Abstract
A novel series of 3-((2-chloroquinolin-3-yl)methylene)indolin-2-ones were synthesized, using the ‘molecular hybridization approach’ and evaluated for anticancer efficacy. Eleven 3-((2-chloroquinolin-3-yl)methylene)indolin-2-ones (LM01 to LM11) were synthesized and evaluated for in vitro cytotoxic efficacy in cancer (ovarian, prostate and colon) and two non-cancerous cell lines. Among the 3-((2-chloroquinolin-3-yl)methylene)indolin-2-one derivatives, LM08, with a 6-Cl substitution in the 3-quinolinyl moiety, had selective and potent cytotoxic efficacy in the ovarian cancer cell line A2780. Further mechanistic investigations indicated that LM08 significantly inhibited the clonogenic survival of A2780 cancer cells, which was mediated by inducing apoptosis.
Keywords: Natural product chemistry, Pharmaceutical science, Pharmaceutical chemistry
1. Introduction
Despite several decades of intensive research, cancer still remains the leading cause of human deaths worldwide, accounting for an estimated 8.2 million deaths (around 13% of all deaths) in 2012 [1]. There are more than 200 types of cancer, making therapy extremely complicated and often inefficient [2]. Although chemotherapy is the mainstay for cancer treatment, the use of available chemotherapeutics is often limited due to severe or problematic adverse effects [3] and the development of multidrug resistance [4]. Consequently, there is an urgent need for novel, efficacious anticancer compounds with reduced toxicity.
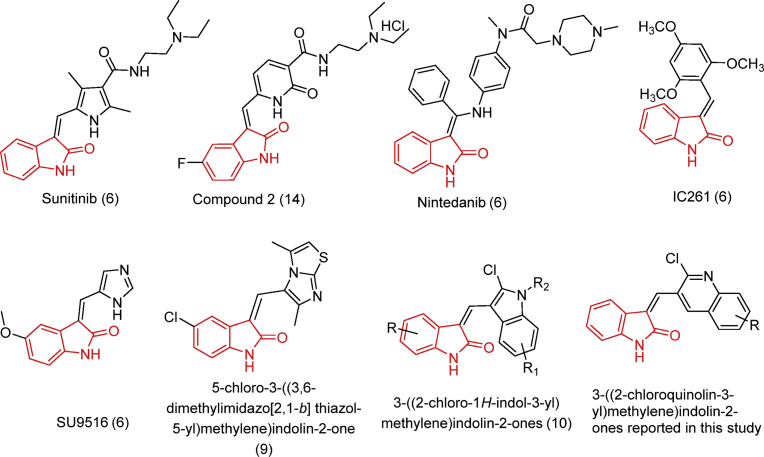
1H-indole-2,3-dione (also known as isatin) is a scaffold of significant interest because of its broad spectrum biological properties [5], and its cytotoxic and antineoplastic efficacies have been widely investigated [6]. 1H-indole-2,3-dione and its derivatives inhibit cancer cell proliferation and tumor growth by interacting with a variety of intracellular targets such as DNA, telomerase, tubulin, P-glycoprotein, protein kinases and phosphatases [6,7]. The aryl/heteroarylidene indolin-2-ones scaffold has recently become a versatile template for discovering novel kinase inhibitors for clinical use in cancer therapy (Fig. 1) [6,8,9,10]. The success of aryl/heteroarylidene indolin-2-one as a new class of antineoplastic drugs is further supported by the approval of the oxindole, sunitinib maleate (Sutent ®), by the United States Food and Drug Administration for the treatment of advanced renal carcinoma [11], gastrointestinal stromal tumors [12] and neuroendocrine tumors of the pancreas [7,13]. The clinical success of sunitinib has led to several synthetic efforts in the search for anticancer drugs based on the heteroarylidene indolin-2-one scaffold. Furthermore, research has primarily been focused on replacing the pyrrole ring in the C3 position indolin-2-one core of sunitinib with different heterocyclic moieties like imidazole, dihydropyridine, indoles, etc [6]. This strategy has been shown to be successful in many instances. For example, compound 3, an indolinone containing a dihydropyridine instead of a pyrrole ring, is cytotoxic and was 3-fold more efficacious than sunitinib in HCT-116 colon, A549 lung and HepG2 liver cancer cells [7,14].
Fig. 1.
Representative examples of aryl/heteroarylidene indolin-2-ones reported as potent anticancer compounds.
Another class of compounds potentially suitable for anticancer drug discovery are the quinolines and their derivatives [15]. Quinolines are inhibitors of tyrosine kinases, proteasomes, tubulin polymerization and DNA repair [15]. Furthermore, a combination of different bioactive fragments with complementary pharmacophoric functions often produced synergistic effects [16]. Based on these data and our ongoing efforts towards developing efficacious antitumor drugs through a “molecular hybridization approach” [17], we synthesized a series of novel 3-((2-chloroquinolin-3-yl)methylene)indolin-2-one derivatives, which incorporate a 2-chloroquinolinyl moiety at C3 position indolin-2-one as novel anticancer drugs. The present study represents the first systematic study on the synthesis and anticancer efficacy evaluation of 3-((2-chloroquinolin-3-yl)methylene)indolin-2-ones.
2. Results & discussion
2.1. Chemistry
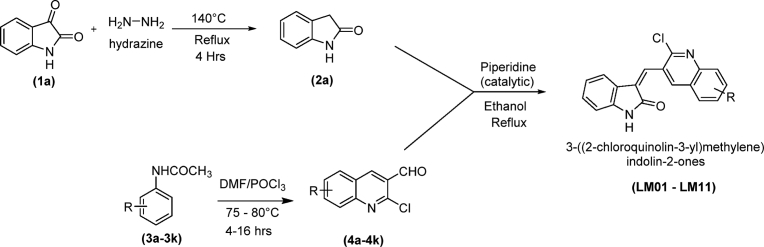
The synthetic protocol used to obtain the desired 3-((2-chloroquinolin-3-yl)methylene)indolin-2-ones (LM01-LM11) is illustrated in Scheme 1. The key intermediates, 2-chloroquinoline-3-carbaldehydes (4a-4k), were synthesized from commercially available acetanilides (3a–3k) using the Vilsmeier-Haack reaction as previously described [18,19]. Another intermediate, indolin-2-one (2a), was obtained using the Wolff-Kishner reduction of isatin (1a) with hydrazine hydrate [20]. The reaction of indolin-2-one (2a) with 2-chloroquinoline-3-carbaldehydes (4a-4k) in ethanol, in the presence of piperidine (catalytic), yielded the target compounds, 3-((2-chloroquinolin-3-yl)methylene)indolin-2-ones (LM01-LM11). The structures of the newly synthesized compounds were confirmed by microanalyses, IR, MASS and NMR spectral experiments.
Scheme 1.
Synthesis of 3-((2-chloroquinolin-3-yl)methylene)indolin-2-one derivatives (LM01 to LM11).
2.2. Anticancer efficacy evaluation
The synthesized compounds (LM01-LM11) were evaluated for in vitro cytotoxic efficacy in ovarian cancer (OV2008, A2780) colon carcinoma (HCT-116 and HT29), prostate cancer (PC3 and DU-145), human primary embryonic kidney (HEK293/pcDNA3.1) mouse fibroblast (NIH/3T3), and Chinese hamster ovarian (CHO) cell lines, using the MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) assay [21]. All of the compounds were tested at several concentrations ranging from 0.1 to 100 μM. The concentration of the tested compounds that produces a 50% inhibition of cell growth (IC50) was calculated. Table 1 summarizes the IC50 values obtained for the compounds evaluated in the cancer cell lines used in this study.
Table 1.
Cytotoxic efficacy of 3-((2-chloroquinolin-3-yl)methylene)indolin-2-ones on various cell lines (cancerous and non-cancerous).
| Compound Code | R | IC50 (μM)a |
||||
|---|---|---|---|---|---|---|
| OV2008 |
A2780 |
CHO |
PC3 |
DU-145 |
||
| Ovarian | Prostate | |||||
| LM01 | H | 55.3 | >100 | >100 | >100 | 77.5 |
| LM02 | 6-CH3 | 59.7 | >100 | 80.0 | >100 | >100 |
| LM03 | 6,7-di CH3 | NA | >100 | NA | >100 | >100 |
| LM04 | 6-OCH3 | NA | >100 | NA | >100 | 47.2 |
| LM05 | 7-OCH3 | NA | >100 | NA | 98.1 | >100 |
| LM06 | 6,7-di OCH3 | NA | >100 | NA | >100 | >100 |
| LM07 | 8-OCH3 | NA | 31.9 | NA | >100 | 11.0 |
| LM08 | 6-Cl | 48.8 | 7.7 | 38.5 | 91.1 | 91.2 |
| LM09 | 7-Cl | 49.1 | 22.7 | 39.2 | >100 | 54.9 |
| LM10 | 6-Br | NA | >100 | NA | >100 | >100 |
| LM11 |
– |
NA |
>100 |
NA |
>100 |
95.7 |
| Compound Code | R | IC50 (μM)a |
||||
| HCT-116 |
HT-29 |
HEK293/pcDNA.3.1 |
3T3 |
|||
| Colon |
Normal |
|||||
| LM01 | H | >100 | >100 | 64.5 | >100 | |
| LM02 | 6-CH3 | 52.1 | >100 | 73.4 | >100 | |
| LM03 | 6,7-di CH3 | >100 | >100 | 97.4 | >100 | |
| LM04 | 6-OCH3 | 60.9 | >100 | 39.9 | >100 | |
| LM05 | 7-OCH3 | 56.2 | >100 | >100 | >100 | |
| LM06 | 6,7-di OCH3 | 75.0 | >100 | >100 | >100 | |
| LM07 | 8-OCH3 | >100 | >100 | 80.8 | >100 | |
| LM08 | 6-Cl | 77.8 | >100 | 64.3 | >100 | |
| LM09 | 7-Cl | 75.2 | >100 | 63.2 | >100 | |
| LM10 | 6-Br | 41.3 | >100 | 50.5 | >100 | |
| LM11 | – | >100 | >100 | 75.3 | >100 | |
IC50 values are represented as the mean of three independent experiments performed in triplicate. A mean IC50 value of 100 μM was the cut off.
Among the 11 compounds tested for cytotoxic efficacy, compound LM08, with a 6-Cl substitution in the 3-quinolinyl moiety, had significant in vitro cytotoxic efficacy in the three tested ovarian tumor cell lines, with IC50 values ranging from 7.7 to 48.8 μM. Interestingly, compound LM09, a positional isomer of LM08, also showed a similar, but reduced efficacy profile in ovarian cancer cells, indicating that the chlorine substitution in the benzo ring of the 3-quinolinyl moiety may affect the cytotoxic efficacy of the 3-((2-chloroquinolin-3-yl)methylene)indolin-2-one derivatives on ovarian cancer cells. Other than the chloro-substituted derivatives, LM01, an unsubstituted derivative and compound LM02, a 6-methyl substituted derivative, also had cytotoxic efficacy (IC50 ∼ 50 μM) in OV2008 cells, and LM07, an 8-methoxyl substituted derivative, had cytotoxicity (IC50 = 32 μM) in A2780 ovarian cancer cells.
In the two prostate cancer cell lines, PC3 and DU-145, the growth of the PC3 cells was decreased by only two of the 3-((2-chloroquinolin-3-yl)methylene)indolin-2-one derivatives that had 6-OCH3 (LM05) and 8-OCH3 (LM08) substitutions at high concentrations (IC50∼ 90 μM). In contrast, DU-145 cells were significantly inhibited by six compounds in the series, LM01, LM04, LM07-09, and LM11. Compound LM07, containing an 8-methoxyl substitution in the 3-quinolinyl moiety, had the greatest cytotoxic efficacy in DU-145 cells, with an IC50 value of 11 μM. The remaining five compounds had low to moderate cytotoxic efficacy in DU-145 cells, with IC50 values ranging from 47 to 96 μM.
In the colon cancer cells lines, HT-29 and HCT-115, none of the synthesized compounds produced cytotoxicity in HT-29 cancer cells, even at the maximum concentration tested (100 μM). In contrast, seven of the 3-((2-chloroquinolin-3-yl)methylene)indolin-2-one derivatives had cytotoxic efficacy in HCT-115 cells. Compound LM10, with a 6-bromo substitution in the benzo ring of the 3-quinolinyl moiety, had the highest cytotoxic efficacy, with an IC50 value of 41.3 μM. It is important to note that none of 3-((2-chloroquinolin-3-yl)methylene)indolin-2-ones, with the exception of LM04 and LM10, produced significant cytotoxicity in human embryonic kidney cells at a concentration of less than 50 μM. Furthermore, all of the 3-((2-chloroquinolin-3-yl)methylene)indolin-2-ones were non-cytotoxic in mouse fibroblast (3T3) cells at the maximum concentration used, suggesting that most of the compounds studied in this study were non-lethal in the normal cell lines.
Our results indicate that compound LM08 may be a potential candidate for further investigations in terms of its potent and selective in vitro cytotoxicity in A2780 ovarian cancer cells. Subsequently, we determined the efficacy of LM08 (10 or 20 μM) to inhibit the clonogenic survival of A2780 cells. The results (Fig. 2) indicate that LM08, at 10 or 20 μM, while negatively impacting the growth and survival of A2780 cells, also significantly inhibited the colony formation of A2780 cells.
Fig. 2.
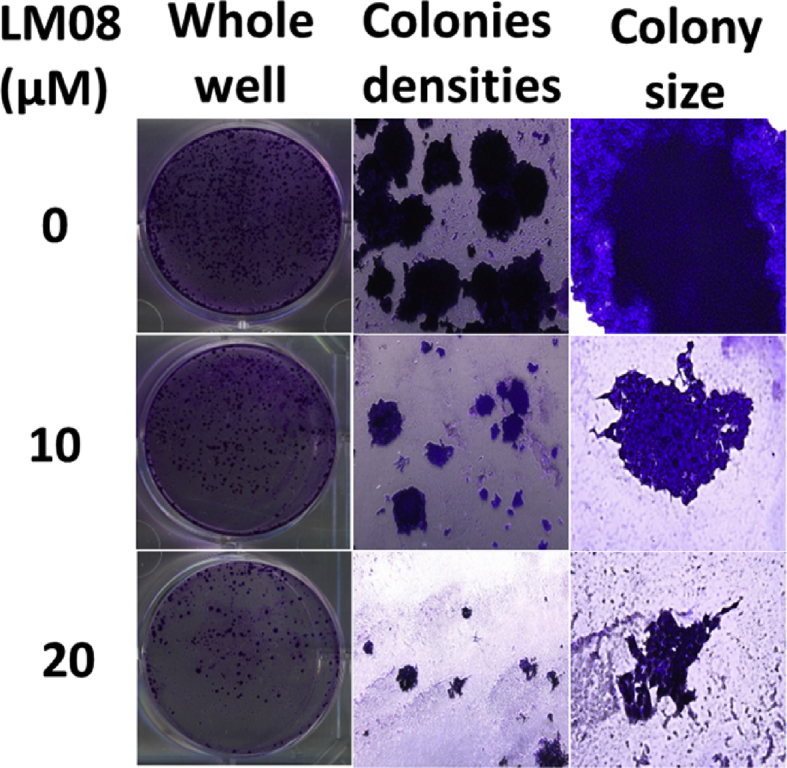
LM08 inhibits colony formation in A2780 cells. The cells were incubated with different concentrations of LM08 (10 or 20 μM) or vehicle. The pictures show the effect of LM08 on A2780 cells colony density and colony size. Each experiment was repeated independently in triplicate.
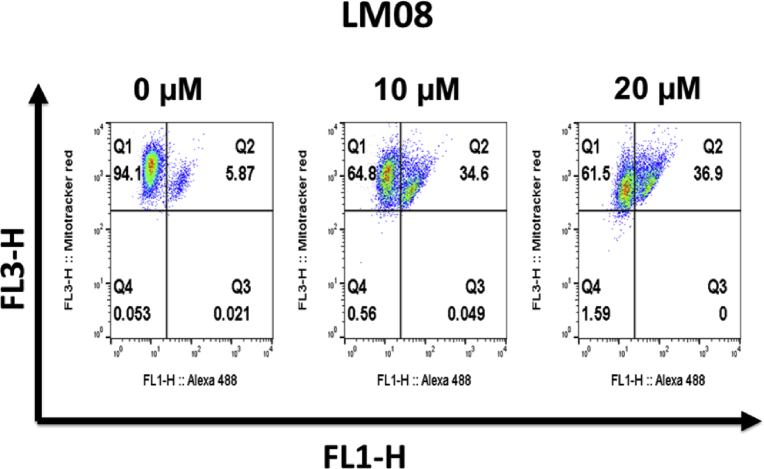
A number of clinically approved anticancer drugs have been reported to produce cytotoxic effects by inducing apoptosis [22]. Therefore, we assessed the apoptosis-inducing potential of LM08 in A2780 cells using the Alexa Fluor 488 annexin V kit for flow cytometry assay.
As shown in Fig. 3, the untreated cells (i.e. control cells) were primarily healthy, viable cells that showed little or no apoptosis (Q1: 94.1%, Q2: 5.87 %). However, there was a significant increase in annexin red fluorescence in cells incubated with LM08, indicating a significant shift in the number of cells showing apoptosis at 10 μM (Q1: 64.8%, Q2: 34.6%) and 20 μM (Q1: 61.5%, Q2: 36.9%). These data indicate that apoptosis induction is one of the mechanisms by which LM08 induces cytotoxicity in A2780 ovarian cells.
Fig. 3.
LM08 induces apoptosis in A2780 cells. A2780 cells in complete medium were incubated with LM08 at 10 or 20 μM or vehicle for 24 h. Cells were then incubated with annexin red dye and analyzed by flow cytometry. The representative results for A2780 cells are from at least two independent experiments, each performed in triplicate.
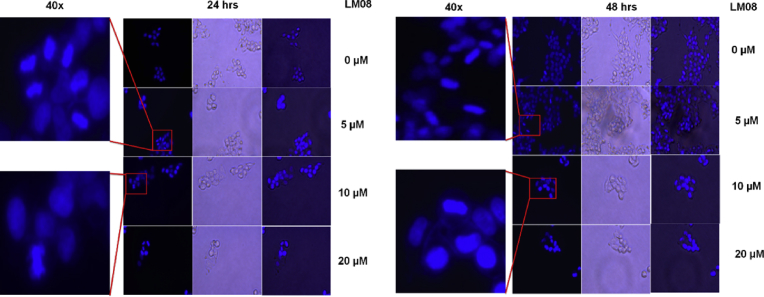
Nuclear condensation is one of the prominent hallmarks of apoptotic cell death [23]. Therefore, we determined the effects of LM08 on the nuclear morphology of A2780 cells using DAPI staining. The nuclear changes in A2780 cells after incubation with different concentrations of LM08 (0, 5,10 or 20 μM) for 24 and 48 h were visualized and recorded (Fig. 4). The results, as shown in Fig. 4, confirm the development of nuclear condensation in A2780 cells incubated with 5, 10, or 20 μM of LM08 at 24 and 48 h, whereas the control cells had a normal nuclear shape, consisting of an oval, non-condensed, shape. Thus, these results further validate the concept that LM08-induced cytotoxicity in A2780 cells is mediated by apoptosis.
Fig. 4.
The effect of LM08 on the nuclear morphology of A2780 cells. The nuclear morphology changes in A2780 cells upon incubation with 5 or 10 μM of LM08 or vehicle for 24 or 48 h, respectively, were detected using DAPI staining. Condensed and fragmented nuclei were observed under an EVOS fluorescent microscope at 40X.
3. Conclusion
In conclusion, a novel series of 3-((2-chloroquinolin-3-yl)methylene)indolin-2-one derivatives was designed, synthesized and evaluated for in vitro cytotoxic efficacy in ovarian, colon and prostate cancer cells and two non-cancerous cell lines. Among the 3-((2-chloroquinolin-3-yl)methylene)indolin-2-one derivatives, LM08, with a 6-Cl substitution in the 3-quinolinyl moiety, displayed selective and potent cytotoxic efficacy in the ovarian cancer cell line, A2780. Mechanistic experiments indicated that LM08 significantly inhibited the clonogenic survival of A2780 cells, and this effect was mediated by apoptosis. Based on the results of this study, LM08 may be an suitable molecule for the discovery of novel and selective anticancer drugs for the treatment of ovarian cancer.
4. Materials & methods
4.1. Chemistry
All reagents and solvents used in this study were obtained from commercial suppliers and used without further purification. The reaction progress was monitored by TLC, using silica gel 60 F-254 (0.25 mm) plates and the developed plates were visualized with UV light. Melting points were determined in open glass capillaries using a Veego digital melting point apparatus and are uncorrected.1H NMR spectra were recorded using dilute solutions of CDCl3 or DMSO-d6 on a Bruker Avance II 400 spectrometer. Chemical shifts (δ) are reported in ppm relative to trimethylsilane, and coupling constants (J) are reported in Hz. Electrospray ionization mass spectra (ESI-MS) were acquired using an Applied BiosystemQtrap 3200 MS/MS system. Infrared (IR) spectral data were obtained using a Schimadzu FT-IR 8400S IR spectrophotometer with an ATR accessory. Elemental analyses were performed with a Vario Microcube CHNS analyzer (Elementar, NJ, USA).
4.1.1. Synthesis of indolin-2-one (2a)
Isatin (0.05 mol) was dissolved in 30 ml of hydrazine hydrate (0.6 mol) and refluxed at 140 °C for 4 hrs. The reaction mixture was poured into ice-water, acidified with 6N HCl and left at room temperature for 2 days to yield pure crystals of Indolin-2-one.
52 % yield, M.P. 127–128 °C, FT-IR (ATR) cm−1: 3202 (NH), 3028 (Aromatic C–H), 2839 (Aliphatic C–H), 1689(C=O), 1616 (C=C). 1H NMR (400 MHz, Chloroform-d) δ 9.27 (br, s, 1H), 7.35–7.12 (m, 2H), 7.01 (t, J = 7.3 Hz, 1H), 6.91 (d, J = 7.9 Hz, 1H), 3.54 (s, 2H). MS-API [M + H]+134 (calculated 133.05).
4.1.2. General procedure for the synthesis of 2 chloro 3 formyl quinolines (4a–4e)
Acetanilide and substituted acetanilides, 4a–4c (0.05 mol) were dissolved in 9.6 ml of dimethyl formamide (0.125 mol) and 32 ml of phosphorus oxychloride (0.35 mol) was added to this solution gradually at 0 °C. The reaction mixture was placed in a round bottom flask (RBF equipped with reflux condenser fitted with a drying tube) and heated for 4–16 hrs on oil bath at 75–80 °C. The solution was cooled to room temperature and then poured on 100 ml of ice water. The precipitate was collected by filtration and recrystallized from ethyl acetate.
2-Chloroquinoline-3-carbaldehyde (4a): 72 % yield, M.P. 148–150 °C (Lit.149 °C). FT-IR (ATR) cm−1: 3044 (Aromatic C–H), 2870 (aldehyde C–H), 1684(C=O), 1574(C=N), 760 (C–Cl), 1H NMR (400 MHz, Chloroform-d) δ 10.57 (s, 1H), 8.77 (s, 1H), 8.08 (d, J = 8.5 Hz, 1H), 7.99 (d, J = 8.1 Hz, 1H), 7.90 (t, J = 7.7 Hz, 1H), 7.66 (t, J = 8.0 Hz, 1H). MS-API [M + H]+192 (calculated 191.01).
2-chloro-6-methylquinoline-3-carbaldehyde(4b): 75 % yield, M.P. 122–123 °C (Lit.123 °C). IR (ATR) cm−1: 3051 (Aromatic C–H), 2873 (aldehyde C–H), 1686 (C=O), 1576 (C=N), 752 (C–Cl). 1H NMR (400 MHz, Chloroform-d) δ 10.55 (s, 1H), 8.66 (s, 1H), 7.96 (d, J = 8.5 Hz, 1H), 7.79–7.63 (m, 2H), 2.57 (s, 3H). MS-API [M + H]+206 (calculated 205.03).
2-chloro-6,7-dimethylquinoline-3-carbaldehyde (4c): 77 % yield, M.P. 156–157 °C (Lit. 156–157 °C). IR (ATR) cm−1: 3055 (Aromatic C–H), 2893(aldehyde C–H), 1682 (C=O), 1576 (C=N), 750 (C–Cl). 1H NMR (700 MHz, Chloroform-d) δ 10.52 (s, 1H), 8.63 (s, 1H), 7.83 (s, 1H), 7.69 (s, 1H), 2.51 (s, 3H), 2.47 (s, 3H).
2-Chloro-6-methoxyquinoline-3-carbaldehyde(4d): 63% yield, M.P. 145–146 °C (Lit.146 °C). IR (ATR) cm−1: 3053 (Aromatic C–H), 2829 (aldehyde C–H), 1680(C=O), 1574(C=N), 1227, 1026 (C–O–C), 766 (C–Cl). 1H NMR (400 MHz, Chloroform-d) δ 10.53 (s, 1H), 8.63 (s, 1H), 7.95 (d, J = 9.2 Hz, 1H), 7.50 (ddd, J = 9.3, 2.9, 1.0 Hz, 1H), 7.18 (s, 1H), 3.94 (s, 3H). MS-API [M + H]+222 (calculated 221.02).
2-Chloro-7-methoxyquinoline-3-carbaldehyde(4e): 78% yield, M.P. 195–196 °C (Lit 196 °C). IR (ATR) cm−1: 3053 (Aromatic C–H), 2879 (aldehyde C–H), 1688 (C=O), 1583(C=N), 1240, 1043 (C–O–C), 760 (C–Cl). 1H NMR (400 MHz, Chloroform-d) δ 10.51 (s, 1H), 8.66 (s, 1H), 7.85 (d, J = 9.0 Hz, 1H), 7.38 (s, 1H), 7.27 (dd, J = 9.0, 2.5 Hz, 1H), 3.98 (s, 3H). MS-API [M + H]+222 (calculated 221.02).
2-Chloro-8-methoxyquinoline-3-carbaldehyde (4f): 12% yield, M.P. 190–191 °C (Lit. 190 °C). IR (ATR) cm−1: 3047 (Aromatic (C–H), 2862 (aldehyde C–H), 1684 (C=O), 1574 (C=N), 1267, 1036 (C–O–C), 763 (C–Cl). 1H NMR (400 MHz, Chloroform-d) δ 10.57 (s, 1H), 8.72 (s, 1H), 7.64–7.46 (m, 2H), 7.32–7.17 (m, 1H), 4.10 (s, 3H). MS-API [M + H]+222 (calculated 221.02).
2-chloro-6,7-dimethoxyquinoline-3-carbaldehyde (4g): 68% yield, M.P. 222°–224 °C (Lit. 222°–224 °C). IR (ATR) cm−1: 3055 (Aromatic (C–H), 2893 (aldehyde C–H), 1682 (C=O), 1576 (C=N), 1248, 1051 (C–O–C), 750 (C–Cl). 1H NMR (400 MHz, Chloroform-d) δ 10.51 (s, 1H), 8.57 (s, 1H), 7.38 (s, 1H), 7.15 (s, 1H), 4.06 (s, 3H), 4.03 (s, 3H). MS-API [M + H]+252.03 (calculated 251.03).
2,6-dichloroquinoline-3-carbaldehyde (4h): 12% yield, M.P. 191–192 °C (Lit.191 °C), IR (ATR) cm−1: 3065 (Aromatic (C–H), 2883 (aldehyde C–H), 1686 (C=O), 1572 (C=N), 766 (C–Cl). 1H NMR (500 MHz, Chloroform-d) δ 10.58 (s, 1H), 8.70 (s, 1H), 8.05 (d, J = 9.0 Hz, 1H), 7.99 (d, J = 2.3 Hz, 1H), 7.84 (dd, J = 9.0, 2.3 Hz, 1H). MS-API [M + H]+226 (calculated 224.97).
6-bromo-2-chloroquinoline-3-carbaldehyde (4i):22% yield, M.P. 187–188 °C (Lit. 188 °C), IR (ATR) cm−1: 3053, 2912 (Aromatic (C–H), 1690 (C=O), 1574 (C=N), 1045 (C–Br), 760 (C–Cl). 1H NMR (400 MHz, Chloroform-d): d (ppm) 10.55 (s, 1H), 8.73 (s, 1H), 8.07 (s, 1H), 7.93 (d, J = 8.5 Hz, 1H), 7.61 (d, J = 8.8 Hz, 1H). MS-API [M + H]+270 (calculated 268.92).
2,7-dichloroquinoline-3-carbaldehyde (4j): 26% yield, M.P. 159–160 °C (Lit. 160 °C), IR (ATR) cm−1: 3053 (Aromatic (C–H), 2847 (aldehyde C–H), 1676 (C=O), 1578 (C=N), 752 (C–Cl). 1H NMR (400 MHz, Chloroform-d) δ 10.53 (s, 1H), 8.72 (s, 1H), 8.06 (s, 1H), 7.91 (d, J = 8.8 Hz, 1H), 7.60 (dd, J = 8.7, 2.1 Hz, 1H). MS-API [M + H]+226 (calculated 224.97).
2-chlorobenzo[h]quinoline-3-carbaldehyde (4k):82% yield, M.P. 210–211 °C (Lit. 210–212 °C), IR (ATR) cm−1: 3038 (Aromatic (C–H), 2866 (aldehyde C–H), 1682 (C=O), 1574 (C=N), 752 (C–Cl). 1H NMR (400 MHz, Chloroform-d) δ 10.61 (s, 1H), 9.47–9.01 (m, 1H), 8.72 (s, 1H), 8.12–7.60 (m, 5H). MS-API [M + H]+242 (calculated 241.03).
4.1.3. General procedure of 3-((2-chloroquinolin-3-yl)methylene)indolin-2-ones (LM01-LM11)
To a stirred a solution of indolin-2-one (1 mmol) in 5 ml of ethanol, 2–3 drops of piperidine and 2-chloroquinoline-3-carbaldehydes (1 mmol) were added at 0 °C. The mixture was then heated under reflux for 4–6 hrs. After the completion of the reaction, based on a single spot in TLC (CHCl3: MeOH, 9:1), the precipitated solid was filtered and washed with cold ethanol to give the corresponding 3-((2-chloroquinolin-3-yl)methylene)indolin-2-ones.
3-((2-chloroquinolin-3-yl)methylene)indolin-2-one (LM01):bright yellow crystals, 88 % yield, M.P. >275 °C, FT-IR (ATR) cm−1: 3181 (NH), 3009 (Aromatic C–H), 1709(C=O), 1607, 1584 (C=C, C=N), 775 (C–Cl). 1H NMR (400 MHz, Chloroform-d) δ 8.56 (s, 1H), 8.13 (d, J = 8.4 Hz, 1H), 8.02 (br, s, 1H), 7.92 (s, 1H), 7.90–7.82 (m, 2H), 7.67 (ddd, J = 8.2, 7.0, 1.2 Hz, 1H), 7.33–7.24 (m, 3H), 6.95 (d, J = 7.6 Hz, 1H), 6.84 (td, J = 7.7, 1.0 Hz, 1H). MRMS-API [M + H]+307 (calculated 306.06), Anal. Calcd forC18H11N2; C, 70.58; H, 3.61; N, 9.13; Found: C, 70.66; H, 3.64; N, 9.28.
3-((2-chloro-6-methylquinolin-3-yl)methylene)indolin-2-one (LM02): 92 % yield, M.P. >275 °C, FT-IR (ATR) cm−1: 3159 (NH), 3086 (Aromatic C–H), 2812 (Aliphatic C–H), 1707 (C=O), 1609, 1584 (C=C, C=N), 777 (C–Cl).1H NMR (400 MHz, Chloroform-d) δ 8.46 (s, 1H), 8.01 (d, J = 8.5 Hz, 2H), 7.92 (s, 1H), 7.68 (dd, J = 8.6, 1.9 Hz, 1H), 7.62 (s, 1H), 7.32–7.25 (m, 3H), 6.94 (d, J = 7.8 Hz, 1H), 6.84 (t, J = 7.7 Hz, 1H), 2.60 (s, 3H). MRMS-API [M + H]+321 (calculated 320.07). Anal. Calcd for C19H13N2; C, 71.14; H, 4.08; N, 8.73, Found: C, 71.22; H, 4.12; N, 8.82.
3-((2-chloro-6,7-dimethylquinolin-3-yl)methylene)indolin-2-one (LM03): 89 % yield, M.P. >275 °C, FT-IR (ATR) cm−1: 3159 (NH), 3086, 3015 (Aromatic C–H), 1709 (C=O), 1609, 1587 (C=C, C=N), 777 (C–Cl).1H NMR (400 MHz, Chloroform-d) δ 8.43 (s, 1H), 7.94 (d, J = 13.5 Hz, 2H), 7.88 (s, 1H), 7.59 (s, 1H), 7.38–7.20 (m, 4H), 6.94 (d, J = 7.8 Hz, 1H), 6.89–6.77 (m, 1H), 2.54 (s, 3H), 2.50 (s, 3H). MRMS-API [M + H]+335.2 (calculated 334.09). Anal. Calcd forC19H13N2; C, 71.75; H, 4.52; N, 8.37 Found: C, 71.82; H, 4.58; N, 8.42.
3-((2-chloro-6-methoxyquinolin-3-yl)methylene)indolin-2-one (LM04): 84 % yield, M.P. >275 °C, FT-IR (ATR) cm−1: 3161 (NH), 3094, 3015 (Aromatic C–H), 2895 (Aliphatic C–H), 1711 (C=O), 1614, 1584 (C=C, C=N), 1231, 1051 (C–O–C), 775 (C–Cl). 1H NMR (400 MHz, Chloroform-d) δ 8.42 (s, 1H), 8.01 (d, J = 9.3 Hz, 1H), 7.90 (s, 1H), 7.49 (dd, J = 9.3, 2.8 Hz, 2H), 7.32–7.25 (m, 2H), 7.10 (d, J = 2.8 Hz, 1H), 6.92 (d, J = 7.8 Hz, 1H), 6.85 (t, J = 7.7 Hz, 1H), 3.97 (s, 3H). MRMS-API [M + H]+337.2 (calculated 336.07), Anal. Calcd forC19H13N2; C, 67.76; H, 3.89; N, 8.32 Found: C, 67.83; H, 4.02; N, 8.39.
3-((2-chloro-7-methoxyquinolin-3-yl)methylene)indolin-2-one (LM05): 81 % yield, M.P. >275 °C, FT-IR (ATR) cm−1: 3184 (NH), 3084 (Aromatic C–H), 2974 (Aliphatic C–H), 1711 (C=O), 1605 (C=C, C=N), 1232, 1047 (C–O–C), 775 (C–Cl). 1H NMR (400 MHz, Chloroform-d) δ 8.47 (s, 1H), 7.91 (s, 1H), 7.74 (d, J = 8.9 Hz, 1H), 7.49 (s, 1H), 7.45 (d, J = 2.5 Hz, 1H), 7.34 (d, J = 7.7 Hz, 1H), 7.32–7.22 (m, 2H), 6.91 (d, J = 7.9 Hz, 1H), 6.85 (t, J = 7.6 Hz, 1H), 4.01 (s, 3H). MRMS-API [M + H]+337.2 (calculated 336.07), Anal. Calcd forC19H13N2; C, 67.76; H, 3.89; N, 8.32 Found: C, 67.82; H, 4.10; N, 8.42.
3-((2-chloro-6,7-dimethoxyquinolin-3-yl)methylene)indolin-2-one (LM06): 77 % yield, M.P. >275 °C, FT-IR (ATR) cm−1: 3190 (NH), 3015 (Aromatic C–H), 1711 (C=O), 1614 (C=C, C=N), 1238, 1057 (C–O–C), 775 (C–Cl). 1H NMR (400 MHz, Chloroform-d) δ 8.38 (s, 1H), 7.92 (s, 1H), 7.53 (s, 1H), 7.45 (s, 1H), 7.35 (d, J = 8.4 Hz, 1H), 7.29–7.27 (m, 1H), 7.07 (s, 1H), 6.92 (d, J = 7.8 Hz, 1H), 6.87 (t, J = 7.9 Hz, 1H), 4.09 (s, 3H), 4.06 (s, 3H). MRMS-API [M + H]+367.2 (calculated 366.08), Anal. Calcd forC20H15N2; C, 65.49; H, 4.12; N, 7.64 Found: C, 65.72; H, 4.24; N, 7.73.
3-((2-chloro-8-methoxyquinolin-3-yl)methylene)indolin-2-one (LM07): 82 % yield, M.P. >275 °C, FT-IR (ATR) cm−1: 3296 (NH), 3001 (Aromatic C–H), 1713 (C=O), 1612, 1564 (C=C, C=N), 1269, 1044 (C–O–C), 770 (C–Cl). 1H NMR (400 MHz, Chloroform-d) δ 8.48 (s, 1H), 8.00 (s, 1H), 7.89 (s, 1H), 7.56 (t, J = 7.9 Hz, 1H), 7.41 (d, J = 8.1 Hz, 1H), 7.23 (d, J = 7.2 Hz, 2H), 7.18 (d, J = 7.8 Hz, 1H), 6.91 (d, J = 7.9 Hz, 1H), 6.80 (t, J = 7.6 Hz, 1H), 4.12 (s, 3H). MRMS-API [M + H]+337.2 (calculated 336.07), Anal. Calcd forC19H13N2; C, 67.76; H, 3.89; N, 8.32; Found: C, 68.04; H, 3.99; N, 8.52.
3-((2,6-dichloroquinolin-3-yl)methylene)indolin-2-one(LM08): 87 % yield, M.P. >275 °C, FT-IR (ATR) cm−1: 3144 (NH), 3026 (Aromatic C–H), 1699 (C=O), 1614, 1551 (C=C, C=N), 779 (C–Cl). 1H NMR (400 MHz, DMSO-d6) δ 10.61 (s, 1H), 8.94 (s, 1H), 8.16 (d, J = 2.4 Hz, 1H), 7.98 (d, J = 9.0 Hz, 1H), 7.91–7.83 (m, 2H), 7.75 (d, J = 7.5 Hz, 1H), 7.26 (t, J = 7.7 Hz, 1H), 7.00 (t, J = 7.5 Hz, 1H), 6.82 (d, J = 7.7 Hz, 1H). MRMS-API [M + H]+341 (calculated 340.02), Anal. Calcd forC19H13N2; C, 63.36; H, 2.95; N, 8.21; Found: C, 63.52; H, 3.08; N, 8.36.
3-((2,7-dichloroquinolin-3-yl)methylene)indolin-2-one (LM09): 86 % yield, M.P. >275 °C, FT-IR (ATR) cm−1: 3169 (NH), 3071 (Aromatic C–H), 1699 (C=O), 1614, 1551 (C=C, C=N), 789 (C–Cl). 1H NMR (400 MHz, Chloroform-d) δ 9.38 (s, 1H), 8.01 (s, 1H), 7.87 (t, J = 4.5 Hz, 2H), 7.73–7.42 (m, 4H), 7.11 (t, J = 7.7 Hz, 1H), 6.87 (d, J = 7.9 Hz, 1H). MRMS-API [M + H]+341 (calculated 340.02), Anal. Calcd forC19H13N2; C, 63.36; H, 2.95; N, 8.21; Found: C, 63.66; H, 3.12; N, 8.33.
3-((6-bromo-2-chloroquinolin-3-yl)methylene)indolin-2-one (LM10): 92 % yield, M.P. >275 °C, FT-IR (ATR) cm−1: 3144 (NH), 3063, 3026 (Aromatic C–H), 1701 (C=O), 1614, 1553 (C=C, C=N), 785 (C–Cl). 1H NMR (400 MHz, DMSO-d6) δ 10.61 (s, 1H), 8.93 (s, 1H), 8.32 (s, 1H), 8.06–7.81 (m, 3H), 7.75 (d, J = 7.2 Hz, 1H), 7.26 (t, J = 7.4 Hz, 1H), 7.00 (t, J = 7.2 Hz, 1H), 6.82 (d, J = 7.5 Hz, 1H). MRMS-API [M + H]+385 (calculated 383.97), Anal. Calcd forC18H10N2;C, 56.06; H, 2.61;N, 7.26; Found: C, 56.34; H, 2.83; N, 7.65.
3-((2-chlorobenzo[h]quinolin-3-yl)methylene)indolin-2-one (LM11): 89% yield, M.P. >275 °C, FT-IR (ATR) cm−1: 3140 (NH), 3071, 3024 (Aromatic C–H), 1699 (C=O), 1616, 1583 (C=C, C=N), 787 (C–Cl). 1H NMR (400 MHz, DMSO-d6) δ 10.62 (s, 1H), 9.12 (s, 1H), 9.09–8.96 (m, 1H), 8.33–7.67 (m, 8H), 7.26 (t, J = 7.7 Hz, 1H), 7.01 (t, J = 7.6 Hz, 1H), 6.84 (d, J = 7.8 Hz, 1H). MRMS-API [M + H]+357.2 (calculated 356.07), Anal. Calcd forC22H13N2; C, 74.06; H, 3.67; N, 7.85; Found: C, 74.57; H, 3.79; N, 7.92.
4.2. Anticancer activity evaluation
4.2.1. Cell lines and culture
Ovarian cancer (OV2008, A2780), colon carcinoma (HCT-116 and HT29), prostate cancer (PC3 and DU-145), normal human primary embryonic kidney (HEK293/pcDNA3.1) normal mouse fibroblast (NIH/3T3) and normal chinse hamster ovarian (CHO) cell lines were all used for cell cytotoxicity assay (MTT). All the cells were grown as monolayers in culture flasks using complete culture medium (DMEM) and 4.5 g of glucose, 10% FBS and 1% penicillin/streptomycin).
4.2.2. Cell cytotoxicity by MTT assay
The effect of the compounds on the cell survival was determined in different cancer and normal cell lines using the MTT assay. The MTT assay was conducted as previously as described [24,25]. The seeded cells in 96 well plates were incubated with different analogs at different concentrations for 72 hrs. The cells were further incubated with MTT for 4 hrs to produce the formation of the purple formazan crystals, which is indicative of viable cells. DMSO was added to dissolve the crystals and the absorbance was determined at 570 nm wavelength as described previously [26]. The IC50 was calculated and the selectivity of the compounds was determined by comparing survival in normal cells.
4.2.3. Evaluation of cell cytotoxicity by colony formation assay
The survival of ovarian cancer cells (A2780) in the presence of LM08 was further determined by colony formation assay as previously described in detail [26,27,28]. Briefly, A2780 cells were incubated with vehicle (0 μM) or LM08 (10 or 20 μM) for 24 hrs. The cells were reseeded at a very low density in six well plates to determine their efficacy to inhibit the formation of new cancer colonies over a period of 2 weeks. The colonies were then fixed and stained. The number of colonies for each treatment was counted under EVOS microscope (Themo Fisher Scientific, Wayne, MI, USA) and colony formation rate was calculated.
4.2.4. Annexin V based apoptosis assay
The induction of apoptosis in A2780 cells was evaluated using MitoTracker Red and Alexa Fluor 488 annexin V kits in combination with flow cytometry (Molecular Probes Inc., Invitrogen, Eugene, OR) as previously described [24] and according to manufacturer instructions. The cells were incubated with vehicle (0 μM) or LM08 (10 or 20 μM). The fluorescence of annexin V (499/521 nm) and MitoTracker Red (579/599 nm) was detected by flow cytometry (BD Accuri™ C6 flow cytometer and analyzed using FCS express 5 plus De Novo software) as shown earlier [28].
4.2.5. Nuclear condensation-based apoptosis assay
The detection of the nuclear changes, including nuclear condensation, to further confirm apoptosis, was conducted as previously described [29]. The A2780 were seeded and incubated with vehicle (0 μM) or LM08 (5, 10, or 20 μM) for 24 or 48 hrs. The cells were fixed and stained with DAPI nuclear staining. Finally, an EVOS microscope was used to detect the blue fluorescent staining of the nuclei and observe the nuclear changes produced by vehicle or LM08.
Declarations
Author contribution statement
Chandrabose Karthikeyan: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Haneen Amawi, Veronica Jones: Performed the experiments.
Charles Ashby, Vishwa Khare: Analyzed and interpreted the data; Wrote the paper.
Piyush Trivedi: Contributed reagents, materials, analysis tools or data. Critically edited the manuscript.
Hari Moorthy: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Amit Tiwari: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Amit Tiwari was supported by University of Toledo start-up funds (110760).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors gratefully acknowledge IISER-Bhopal for the NMR spectral analysis of the compounds used in this study. We also thank the Council of Scientific and Industrial Research (CSIR), New Delhi, India for providing a Senior Research Fellowship (C. K.). The study was supported by the University of Toledo start up funds (110760) to A.K.T.
Contributor Information
Chandrabose Karthikeyan, Email: karthikeyan_c@igntu.ac.in.
Amit K. Tiwari, Email: amit.tiwari@utoledo.edu.
References
- 1.World Cancer Report 2014. World Health Organization; 2014. Chapter 1.1. [Google Scholar]
- 2.Belal A. Design, synthesis and anticancer activity evaluation of some novel pyrrolo[1,2-a]azepine derivatives. Arch. Pharm. 2014;347(7):515–522. doi: 10.1002/ardp.201400004. Epub 2014/04/15PubMed PMID: 24729464. [DOI] [PubMed] [Google Scholar]
- 3.Kurumurthy C., Veeraswamy B., Sambasiva Rao P., Santhosh Kumar G., Shanthan Rao P., Loka Reddy V. Synthesis of novel 1,2,3-triazole tagged pyrazolo[3,4-b]pyridine derivatives and their cytotoxic activity. Bioorg. Med. Chem. Lett. 2014;24(3):746–749. doi: 10.1016/j.bmcl.2013.12.107. Epub 2014/01/16PubMed PMID: 24424132. [DOI] [PubMed] [Google Scholar]
- 4.Wang S., Folkes A., Chuckowree I., Cockcroft X., Sohal S., Miller W. Studies on pyrrolopyrimidines as selective inhibitors of multidrug-resistance-associated protein in multidrug resistance. J. Med. Chem. 2004;47(6):1329–1338. doi: 10.1021/jm031011g. Epub 2004/03/05PubMed PMID: 14998323. [DOI] [PubMed] [Google Scholar]
- 5.Pakravan P., Kashanian S., Khodaei M.M., Harding F.J. Biochemical and pharmacological characterization of isatin and its derivatives: from structure to activity. Pharmacol. Rep. : PR. 2013;65(2):313–335. doi: 10.1016/s1734-1140(13)71007-7. Epub 2013/06/08. PubMed PMID: 23744416. [DOI] [PubMed] [Google Scholar]
- 6.Vine K.L., Matesic L., Locke J.M., Ranson M., Skropeta D. Cytotoxic and anticancer activities of isatin and its derivatives: a comprehensive review from 2000-2008. Anti Cancer Agents Med. Chem. 2009;9(4):397–414. doi: 10.2174/1871520610909040397. Epub 2009/05/16. PubMed PMID: 19442041. [DOI] [PubMed] [Google Scholar]
- 7.Vine K.L., Matesic L., Locke J.M., Skropeta D. 2013. Recent Highlights in the Development of Isatin-Based Anticancer Agents. [Google Scholar]
- 8.Prakash C.R. Indolin-2-Ones in clinical trials as potential kinase inhibitors: a review. Pharmacol. Pharm. 2012;03(01):62–71. [Google Scholar]
- 9.Andreani A., Burnelli S., Granaiola M., Leoni A., Locatelli A., Morigi R. Antitumor activity of new substituted 3-(5-Imidazo[2,1-b]thiazolylmethylene)-2-indolinones and 3-(5-Imidazo[2,1-b]thiadiazolylmethylene)-2-indolinones: selectivity against colon tumor cells and effect on cell cycle-related events. J. Med. Chem. 2008;51(23):7508–7513. doi: 10.1021/jm800827q. [DOI] [PubMed] [Google Scholar]
- 10.Andreani A., Burnelli S., Granaiola M., Leoni A., Locatelli A., Morigi R. Substituted E-3-(2-chloro-3-indolylmethylene)1,3-dihydroindol-2-ones with antitumor activity. Effect on the cell cycle and apoptosis. J. Med. Chem. 2007;50(14):3167–3172. doi: 10.1021/jm070235m. [DOI] [PubMed] [Google Scholar]
- 11.Motzer R.J., Michaelson M.D., Redman B.G., Hudes G.R., Wilding G., Figlin R.A. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2006;24(1):16–24. doi: 10.1200/JCO.2005.02.2574. Epub 2005/12/07PubMed PMID: 16330672. [DOI] [PubMed] [Google Scholar]
- 12.Prenen H., Cools J., Mentens N., Folens C., Sciot R., Schoffski P. Efficacy of the kinase inhibitor SU11248 against gastrointestinal stromal tumor mutants refractory to imatinib mesylate. Clin. Cancer Res. : An Off. J. Am. Assoc. Cancer Res. 2006;12(8):2622–2627. doi: 10.1158/1078-0432.CCR-05-2275. Epub 2006/04/28PubMed PMID: 16638875. [DOI] [PubMed] [Google Scholar]
- 13.Raymond E., Hammel P., Dreyer C., Maatescu C., Hentic O., Ruszniewski P. Sunitinib in pancreatic neuroendocrine tumors. Targeted Oncol. 2012;7(2):117–125. doi: 10.1007/s11523-012-0220-2. Epub 2012/06/05PubMed PMID: 22661319. [DOI] [PubMed] [Google Scholar]
- 14.Zou H., Zhang L., Ouyang J., Giulianotti M.A., Yu Y. Synthesis and biological evaluation of 2-indolinone derivatives as potential antitumor agents. Eur. J. Med. Chem. 2011;46(12):5970–5977. doi: 10.1016/j.ejmech.2011.10.009. Epub 2011/10/25PubMed PMID: 22019188. [DOI] [PubMed] [Google Scholar]
- 15.Solomon V.R., Lee H. Quinoline as a privileged scaffold in cancer drug discovery. Curr. Med. Chem. 2011;18(10):1488–1508. doi: 10.2174/092986711795328382. Epub 2011/03/25. PubMed PMID: 21428893. [DOI] [PubMed] [Google Scholar]
- 16.Viegas-Junior C., Danuello A., da Silva Bolzani V., Barreiro E.J., Fraga C.A. Molecular hybridization: a useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007;14(17):1829–1852. doi: 10.2174/092986707781058805. Epub 2007/07/14. PubMed PMID: 17627520. [DOI] [PubMed] [Google Scholar]
- 17.Karthikeyan C., Solomon V.R., Lee H., Trivedi P. Design, synthesis and biological evaluation of some isatin-linked chalcones as novel anti-breast cancer agents: a molecular hybridization approach. Biomed. Prev. Nutr. 2013;3(4):325–330. [Google Scholar]
- 18.Karthikeyan C., Lee C., Moore J., Mittal R., Suswam E.A., Abbott K.L. IND-2, a pyrimido[1″,2″:1,5]pyrazolo[3,4-b]quinoline derivative, circumvents multi-drug resistance and causes apoptosis in colon cancer cells. Bioorg. Med. Chem. 2015;23(3):602–611. doi: 10.1016/j.bmc.2014.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meth-Cohn O., Narine B., Tarnowski B. A versatile new synthesis of quinolines and related fused pyridines, Part 5. The synthesis of 2-chloroquinoline-3-carbaldehydes. J. Chem. Soc., Perkin Trans. 1981;1(0):1520–1530. [Google Scholar]
- 20.Olgen S., Gotz C., Jose J. Synthesis and biological evaluation of 3-(substituted-benzylidene)-1,3-dihydro-indolin derivatives as human protein kinase CK2 and p60(c-Src) tyrosine kinase inhibitors. Biol. Pharm. Bull. 2007;30(4):715–718. doi: 10.1248/bpb.30.715. Epub 2007/04/06. PubMed PMID: 17409508. [DOI] [PubMed] [Google Scholar]
- 21.Carmichael J., DeGraff W.G., Gazdar A.F., Minna J.D., Mitchell J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47(4):936–942. Epub 1987/02/15. PubMed PMID: 3802100. [PubMed] [Google Scholar]
- 22.Hickman J.A. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992;11(2):121–139. doi: 10.1007/BF00048059. Epub 1992/09/01. PubMed PMID: 1327566. [DOI] [PubMed] [Google Scholar]
- 23.Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. Epub 2007/06/15PubMed PMID: 17562483; PubMed Central PMCID: PMCPMC2117903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manivannan E., Amawi H., Hussein N., Karthikeyan C., Fetcenko A., Narayana Moorthy N.S.H. Design and discovery of silybin analogues as antiproliferative compounds using a ring disjunctive – based, natural product lead optimization approach. Eur. J. Med. Chem. 2017;133:365–378. doi: 10.1016/j.ejmech.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 25.Marie Kirwen E., Batra T., Karthikeyan C., Deora G.S., Rathore V., Mulakayala C. 2,3-Diaryl-3H-imidazo[4,5-b]pyridine derivatives as potential anticancer and anti-inflammatory agents. Acta Pharm. Sin. B. 2017;7(1):73–79. doi: 10.1016/j.apsb.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hussein N., Ashby C.R., Jr., Amawi H., Nyinawabera A., Vij A., Khare V.M. Cariprazine, a dopamine D(2)/D(3) receptor partial agonist, modulates ABCG2-mediated multidrug resistance in cancer. Cancers. 2018;10(9) doi: 10.3390/cancers10090308. Epub 2018/09/06PubMed PMID: 30181510; PubMed Central PMCID: PMCPMC6162716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amawi H., Hussein N.A., Karthikeyan C., Manivannan E., Wisner A., Williams F.E. HM015k, a novel silybin derivative, multi-targets metastatic ovarian cancer cells and is safe in zebrafish toxicity studies. Front. Pharmacol. 2017;8:498. doi: 10.3389/fphar.2017.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karthikeyan C., Amawi H., Viana A.G., Sanglard L., Hussein N., Saddler M. lH-Pyrazolo[3,4-b]quinolin-3-amine derivatives inhibit growth of colon cancer cells via apoptosis and sub G1 cell cycle arrest. Bioorg. Med. Chem. Lett. 2018;28(13):2244–2249. doi: 10.1016/j.bmcl.2018.05.045. Epub 2018/06/02PubMed PMID: 29853331. [DOI] [PubMed] [Google Scholar]
- 29.Amawi H., Karthikeyan C., Pathak R., Hussein N., Christman R., Robey R. Thienopyrimidine derivatives exert their anticancer efficacy via apoptosis induction, oxidative stress and mitotic catastrophe. Eur. J. Med. Chem. 2017;138:1053–1065. doi: 10.1016/j.ejmech.2017.07.028. [DOI] [PubMed] [Google Scholar]