Abstract
In the present study, we have established a novel transgenic mouse and transgenic rats with dual reporters of EGFP and ELuc. In these transgenic (Tg) rodents, both GFP fluorescent and luciferase luminescent signals were ubiquitously detected in the heart, liver, kidney and testis, while only the GFP signal was detected in the brain. This expression system is based on a P2A linked EGFP/ELuc protein allowing both signals to be generated simultaneously. Microscopy experiments, FCM, and luciferase assays showed strong expression in freshly isolated ADSCs from Tg rodents upon transplantation of Tg rat-derived ADSCs into wild-type-mice. The ELuc transgene signal was observed and traced in vivo, and EGFP positive cells could be recovered from ELuc positive tissues in engraftment sites of wild-type mice for multiple analysis. These dual reporter Tg rodents are a useful reconstituted model system of regenerative medicine and are a valuable tool to study stem cells.
Keywords: In vivo imaging, Fluorescence, Luminescence, Reporter, Transplantation, Stem cells
Abbreviations: EGFP, enhanced green fluorescence protein; GFP, green fluorescence protein; Tg, transgenic; ADSCs, adipose derived stem cells; FCM, flow cytometry; MSCs, mesenchymal stem cells; RT, room temperature
Highlights
-
•
Establishment of dual reporter transgenic mice and rats, which express luciferase and GFP in all organs.
-
•
Both luciferase and GFP signals were detected by in vivo imaging using their respective antibodies.
-
•
Isolated mesenchymal stem cells from transgenic rodents showed both luciferase and GFP signals.
-
•
Implantation of transgenic mesenchymal stem cells enables cell tracking in vivo.
1. Introduction
Adult tissue stem cells present the capability of both self-renewal and differentiation [1]. Additionally, due to their intrinsically limited proliferation, it has been shown that adult tissue stem cells could be autologously transplanted to patients with various diseases to compensate for organ dysfunction, with a lessened chance of tumor formation [2]. Adipose-derived stem cells (ADSCs) existing in adipose tissues are derived from mesenchymal stem cells (MSCs). It has been reported that they can directly differentiate not only into adipose tissue but also into various other tissues such as vasculature, bone and cartilage [3,4]. They also can secrete several cytokines to protect cells via actions of angiogenesis and anti-inflammation [5]. The simplicity of their isolation via liposuction, their abundance, and lack of ethical controversy make ADSCs a beneficial model to study regenerative medicine [6].
Recently, many clinical trials have incorporated ADSCs to aid in tissue reconstruction, such as in breast reconstruction after surgical operation in patients with breast cancer [7]. There are many clinical trials showing that ADSC transplantations associated with transplantation of adipose tissue can lead to successful breast reconstruction after surgical operation in patients with breast cancer [8]. In the clinical setting, transplanted ADSCs are expected to differentiate to adipose tissues and while simultaneously secreting several cytokines to protect the transplanted adipose tissues. However, it remains unknown whether they remain in the engraftment site or not, how long they stay there, and whether they differentiate into host tissue cells [9]. To address these issues, appropriate and stable marking for ADSCs is required for their visualization in deep tissue in vivo and collection to evaluate their cell fate.
Fluorescence imaging techniques that use green fluorescent protein (GFP) as a probe are widely utilized for tracking the cell fate of transplanted stem cells, although the limitation of transparency of GFP signal does not allow imaging of deep tissue in vivo. To address this problem, bioluminescence imaging is available [10]. Luminescence proteins react with the substrate of luciferin and ATP to generate photons without excitation. Circulating luciferin via intra-venous injection allows the visualization of luminescence signal in deep tissue, providing the highest sensitivity in vivo imaging [11].
In this study, we developed dual reporter transgenic rodents with genetically encoded GFP and luciferase wherein both proteins are expressed through P2A alliance, which enables both bioluminescence and fluorescence imaging from their organ and live cells. P2A peptide is “self-cleaving” 22 amino acid peptide, identified in the porcine teschovirus-1 2A [12]. Therefore, the EGFP and ELuc transgenes are transcribed to single mRNA and translated to single peptide. The single peptide is cleaved into two peptides after the translation. Then, each cleaved peptides work as proteins. In vivo, we successfully tracked the luminescent signal from transgenic (Tg) rat-derived ADSCs transplanted into a model rat of reconstructing breast.
2. Materials and methods
2.1. Plasmid construction
DNA constructs were generated using standard methods with restriction enzymes, klenow fragment and ligase (New England BioLabs/TaKaRa). EGFP gene was cloned from pEGFP-N1 (Clontech) and ELuc gene [13] cloned from pELuc-test (Toyobo) and inserted to pCAGGS vector which contain CAG promoter [14,15]. P2A sequence [16] was synthesized and inserted between EGFP and ELuc gene.
2.2. Generation of transgenic mice and rats
All experimental protocols were approved by Institutional Animal Care and Use Committee of Tottori University. We outsourced microinjections and generation of transgenic mice and rats to animal facility in Tottori University Medical Center.
2.3. In vivo imaging
We used 3-5 days-old mice and rats for whole body imaging and 6-8 weeks-old mice and rats for organs imaging (both males and females). 100 mM D-luciferin (Toyobo) was intraperitoneal injected into mice and rats (150 mg/kg). Animals were anesthetized with isoflurane right after D-luciferin injections, and bioluminescence images of the mice and rats were obtained using the IVIS 200 imaging system (Xenogen). Fluorescence images were taken immediately after bioluminescence imaging. For ex vivo analysis following the D-luciferin injection, mice and rats were sacrificed using the standard procedure approved in our animal protocol and the organs (brain, heart, liver, kidneys, testis) isolated from the mice and rats. Then, bioluminescence and fluorescence imaging were performed using IVIS imaging system. To detect the ELuc positive cells from fat graft, we directly injected D-luciferin into the fat graft and performed bioluminescence analysis in mammal reconstruction model mice.
2.4. Immunohistochemistry
The organs (liver, kidney, heart, muscle) were dissected from transgenic mice and rats and embedded in Tissue-Tek 4583 Optimal Cutting Temperature compound (Sakura Finetek Japan Co.) and snap-frozen in liquid nitrogen for frozen sections. Multiple cryostat sections (8 μm thick) were prepared for immunostaining. The sections were stained with α-GFP (1:100; MEDICAL & BIOLOGICAL LABORATORIES CO., LTD.) and α-ELuc (1:100; kindly provided from Dr. Nakajima) antibodies after fixation and H2O2 treatment. VECSTAIN ABC Kits (VECTOR) was used to detect the signals. The samples were imaged using All-IN-ONE fluorescence microscope BZ-9000 (KEYENCE).
2.5. Western blot analysis
The protein samples were extracted from transgenic organs (liver, kidney, heart, muscle) using extraction buffer ((1X phosphate-buffered saline, 1% Nonidet P-40 (NP-40), 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS) and an EDTA-free protease inhibitor cocktail tablet)). The equal volume of 2X SDS sample buffer (BioRad) was added to the lysate samples and boiled for 5min. The protein samples were subjected to 10% SDS-polyacrylamide gel electrophoresis, and then electro-transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% non-fat milk powder in TBS (50 mM Tris n ad 150 mM NaCl, pH7.5) plus 0.1% Tween. Then, the membranes were incubated with following primary antibodies: α-GFP (Abcam, 1/10000 dilution), α-ELuc (kindly provided from Dr. Nakajima, 1/20000 dilution), α-β-tubulin (Abcam, 1/5000 dilution) and horseradish-peroxidase (HRP)-conjugated secondary antibodies: α-rabbit IgG (GE Healthcare, 1/3000 dilution) and α-mouse IgG (GE Healthcare, 1/3000 dilution). The protein signals were detected using Pierce™ ECL Plus Western Blotting Substrate (Thermo Fisher Scientific).
2.6. ADSCs isolation and culture
ADSCs were obtained from inguinal subcutaneous fat tissue 8 weeks-old transgenic mice and rats or wild-type Lewis rats. Adipose tissues were minced and digested with 1 mg/mL type Ⅰ collagenase (Wako) (37 °C, 1hr). The digested tissues were filtered through a 100 μm filter (BD Biosciences). Then, they were centrifuged (2,000 rpm, 4 °C, 5min) and washed with D-PBS(-) (Wako) to remove residual adipocytes. The ADSCs were finally suspended to ADSCs culture medium (Dulbecco's Modified Eagle Medium; DMEM (SIGMA) supplemented with 20% Fetal Bovine Serum; FBS (Corning) plus 1% Penicillin-Streptomycin-Glutamine (Wako)) and harvested to culture dish (Corning). ADSCs were cultured under normoxia using incubator (Sanyo)(20% O2, 5% CO2 at 37 °C). ADSCs were passaged using 0.25% trypsin-EDTA (Wako) every two to three days.
2.7. Fluorescence imaging
The mice and rat ADSCs were harvested and plated on glass-bottom dishes (Greiner) and GFP signals were imaged with GFP (470 nm) filters and Nikon element using ECLIPSE Ti fluorescence microscope (Nikon).
2.8. Flow cytometry (FCM) analysis
The mouse and rat ADSCs were dissociated to single cells by treatment with 0.25% trypsin-EDTA (37 °C, 1min). The transplanted rat ADSCs were isolated from the fat graft of reconstruction model mice. The fat graft was minced and dissociated with 1 mg/mL type Ⅰ collagenase (Wako) (37 °C, 30-60min). The digested tissues were filtered through a 100 μm filter (BD Biosciences). Cells were centrifuged and washed with PBS twice and finally resuspended in MACS Buffer (Milteny Biotec). Dissociated cells were filtered using a 0.35 μm filter (BD Biosciences) and stained with propidium iodide solution (Sigma, final 0.1μg/mL) for detecting dead cells. Flow cytometry analysis was performed using BD LSRFortessa X-20 (Becton Dickinson).
2.9. Luciferase assay
ADSCs were plated in 96 well white clear bottom plate (Corning). 0.2 mM D-luciferin was directly added to culture wells and measured luciferase activity in live cell measurement condition. Emerald Luc Luciferase Assay Reagent Neo (Toyobo) was used for dissolving cell analysis. Luciferase (Luc) activity levels were measured using TECAN infinite F500 (TECAN).
2.10. Immunocytohemistry
The ADSCs were fixed in 4% paraformaldehyde (PFA, Wako) (4 °C, 30min) washed with PBS three times, treated with 0.1% Triton-X100 (room temperature; RT, 30min), and then incubated in blocking buffer (1% bovine serum albumin; BSA in PBST (PBS supplemented with 0.1% Tween)) (Sigma) (RT, 30min). Then, the cells were incubated with primary antibodies; α-GFP (1:400 dilution; ThermoFisher) and α-ELuc (1:500 dilution; kindly provided from Dr. Nakajima) antibodies in blocking buffer (4 °C, over night) and followed by incubation with secondary antibodies; AlexaFluor 488-conjugated α-chicken IgY (1:500 dilution; ThermoFisher) and AlexaFluor 546-conjugated α-rabbit IgG (1:500 dilution; ThermoFisher) in blocking buffer (RT, 1hr). Nuclei were counter stained counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and mounted using ProLong Diamond Antifade Mountant (ThermoFisher), and samples were analyzed under ECLIPSE Ti fluorescence microscope (Nikon).
2.11. Cell transplantation
The ADSCs were isolated from transgenic rats and the inguinal subcutaneous fat tissues were obtained from 8 weeks-old Lewis rats. The ADSCs extraction was done as previously described (section 2.6). The fat tissues were minced and mixed with ADSCs at a particular ratio (3.5 × 106 or 0.7 × 106 ADSCs per 0.7g fat for each graft). The BALB/cAJcl-nu/nu mice (males, 8 weeks-old) were anesthetized by intraperitoneal injection of xylazine hydrochloride (5 mg/kg; Bayer) and ketamine hydrochloride (80 mg/kg; Daiichi-Sankyo Pharmaceuticals). Then, the ADSCs and fats mixture was subcutaneously implanted into the back of mice using a special needle (Cytori). One week after implantation, the mice were analyzed via in vivo bioluminescence imaging and sacrificed for FCM assay.
3. Results
3.1. Establishment of transgenic rodents harboring dual reporter genes
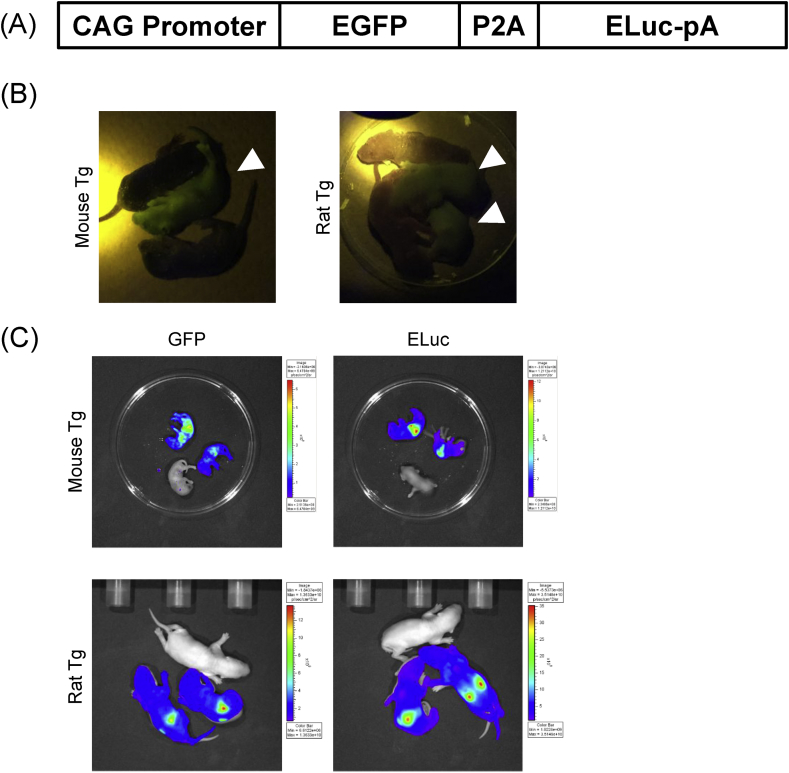
The transgenic mouse and rats (Tg rodents) lines allowing us to observe both fluorescent and luminescent signals simultaneously were established using the expression vector harboring EGFP and ELuc dual reporter genes (Fig. 1A). The constructed plasmid vector which is driven by CAG promoter contains EGFP and ELuc reporter genes separated with P2A alliance so that both reporter proteins can express ubiquitously in the same cells. We generated the Tg rodents by injecting this plasmid into fertilized eggs, which resulted in the generation of 1 mouse line and 5 rats lines. F1 animals were developed by crossing these lines with wild-type animals, and germline transmission of the reporter genes was confirmed.
Fig. 1.
Establishment of dual reporter transgenic rodents. (A) Schematic representation of the EGFP/ELuc dual reporter. (B) Representative images of living transgenic mouse and rats expressing GFP with cohort controls. GFP signals were excited by hand-held UV light (mouse n = 3/rats n = 3). White arrowheads show positive pups. (C) Representative images of living transgenic mouse and rats expressing both GFP and ELuc in pups with cohort controls. GFP signals were excited using an in vivo imaging system with blue excitation light (488 nm) (left). ELuc signals were detected by in vivo imaging system (right) (mouse n = 3/rats n = 3). . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Newborn rodents were taken directly to analyze EGFP expression via fluorescence. The whole bodies of the Tg rodents were green under 488 nm lights (Fig. 1B). In addition to the EGFP, we also confirmed ELuc bioluminescence signals in bodies of Tg rodents 30 min after administration of D-luciferin intraperitoneally (Fig. 1C). These results indicated the successful development of a transgenic mouse and transgenic rats lines, which express both GFP and luciferase ubiquitously and simultaneously, both of which proliferated for 5 generations.
3.2. Protein expression of GFP and ELuc in various organs of transgenic rodents
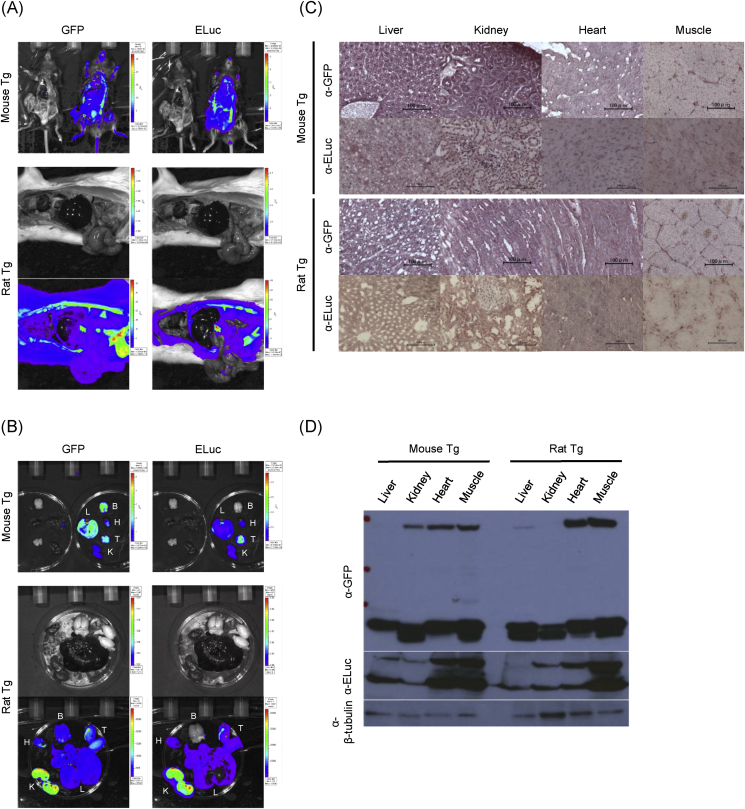
To examine the expression pattern of EGFP and ELuc in newly developed Tg rodents, various organs were removed and examined using ex vivo imaging. EGFP signals were detected in all intra-abdominal organs and outer skin. Intraperitoneal injection of D-luciferin resulted in detectable ELuc bioluminescent signals in all intra-abdominal organs (Fig. 2A). In addition, both signals were detected in isolated liver, kidney, heart and testis, while only EGFP was detected in the brain (Fig. 2B). For detailed analysis of EGFP and ELuc expression in Tg rodents, we stained the tissue sections from various organs with α-GFP and α-ELuc antibodies. The proteins of both GFP and ELuc were simultaneously observed at almost all cells in sections from liver, kidney, heart and muscle (Fig. 2C). These results indicated that co-expression of EGFP and ELuc in single cells does not negate each other since we can detect the almost same expression pattern of EGFP fluorescence and ELuc luminescence in various organs (Fig. 2A and B).
Fig. 2.
EGFP-2A-Eluc transgenic mouse and rats express GFP fluorescence and ELuc luciferase in all organs. (A) GFP fluorescence and bioluminescence images of intraperitoneal organs taken from transgenic mouse and rats. All organs expressed both GFP and ELuc signals (Experiments were performed in two to three times independent experiments, each with similar results). (B) Representative images of GFP fluorescence and ELuc bioluminescence in transgenic mouse and rat organs (B; brain, H; heart, L; liver, K; kidney, T; testis) (mouse n = 3/rats n = 3). (C) Representative pictures of transgenic mouse and rat tissue sections stained with GFP or ELuc antibody respectively (liver, kidney, heart, muscle) (mouse n = 3/rats n = 3). Scale bars: 100μm. (D) Exogenous GFP and ELuc expression in transgenic mouse and rat organs were confirmed by western blotting using α-GFP and α-ELuc antibody. α-β-tubulin was used as a loading control. (n = 3).
We then tested whether individual EGFP and ELuc were produced from a singular protein prior to their cleavage via P2A. α-GFP western blot analysis of liver, kidney, heart, and muscle showed a 60kd band, the estimated size of the linked EGFP-Eluc protein, in addition to a 27 kd band, for the individual EGFP protein. Moreover, the same linking protein and 37 kd individual Eluc protein were produced in all organs examined (Fig. 2D). These results suggested that EGFP-ELuc linking protein was produced from single mRNA and processed properly at P2A sequence as expected from the plasmid vector design. Thus, the protein expression of GFP and ELuc in various tissues were consistent with the distribution of both fluorescent and bioluminescent signals observed in the ex vivo imaging.
3.3. Mesenchymal stem cells derived from Tg rodents express both GFP and ELuc in single cells
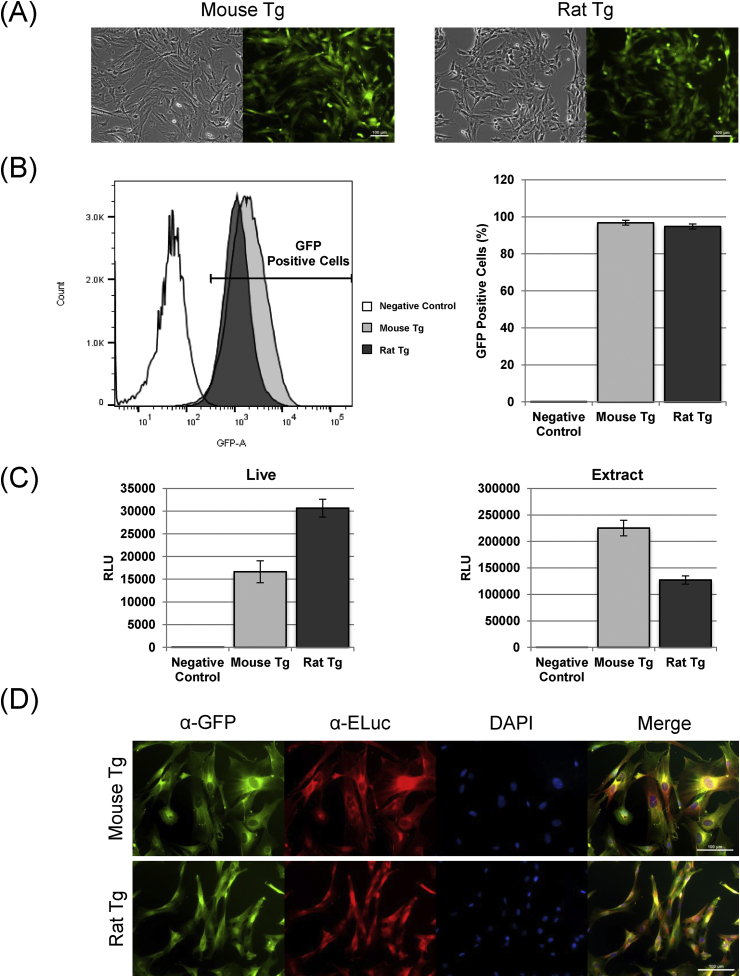
We examined whether GFP and ELuc signals of mesenchymal stem cells (adipose derived stem cells; ADSCs) obtained from the subcutaneous adipose tissue of Tg rodents could be used to monitor the fate of the transplanted cells. First, we showed that freshly isolated ADSCs expressed GFP fluorescence. The cytoplasm of all ADSCs were uniformly expressed GFP fluorescence signal (Fig. 3A). Flow cytometry analysis indicated approximately 98% and 99% ADSCs obtained from mouse and rat, respectively, were GFP positive (Fig. 3B). These primary ADSCs from Tg rodents can be clearly distinguished by GFP fluorescence signal. Next, we examined the signals of ELuc in living and extracted cells from both Tg mouse and rat adipose tissues after exposure to luciferin. Strong ELuc bioluminescent signals were detected in living ADSCs and were consistently observed after extraction into cell lysates (Fig. 3C). Finally, we confirmed the simultaneous expression of GFP and ELuc in ADSCs using immunofluorescence. All ADSCs obtained from both Tg mouse and rats expressed the protein of both GFP and ELuc simultaneously (Fig. 3D). These results demonstrated that isolated primary ADSCs from Tg rodents were characterized showing ubiquitously expression of both GFP and ELuc signals. These results also indicate that GFP and ELuc proteins do not interfere each other.
Fig. 3.
Differential expression pattern of GFP fluorescence and ELuc luciferase in EGFP-2A-ELuc transgenic mouse and rats. (A) Bright field (left) and GFP fluorescence (right) images of isolated ADSCs from transgenic mice (left) or rats (right). All cells indicated GFP fluorescence. Scale bars: 100 μm. (B) FCM analysis of GFP expressing ADSCs established from transgenic mouse (gray column) and rat (black column). Wild-type rat ADSCs used as negative control (white column). Each bar represents the mean ± S.D. (n = 4). (C) Luciferase signals detected from living and lysed ADSCs derived from transgenic mice (gray column) and rats (black column) respectively. Wild-type rat ADSCs used as negative control (white column). Each bar represents the mean ± S.D. (n = 11–14 in each group). (D) Immunofluosrescence analysis of transgenic mouse and rat ADSCs using α-GFP (green) and α-ELuc (red) antibodies showed the expression of exogenous both GFP and ELuc in individual cells. The cells were counterstained with DAPI (blue). Scale bars: 100 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. Tracking signals of GFP/ELuc from implanted cells in vivo
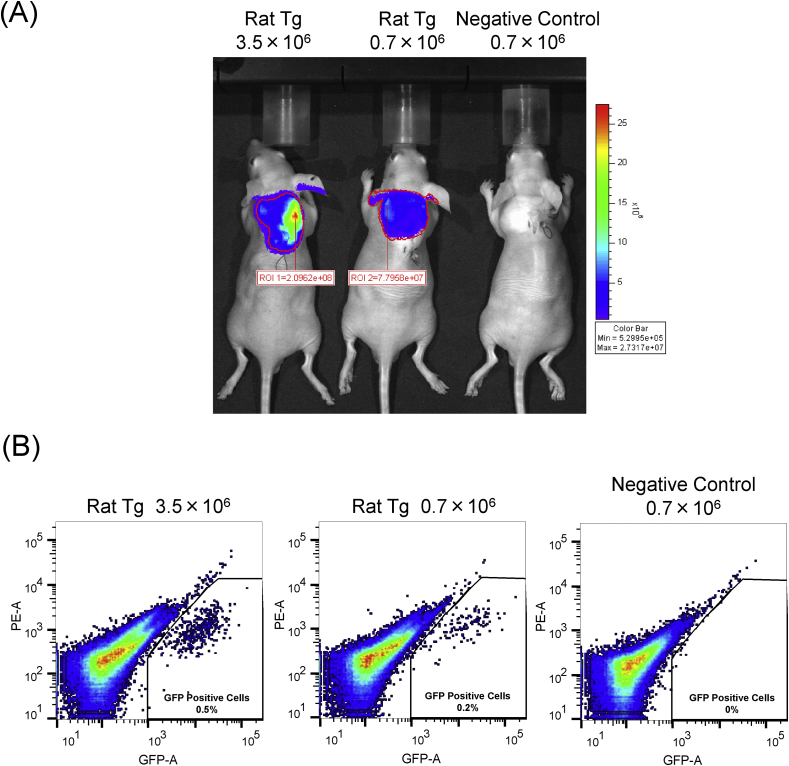
We elucidated whether signals of GFP/ELuc in ADSCs obtained from Tg rats allow us to monitor or trace their fate in vivo after transplantation. Tg rat-derived ADSCs were transplanted into wild-type mice as a model for breast reconstruction. 1 week after transplantation, injection of D-luciferin into the fat graft visualized the ELuc signal at the engraftment site observable through in vivo imaging (Fig. 4A). The ELuc signal observed was found to be dependent on the number of transplanted ADSCs. Indeed, the intensity on ELuc signals derived from transplanted ADSCs at 3.5 × 106 cells was stronger than those at seen from 0.7 × 106 cells. We were also able to recover GFP positive implanted ADSCs from ELuc positive tissues in these breast reconstruction models, although less than 1% of cells were GFP positive in FCM analysis (Fig. 4B). The number of recovered GFP-positive cells was also dependent on the number of ADSCs transplanted into model mice. Thus, we can examine the nature and property of ADSCs before and after transplantation.
Fig. 4.
Quantification of transplanted cells in mammal reconstruction mice model.(A) Bioluminescence images of the reconstruction model of mice implanted EGFP-P2A-ELuc transgenic rat ADSCs. The numbers of remaining ADSCs in the fat graft were detected using in vivo bioluminescence imaging (n = 2). (B) FCM analysis of residual EGFP positive cells in the reconstruction model isolated from fat graft (individual, n = 2).
4. Discussion
In the present study, we have established a new transgenic mouse and rat model with both GFP and ELuc dual reporter genes. We found that 1) the GFP/ELuc-transgene signal and proteins were ubiquitously expressed in the heart, liver, kidney and testis, while only the GFP transgene was expressed in brain, 2) the GFP/ELuc-transgene signal and proteins were expressed in live ADSCs, 3) the GFP/ELuc-transgene signal was observed in transplanted ADSCs in vivo.
The most prominent finding is the generation of dual reporter rats with GFP and ELuc markers to label freshly isolated ADSCs from adipose tissue. Previously it has been reported that the lentiviral transduction of pSIN-dual-LUC-GFP2 reporter gene into ADSCs enabled tracking of implanted cells in real time [17]. Since lentiviral transduction into ADSCs requires a cell culturing period, it is difficult to label and monitor the freshly isolated ADSCs using this method. Additionally prolonged culture of ADSCs may result in changes of various physiological properties of ADSCs. In our previous report, the freshly isolated ADSCs showed remarkable angiogenesis with abundant expression of angiogenic factors, while cultured ADSCs showed weaker angiogenesis [18]. In addition, the efficiency of lentiviral transduction into cells is variable in each experiment. In the present study, we collected the freshly isolated ADSCs from the dual reporter transgenic rats and immediately transplanted these cells into the rat breast reconstruction model. We successfully tracked both fluorescence and luminescent signals of the transplanted ADSCs, which enabled in vivo imaging and cellular analysis of collected cells simultaneously. These results demonstrated the advantage of our transgenic rodents for monitoring transplanted stem cells.
Our established dual reporter rodents have several unique features compared to previous model systems such as β-galactosidase (LacZ) and green fluorescent protein (GFP) -Tg rats [19]. The dual reporter rodents can be monitored by both GFP and ELuc bioluminescence proteins, which allows us to track living cells in vivo and in vitro (Fig. 2, Fig. 3). The most common tool to determine cell fate in vivo is LacZ, because of the protein's stability and high sensitivity. However, the necessity to fix and stain for LacZ detection prevents continuous live observation of transplanted cells in vivo and in vitro on a real-time basis to determine cellular localization and cellular lineage. Our dual rodents encoded with the ELuc expression system in replacement of LacZ, reveals the image of opaque deep tissues in vivo. Indeed, transplanted ADSCs derived from the dual rats were easily monitored in adult recipient mouse through ELuc signal (Fig. 4). Albeit due to the limited use of in vivo imaging, GFP has a long history as an in vitro cell reporter because of its advantageous nature as a non-invasive molecule to observe the fluorescence from intact live cells (Fig. 3). These features suggested that the dual reporter rodents consisted from GFP and ELuc give us better tools for visualizing and tracking the transplanted cells into tissues.
As expected from the utilization of the CAG promoter to drive reporter expression in dual reporter rodents, GFP and ELuc signals were detected all tissues examined with the exception of ELuc in the brain (Fig. 2B). Lack of ELuc signal in the brain is not due to a lack of gene expression but rather the blood-brain barrier preventing luciferin substrate access [20]. In addition, ADSCs dissected from Tg rodents also expressed GFP signal as well as ELuc strongly (Fig. 3D). Previous dual reported systems with two ubiquitous promoter expression units separately control each reporter expression, which might cause a different expression pattern between them. In other systems, two reporter proteins were synthesized from single mRNA through ribosome re-entry at IRES element in the mRNA, in which quantitative difference between two reporters was observed [21]. In our dual rodents, ELuc/GFP proteins were synthesized as a linking protein cleaved by processing through 2A peptide. Indeed, Tg rodents provided EGFP and Eluc expression together with both fluorescent and bioluminescent signals detected in vivo and in vitro. Therefore, in two different aspect of analysis such as in vivo tracking using ELuc and in vitro cell isolation through visualization with GFP, cell identity for transplanted cells can always be assured.
The dual reporter rodents described in this study provide us a useful tool to monitor not only transplanted ADSCs in vivo, but also various stem cells from many different tissues.
Acknowledgements
We appreciate Dr. Yoshihiro Nakajima and Dr. Mayu Yasunaga for gifting anti-ELuc antibody. We also thank to Yumiko Inoue for experimental assistance, Ramsey Bekdash and Jose Quejada for editing the manuscripts. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technologies (15K21170/25893134/17K08539/17K11539/16K11367/) of Japan.
Footnotes
Transparency document related to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100645.
Transparency document
References
- 1.Xin T., Greco V., Myung P. Hardwiring stem cell communication through tissue structure. Cell. 2016;164:1212–1225. doi: 10.1016/j.cell.2016.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trounson A., McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., Benhaim P., Lorenz H.P., Hedrick M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 4.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H., Alfonso Z.C., Fraser J.K., Benhaim P., Hedrick M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locke M., Feisst V., Dunbar P.R. Concise review: human adipose-derived stem cells: separating promise from clinical need. Stem Cell. (Dayton, Ohio) 2011;29:404–411. doi: 10.1002/stem.593. [DOI] [PubMed] [Google Scholar]
- 6.Naderi N., Combellack E.J., Griffin M., Sedaghati T., Javed M., Findlay M.W., Wallace C.G., Mosahebi A., Butler P.E., Seifalian A.M., Whitaker I.S. The regenerative role of adipose-derived stem cells (ADSC) in plastic and reconstructive surgery. Int. Wound J. 2017;14:112–124. doi: 10.1111/iwj.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindroos B., Suuronen R., Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. 2011;7:269–291. doi: 10.1007/s12015-010-9193-7. [DOI] [PubMed] [Google Scholar]
- 8.Mestak O., Sukop A., Hsueh Y.S., Molitor M., Mestak J., Matejovska J., Zarubova L. Centrifugation versus PureGraft for fatgrafting to the breast after breast-conserving therapy. World J. Surg. Oncol. 2014;12:178. doi: 10.1186/1477-7819-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross R.J., Shayan R., Mutimer K.L., Ashton M.W. Autologous fat grafting: current state of the art and critical review. Ann. Plast. Surg. 2014;73:352–357. doi: 10.1097/SAP.0b013e31827aeb51. [DOI] [PubMed] [Google Scholar]
- 10.Contag C.H., Bachmann M.H. Advances in in vivo bioluminescence imaging of gene expression. Annu. Rev. Biomed. Eng. 2002;4:235–260. doi: 10.1146/annurev.bioeng.4.111901.093336. [DOI] [PubMed] [Google Scholar]
- 11.Badr C.E., Tannous B.A. Bioluminescence imaging: progress and applications. Trends Biotechnol. 2011;29:624–633. doi: 10.1016/j.tibtech.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Felipe P., Luke G.A., Hughes L.E., Gani D., Halpin C., Ryan M.D. E unum pluribus: multiple proteins from a self-processing polyprotein. Trends Biotechnol. 2006;24:68–75. doi: 10.1016/j.tibtech.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Nakajima Y., Yamazaki T., Nishii S., Noguchi T., Hoshino H., Niwa K., Viviani V.R., Ohmiya Y. Enhanced beetle luciferase for high-resolution bioluminescence imaging. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyazaki J., Takaki S., Araki K., Tashiro F., Tominaga A., Takatsu K., Yamamura K. Expression vector system based on the chicken beta-actin promoter directs efficient production of interleukin-5. Gene. 1989;79:269–277. doi: 10.1016/0378-1119(89)90209-6. [DOI] [PubMed] [Google Scholar]
- 15.Niwa H., Yamamura K., Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 16.Kim J.H., Lee S.R., Li L.H., Park H.J., Park J.H., Lee K.Y., Kim M.K., Shin B.A., Choi S.Y. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munoz M.F., Arguelles S., Guzman-Chozas M., Guillen-Sanz R., Franco J.M., Pintor-Toro J.A., Cano M., Ayala A. Cell tracking, survival, and differentiation capacity of adipose-derived stem cells after engraftment in rat tissue. J. Cell. Physiol. 2018;233:6317–6328. doi: 10.1002/jcp.26439. [DOI] [PubMed] [Google Scholar]
- 18.Harada Y., Yamamoto Y., Tsujimoto S., Matsugami H., Yoshida A., Hisatome I. Transplantation of freshly isolated adipose tissue-derived regenerative cells enhances angiogenesis in a murine model of hind limb ischemia. Biomed. Res. 2013;34:23–29. doi: 10.2220/biomedres.34.23. [DOI] [PubMed] [Google Scholar]
- 19.Inoue H., Ohsawa I., Murakami T., Kimura A., Hakamata Y., Sato Y., Kaneko T., Takahashi M., Okada T., Ozawa K., Francis J., Leone P., Kobayashi E. Development of new inbred transgenic strains of rats with LacZ or GFP. Biochem. Biophys. Res. Commun. 2005;329:288–295. doi: 10.1016/j.bbrc.2005.01.132. [DOI] [PubMed] [Google Scholar]
- 20.Lee K.H., Byun S.S., Paik J.Y., Lee S.Y., Song S.H., Choe Y.S., Kim B.T. Cell uptake and tissue distribution of radioiodine labelled D-luciferin: implications for luciferase based gene imaging. Nucl. Med. Commun. 2003;24:1003–1009. doi: 10.1097/00006231-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Szymczak A.L., Vignali D.A. Development of 2A peptide-based strategies in the design of multicistronic vectors. Expert Opin. Biol. Ther. 2005;5:627–638. doi: 10.1517/14712598.5.5.627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.