Abstract
Background
Symptomatic infection with pinworm (Enterobius vermicularis), a human pathogen, is clinically relevant in Germany, with an estimated prevalence in childhood of 2–20%. Enterobiasis can cause major mental distress. There is little systematically verified knowledge on the treatment of this condition, and there is no corresponding German guideline. This review is, therefore, intended as a summary of the current state of knowledge.
Methods
This review is based on pertinent publications retrieved by a selective search in PubMed for literature appearing from 1 January 1990 to 5 February 2019 and containing the search terms “enterobiasis,” “oxyuriasis,” “Enterobius vermicularis,” “pinworm,” and “threadworm.”
Results
More than one billion people worldwide are thought to be infected with pinworm. Estimates of its prevalence among kindergarten and primary-school pupils in Europe are generally near 20%. Infants (<2 years of age), adolescents (>14 years of age), and adults are only sporadically affected. The main risk factors are age 4–11 years, uncontrolled anus-finger-mouth contact, nail-biting (onychophagia/perionychophagia), unsupervised body hygiene, and poor compliance with basic hand hygiene. No large-scale, randomized, controlled trials of treatment are available. The approved antihelminthic agents are mebendazole, pyrantel embonate, and pyrvinium embonate (success rates up to >90%). For recurrent infections, prolonged treatment for up to 16 weeks (a “pulse scheme”) is recommended.
Conclusion
In nearly all cases, antihelminthic treatment along with attention to hygienic measures can successfully eradicate pinworm infection and prevent recurrence and autoinfection. The involvement of all persons living in the patient’s household, including sexual partners, is a prerequisite to the lasting success of treatment.
Due to its widespread prevalence in children—and occasional occurrence in adults—symptomatic pinworm infection (enterobiasis) remains a relevant problem in Germany, despite the fact that infection rates have declined since the reunification. A 1978 investigation in daycare centers in Schwerin, Germany, found pinworms in 29% of children aged 1–3 years, 64% of 4- to 7-year-olds, and 28% of teachers. A follow-up investigation conducted in 1997 found a drop in these rates to 2%, 3.4%, and 0.7%, respectively (1). However, there have also been isolated signs of locally clustered detection rates over the past 10 years, for instance in the greater Berlin area (2). Although pinworm infection follows a harmless course in the majority of cases and is even asymptomatic in approximately 40% of cases (3), it is not uncommon for enterobiasis to be associated with considerable psychological distress for children and adults. For this reason, affected patients are often too embarrassed to seek medical advice (4). A lack of knowledge about how infection is transmitted and can be prevented, unsuccessful attempts at treatment, as well as a relatively high recurrence/autoinfection rate can lead to resignation on the part of the patient.
Despite a high disease incidence, there is surprisingly scant systematically proven knowledge on treatment—not to mention a German-language guideline; therefore, since general practitioners, as well as infectious disease and pediatric outpatient departments, are nevertheless regularly confronted with enterobiasis, this article aims to summarize the current state of knowledge in order to ensure optimal diagnosis and treatment.
Methods
The article is based on a selective literature search in PubMed for relevant publications during the period 1 January 1990 to 5 February 2019 using the search terms “enterobiasis,” “oxyuriasis,” “Enterobius vermicularis,” “pinworm,” and “threadworm.” A number of older publications, textbooks, as well as the authors‘ clinical experience were also taken into consideration.
Pathogen/etiology
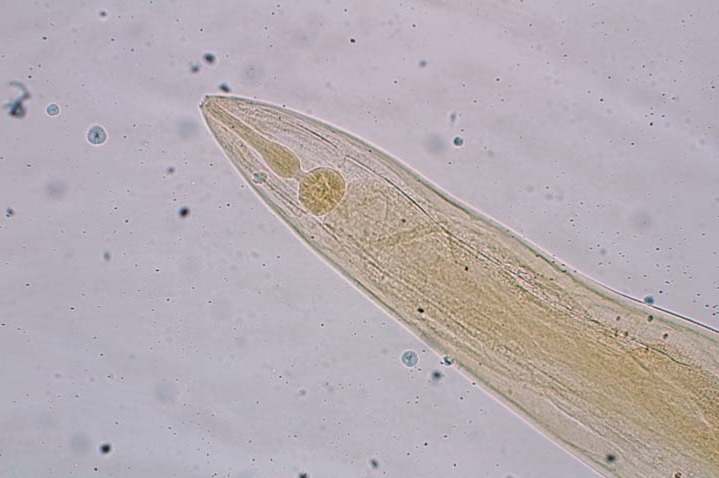
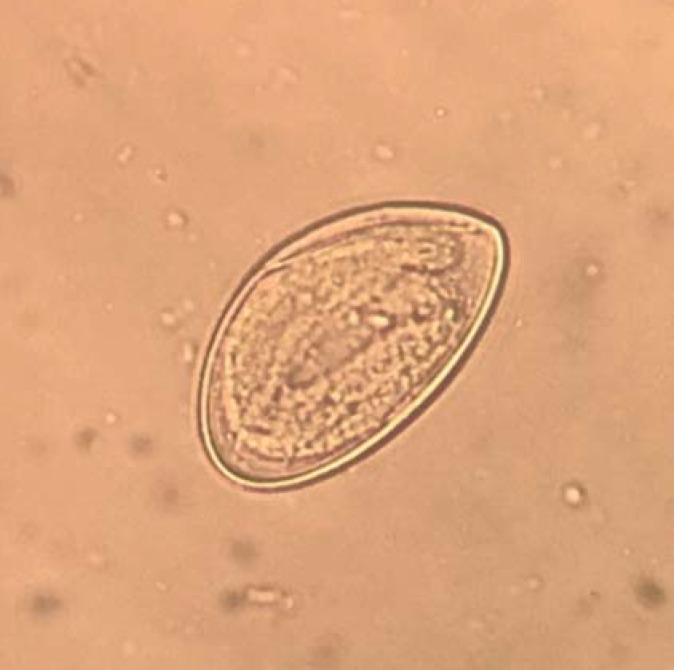
Enterobius (syn. Oxyuris) vermicularis is a human-pathogenic intestinal parasite belonging to the nematodes (Nematoda). Synonyms include “threadworm” and “seatworm.” Symptomatic pinworm infection is referred to as enterobiasis (older term: oxyuriasis) (5). The first description of the typically configured worm eggs by the Swedish natural scientist, Carl von Linné, dates back 1758 (6). One can assume that E. vermicularis has been successfully established as a parasite in the host organism since the evolution of human hominids. Fossil findings confirm that their co-existence stretches back over many thousands of years (7– 9). The distinguishing feature of these worms is their eponymous “thread-like” appearance (Fig. 1). The females are 9–12 mm long with a diameter of approximately 0.5 mm, while the males are shorter (3–5 mm) but visible to the naked eye. Striking whitish-beige in color, they are typically round in shape and move with a vigorous worm-like crawling motion. The head section is rounded and contains a muscular esophagus and bulb (eFigure); in females, the tail section is narrow and sharply tapered. The extensive uterine reproductive system of the fertilized female worm is often completely filled with eggs (>10 000/worm) (6, 7). Like all nematodes, E. vermicularis has a thick outer protective covering (cuticle). The double-layered, elongate-oval eggs (Figure 2) are 50–60 × 25 µm in size, translucent, and asymmetrical in shape (“slice of bread” shape). The first larval stage in the egg can often be well visualized. The parasite eggs are able to survive for a number of days outside the body (tenacity) (10– 12).
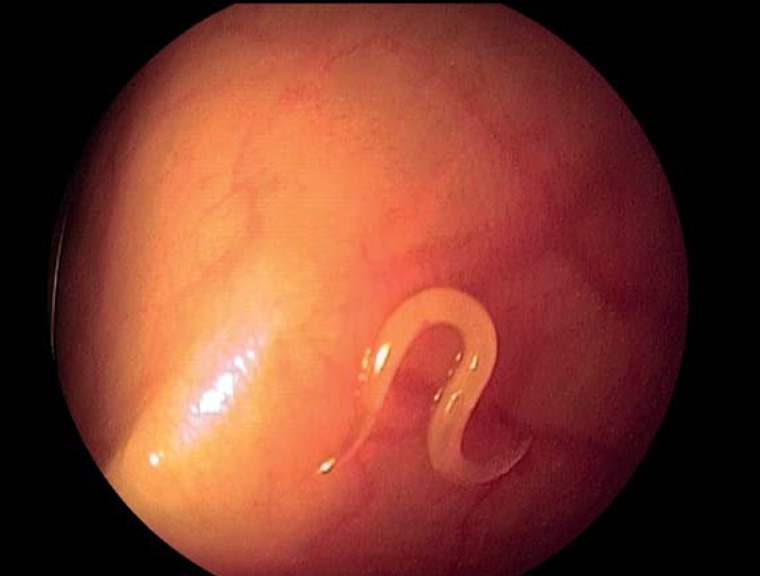
Figure 1.
Colonoscopic detection of Enterobius vermicularis in the cecum of an adult patient that holidayed on an organic farm
eFigure.
A microscopic specimen of an adult Enterobius vermicularis: the head section is rounded and contains a muscular esophagus and bulb (100x magnification).
Figure 2.
Microscopic detection of an Enterobius vermicularis egg in stool (400x magnification)
Life cycle
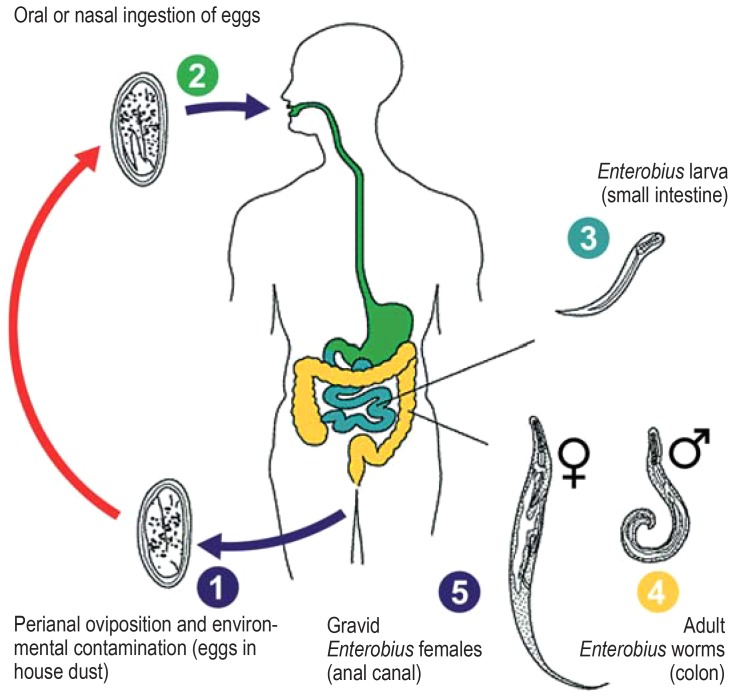
E. vermicularis has a simple direct life cycle (Figure) (13), which takes place in the gastrointestinal lumen. Infection occurs via oral ingestion of infective eggs—the larvae contained in the eggs become infective within as little as 4–6 h following oviposition. Once in the digestive system of the host organism, the external membrane of the eggs softens. After passing through the pylorus, the pinworm larvae hatch in the small intestine. After molting twice, the worms copulate and then migrate downwards to the large intestine, where they can be found in large numbers particularly in the cecum, appendix, or ascending colon. The males die soon after copulation; the gravid females, on the other hand, have an overall life span of up to 100 days, reaching the anal canal by means of active migration (14). The time interval between ingestion of infective eggs and oviposition by the adult female pinworm is 2–6 weeks (15). Oviposition occurs primarily while the host is resting, i.e., predominantly at night while they sleep. It involves the females leaving the anus and fixing their eggs to tissue in the perianal area with an adhesive matrix. The migratory movements of the worms during this process often cause uncomfortable pruritus, as a result of which children in particular tend to relieve the itching by using their fingers to scratch the perianal region. The disintegration of the worm cuticle caused by fingers and finger nails leads to a massive release and spreading of eggs into underwear and the surrounding area. The contaminated hands of the host play an important role in maintaining the chain of infection (autoinfection) (16). The already-hatched larvae can also enter the anus retrogradely, thereby causing renewed infection (retrograde infection) (17). Very rarely, incidental involvement of other organs occurs (an estimated <1% of cases) (18– 20). The eggs always need suitable conditions outside the gastrointestinal tract (low temperatures, high oxygen concentration) in order to mature. No intraluminal propagation occurs.
Epidemiology
Rough estimates put the worldwide E. vermicularis infection rate at more than a billion people (12, 21, 22). Pinworm infection is also common in moderate climates and industrial countries, where it is seen at all social levels (23). Frequency analyses in children have been conducted for some European countries: a Norwegian study (24) found that 18% of 395 children tested positive for Enterobius eggs using the Scotch tape test, with the highest prevalence (34%) among 6- to 11-year-olds (only two of 72 children that tested positive were known to have prior infection). Using the same detection method, a Swedish investigation (25) in 4- to 10-year-olds revealed an infection rate of 28.5% (49/172), while a large Estonian study of 954 kindergarten children (26) found a comparable prevalence of 24.4%.
A recent German study conducted in the greater Berlin area showed that the rate of positive detections doubled during the period 2007–2017 (from 12.7% to 23.6%), with a seasonal peak between October and December (2). No precise data are available for adults. A retrospective Romanian investigation (27) put the mean annual incidence for the period 1993–2006 at 777 per 100 000 inhabitants (independent of age). According to experience, young children (<2 years), older children (>14 years), and adults are affected significantly less frequently.
Risk factors and modes of transmission
A number of studies have identified major risk factors for pinworm infection (2, 16, 22, 25, 28, 29): children aged 4–11 years are particularly frequently affected, with male subjects sometimes being affected more often. A large percentage of children attend kindergarten or primary school during this phase. Close social contact, putting toys or writing utensils in the mouth, as well as nail biting (onychophagia/perionychophagia) play an important role in exposure to E. vermicularis at this age. Scratching in the perianal region, unchecked anus-finger-mouth contact, independent and unsupervised personal hygiene practices, as well as low compliance with regard to hand-washing prior to eating are all factors associated with significantly higher infection rates. The type of home construction, cleaning methods, or sharing a bedroom with other children or siblings were not associated with higher case numbers in individual studies (28). There are no data on risk factors in adults. With regard to the question of whether men who have sex with men (MSM) represent a special risk group, there are only individual older studies that point to this being the case (30, 31). Transmission in heterosexual partnerships is also relevant (32).
The considerable potential for E. vermicularis transmission is explained by the tenacity and adhesive property of the eggs, which adhere particularly well to hands and under fingernails (16, 25), thereby easily maintaining the chain of infection (continuous exposure, contact infection, autoinfection). The epidemiological relevance of persistent infective pinworm eggs in the environment (e.g., in house dust) is repeatedly emphasized in secondary sources. However, this hypothesis is difficult to confirm in studies: experiments show that, at room temperature, eggs are no longer infective after as little as 5 days (10). Since E. vermicularis is a strictly human-pathogenic parasite, domestic pets do not play a role as natural reservoirs of infection.
Symptoms/clinical picture
Approximately 40% of affected individuals are oligo- or asymptomatic (3, 4, 33). If autoinfection does not occur, pinworm infection is self-limiting due to the short life span of adult worms (11, 13). The main symptom of infection is pronounced (peri-)anal pruritus, which occurs primarily at nighttime while the affected individual sleeps. This can lead to disturbed sleep, childhood enuresis (in up to 53% of cases) and impaired concentration during the day (34– 36). In some cases, childhood developmental disorders have been linked to enterobiasis (36, e1).
Scratching in the perianal region can cause ulceration (excoriation) that shows a tendency toward bacterial superinfection. Anal dermatitis, perianal folliculitis, or ischiorectal abscess may develop. Very rarely, pinworms also migrate to the vaginal area, where they can cause vulvovaginitis (37) or be indirectly responsible for urinary tract infections due to adherent enterobacteria such as Escherichia coli (38). The role of E. vermicularis in relation to the pathogenesis of some cases of acute appendicitis has been the subject of controversy for many years, despite the fact that no causality has been reliably demonstrated (39, 40).
Extraintestinal infection patterns in the vagina, urinary bladder, peritoneum, kidneys, liver, and eye have been described in isolated cases (e2– e6). Enterobiasis can sometimes also overlap with the clinical picture of chronic inflammatory bowel disease (e7, e8). Invasive systemic infections do not occur even in severely immunosuppressed patients. Alongside the intense itch, the disease is also characterized by marked psychosocial strain.
Diagnosis
In addition to the typical patient history involving the cardinal symptom, i.e., intermittent (peri-)anal pruritus, an inspection of clothes and linen, the anal area, as well as stool can yield diagnostic information. The moving worm-like parasites are sometimes visible on underwear, bed sheets, or directly on the anal verge. In the case of severe infestation, the worms may be visibly expelled with the stool. Occasionally, individual adult worms are visualized on proctoscopy or colonoscopy (Figure 1, eVideo). A worm identified macroscopically constitutes evidence of infection.
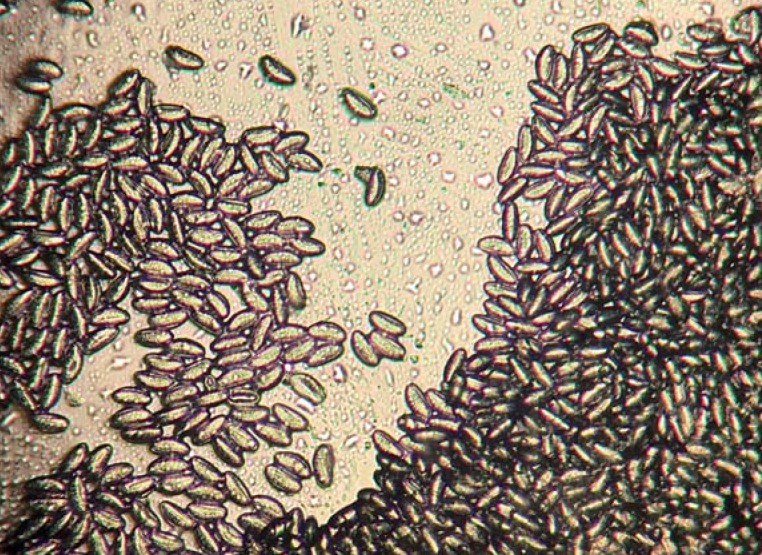
Many affected individuals do not observe visible pinworms (low parasite burden and no symptoms). Since oviposition occurs on the anal folds, the worm eggs that are invisible to the naked eye can be swabbed using commercially available adhesive cellulose tape (“Scotch tape”) in the morning prior to defecation and before washing the genital area (Scotch tape test). To this end, one presses the adhesive side of the tape (previously cut to size, e.g., 10 × 2 cm) against the anal and perianal region several times consecutively with the buttocks spread apart. The tape is then affixed to a suitable slide, adhesive side down. Microscopic detection of the characteristic worm eggs (Figure 3) confirms infection. The slides do not need to be stored, prepared, or preserved in any particular manner. Microbiological laboratories and pharmacies now offer readymade diagnostic kits. Alternatively, one can swab the anal region with a cotton swab and then place this in physiological saline solution. The solution can then be used in the microscopic inspection for worm eggs (or in molecular genetic detection methods). This diagnostic technique should be performed on three different days in order to increase its sensitivity (from around 50% in the case of a one-off Scotch tape test to approximately 90% if performed on three separate days [e9, e10]).
Figure 3.
Microscopic detection of Enterobius vermicularis eggs in a Scotch tape specimen (100x magnification)
Stool microscopy is not a helpful diagnostic tool, since the worm eggs are deposited outside the intestine. Serological methods are of no diagnostic relevance. Similarly, neither blood eosinophilia nor elevated immunoglobulin E levels are generally expected due to the low invasiveness of the worms.
Treatment
There are a number of highly effective and well-tolerated anthelmintic agents for the treatment of enterobiasis (Table). Substances that have been approved since the 1970s include mebendazole, pyrantel embonate, and pyvinium embonate. These are able to achieve eradication rates of >90% (e11). However, larger controlled studies on the individual drugs and treatment modes are lacking. Experience has shown that achieving initial treatment success is not the challenge, but rather the prevention of reinfection or autoinfection.
Table. Treatment of enterobiasis.
| Approved active substances (references) | Clinical picture | Dose | Repeat treatment | Success rate (%) | Side effects, interactions | Comments |
| Mebendazole (4, e11– e13) |
Initial infection | 100–200 mg (single dose), approved from 2 years of age | After 14 and 28 days | 90–100 | Generally well tolerated; occasional abdominal symptoms (nausea, vomiting, diarrhea, flatulence, cramps); vigilance required for interactions with metronidazole, cimetidine, praziquantel, and dexamethasone | Treatment of first choice in children >2 years and adults without extraintestinal infection; ovicidal; drug of first choice (off-label) during pregnancy and lactation with careful benefit–risk assessment and careful monitoring required |
| Chronic recurrent course | Every 14 days for a period of 16 weeks | No data | Medication to be extended to all household members and sexual partners | |||
| Pyrantel embonate (4, e11– e13) |
Initial infection | 10 mg/kg body weight (BW) (single dose), approved from 7 months of age | After 14 and 28 days | 90–100 | Maximum dose 1 g; generally well tolerated, occasional loss of appetite, insomnia, headache, dizziness, nausea, vomiting, diarrhea; transaminase elevation common; contraindicated in liver damage; interacts with piperazine and theophylline | Restrictions on use: infants <6 months; dosed according to body weight |
| Pyrvinium embonate (4, e11– e13) |
Initial infection | 5 mg/kg BW (single dose), approved from the age of 4 month | After 14 and 28 days | No data | Maximum dose 400 mg; generally well-tolerated; occasional abdominal symptoms; possible reddening of stool; contraindicated in liver damage, kidney failure and chronic inflammatory bowel disease | Restriction on use: infants <3 months; dosed according to body weight |
| Other active substances (references) | Clinical picture | Dose | Repeat treatment | Success rate (%) | Side effects, interactions | Comments |
| Albendazole (4, 14, e11– e13) |
Initial infection | 200–400 mg (single dose), children >2 years and weighing more than 10 kg receive 400 mg, children aged 1 year and weighing less than 10 kg receive 200 mg | After 14 and 28 days | 90–100 | Generally well-tolerated, occasional abdominal symptoms; potentially teratogenic and hepatotoxic; interacts with metronidazole, cimetidine, praziquantel, and dexamethasone | Generally only used for extraintestinal (urogenital) infection or recurrence (off-label); ovicidal; not suitable for children <6 years; less safety data than for mebendazole; cost-intensive, only available in 60-tablet pack in germany; approved for enterobiasis treatment in switzerland |
| Chronic recurrent course | Every 14 days for a period of 16 weeks | No data | Medication to be extended to all household members and sexual partners | |||
| Extraintestinal infection (urogenital) | After 14 and 28 days | Superior to mebendazole in extraintestinal infection due to systemic effect | ||||
| Ivermectin (e18, e19) |
Extraintestinal infection (urogenital) | 200 µg/kg BW (single dose) | After 14 and 28 days | 53–85 | Transient hypereosinophilia, transamine elevation | Generally only used in exceptional cases for extraintestinal (urogenital) infection (off-label); no safety guidelines for children <15 kg bw |
Only the two benzimidazole derivates, mebendazole and albendazole, are both adulticidal and ovicidal and are therefore considered to be the most effective drugs. However, albendazole, which is not approved for the treatment of enterobiasis, is costly and poses concerns with regard to teratogenicity and possible hepatotoxicity (14, e11– e13). The anthelmintic efficacy of benzimidazole is based on binding to ß-tubulin, which inhibits tubulin polymerization (e14) and depletes the worms’ glycogen reserves. Due to the structural similarity between parasitic and human ß-tubulins, side effects (Table) may be seen in some cases (particularly those involving prolonged and high-dose administration) and are due to impaired cell division.
In most cases, the above-mentioned drugs are well-tolerated, causing at most harmless and short-term side effects such as headache, abdominal pain, and diarrhea. In contrast to albendazole, only 7% of mebendazole is absorbed when administered orally (e11): the active substance largely remains in the intestinal lumen, which leads to fewer systemic side effects and is beneficial in the case of purely intestinal enterobiasis. There are a number of different dose regimens and various intervals between administrations (Table). Repeat administration following 14 and 28 days is recommended for initial infections (e13), since autoinfection is otherwise promoted by infective eggs (recurrence prevention). The majority of residual Enterobius eggs (in house dust) are no longer infective 5 days following oviposition (10). In the case of persistent infection of the urogenital system, treatment with the systemically effective substance albendazole (or ivermectin) can be considered in specialized centers (e15– e17). The literature reports eradication rates of 94%–100% for albendazole (e11) and 53%–85% for ivermectin (e18, e19). Intestinal lavage, “garlic cures,” Karlovy Vary salts, and appendectomy (in the absence of appendicitis) are all obsolete.
None of the anthelmintic agents discussed here is approved for use during pregnancy, or their use is recommended only after a rigorous benefit–risk analysis. However, the specialist literature, as well as experience in daily practice, suggests that, due to possible complications of enterobiasis and severely impaired well-being, treatment should not be withheld from pregnant and breast-feeding women (e12). Given its low systemic effect and the meanwhile relatively extensive evidence on its use during pregnancy and lactation, mebendazole is also the drug of choice under careful prenatal supervision.
In the case of chronic recurrent infection, simultaneously treating all (including asymptomatic) members of a household (parents, siblings, grandparents, fellow occupants) has proved a successful approach. All individuals included in treatment should also be informed about the clinical picture and modes of transmission of enterobiasis and encouraged to take special preventive measures (Box) during treatment (4, e20). These include thorough hand-washing before meals and after using the toilet, avoiding scratching in the anogenital area and anus-finger-mouth contact (where necessary, use of antipruritic ointments), daily washing of the genital region (from front to back), regular changes of underwear and sleeping garments, as well as strictly exclusive use of towels and flannels by the same person. Onychophagia should be avoided and anal–oral or anogenital–oral sexual practices should be abstained from. Hygiene measures over and above these (e.g., excessive personal hygiene) are not beneficial.
Box. Hygiene measures during the period of worm migration outside the rectum and anal pruritus.
Hands should be washed using soap following defecation and prior to eating
Prevention of nail biting (onychophagia/perionychophagia): fingernails should be kept as short as possible and regularly scrubbed
Underwear should be changed daily and washed at temperatures of at least 40 °C
Towels and flannels to be used exclusively by the same person
Bedclothes should be changed/washed following treatment
Daily washing of the anal region, particularly in the morning after getting up; if necessary, external agents (e.g., zinc ointment) should be applied
Household members and sexual partners should also be treated
All those involved should be informed about the clinical picture and modes of transmission
Treatment-refractory cases or multiple recurrences can be managed with repeat treatment (“pulse regimen”) (14, e13): Patients and all household members or sexual partners receive a single dose of preferably mebendazole every 14 days for a period of 16 weeks. The authors have achieved good results with this intensified treatment schedule.
Figure.
Life cycle of Enterobius vermicularis (modified from [13])
Key messages.
Enterobiasis is one of the commonest worm diseases in humans, occurring predominantly in children and, albeit far more rarely, also in adults.
The pinworm lays its eggs primarily at nighttime in the (peri-)anal region, often causing intense anal pruritus. Pinworm eggs have high potential for transmission (fingernail and environmental contamination, eggs in house dust).
The diagnostic method of choice is the perianal “Scotch tape specimen,” which is taken in the morning prior to the first bowel movement and washing of the genital area (sensitivity of three samples taken consecutively and analyzed microscopically: around 90%).
Mebendazole should be used as first-line treatment. Due to the high risk of recurrence, repeat administration after 14 and 28 days is recommended even in the case of initial infection.
Treating all members of a household, as well as sexual partners, with single-dose mebendazole at 14-day intervals for a period of 16 weeks in collaboration with a specialized center is recommended in the case of chronic recurrent infection.
Acknowledgments
Translated from the original German by Christine Schaefer-Tsorpatzidis
Footnotes
Conflict of interests The authors state that there are no conflicts of interest.
References
- 1.Gauert B. Eine vergleichende Untersuchung über Vorkommen und Verbreitung von Intestinalparasiten in Kindertagesstätten der Landeshauptstadt Schwerin. Gesundheitswesen. 1998;60:301–306. [PubMed] [Google Scholar]
- 2.Friesen J, Bergmann C, Neuber R, et al. Detection of Enterobius vermicularis in greater Berlin, 2007-2017: seasonality and increased frequency of detection. Eur J Clin Microbiol Infect Dis. 2019 doi: 10.1007/s10096-019-03495-1. doi: 10.1007/s10096-019-03495-1. [DOI] [PubMed] [Google Scholar]
- 3.Kubiak K, Dzika E, Paukszto L. Enterobiasis epidemiology and molecular characterization of Enterobius vermicularis in healthy children in north-eastern Poland. Helminthologia. 2017;54:284–291. [Google Scholar]
- 4.Ibarra J. Threadworms: a starting point for family hygiene. Br J Community Nurs. 2001;6:414–420. doi: 10.12968/bjcn.2001.6.8.7058. [DOI] [PubMed] [Google Scholar]
- 5.Deplazes P, Eckert J, von Samson-Himmelstjerna G, Zahner H. Enke Verlag. 3. Stuttgart: 2013. Lehrbuch der Parasitologie für die Tiermedizin. [DOI] [PubMed] [Google Scholar]
- 6.Despommier DD, Gwadz RW, Hotez PJ. Enterobius vermicularis (Linnaeus 1758) In: Despommier DD, Gwadz RW, Hotez PJ, editors. Parasitic diseases. New York Springer: 1995. pp. 2–6. [Google Scholar]
- 7.Cook GC. Enterobius vermicularis infection. Gut. 1994;35:1159–1162. doi: 10.1136/gut.35.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinhard KJ, Araújo A, Morrow JJ. Temporal and spatial distribution of Enterobius vermicularis (Nematoda: Oxyuridae) in the prehistoric Americas. Korean J Parasitol. 2016;54:591–603. doi: 10.3347/kjp.2016.54.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaeger LH, Gijón-Botella H, Del Carmen Del Arco-Aguilar M, et al. Evidence of helminth infection in guanche mummies: integrating paleoparasitological and paleogenetic investigations. J Parasitol. 2016;102:222–228. doi: 10.1645/15-866. [DOI] [PubMed] [Google Scholar]
- 10.Hulínská D. Morphogenesis and viability of larvae in the eggs of Enterobius vermicularis. Folia Parasitol. 1974;21:225–232. [PubMed] [Google Scholar]
- 11.Mehlhorn H. Spektrum Akademischer Verlag. 7. Heidelberg: 2012. Die Parasiten des Menschen: Erkrankungen erkennen, bekämpfen und vorbeugen. [Google Scholar]
- 12.Richter J, Häussinger D, Mehlhorn H. Madenwurminfektion: Eine häufige, aber wenig beachtete Parasitose. Dtsch Arztebl. 2003;100 [Google Scholar]
- 13.Centers for Disease Control and Prevention. Enterobiasis. www.cdc.gov/parasites/pinworm/ (last accessed on 5 October 2018) [Google Scholar]
- 14.Schubert S. Familienbehandlung mit Albendazol (Zentel) bei chronisch-rezidivierendem Enterobius vermicularis-Befall des Erwachsenen. Mitt Österr Ges Tropenmed Parasitol. 1991;13:191–198. [Google Scholar]
- 15.Chiodini PL, Moody AH, Manser DW. Churchill Livingstone. 4. Edinburgh: 2001. Atlas of medical helminthology and protozoology. [Google Scholar]
- 16.Cranston I, Potgieter N, Mathebula S, Ensink JHJ. Transmission of Enterobius vermicularis eggs through hands of school children in rural South Africa. Acta Trop. 2015;150:94–96. doi: 10.1016/j.actatropica.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Schüffner W, Swellengrebel NH. Retrofection in oxyuriasis: a newly discovered mode of infection with Enterobius vermicularis. J Parasitol. 1949;35:138–146. [PubMed] [Google Scholar]
- 18.Tornieporth NG, Disko R, Brandis A, Barutzki D. Ectopic Enterobiasis: a case report and review. J Infect. 1992;24:87–90. doi: 10.1016/0163-4453(92)91122-r. [DOI] [PubMed] [Google Scholar]
- 19.Powell G, Sarmah P, Sethi B, Ganesan R. Enterobius vermicularis infection of the ovary. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-201146. doi: 10.1136/bcr-2013-201146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai CY, Junod R, Jacot-Guillarmod M, Beniere C, Ziadi S, Bongiovanni M. Vaginal Enterobius vermicularis diagnosed on liquid-based cytology during Papanicolaou test cervical cancer screening: a report of two cases and a review of the literature. Diagn Cytopathol. 2018;46:179–186. doi: 10.1002/dc.23812. [DOI] [PubMed] [Google Scholar]
- 21.Norhayati M, Fatmah MS, Yusof S, Edariah AB. Intestinal parasitic infections in man: a review. Med J Malaysia. 2003;58:296–305. quiz 306. [PubMed] [Google Scholar]
- 22.Li HM, Zhou CH, Li ZS, et al. Risk factors for Enterobius vermicularis infection in children in Gaozhou, Guangdong, China. Infect Dis Poverty. 2015;4 doi: 10.1186/s40249-015-0058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dutto M, Montù D, Raineri G. Enterobiasis in pediatric subjects in north-western Italy: a study of home remedies. Ann Ig. 2012;24:81–84. [PubMed] [Google Scholar]
- 24.Bøås H, Tapia G, Sødahl JA, Rasmussen T, Rønningen KS. Enterobius vermicularis and risk factors in healthy Norwegian children. Pediatr Infect Dis J. 2012;31:927–930. doi: 10.1097/INF.0b013e318258cdb5. [DOI] [PubMed] [Google Scholar]
- 25.Herrström P, Henricson KA, Råberg A, Karlsson A, Högstedt B. Allergic disease and the infestation of Enterobius vermicularis in Swedish children 4-10 years of age. J Investig Allergol Clin Immunol. 2001;11:157–160. [PubMed] [Google Scholar]
- 26.Remm M. Distribution of Enterobiasis among nursery school children in SE Estonia and of other helminthiases in Estonia. Parasitol Res. 2006;99:729–736. doi: 10.1007/s00436-006-0220-1. [DOI] [PubMed] [Google Scholar]
- 27.Neghina R, Dumitrascu V, Neghina AM, et al. Epidemiology of ascariasis, Enterobiasis and giardiasis in a Romanian western county (Timis), 1993-2006. Acta Trop. 2013;125:98–101. doi: 10.1016/j.actatropica.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Chen KY, Yen CM, Hwang KP, Wang LC. Enterobius vermicularis infection and its risk factors among pre-school children in Taipei, Taiwan. J Microbiol Immunol Infect. 2018;51:559–564. doi: 10.1016/j.jmii.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Yao Z, Hou Y, et al. Prévalence d‘Enterobius vermicularis chez les enfants d‘âge préscolaire en 2003 et 2013 dans la ville de Xinxiang, province du Henan, Chine centrale. Parasite. 2016;23 doi: 10.1051/parasite/2016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waugh MA. Threadworm infestation in homosexuals. Trans St Johns Hosp Dermatol Soc. 1972;58:224–225. [PubMed] [Google Scholar]
- 31.Waugh MA. Letter: sexual transmission of intestinal parasites. Br J Vener Dis. 1974;50:157–158. doi: 10.1136/sti.50.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdolrasouli A, Hart J. Oral-anal intercourse and sexual transmission of Enterobius vermicularis; do we need to screen for other intestinal parasites? Int J STD AIDS. 2009;20 doi: 10.1258/ijsa.2009.009263. [DOI] [PubMed] [Google Scholar]
- 33.Gatherer A. School nurses: a common problem. Community Outlook. 1978;74:303–304. [PubMed] [Google Scholar]
- 34.Otu-Bassey IB, Ejezie GC, Epoke J, Useh MF. Enterobiasis and its relationship with anal itching and enuresis among school-age children in Calabar, Nigeria. Ann Trop Med Parasitol. 2005;99:611–616. doi: 10.1179/136485905X51481. [DOI] [PubMed] [Google Scholar]
- 35.Devera R, Pérez C, Ramos Y. [Enterobiasis in students from Ciudad Bolivar, Venezuela] Bol Chil Parasitol. 1998;53:14–18. [PubMed] [Google Scholar]
- 36.Zhao YE, Zhang H, Chang Y, Xun M, Wu XH. The relationship between the infection of pinworm and personal-social factors and its influence on the children´s growth. Chin J Parasit Dis Con. 2001;14:268–271. [Google Scholar]
- 37.Eder IB, Wendt S, Lipek T. Extraintestinal oxyuriasis. Dtsch Arztebl Int. 2018;115 doi: 10.3238/arztebl.2018.0326b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ok UZ, Ertan P, Limoncu E, Ece A, Ozbakkaloglu B. Relationship between pinworm and urinary tract infections in young girls. APMIS. 1999;107:474–476. doi: 10.1111/j.1699-0463.1999.tb01582.x. [DOI] [PubMed] [Google Scholar]
- 39.Fleming CA, Kearney DE, Moriarty P, Redmond HP, Andrews EJ. An evaluation of the relationship between Enterobius vermicularis infestation and acute appendicitis in a paediatric population—a retrospective cohort study. Int J Surg. 2015;18:154–158. doi: 10.1016/j.ijsu.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Vleeschouwers W, Hofman P, Gillardin JP, Meert V, van Slycke S. Appendicitis-like clinical image elicited by Enterobius vermicularis: case report and review of the literature. Acta Chir Belg. 2013;113:139–142. [PubMed] [Google Scholar]
- E1.Romanenko NA, Sergiev VP, Chernyshenko AI, et al. New approach to the eradication of Enterobiasis in children. Med Parazitol (Moskow) 1997;1:3–5. [PubMed] [Google Scholar]
- E2.Kiliç S, Ekinci S, Orhan D, Senocak ME. Enterobius granuloma: an unusual cause of omental mass in an 11-year-old girl. Turk J Pediatr. 2014;56:189–191. [PubMed] [Google Scholar]
- E3.Sammour ZM, Gomes CM, Tome ALF, Bruschini H, Srougi M. Prolonged irritative voiding symptoms due to Enterobius vermicularis bladder infestation in an adult patient. Braz J Infect Dis. 2008;12 doi: 10.1590/s1413-86702008000400020. [DOI] [PubMed] [Google Scholar]
- E4.Serpytis M, Seinin D. Fatal case of ectopic Enterobiasis: Enterobius vermicularis in the kidneys. Scand J Urol Nephrol. 2012;46:70–72. doi: 10.3109/00365599.2011.609834. [DOI] [PubMed] [Google Scholar]
- E5.Furnée EJB, Spoto C, de Graaf MJ, Smakman N. Enterobius vermicularis infection of the liver in a patient with colorectal carcinoma with suspected liver metastasis. BMJ Case Rep. 2015 doi: 10.1136/bcr-2015-212271. doi: 10.1136/bcr-2015-212271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Babady NE, Awender E, Geller R, et al. Enterobius vermicularis in a 14-year-old girl‘s eye. J Clin Microbiol. 2011;49:4369–4370. doi: 10.1128/JCM.05475-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E7.Liu LX, Chi J, Upton MP, Ash LR. Eosinophilic colitis associated with larvae of the pinworm Enterobius vermicularis. Lancet. 1995;346:410–412. doi: 10.1016/s0140-6736(95)92782-4. [DOI] [PubMed] [Google Scholar]
- E8.Al-Saffar F, Najjar N, Ibrahim S, Clark M. Pin worms presenting as suspected crohn‘s disease. Am J Case Rep. 2015;16:737–739. doi: 10.12659/AJCR.895566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Mahmoud AF. Intestinal nematodes (roundworms) Principles and practice of infectious diseases. In: Mandell GL, Douglas RG Jr, Bennett JE, editors. Churchill Livingstone Inc. 4. New York: 1995. pp. 2526–2531. [Google Scholar]
- E10.Jeandron A, Abdyldaieva G, Usubalieva J, et al. Accuracy of the Kato-Katz, adhesive tape and FLOTAC techniques for helminth diagnosis among children in Kyrgyzstan. Acta Trop. 2010;116:185–192. doi: 10.1016/j.actatropica.2010.08.010. [DOI] [PubMed] [Google Scholar]
- E11.Georgiev VS. Chemotherapy of Enterobiasis (Oxyuriasis) Expert Opin Pharmacother. 2001;2:267–275. doi: 10.1517/14656566.2.2.267. [DOI] [PubMed] [Google Scholar]
- E12.Djakovic A, Tappe D, Dietl J. Diagnostik und Therapie von Enterobius vermicularis-Infektionen in der Schwangerschaft: Literaturübersicht und Kasuistik. Z Geburtshilfe Neonatol. 2006;210:147–152. doi: 10.1055/s-2006-947219. [DOI] [PubMed] [Google Scholar]
- E13.Berner R, Bialek R, Forster J, et al. Georg Thieme Verlag. 7. Stuttgart, New York: 2018. DGPI-Handbuch: Infektionen bei Kindern und Jugendlichen. [Google Scholar]
- E14.Oxberry ME, Geary TG, Winterrowd CA, Prichard RK. Individual expression of recombinant alpha- and beta-tubulin from haemonchus contortus: polymerization and drug effects. Protein Expr Purif. 2001;21:30–39. doi: 10.1006/prep.2000.1347. [DOI] [PubMed] [Google Scholar]
- E15.Kashyap B, Samantray JC, Kumar S, Jhamb R, Singh AK, Kaur IR. Recurrent paediatric pinworm infection of the vagina as a potential reservoir for Enterobius vermicularis. J Helminthol. 2014;88:381–383. doi: 10.1017/S0022149X13000345. [DOI] [PubMed] [Google Scholar]
- E16.González P, González FA, Ueno K. Ivermectin in human medicine, an overview of the current status of its clinical applications. Curr Pharm Biotechnol. 2012;13:1103–1109. doi: 10.2174/138920112800399248. [DOI] [PubMed] [Google Scholar]
- E17.Patel B, Sharma T, Bhatt GC, Dhingra Bhan B. Enterobius vermicularis: an unusual cause of recurrent urinary tract infestation in a 7-year-old girl: case report and review of the literature. Trop Doct. 2015;45:132–134. doi: 10.1177/0049475514566872. [DOI] [PubMed] [Google Scholar]
- E18.Naquira C, Jimenez G, Guerra JG, et al. Ivermectin for human strongyloidiasis and other intestinal helminths. Am J Trop Med Hyg. 1989;40:304–309. doi: 10.4269/ajtmh.1989.40.304. [DOI] [PubMed] [Google Scholar]
- E19.Wen LY, Yan XL, Sun FH, Fang YY, Yang MJ, Lou LJ. A randomized, double-blind, multicenter clinical trial on the efficacy of ivermectin against intestinal nematode infections in China. Acta Trop. 2008;106:190–194. doi: 10.1016/j.actatropica.2008.03.007. [DOI] [PubMed] [Google Scholar]
- E20.Blake J. An action plan to prevent and combat threadworm infection. Nurs Times. 2003;99:18–19. [PubMed] [Google Scholar]