Adapt78 gene family as RCAN: Regulators of calcineurin. FASEB J. 2007;21:3023–3028. doi: 10.1096/fj.06-7246com. [DOI] [PubMed] [Google Scholar]
- 54.Rothermel B., Vega R.B., Yang J., Wu H., Bassel-Duby R., Williams R.S. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J Biol Chem. 2000;275:8719–8725. doi: 10.1074/jbc.275.12.8719. [DOI] [PubMed] [Google Scholar]
- 55.Ermak G., Davies K.J. DSCR1(Adapt78)–a Janus gene providing stress protection but causing Alzheimer's disease? IUBMB life. 2003;55:29–31. doi: 10.1080/1521654031000066820. [DOI] [PubMed] [Google Scholar]
- 56.Ermak G., Harris C.D., Davies K.J. The DSCR1 (Adapt78) isoform 1 protein calcipressin 1 inhibits calcineurin and protects against acute calcium-mediated stress damage, including transient oxidative stress. FASEB J. 2002;16:814–824. doi: 10.1096/fj.01-0846com. [DOI] [PubMed] [Google Scholar]
- 57.Ladner C.J., Czech J., Maurice J., Lorens S.A., Lee J.M. Reduction of calcineurin enzymatic activity in Alzheimer's disease: Correlation with neuropathologic changes. J Neuropathol Exp Neurol. 1996;55:924–931. doi: 10.1097/00005072-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Kayyali U.S., Zhang W., Yee A.G., Seidman J.G., Potter H. Cytoskeletal changes in the brains of mice lacking calcineurin A alpha. J Neurochem. 1997;68:1668–1678. doi: 10.1046/j.1471-4159.1997.68041668.x. [DOI] [PubMed] [Google Scholar]
- 59.Cook C.N., Hejna M.J., Magnuson D.J., Lee J.M. Expression of calcipressin1, an inhibitor of the phosphatase calcineurin, is altered with aging and Alzheimer's disease. J Alzheimers Dis. 2005;8:63–73. doi: 10.3233/jad-2005-8108. [DOI] [PubMed] [Google Scholar]
- 60.de la Monte S.M., Hedley-Whyte E.T. Small cerebral hemispheres in adults with Down's syndrome: Contributions of developmental arrest and lesions of Alzheimer's disease. J Neuropathol Exp Neurol. 1990;49:509–520. doi: 10.1097/00005072-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Cork L.C. Neuropathology of Down syndrome and Alzheimer disease. Am J Med Genet Suppl. 1990;7:282–286. doi: 10.1002/ajmg.1320370756. [DOI] [PubMed] [Google Scholar]
- 62.Mann D.M. Alzheimer's disease and Down's syndrome. Histopathology. 1988;13:125–137. doi: 10.1111/j.1365-2559.1988.tb02018.x. [DOI] [PubMed] [Google Scholar]
- 63.Norris C.M. Calcineurin: Directing the damage in Alzheimer disease: An Editorial for 'Neuronal calcineurin transcriptional targets parallel changes observed in Alzheimer disease brain' on page 24. J Neurochem. 2018;147:8–11. doi: 10.1111/jnc.14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reese L.C., Laezza F., Woltjer R., Taglialatela G. Dysregulated phosphorylation of Ca(2+)/calmodulin-dependent protein kinase II-alpha in the hippocampus of subjects with mild cognitive impairment and Alzheimer's disease. J Neurochem. 2011;119:791–804. doi: 10.1111/j.1471-4159.2011.07447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taglialatela G., Hogan D., Zhang W.R., Dineley K.T. Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav Brain Res. 2009;200:95–99. doi: 10.1016/j.bbr.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dineley K.T., Kayed R., Neugebauer V., Fu Y., Zhang W., Reese L.C. Amyloid-beta oligomers impair fear conditioned memory in a calcineurin-dependent fashion in mice. J Neurosci Res. 2010;88:2923–2932. doi: 10.1002/jnr.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spires-Jones T.L., Kay K., Matsouka R., Rozkalne A., Betensky R.A., Hyman B.T. Calcineurin inhibition with systemic FK506 treatment increases dendritic branching and dendritic spine density in healthy adult mouse brain. Neurosci Lett. 2011;487:260–263. doi: 10.1016/j.neulet.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rozkalne A., Hyman B.T., Spires-Jones T.L. Calcineurin inhibition with FK506 ameliorates dendritic spine density deficits in plaque-bearing Alzheimer model mice. Neurobiol Dis. 2011;41:650–654. doi: 10.1016/j.nbd.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hudry E., Wu H.Y., Arbel-Ornath M., Hashimoto T., Matsouaka R., Fan Z. Inhibition of the NFAT pathway alleviates amyloid beta neurotoxicity in a mouse model of Alzheimer's disease. J Neurosci. 2012;32:3176–3192. doi: 10.1523/JNEUROSCI.6439-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Norris C.M., Kadish I., Blalock E.M., Chen K.C., Thibault V., Porter N.M. Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer's models. J Neurosci. 2005;25:4649–4658. doi: 10.1523/JNEUROSCI.0365-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abdul H.M., Sama M.A., Furman J.L., Mathis D.M., Beckett T.L., Weidner A.M. Cognitive decline in Alzheimer's disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci. 2009;29:12957–12969. doi: 10.1523/JNEUROSCI.1064-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sompol P., Furman J.L., Pleiss M.M., Kraner S.D., Artiushin I.A., Batten S.R. Calcineurin/NFAT signaling in activated astrocytes drives network hyperexcitability in abeta-bearing mice. J Neurosci. 2017;37:6132–6148. doi: 10.1523/JNEUROSCI.0877-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hopp S.C., Bihlmeyer N.A., Corradi J.P., Vanderburg C., Cacace A.M., Das S. Neuronal calcineurin transcriptional targets parallel changes observed in Alzheimer disease brain. J Neurochem. 2018;147:24–39. doi: 10.1111/jnc.14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peiris H., Raghupathi R., Jessup C.F., Zanin M.P., Mohanasundaram D., Mackenzie K.D. Increased expression of the glucose-responsive gene, RCAN1, causes hypoinsulinemia, beta-cell dysfunction, and diabetes. Endocrinology. 2012;153:5212–5221. doi: 10.1210/en.2011-2149. [DOI] [PubMed] [Google Scholar]
- 75.Peiris H., Duffield M.D., Fadista J., Jessup C.F., Kashmir V., Genders A.J. A syntenic cross species aneuploidy genetic screen links RCAN1 expression to beta-cell mitochondrial dysfunction in type 2 diabetes. PLoS Genet. 2016;12:e1006033. doi: 10.1371/journal.pgen.1006033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grunblatt E., Koutsilieri E., Hoyer S., Riederer P. Gene expression alterations in brain areas of intracerebroventricular streptozotocin treated rat. J Alzheimers Dis. 2006;9:261–271. doi: 10.3233/jad-2006-9305. [DOI] [PubMed] [Google Scholar]
- 77.Salkovic-Petrisic M., Tribl F., Schmidt M., Hoyer S., Riederer P. Alzheimer-like changes in protein kinase B and glycogen synthase kinase-3 in rat frontal cortex and hippocampus after damage to the insulin signalling pathway. J Neurochem. 2006;96:1005–1015. doi: 10.1111/j.1471-4159.2005.03637.x. [DOI] [PubMed] [Google Scholar]
- 78.de la Monte S.M., Wands J.R. Alzheimer's disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. 2008;2:1101–1113. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shingo A.S., Kanabayashi T., Kito S., Murase T. Intracerebroventricular administration of an insulin analogue recovers STZ-induced cognitive decline in rats. Behav Brain Res. 2013;241:105–111. doi: 10.1016/j.bbr.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 80.de la Monte S.M., Tong M., Vimbela G. Oligodendroglial and neuroglial molecular abnormalities in the intracerebral streptozotocin model of sporadic Alzheimer's disease. J Alzheimers Parkinsonism Dement. 2016;1:1–15. [Google Scholar]
- 81.Tong M., Deochand C., Didsbury J., de la Monte S.M. T3D-959: A multi-faceted disease remedial drug candidate for the treatment of Alzheimer's disease. J Alzheimers Dis. 2016;51:123–138. doi: 10.3233/JAD-151013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Talbot K., Wang H.Y., Kazi H., Han L.Y., Bakshi K.P., Stucky A. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Corder E.H., Saunders A.M., Strittmatter W.J., Schmechel D.E., Gaskell P.C., Small G.W. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 84.Hofman A., Ott A., Breteler M.M., Bots M.L., Slooter A.J., van Harskamp F. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 85.Myers R.H., Schaefer E.J., Wilson P.W., D'Agostino R., Ordovas J.M., Espino A. Apolipoprotein E epsilon4 association with dementia in a population-based study: The Framingham study. Neurology. 1996;46:673–677. doi: 10.1212/wnl.46.3.673. [DOI] [PubMed] [Google Scholar]
- 86.Strittmatter W.J., Saunders A.M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G.S. Apolipoprotein E: High-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beydoun M.A., Beydoun H.A., Gamaldo A.A., Teel A., Zonderman A.B., Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: Systematic review and meta-analysis. BMC Public Health. 2014;14:643. doi: 10.1186/1471-2458-14-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kidd P.M. Alzheimer's disease, amnestic mild cognitive impairment, and age-associated memory impairment: current understanding and progress toward integrative prevention. Altern Med Rev. 2008;13:85–115. [PubMed] [Google Scholar]
- 89.Kirk-Sanchez N.J., McGough E.L. Physical exercise and cognitive performance in the elderly: current perspectives. Clin Interv Aging. 2014;9:51–62. doi: 10.2147/CIA.S39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beydoun N., Bucci J.A., Chin Y.S., Spry N., Newton R., Galvao D.A. Prospective study of exercise intervention in prostate cancer patients on androgen deprivation therapy. J Med Imaging Radiat Oncol. 2014;58:369–376. doi: 10.1111/1754-9485.12115. [DOI] [PubMed] [Google Scholar]
- 91.Derosa G., Maffioli P., Salvadeo S.A., Ferrari I., Ragonesi P.D., Querci F. Effects of sitagliptin or metformin added to pioglitazone monotherapy in poorly controlled type 2 diabetes mellitus patients. Metabolism. 2010;59:887–895. doi: 10.1016/j.metabol.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 92.Benedict C., Frey W.H., 2nd, Schioth H.B., Schultes B., Born J., Hallschmid M. Intranasal insulin as a therapeutic option in the treatment of cognitive impairments. Exp Gerontol. 2011;46:112–115. doi: 10.1016/j.exger.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 93.Claxton A., Baker L.D., Hanson A., Trittschuh E.H., Cholerton B., Morgan A. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer's disease dementia. J Alzheimers Dis. 2015;44:897–906. doi: 10.3233/JAD-141791. [DOI] [PubMed] [Google Scholar]
- 94.de la Monte S.M. Intranasal insulin therapy for cognitive impairment and neurodegeneration: Current state of the art. Expert Opin Drug Deliv. 2013;10:1699–1709. doi: 10.1517/17425247.2013.856877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Freiherr J., Hallschmid M., Frey W.H., 2nd, Brunner Y.F., Chapman C.D., Holscher C. Intranasal insulin as a treatment for Alzheimer's disease: A review of basic research and clinical evidence. CNS drugs. 2013;27:505–514. doi: 10.1007/s40263-013-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reger M.A., Watson G.S., Frey W.H., 2nd, Baker L.D., Cholerton B., Keeling M.L. Effects of intranasal insulin on cognition in memory-impaired older adults: Modulation by APOE genotype. Neurobiol Aging. 2006;27:451–458. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 97.Messier C., Teutenberg K. The role of insulin, insulin growth factor, and insulin-degrading enzyme in brain aging and Alzheimer's disease. Neural Plast. 2005;12:311–328. doi: 10.1155/NP.2005.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baker L.D., Cross D.J., Minoshima S., Belongia D., Watson G.S., Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68:51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Duffy A.M., Holscher C. The incretin analogue D-Ala2GIP reduces plaque load, astrogliosis and oxidative stress in an APP/PS1 mouse model of Alzheimer's disease. Neuroscience. 2013;228:294–300. doi: 10.1016/j.neuroscience.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 100.Craft S. Insulin resistance and cognitive impairment: A view through the prism of epidemiology. Arch Neurol. 2005;62:1043–1044. doi: 10.1001/archneur.62.7.1043-a. [DOI] [PubMed] [Google Scholar]
- 101.Craft S. Insulin resistance syndrome and Alzheimer disease: Pathophysiologic mechanisms and therapeutic implications. Alzheimer Dis Assoc Disord. 2006;20:298–301. doi: 10.1097/01.wad.0000213866.86934.7e. [DOI] [PubMed] [Google Scholar]
- 102.Craft S. Insulin resistance and Alzheimer's disease pathogenesis: Potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4:147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 103.de la Monte S.M., Daiello L.A., Hapel A.J., Tong M., Ott B.R. Altered serum and cerebrospinal fluid inflammatory cascades in mild cognitive impairment and Alzheimer's disease. J Neuroinflam Neurodegen. 2017;1:1–24. Article ID: 100004. [Google Scholar]
- 104.Lee S., Tong M., Hang S., Deochand C., de la Monte S. CSF and brain indices of insulin resistance, oxidative stress and neuro-inflammation in early versus late Alzheimer's disease. J Alzheimers Dis Parkinsonism. 2013;3:128. doi: 10.4172/2161-0460.1000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de la Monte S.M., Tong M., Daiello L.A., Ott B.R. Early-stage Alzheimer's disease is associated with simultaneous systemic and central nervous system dysregulation of insulin-linked metabolic pathways. J Alzheimers Dis. 2019:1–12. doi: 10.3233/JAD-180906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tong M., Yu R., Silbermann E., Zabala V., Deochand C., de la Monte S.M. Differential contributions of alcohol and nicotine-derived nitrosamine ketone (NNK) to white matter pathology in the adolescent rat brain. Alcohol Alcohol. 2015;50:680–689. doi: 10.1093/alcalc/agv102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Longato L., Tong M., Wands J.R., de la Monte S.M. High fat diet induced hepatic steatosis and insulin resistance: Role of dysregulated ceramide metabolism. Hepatol Res. 2012;42:412–427. doi: 10.1111/j.1872-034X.2011.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bosco D., Fava A., Plastino M., Montalcini T., Pujia A. Possible implications of insulin resistance and glucose metabolism in Alzheimer's disease pathogenesis. J Cell Mol Med. 2011;15:1807–1821. doi: 10.1111/j.1582-4934.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Neumann K.F., Rojo L., Navarrete L.P., Farias G., Reyes P., Maccioni R.B. Insulin resistance and Alzheimer's disease: molecular links & clinical implications. Curr Alzheimer Res. 2008;5:438–447. doi: 10.2174/156720508785908919. [DOI] [PubMed] [Google Scholar]
- 110.Umegaki H. Neurodegeneration in diabetes mellitus. Adv Exp Med Biol. 2012;724:258–265. doi: 10.1007/978-1-4614-0653-2_19. [DOI] [PubMed] [Google Scholar]
- 111.Claxton A., Baker L.D., Wilkinson C.W., Trittschuh E.H., Chapman D., Watson G.S. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer's disease. J Alzheimers Dis. 2013;35:789–797. doi: 10.3233/JAD-122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de la Monte S.M. Early intranasal insulin therapy halts progression of neurodegeneration: Progress in Alzheimer therapeutics. Aging health. 2012;8:61–64. doi: 10.2217/ahe.11.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reger M.A., Watson G.S., Green P.S., Wilkinson C.W., Baker L.D., Cholerton B. Intranasal insulin improves cognition and modulates {beta}-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 114.Caccamo A., Oddo S., Sugarman M.C., Akbari Y., LaFerla F.M. Age- and region-dependent alterations in Abeta-degrading enzymes: Implications for Abeta-induced disorders. Neurobiol Aging. 2005;26:645–654. doi: 10.1016/j.neurobiolaging.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 115.Morelli L., Llovera R.E., Mathov I., Lue L.F., Frangione B., Ghiso J. Insulin-degrading enzyme in brain microvessels: proteolysis of amyloid {beta} vasculotropic variants and reduced activity in cerebral amyloid angiopathy. J Biol Chem. 2004;279:56004–56013. doi: 10.1074/jbc.M407283200. [DOI] [PubMed] [Google Scholar]
- 116.Li H., Wu J., Zhu L., Sha L., Yang S., Wei J. Insulin degrading enzyme contributes to the pathology in a mixed model of Type 2 diabetes and Alzheimer's disease: Possible mechanisms of IDE in T2D and AD. Biosci Rep. 2018;38 doi: 10.1042/BSR20170862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hoyer S., Muller D., Plaschke K. Desensitization of brain insulin receptor. Effect on glucose/energy and related metabolism. J Neural Transm Suppl. 1994;44:259–268. doi: 10.1007/978-3-7091-9350-1_20. [DOI] [PubMed] [Google Scholar]
- 118.de la Monte S.M. Metabolic derangements mediate cognitive impairment and Alzheimer's disease: Role of peripheral insulin-resistance diseases. Panminerva Med. 2012;54:171–178. [PMC free article] [PubMed] [Google Scholar]
- 119.Harris C.D., Ermak G., Davies K.J. RCAN1-1L is overexpressed in neurons of Alzheimer's disease patients. FEBS J. 2007;274:1715–1724. doi: 10.1111/j.1742-4658.2007.05717.x. [DOI] [PubMed] [Google Scholar]
- 120.Perluigi M., Pupo G., Tramutola A., Cini C., Coccia R., Barone E. Neuropathological role of PI3K/Akt/mTOR axis in Down syndrome brain. Biochim Biophys Acta. 2014;1842:1144–1153. doi: 10.1016/j.bbadis.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rajasekar N., Nath C., Hanif K., Shukla R. Inhibitory effect of memantine on streptozotocin-induced insulin receptor dysfunction, neuroinflammation, amyloidogenesis, and neurotrophic factor decline in astrocytes. Mol Neurobiol. 2016;53:6730–6744. doi: 10.1007/s12035-015-9576-5. [DOI] [PubMed] [Google Scholar]
- 122.Kukreja L., Kujoth G.C., Prolla T.A., Van Leuven F., Vassar R. Increased mtDNA mutations with aging promotes amyloid accumulation and brain atrophy in the APP/Ld transgenic mouse model of Alzheimer's disease. Mol Neurodegener. 2014;9:16. doi: 10.1186/1750-1326-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ferretti M.T., Partridge V., Leon W.C., Canneva F., Allard S., Arvanitis D.N. Transgenic mice as a model of pre-clinical Alzheimer's disease. Curr Alzheimer Res. 2011;8:4–23. doi: 10.2174/156720511794604561. [DOI] [PubMed] [Google Scholar]
- 124.Kalinin S., Richardson J.C., Feinstein D.L. A PPARdelta agonist reduces amyloid burden and brain inflammation in a transgenic mouse model of Alzheimer's disease. Curr Alzheimer Res. 2009;6:431–437. doi: 10.2174/156720509789207949. [DOI] [PubMed] [Google Scholar]
- 125.Boussaha M., Hannequin D., Verpillat P., Brice A., Frebourg T., Campion D. Polymorphisms of insulin degrading enzyme gene are not associated with Alzheimer's disease. Neurosci Lett. 2002;329:121–123. doi: 10.1016/s0304-3940(02)00586-4. [DOI] [PubMed] [Google Scholar]
- 126.Dorfman V.B., Pasquini L., Riudavets M., Lopez-Costa J.J., Villegas A., Troncoso J.C. Differential cerebral deposition of IDE and NEP in sporadic and familial Alzheimer's disease. Neurobiol Aging. 2010;31:1743–1757. doi: 10.1016/j.neurobiolaging.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carrasquillo M.M., Belbin O., Zou F., Allen M., Ertekin-Taner N., Ansari M. Concordant association of insulin degrading enzyme gene (IDE) variants with IDE mRNA, Abeta, and Alzheimer's disease. PLoS One. 2010;5:e8764. doi: 10.1371/journal.pone.0008764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vepsalainen S., Hiltunen M., Helisalmi S., Wang J., van Groen T., Tanila H. Increased expression of Abeta degrading enzyme IDE in the cortex of transgenic mice with Alzheimer's disease-like neuropathology. Neurosci Lett. 2008;438:216–220. doi: 10.1016/j.neulet.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 129.Liu Y., Liu L., Lu S., Wang D., Liu X., Xie L. Impaired amyloid beta-degrading enzymes in brain of streptozotocin-induced diabetic rats. J Endocrinol Invest. 2011;34:26–31. doi: 10.1007/BF03346691. [DOI] [PubMed] [Google Scholar]
- 130.Tan T., Zhang Y., Luo W., Lv J., Han C., Hamlin J.N.R. Formaldehyde induces diabetes-associated cognitive impairments. FASEB J. 2018;32:3669–3679. doi: 10.1096/fj.201701239R. [DOI] [PubMed] [Google Scholar]
- 131.Kochkina E.G., Plesneva S.A., Vasilev D.S., Zhuravin I.A., Turner A.J., Nalivaeva N.N. Effects of ageing and experimental diabetes on insulin-degrading enzyme expression in male rat tissues. Biogerontology. 2015;16:473–484. doi: 10.1007/s10522-015-9569-9. [DOI] [PubMed] [Google Scholar]
- 132.Ries M., Sastre M. Mechanisms of abeta clearance and degradation by glial cells. Front Aging Neurosci. 2016;8:160. doi: 10.3389/fnagi.2016.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ma D., Doi Y., Jin S., Li E., Sonobe Y., Takeuchi H. TGF-beta induced by interleukin-34-stimulated microglia regulates microglial proliferation and attenuates oligomeric amyloid beta neurotoxicity. Neurosci Lett. 2012;529:86–91. doi: 10.1016/j.neulet.2012.08.071. [DOI] [PubMed] [Google Scholar]
- 134.Serrano-Perez M.C., Martin E.D., Vaquero C.F., Azcoitia I., Calvo S., Cano E. Response of transcription factor NFATc3 to excitotoxic and traumatic brain insults: Identification of a subpopulation of reactive astrocytes. Glia. 2011;59:94–107. doi: 10.1002/glia.21079. [DOI] [PubMed] [Google Scholar]
- 135.Canellada A., Ramirez B.G., Minami T., Redondo J.M., Cano E. Calcium/calcineurin signaling in primary cortical astrocyte cultures: Rcan1-4 and cyclooxygenase-2 as NFAT target genes. Glia. 2008;56:709–722. doi: 10.1002/glia.20647. [DOI] [PubMed] [Google Scholar]
- 136.Mitchell A.N., Jayakumar L., Koleilat I., Qian J., Sheehan C., Bhoiwala D. Brain expression of the calcineurin inhibitor RCAN1 (Adapt78) Arch Biochem Biophys. 2007;467:185–192. doi: 10.1016/j.abb.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 137.Badia M.C., Lloret A., Giraldo E., Dasi F., Olaso G., Alonso M.D. Lymphocytes from young healthy persons carrying the ApoE4 allele overexpress stress-related proteins involved in the pathophysiology of Alzheimer's disease. J Alzheimers Dis. 2013;33:77–83. doi: 10.3233/JAD-2012-120973. [DOI] [PubMed] [Google Scholar]
- 138.Bjork B.F., Katzov H., Kehoe P., Fratiglioni L., Winblad B., Prince J.A. Positive association between risk for late-onset Alzheimer disease and genetic variation in IDE. Neurobiol Aging. 2007;28:1374–1380. doi: 10.1016/j.neurobiolaging.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 139.Zuo X., Jia J. Promoter polymorphisms which modulate insulin degrading enzyme expression may increase susceptibility to Alzheimer's disease. Brain Res. 2009;1249:1–8. doi: 10.1016/j.brainres.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 140.Bartl J., Scholz C.J., Hinterberger M., Jungwirth S., Wichart I., Rainer M.K. Disorder-specific effects of polymorphisms at opposing ends of the insulin degrading enzyme gene. BMC Med Genet. 2011;12:151. doi: 10.1186/1471-2350-12-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Prince J.A., Feuk L., Gu H.F., Johansson B., Gatz M., Blennow K. Genetic variation in a haplotype block spanning IDE influences Alzheimer disease. Hum Mutat. 2003;22:363–371. doi: 10.1002/humu.10282. [DOI] [PubMed] [Google Scholar]