Abstract
Background
Although evidence from animal and human studies indicates opioid analgesics increase susceptibility to infections, it is unclear whether the risk varies by specific opioid. We compared the risk of serious infection among patients initiating long-acting opioid analgesics with and without previously reported immunosuppressive properties.
Methods
We conducted a retrospective cohort study of Tennessee Medicaid enrollees age ≥18 years initiating long-acting opioids (1995–2015). Hospitalizations for serious infection were identified using validated coding algorithms. We used multivariable Poisson regression models to calculate adjusted incidence rate ratios (aIRR) and 95% confidence intervals (CI) to compare the infection risk among patients using long-acting opioids with known immunosuppressive properties (morphine, fentanyl, methadone) to the infection risk among patients using long-acting opioids without known immunosuppressive properties (oxycodone, oxymorphone, tramadol) accounting for demographics, opioid dose, comorbidities and pain conditions, medication use, frailty indicators, and healthcare encounter history using exposure propensity scores. We further compared users of individual long-acting opioids to long-acting morphine users (considered the prototypical immunosuppressive opioid).
Results
Among the 61 240 patients initiating opioids with immunosuppressive properties and 22 811 patients initiating opioids without immunosuppressive properties, we identified 1906 serious infections. Nonimmunosuppressive opioid users had a lower rate of infections than immunosuppressive opioid users (aIRR:0.78 [CI: 0.66–0.91]). Among individual opioids, oxycodone users had a lower rate of infection than morphine users (aIRR:0.73 [CI: 0.60–0.89]). There were no significant differences in the infection risk between other opioids and morphine.
Conclusion
The risk of serious infections among long-acting opioid users varies by opioid type. Providers should carefully consider the risk of serious infections when making pain management decisions.
Keywords: opioid analgesics, serious infections
Patients using nonimmunosuppressive opioids had a lower rate of serious infections than immunosuppressive opioid users. Among individual opioids, oxycodone users had a lower rate of infection than morphine users. The infection risk among long-acting opioid users varies by opioid type.
The opioid epidemic in the United States was recently declared a public health emergency [1, 2]. Opioid use is associated with increased morbidity and mortality due to overdose, adverse respiratory outcomes, cardiovascular events, as well as serious infections [3–10].
Evidence from animal and in vitro experimental studies indicates that certain opioids (ie, morphine, methadone, and fentanyl) can disrupt immune responses and increase susceptibility to infections [11–14]. However, similar effects have not been documented with other opioids, including oxycodone, oxymorphone, and tramadol [11, 12, 15]. Existing evidence from randomized controlled trials is insufficient to elucidate the relevance of this opioid-induced immunosuppression on clinical outcomes due to limited sample sizes and incomplete reporting of infections [11, 14, 16]. Thus, whether the risk of serious infection varies by specific opioid formulation remains unclear.
The risk of opioid-related adverse outcomes is particularly worrisome among users of long-acting opioid formulations, typically prescribed for chronic pain, due to their potency and increased toxicity [5, 17, 18]. As multiple formulations of long-acting opioids are available, determining if the risk of serious infections varies by specific formulation could inform pain pharmacotherapy. Therefore, we conducted a retrospective cohort study to compare the risk of serious infection among patients initiating the use of different long-acting opioid analgesics, specifically those with and without previously reported immunosuppressive properties.
METHODS
Study Population
We conducted a retrospective cohort study among Tennessee Medicaid (TennCare) enrollees initiating the use of long-acting opioids from 1 January 1995 through 30 September 2015 (Supplement Section 1). The study was approved by the institutional review boards of Vanderbilt University and the Tennessee Department of Health, and by the Division of TennCare.
The cohort included adults aged ≥18 years initiating use of long-acting opioids, defined as filling a prescription for a long-acting opioid after 180 days with no long-acting opioid use. We required continuous enrollment in TennCare during the 366-day baseline period before the qualifying prescription fill and ≥ 1 clinical healthcare encounter during baseline. We excluded patients with conditions likely to reduce follow-up, increase the risk of infection independent of opioid use, or increase the possibility of misclassification of the opioid exposure (ie, patients with cancer, cystic fibrosis, chronic liver, lung and respiratory disease, end-stage renal disease, human immunodeficiency virus, immune disorders, organ transplantation, stroke, substance abuse, overdose and those using nonstudy opioids; Supplement Section 1; Supplementary Table S1). Patients could not enter the cohort until at least 30 days post-discharge from the most recent hospitalization. Patients who filled 2 different long-acting study opioids on the same qualifying date were excluded.
Exposure and Covariates
Study opioids consisted of oral and transdermal formulations of long-acting opioid analgesics (Supplement Section 1). For the primary analysis, opioids were classified based on experimental animal and in vitro studies as those with immunosuppressive properties (fentanyl, methadone, and morphine) and those without previously recognized immunosuppressive properties (oxycodone, oxymorphone, and tramadol) [11, 13, 15]. Secondary analyses focused on individual opioid formulations using morphine as the reference (the prototypical opioid with immunosuppressive properties) [11, 13, 15]. The daily dose of opioid use (in morphine milligram equivalents [MME]) was calculated using standard conversion factors (Supplementary Table S2).
We measured covariates in the 366-day baseline period including and before t0 for all episodes of a filled long-acting opioid prescription. Covariates included demographics (age, sex, and race/ethnicity), pain conditions, comorbidities, and medication use (including short-acting opioid use), frailty indicators, and healthcare use history (Supplementary Table S1). Because only individuals enrolled with full benefits that demonstrated active use of healthcare services were eligible, lack of evidence for a covariate was counted as not present, rather than missing.
Follow-up
Patients entered the cohort on the earliest qualifying prescription fill date (t0) for a long-acting study opioid that met all the above requirements. Follow-up continued from t0 through the earliest of the end of the study (30 September 2015), identification of a serious condition or substance abuse diagnosis, loss of enrollment, a prescription fill for a non-study opioid or a different long-acting opioid, the 180th day without availability of a long-acting study opioid, date of death, or the date of hospitalization for serious infection (Supplementary Table S1). We characterized each person-day of follow-up according to the prescribed days supply of filled long-acting opioids, based on pharmacy records. We classified follow-up person-time covered by filled medication prescriptions as current use, person-time from 1 to 90 days after the most recent end of opioid supply as recent use, and all other person-time as past use. Current use in the first 30 days after t0 was further classified as new use. Additional follow-up details are summarized in Supplement Section 1.
Outcome
Hospitalizations for serious infection were identified using an algorithm based on International Classifications of Diseases-Clinical Modification 9th-revision (ICD9-CM) diagnosis codes previously validated in the study population with a positive predictive value of 90.2% [19]. Infections identified included pneumonia, bacteremia/sepsis, pyelonephritis, meningitis/encephalitis, osteomyelitis/septic arthritis, endocarditis, and cellulitis (Supplementary Table S1) [10, 20, 21].
Statistical Analysis
Primary and Secondary Analyses
We used a multivariable Poisson regression model with robust standard errors to compare the relative incidence of hospitalizations for serious infection during periods of current use between those using opioids with and without previous evidence of immunosuppression [22, 23]. We calculated incidence rate ratios (IRR) and 95% confidence intervals (CIs) accounting for study covariates using exposure propensity scores, calendar year of the episode, and restricted cubic splines of patient age and opioid dose. Calculated propensity scores were included in the outcome regression model using restricted cubic splines to relax the assumption of the correct specification of the summary covariate’s functional form [24, 25]. Secondary analyses compared the risk of serious infections between the current use of morphine and other opioids (fentanyl, oxycodone, methadone, and oxymorphone). We calculated separate propensity scores for each of these assessments.
Sensitivity and Exploratory Analyses
Details of additional sensitivity analyses are described in Supplement Section 1. In summary, we used inverse-probability of treatment weighting (IPTW) based on the propensity score as a different covariate balancing strategy, compared the risk of infections during periods of current use to past use by immunosuppressive properties of the opioid using the Centers for Disease Control and Prevention (CDC)-defined opioid dose categories (<50 MME/day, 50–90 MME/day, and ≥90 MME/day) [4], assessed the possibility of protopathic bias, and repeated the primary analysis excluding tramadol users. We conducted post-hoc sensitivity analyses accounting for cumulative dose of past glucocorticoid use, characteristics of the patient’s geographical setting, and the potential interaction between treatment groups and opioid dose (Supplement Section 1). Statistical analyses and manuscript preparation were performed using SAS version 9.4 (SAS Institute), Stata Version 15.1 (StataCorp LP), and StatTag (Northwestern University) [26].
RESULTS
Study Population and Episodes of Long-acting Opioid Use
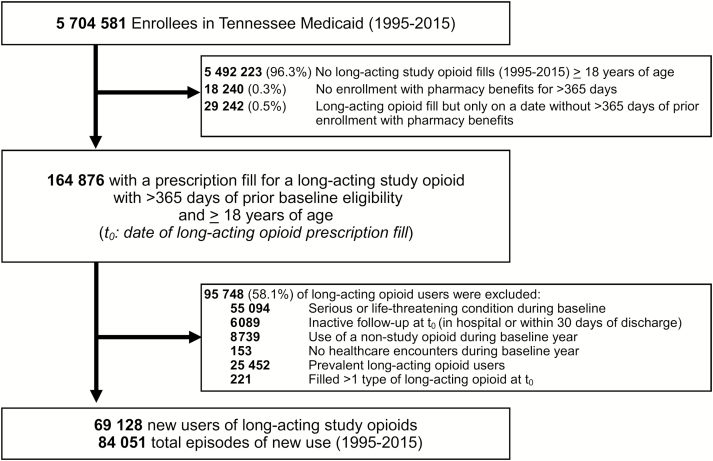
After selection criteria were applied, we identified 69128 unique patients who contributed 84051 new episodes of long-acting study opioid use (Figure 1). Major reasons for exclusion among eligible long-acting opioid users (n = 164876) were the presence of serious/life-threatening conditions and substance abuse, prevalent opioid use and nonstudy opioid use (Figure 1).
Figure 1.
Identifying a retrospective cohort of patients initiating long-acting opioids, Tennessee Medicaid (1995–2015).
The majority of patients initiated the use of long-acting morphine (45.2%), followed by fentanyl (20.9%), oxycodone (19.7%), methadone (6.7%), oxymorphone (4.6%), and tramadol (2.9%) (Supplementary Table S3a). The most common conditions associated with pain were trauma, back, and musculoskeletal pain. The most common comorbidities were hypertension, chronic obstructive pulmonary disease, diabetes, prior infections, lipid disorders, and peripheral artery disease. The most common medications used during baseline were short-acting opioids, antibiotics, antidepressants, anticonvulsants, benzodiazepines, bronchodilators, glucocorticoids, proton-pump inhibitors and nonsteroidal anti-inflammatory drugs (Table 1 and Supplementary Table S4). The full list of conditions and medication use is reported in Supplementary Table S4.
Table 1.
Baseline Characteristics of New Long-acting Opioid Users for Selected Variables of Interesta by Treatment Group, Tennessee Medicaid (1995–2015)
| Nonimmunosuppressive (N = 22811) |
Immunosuppressive (N = 61240) |
|||
|---|---|---|---|---|
| Age, y | ||||
| Mean (SD) | 49.1 | (15.3) | 53.7 | (17.3) |
| Median (IQR) | 48.0 | (21.0) | 51.0 | (23.0) |
| Characteristics | No. | (%) | No. | (%) |
| Sex | ||||
| Female | 13760 | (60.3) | 39233 | (64.1) |
| Race | ||||
| White | 18134 | (79.5) | 49090 | (80.2) |
| Black | 2109 | (9.3) | 5818 | (9.5) |
| Hispanic/Asian/Other | 2568 | (11.3) | 6332 | (10.3) |
| Selected comorbidities | ||||
| Cardiovascular diseaseb | 2237 | (9.8) | 7162 | (11.7) |
| Chronic obstructive pulmonary disease | 3156 | (13.8) | 9835 | (16.1) |
| Diabetes | 3315 | (14.5) | 9857 | (16.1) |
| Hypertension | 6266 | (27.5) | 18965 | (31.0) |
| Infection history | 8269 | (36.3) | 23755 | (38.8) |
| Lipid disorders | 2119 | (9.3) | 6501 | (10.6) |
| Peripheral artery disease | 2305 | (10.1) | 7003 | (11.4) |
| Tobacco use | 732 | (3.2) | 1835 | (3.0) |
| Selected pain or pain-inducing conditions | ||||
| Abdominal pain | 1549 | (6.8) | 4391 | (7.2) |
| Back pain | 8356 | (36.6) | 25342 | (41.4) |
| Dental pain | 259 | (1.1) | 519 | (0.8) |
| External causes of injury | 882 | (3.9) | 2253 | (3.7) |
| Musculoskeletal pain | 7402 | (32.4) | 21516 | (35.1) |
| Neuropathic pain | 1805 | (7.9) | 5716 | (9.3) |
| Pain not specified | 1349 | (5.9) | 3649 | (6.0) |
| Trauma | 2857 | (12.5) | 6840 | (11.2) |
| Selected medication use history | ||||
| Short-acting hydrocodone | 5667 | (24.8) | 19082 | (31.2) |
| Short-acting oxycodone | 3323 | (14.6) | 6724 | (11.0) |
| Other short-acting opioids | 5294 | (23.2) | 12606 | (20.6) |
| More than one short-acting opioid | 8527 | (37.4) | 22828 | (37.3) |
| Antibiotics | 16252 | (91.2) | 45231 | (73.9) |
| Antifungals | 2795 | (12.3) | 8266 | (13.5) |
| Antipsychotics | 2493 | (10.9) | 9902 | (16.2) |
| Benzodiazepines | 10842 | (47.5) | 30335 | (49.5) |
| Bronchodilators, beta agonist | 5444 | (23.9) | 15682 | (25.6) |
| Bronchodilators, other | 2822 | (12.4) | 9297 | (15.2) |
| Diabetes, hypoglycemic medications | 3253 | (14.3) | 9634 | (15.7) |
| Diabetes, insulin | 1728 | (7.6) | 5708 | (9.3) |
| Disease-modifying antirheumatic drugs | 440 | (1.9) | 1214 | (2.0) |
| Influenza vaccine | 3230 | (14.2) | 10041 | (16.4) |
| Glucocorticoids | 9826 | (43.1) | 27859 | (45.5) |
| Nonsteroidal anti-inflammatory drugs | 13361 | (58.6) | 35402 | (57.8) |
| Pneumococcal vaccine | 534 | (2.3) | 1657 | (2.7) |
| Proton pump inhibitors | 6675 | (29.3) | 21566 | (35.2) |
| Sedatives | 2982 | (13.1) | 9114 | (14.9) |
| Selected frailty indicators | ||||
| Ambulation devices | 1553 | (6.8) | 5080 | (8.3) |
| Continuous/bilevel positive airway pressure | 738 | (3.2) | 2303 | (3.8) |
| Decubitus/pressure ulcers | 719 | (3.2) | 2929 | (4.8) |
| Enteral and parenteral nutrition | 235 | (1.0) | 1234 | (2.0) |
| Impaired mobility | 321 | (1.4) | 895 | (1.5) |
| Incontinence | 530 | (2.3) | 1982 | (3.2) |
| Oxygen supplementation | 1316 | (5.8) | 4445 | (7.3) |
| Rehabilitation | 909 | (4.0) | 2177 | (3.6) |
| Healthcare utilization | ||||
| ED visits in prior year | ||||
| 0 | 10172 | (44.6) | 27984 | (45.7) |
| 1 | 5566 | (24.4) | 15096 | (24.7) |
| 2 | 2837 | (12.4) | 7361 | (12.0) |
| ≥ 3 | 4236 | (18.6) | 10799 | (17.6) |
| Outpatient visits in past year | ||||
| 0 | 10270 | (45.0) | 26095 | (42.6) |
| 1 | 4166 | (18.3) | 10798 | (17.6) |
| 2 | 4135 | (18.1) | 11812 | (19.3) |
| ≥ 3 | 4240 | (18.6) | 12535 | (20.5) |
| Hospitalizations in past year | ||||
| 0 | 18297 | (80.2) | 46788 | (76.4) |
| 1 | 3944 | (17.3) | 12364 | (20.2) |
| ≥ 2 | 570 | (2.5) | 2088 | (3.4) |
Abbreviations: ED, emergency department; IQR, interquartile range; PS, propensity-score; SD, standard deviation.
aFull list of covariates is included in Supplementary Table S4.
bCardiovascular disease is an indication of at least one of the following: heart failure, acute myocardial infarction, carotid revascularization, coronary heart disease (ie, angina, coronary revascularization, stents, use of long-acting nitrates), cor pulmonale, and congenital heart defects.
A total of 1906 hospitalizations for serious infections were identified during 55663 person-years of follow-up (3.42 hospitalizations for serious infections per 100 person-years). The most common infections were pneumonia (56.1%), cellulitis (17.9%), bacteremia without pneumonia (15.3%), pyelonephritis (6.1%), and septic arthritis/osteomyelitis (3.8%).
Comparison of Opioids With and Without Previously Reported Immunosuppressive Properties
Patients initiating immunosuppressive opioids were slightly older and more likely to be female compared to those initiating opioids without known immunosuppressive properties (Table 1). The mean starting dose of the opioids was similar among those initiating with immunosuppressive versus non-immunosuppressive opioids (72.6 MME/day versus 75.9 MME/day, respectively). The distribution of the propensity score had substantial overlap between the opioid groups (Supplementary Figure 1).
The crude incidence rate of infection-related hospitalizations was higher among patients using immunosuppressive opioids compared to those using opioids without known immunosuppression. In the adjusted analysis, the current use of opioids without known immunosuppressive properties was associated with a 22% lower rate of serious infections compared to the current use of immunosuppressive opioids (IRR: 0.78 [95% CI: 0.66–0.91]) (Table 2).
Table 2.
Crude and Adjusted Incidence Rate Ratios for Serious Infections by Immunosuppressive Properties of the Long-acting Opioid Among Tennessee Medicaid Enrollees (1995–2015)
| Opioid Type | Infections During Current Use (n) | Incidence per 100 Person-years of Current Use | Crude IRR (95% CI) |
PS Spline-adjusted IRR (95% CI)a |
IPTW-adjusted IRR (95% CI)b |
|||
|---|---|---|---|---|---|---|---|---|
| Previously described immunosuppressive properties | ||||||||
| Immunosuppressivec | 1012 | 4.28 | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) |
| Nonimmunosuppressived | 205 | 2.46 | 0.58 | (0.49, 0.67) | 0.78 | (0.66, 0.91) | 0.79 | (0.67, 0.92) |
Abbreviations: CI, confidence interval; IPTW, inverse-probability of treatment weighting; IRR, incidence rate ratio.
aAdjusted for cubic spline of the propensity score for treatment with non-immunosuppressive opioids, cubic spline of the cumulative opioid dose, and cubic spline of age, and calendar year.
bAdjusted for cubic spline of the cumulative dose, cubic spline of age, calendar year, and using IPTW with the propensity score for treatment with nonimmunosuppressive opioids.
cOpioids with evidence of immunosuppressive properties in experimental studies: morphine, fentanyl, methadone.
dOpioids without evidence or weak evidence of immunosuppressive properties in experimental studies: oxycodone, oxymorphone, tramadol.
Infection-related hospitalization rates were higher during periods of current compared to past use (IRR:1.50 [95% CI: 1.29–1.74]) and was highest in the first 30 days after opioid initiation (new current use) (IRR:1.70 [95% CI: 1.50–1.94]) compared to prevalent current use. There were no statistically significant differences in the risk of hospitalization for infections between those initiating nonimmunosuppressive opioids compared to immunosuppressive opioids during periods of recent and past opioid use (IRR:0.87 [95% CI: 0.69–1.08] and IRR:0.79 [95% CI: 0.55–1.12], respectively). Additionally, the risk of infection associated with the new use versus past use was higher for immunosuppressive opioids (IRR: 2.51 [95% CI: 2.09–3.02]) compared to non-immunosuppressive opioids (IRR: 1.55 [95% CI: 1.12–2.14]). Similar findings were observed when comparing prevalent to past use for immunosuppressive and non-immunosuppressive opioids (IRR: 1.39 [95% CI: 1.19–1.62] and IRR: 1.14 [95% CI: 0.92–1.41], respectively).
Individual Opioids
Baseline patients’ characteristics differed according to opioid type. Of note, fentanyl users were older and more likely to be female compared to those using morphine (Supplementary Table S6). Oxycodone users were similar in age and sex compared to morphine users (Supplementary Table S6). Morphine and oxycodone users had the most follow-up in the study compared to fentanyl, methadone, and oxymorphone users, whereas tramadol users had the least follow-up in the study (Supplementary Table S3a). Oxycodone and tramadol users the most likely to end follow-up due to opioid discontinuation, whereas fentanyl users were the most likely to die during follow-up (Supplementary Table S5a and S5b). Because tramadol users only had 7 serious infections during periods of current use, this group was not compared to morphine users.
The propensity score distribution overlap for each opioid relative to morphine is shown in Supplementary Figure 2. In the adjusted analyses, the rates during periods of current fentanyl and methadone (both immunosuppressive) use compared to current morphine use were not statistically significantly different (Table 3), whereas oxycodone (without known immunosuppressive properties) users had a significantly lower rate of infections compared to morphine (immunosuppressive) users (IRR: 0.73 [95% CI: 0.60–0.89]). The rate of serious infections was similar among oxymorphone users compared to morphine users (Table 3).
Table 3.
Crude and Adjusted Incidence Rate Ratios for Serious Infections by Individual Opioids Among Tennessee Medicaid Enrollees (1995–2015)
| Opioid Type | Infections During Current Use (n) | Incidence per 100 Person-years of Current Use | Crude IRR (95% CI) |
Spline-adjusted IRRa (95% CI) |
IPTW-adjusted IRRb (95% CI) |
|||
|---|---|---|---|---|---|---|---|---|
| Opioids with previously recognized immunosuppressive properties | ||||||||
| Morphine | 459 | 2.91 | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) |
| Fentanyl | 501 | 8.95 | 3.08 | (2.71, 3.50) | 1.06 | (0.88, 1.28) | 0.89 | (0.73, 1.08) |
| Methadone | 52 | 2.30 | 0.79 | (0.59, 1.06) | 0.83 | (0.61, 1.13) | 0.82 | (0.60, 1.11) |
| Opioids without previously recognized immunosuppressive properties | ||||||||
| Oxycodone | 179 | 2.84 | 0.98 | (0.82, 1.16) | 0.73 | (0.60, 0.89) | 0.72 | (0.59, 0.87) |
| Oxymorphone | 19 | 1.17 | 0.40 | (0.25, 0.64) | 0.95 | (0.58, 1.56) | 1.05 | (0.56, 1.97) |
Abbreviations: CI, confidence interval; IPTW, inverse-probability of treatment weighting; IRR, incidence rate ratio.
aAdjusted for cubic spline of the propensity score for treatment with each opioid, cubic spline of the cumulative opioid dose, and cubic spline of age and calendar year (2-year intervals).
bAdjusted for cubic spline of the cumulative dose, cubic spline of age, calendar year (2-year intervals), and using IPTW with the propensity score for treatment with each opioid.
Sensitivity Analyses (Described in Supplement Section 2)
The results of the IPTW analysis were consistent with the propensity score analyses for the primary comparison and the individual opioid comparisons (Tables 2 and 3). In the sensitivity analysis using CDC-defined opioid dosing categories, the risk of infection was higher for immunosuppressive opioids within each dosing category, and the association increased as the dose increased for immunosuppressive opioids. In contrast, this was not consistently observed for opioids without previously described immunosuppressive properties (Supplementary Table S7). The results from the sensitivity analyses accounting for the cumulative dose of past glucocorticoid use, possible protopathic bias, excluding tramadol users and accounting for residence county and rurality characteristics were similar to those from the primary analysis (Supplementary Table S8), and no interaction was observed between dose and opioid type (P = .28).
DISCUSSION
Prescription opioid use is a risk factor for infections among humans, with the greatest risk reported among users of long-acting opioids [8–10]. Experimental evidence from animal models and human studies suggest that different opioids exhibit different immunosuppressive properties [11, 13, 27]. However, whether the risk of serious infections differs for individual opioids among humans remained unclear. Our findings demonstrate that among patients initiating long-acting opioid analgesic use, patients using opioids without previously reported immunosuppressive properties had a lower risk of hospitalization for serious infections compared to immunosuppressive opioids. Furthermore, oxycodone users had a significantly lower risk of hospitalization for serious infection relative to morphine users, whereas no differences in risk were observed for other opioids.
Morphine, as the prototypical opioid used in experimental and in vitro studies, has well-recognized immunosuppressive properties, with only limited evidence of immunosuppression for methadone and fentanyl (Supplement Section 3) [11, 13, 27]. Immunosuppressive effects have not been observed in experimental studies among other opioid analgesics (eg, oxycodone, oxymorphone, hydrocodone, tramadol) [11, 13, 27]. As the precise immunosuppressive mechanism of opioids requires further study, our group of “nonimmunosuppressive” opioids may include some opioids with previously unrecognized immunosuppressive properties [11, 13, 15]. Importantly, our study could not directly evaluate the mechanism for the higher risk of infections associated with use of immunosuppressive opioids.
Although previous studies have demonstrated that the immunosuppressive properties of certain opioids are associated with an increased risk of serious infections in humans, few studies have assessed whether the use of different opioids is associated with a different risk of infection [8–10, 27–29]. In a study among community-dwelling older adults, the association between opioid use and pneumonia was stronger among users of immunosuppressive opioids (odds ratio [OR]:1.88 [95% CI: 1.26–2.79]) than users of nonimmunosuppressive opioids (OR: 1.23 [95% CI: 0.89–1.69]) compared to nonusers [8]. Among patients with rheumatoid arthritis, the strongest association between opioid use and the risk of infections was observed among periods of immunosuppressive opioid use compared to nonuse (IRR: 1.72 [95% CI: 1.33–2.23]), with a more modest association during periods of non-immunosuppressive opioid use (IRR: 1.37 [95% CI: 1.15–1.62]) [10]. Furthermore, among Tennessee Medicaid enrollees, opioids with known immunosuppressive properties had the strongest association with laboratory-confirmed invasive pneumococcal disease([OR: 1.74 [95% CI: 1.20–2.53]) compared to opioids without known immunosuppressive properties (OR: 1.55 [95% CI: 1.27–1.88]) [9]. Unlike those previous studies that used unexposed groups as reference for comparisons, our study compared patients using different long-acting opioids and demonstrated that the risk of hospitalization for serious infection was 22% lower among those initiating opioids without previously reported immunosuppressive properties compared to those with such properties. Importantly, we also observed that the risk of serious infections associated with the current use of immunosuppressive opioids increased as the dose increased, but that the risk did not increase as the dose of nonimmunosuppressive opioids increased.
An important strength of this study was the use of validated definitions for identifying hospitalizations for serious infection using administrative data in the TennCare population. These definitions have a high positive predictive value (90.2%) compared with medical chart reviews [19]. When the incidence of the outcome is low, as in our study, the PPV approximates the specificity, such that the ICD9 coding algorithms had a high specificity for the identification of hospitalizations for serious infections. Nondifferential outcome misclassification between the exposure groups (ie, immunosuppressive vs nonimmunosuppressive opioid users) in this setting will bias the risk ratio estimate toward the null [27–29].
We focused this study on comparisons of long-acting opioid formulations only, rather than including comparisons with short-acting formulations, as previous evidence indicated that use of long-acting opioid formulations was associated with the highest risk of infection and due to concerns of comparability between those using long-acting and short-acting opioids. Additionally, by excluding individuals with serious life-threatening conditions and with evidence of opioid use disorders, we were able to focus the comparisons among individuals using long-acting opioid analgesics for chronic pain conditions. We observed consistent results when we applied several different analytical strategies to control for confounding, including the use of exposure propensity scores, to help balance out any differences in the large number of covariates between comparison groups. The new-user design helped with the comparability of study groups by equalizing the beginning of follow-up and ensuring all events that occurred after initiation of opioid use were appropriately classified [30]. Nevertheless, in spite of the use of active comparator groups and the use of propensity scores, we cannot completely rule out the possibility of residual confounding.
An important limitation in our study was that prescription opioid use was defined based on filled pharmacy prescriptions but not directly observed. It is possible that patients did not complete their full prescription or did not take their opioids as prescribed. To reduce this potential misclassification, we classified intervals covered by prescription days of supply as current use (representing the periods with highest likelihood of prescription opioid exposure) and compared the rate of infections during periods of current use in the primary analysis. Although we excluded patients with evidence of substance or alcohol use disorders, we were unable to account for illicit opioid use. We were also unable to examine the risk for individual infection types (ie, bacterial, viral, or fungal) due to the challenge of accurately determining disease etiology in routine clinical practice or in research studies [31]. Although some reports have reported an association between conditions or procedures that induce pain with immunosuppression, we specifically accounted for pain-inducing conditions in our analyses to reduce concerns of residual confounding due to pain. Furthermore, although differences in pain control could theoretically contribute to the risk of infection among different opioid users, there is no evidence to suggest that the efficacy of pain control is different for patients using opioids with and without previously recognized immunosuppressive properties.
The selection of one of the multiple long-acting opioids available needs to consider both the safety and effectiveness of each formulation. In head-to-head randomized controlled trials, few differences in effectiveness or safety have been observed among different long-acting opioids, although small sample sizes likely limited the detection of differences in these trials [31]. Although not reported in every study, some trials suggest a lower rate of gastrointestinal complications associated with the use of long-acting oxycodone and fentanyl compared to long-acting morphine [31–34]. In addition, a retrospective observational study found that long-acting oxycodone users had a lower risk of all-cause hospitalizations and all-cause mortality than users of long-acting morphine, though morphine had a lower risk of hospitalizations and overdose compared to fentanyl and methadone, respectively [35]. Other studies have offered conflicting evidence regarding an increased risk of all-cause mortality among methadone users compared to morphine users [36, 37]. Our observations of differential infection risk with different opioid formulations should be considered among other safety and effectiveness concerns by clinicians and patients when making pain management decisions [28].
In conclusion, patients using long-acting opioids previously described as immunosuppressive had a higher risk of serious infections compared with patients using nonimmunosuppressive long-acting opioids. In addition, patients using long-acting oxycodone had a significantly lower risk of serious infection compared with those using long-acting morphine, the prototypical immunosuppressive opioid. These findings are consistent with results from animal and in vitro experimental studies showing immunosuppressive properties only in certain opioids, and provide further evidence that cautious, judicious and informed opioid selection for pain management is of utmost importance.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. A. D. W. planned the statistical analysis, analyzed and interpreted the data, and drafted and revised the paper. M. R. G., W. S., C. M. S., and R. A. G. planned the statistical analysis, interpreted the data, and revised the paper. E. F. M. prepared the data and revised the paper. C. G. G. initiated the project, acquired the data from TennCare, planned the statistical analysis, interpreted the data, and revised the paper.
Acknowledgments. We are indebted to the Tennessee Division of TennCare of the Department of Finance and Administration, which provided data for the study. We are also indebted to the Tennessee Department of Health for providing data for the study.
Disclaimer. The funder of the study had no role in the study design, data analysis, data interpretation, or writing of the report. The corresponding author had final responsibility for the decision to submit for publication.
Financial support. The study was supported by the National Institute of Aging of the National Institutes of Health, through grants R03 AG042981 and R01 AG043471.
Potential conflicts of interest. C. G. G. has received consulting fees from Pfizer, Sanofi, and Merck, and received research support from Sanofi-Pasteur, Campbell Alliance, the Centers for Disease Control and Prevention, the National Institutes of Health, the Food and Drug Administration, and the Agency for Health Care Research and Quality. W. S. has received personal fees from Pfizer, Merck, Novavax, Dynavax, Sanofi-Pasteur, GlaxoSmithKline, and Seqirus, and received research support from the Centers for Disease Control and Prevention. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med 2010; 363:1981–5. [DOI] [PubMed] [Google Scholar]
- 2. Dart RC, Surratt HL, Cicero TJ, et al. . Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med 2015; 372:241–8. [DOI] [PubMed] [Google Scholar]
- 3. Solomon DH, Rassen JA, Glynn RJ, et al. . The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med 2010; 170:1979–86. [DOI] [PubMed] [Google Scholar]
- 4. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain: United States, 2016. MMWR Recomm Rep 2016; 65:1–49. [DOI] [PubMed] [Google Scholar]
- 5. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA 2016; 315:2415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ekstrom MP, Bornefalk-Hermansson A, Abernethy AP, Currow DC. Safety of benzodiazepines and opioids in very severe respiratory disease: national prospective study. BMJ 2014; 348:g445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vozoris NT, Wang X, Fischer HD, et al. . Incident opioid drug use among older adults with chronic obstructive pulmonary disease: a population-based cohort study. Br J Clin Pharmacol 2016; 81:161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dublin S, Walker RL, Jackson ML, et al. . Use of opioids or benzodiazepines and risk of pneumonia in older adults: a population-based case-control study. J Am Geriatr Soc 2011; 59:1899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wiese AD, Griffin MR, Schaffner W, Stein CM, Grijalva CG. Opioid analgesic use and risk for invasive pneumococcal diseases. Ann Intern Med 2018; 169:355. [DOI] [PubMed] [Google Scholar]
- 10. Wiese AD, Griffin MR, Stein CM, Mitchel EF Jr, Grijalva CG. Opioid analgesics and the risk of serious infections among patients with rheumatoid arthritis: a self-controlled case series study. Arthritis Rheumatol 2016; 68:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Plein LM, Rittner HL. Opioids and the immune system: friend or foe. Br J Pharmacol 2018; 175:2717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sacerdote P, Franchi S, Panerai AE. Non-analgesic effects of opioids: mechanisms and potential clinical relevance of opioid-induced immunodepression. Curr Pharm Des 2012; 18:6034–42. [DOI] [PubMed] [Google Scholar]
- 13. Sacerdote P. Opioid-induced immunosuppression. Curr Opin Support Palliat Care 2008; 2:14–8. [DOI] [PubMed] [Google Scholar]
- 14. Brack A, Rittner HL, Stein C. Immunosuppressive effects of opioids–clinical relevance. J Neuroimmune Pharmacol 2011; 6:490–502. [DOI] [PubMed] [Google Scholar]
- 15. Sacerdote P. Opioids and the immune system. Palliat Med 2006; 20(Suppl 1):s9–15. [PubMed] [Google Scholar]
- 16. Rittner HL, Roewer N, Brack A. The clinical (ir)relevance of opioid-induced immune suppression. Curr Opin Anaesthesiol 2010; 23:588–92. [DOI] [PubMed] [Google Scholar]
- 17. Pimentel CB, Gurwitz JH, Tjia J, Hume AL, Lapane KL. New initiation of long-acting opioids in long-stay nursing home residents. J Am Geriatr Soc 2016; 64:1772–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller M, Barber CW, Leatherman S, et al. . Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med 2015; 175:608–15. [DOI] [PubMed] [Google Scholar]
- 19. Wiese AD, Griffin M, Schaffner W, et al. . Validation of discharge diagnosis codes to identify serious infections among middle age and older adults. BMJ Open 2018; 8:e020857. doi: 10.1136/bmjopen-2017-020857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grijalva CG, Kaltenbach L, Arbogast PG, Mitchel EF Jr, Griffin MR. Initiation of rheumatoid arthritis treatments and the risk of serious infections. Rheumatology (Oxford) 2010; 49:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grijalva CG, Chung CP, Stein CM, et al. . Computerized definitions showed high positive predictive values for identifying hospitalizations for congestive heart failure and selected infections in Medicaid enrollees with rheumatoid arthritis. Pharmacoepidemiol Drug Saf 2008; 17:890–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cooper WO, Hickson GB, Fuchs C, Arbogast PG, Ray WA. New users of antipsychotic medications among children enrolled in TennCare. Arch Pediatr Adolesc Med 2004; 158:753–9. [DOI] [PubMed] [Google Scholar]
- 23. Chan EW, Lau WC, Leung WK, et al. . Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: a population-based study. Gastroenterology 2015; 149:586–95.e3. [DOI] [PubMed] [Google Scholar]
- 24. Ray WA, Stein CM, Daugherty JR, Hall K, Arbogast PG, Griffin MR. COX-2 selective non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease. Lancet 2002; 360:1071–3. [DOI] [PubMed] [Google Scholar]
- 25. Hade EM, Lu B. Bias associated with using the estimated propensity score as a regression covariate. Stat Med 2014; 33:74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Welty LJ, Rasmussen LV, Baldridge AS, Whitney E.. StatTag. Chicago, IL, United States: Galter Health Sciences LIbrary, 2016. [Google Scholar]
- 27. Wiese AD, Grijalva CG. The use of prescribed opioid analgesics and the risk of serious infections. Future Microbiol 2018; 13:849–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dublin S, Von Korff M. Prescription opioids and infection risk: research and caution needed. Ann Intern Med 2018; 168:444–5. [DOI] [PubMed] [Google Scholar]
- 29. Savilampi J, Ahlstrand R, Magnuson A, Geijer H, Wattwil M. Aspiration induced by remifentanil: a double-blind, randomized, crossover study in healthy volunteers. Anesthesiology 2014; 121:52–8. [DOI] [PubMed] [Google Scholar]
- 30. Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003; 158:915–20. [DOI] [PubMed] [Google Scholar]
- 31. Carson S, Thakurta S, Low A, Smith B, Chou R.. Drug class reviews: long-acting opioid analgesics: final update 6 report. Portland, OR: Oregon Health and Science University, Copyright (c) 2011 by Oregon Health and Science University, 2011. [PubMed] [Google Scholar]
- 32. Tassinari D, Sartori S, Tamburini E, et al. . Adverse effects of transdermal opiates treating moderate-severe cancer pain in comparison to long-acting morphine: a meta-analysis and systematic review of the literature. J Palliat Med 2008; 11:492–501. [DOI] [PubMed] [Google Scholar]
- 33. Hale ME, Dvergsten C, Gimbel J. Efficacy and safety of oxymorphone extended release in chronic low back pain: results of a randomized, double-blind, placebo- and active-controlled phase III study. J Pain 2005; 6:21–8. [DOI] [PubMed] [Google Scholar]
- 34. Chou R, Clark E, Helfand M. Comparative efficacy and safety of long-acting oral opioids for chronic non-cancer pain: a systematic review. J Pain Symptom Manage 2003; 26:1026–48. [DOI] [PubMed] [Google Scholar]
- 35. Hartung DM, Middleton L, Haxby DG, Koder M, Ketchum KL, Chou R. Rates of adverse events of long-acting opioids in a state Medicaid program. Ann Pharmacother 2007; 41:921–8. [DOI] [PubMed] [Google Scholar]
- 36. Ray WA, Chung CP, Murray KT, Cooper WO, Hall K, Stein CM. Out-of-hospital mortality among patients receiving methadone for noncancer pain. JAMA Intern Med 2015; 175:420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Krebs EE, Becker WC, Zerzan J, Bair MJ, McCoy K, Hui S. Comparative mortality among Department of Veterans Affairs patients prescribed methadone or long-acting morphine for chronic pain. Pain 2011; 152:1789–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.