Abstract
Background
Clostridioides (formerly Clostridium) difficile infection (CDI) is associated with significant morbidity and mortality, including frequent hospitalizations. However, the impact of CDI after hospital discharge is poorly understood. The purpose of this study was to assess patient discharge disposition and understand CDI-related risk factors for nonhome discharge.
Methods
Using a nationally representative database of Veterans Health Administration (VHA) patients (2003–2014) and a validation database from hospitalized non-VHA patients in Houston, Texas, admission and discharge disposition was obtained for patients with CDI and matched controls. Incidence of and clinical/microbiologic risk factors for nonhome discharge were assessed using these databases.
Results
A total of 15173 VHA patients with CDI and 48599 non-CDI control patients originally admitted from the community were included. Significantly more patients with CDI were discharged to a nonhome location compared with controls (18% vs 8%; P < .0001), most commonly hospice/death (12%) or nursing home/long-term care facility (6%). Results were confirmed using a propensity-matched analysis and a validation cohort of 1941 hospitalized patients with CDI in Houston, Texas. Age, comorbidities, severe CDI, and ribotypes F027, F001, and F053-163 were associated with a nonhome discharge (P < .05 for all).
Conclusions
Hospitalized patients with CDI frequently required a higher level of medical care residence at discharge compared with non-CDI patients. Risk factors for discharge to a higher level of care included CDI disease severity and variables associated with recurrent CDI.
Keywords: outcomes research, epidemiology, anaerobic infections, healthcare-associated infections, strain typing
Patients admitted from the community who are hospitalized with Clostridioides difficile infection (CDI), especially severe CDI, were more frequently discharged to a nonhome location compared to patients without CDI. Certain virulent strains including ribotype 027 were associated with a nonhome discharge.
Clostridioides (formerly Clostridium) difficile is the most common organism implicated in healthcare-associated infections in the United States [1], likely attributed to an epidemic, so-called hypervirulent ribotype 027 strain [2, 3]. Clostridioides difficile infection (CDI) is associated with significant morbidity and mortality, including frequent hospitalizations. CDI-attributable hospital costs have been estimated to be $21448 per case, resulting in a total financial burden of $6.3 billion in the United States in 2015 [4, 5]. One-fifth of hospitalized patients with a first episode of CDI treated with vancomycin or metronidazole will experience recurrent CDI, with risk increasing with each subsequent episode [6–10]. Up to half of these patients with recurrent CDI will be rehospitalized, further contributing to the high patient and economic burden [11]. Despite a heightened awareness of the disease burden during hospitalization, very little is known about how a patient with CDI progresses through the continuum of healthcare after hospital discharge. Specifically, CDI is associated with long-term poor patient outcomes posthospitalization, including decreased quality of life and a high incidence of irritable bowel syndrome [12, 13]. We hypothesized that CDI would increase the likelihood for a nonhome discharge among hospitalized patients. Given that certain C. difficile ribotypes are associated with increased disease severity and outcomes [14], we also hypothesized that certain ribotypes would be associated with increased risk for a nonhome discharge. The purpose of this study was to assess patient discharge disposition and assess CDI-related patient and strain risk factors for a nonhome discharge. To accomplish these aims, we used data available from the nationwide inpatient Veterans Health Administration (VHA) facilities in the United States [15] and an ongoing cohort study of hospitalized patients with CDI in Houston, Texas [16]. In separate analyses, we used the large, nationwide VHA database to assess the incidence of nonhome discharge in hospitalized veterans with CDI compared with non-CDI control patients. Clinical risk factors for a nonhome discharge were also assessed. Incidence and risk factors were validated in the Houston cohort with an additional analysis investigating whether certain strain types are associated with a nonhome discharge.
METHODS
VHA Database
Study Design
This was a retrospective cohort study of patients with their first occurrence of CDI receiving care at any inpatient VHA facility in the United States. Data for this study were obtained from the Department of Veterans Affairs Informatics and Computing Infrastructure, which includes administrative, clinical, laboratory, and pharmacy data repositories that are linked using unique patient identifiers. This study was approved by the institutional review boards at the University of Texas Health Science Center at San Antonio and the South Texas Veterans Health Care System Research and Development Committee.
Study Population
The CDI cohort was created by identifying patients 18–89 years old who had any inpatient or outpatient International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code for CDI (008.45) plus a positive CDI stool test (eg, toxin enzyme immunoassay or nucleic acid amplification test with or without glutamate dehydrogenase antigen test) during the visit or within 7 days of the visit from 1 October 2002 through 30 September 2014. A control group was created by identifying a random sample of VHA patients without a CDI ICD-9-CM code for the duration of the study period and matching 2:1 (control:CDI) by treatment setting (inpatient or outpatient) and fiscal year of visit. Following cohort creation, we limited both cohorts to those patients who were hospitalized, were admitted directly from the community (ie, direct admission) or an outpatient clinic/treatment setting, had complete admission and discharge information, and received active CDI therapy (CDI group only: metronidazole, oral vancomycin, fidaxomicin, rifaximin, nitazoxanide, or fecal microbiota transplantation). Patients with an ICD-9-CM code for CDI (008.45) in the year prior to study inclusion were excluded.
Study Definitions
Data collected included admission and discharge sources for first, second, and third occurrence of CDI. Nonhome discharge was categorized as discharge to nursing home/long-term care facility (LTCF), another hospital, hospice, or death. CDI first recurrence was defined as a second inpatient visit during which a patient received an ICD-9-CM code for CDI, plus a minimum 3-day gap between the visit and the end of active CDI therapy for the initial episode. A second CDI recurrence was defined in the same manner as the first, but using the third CDI diagnosis over the cohort period.
Patient demographics included age, sex, race, and ethnicity. Charlson comorbidities and other relevant diagnoses, as defined by ICD-9-CM codes, were collected for the year prior to the CDI encounter (Supplementary Appendix 1). The Charlson comorbidity score was calculated as modified by Deyo et al [17]. In addition, concomitant infections were collected that occurred during an encounter (between CDI episode start date and end of CDI therapy for CDI patients and during hospitalization for control group), including bacteremia, pneumonia, skin infection, intra-abdominal infection, urinary tract infection, device-related infection, endocarditis, and acute respiratory infection. Other markers of CDI severity that occurred during an encounter were also captured, including sepsis/septicemia, shock, megacolon, prolonged ileus, perforated intestine, acute renal failure, and intensive care unit admission. Patients were considered “severe” if they had any one of these CDI severity indicators, though this definition differs slightly from the CDI clinical practice guidelines (ie, combination of severe and fulminant criteria).
Data on prior and concomitant non-CDI antibiotics (excludes oral vancomycin, metronidazole, fidaxomicin, rifaximin, and nitazoxanide), gastric acid suppressant (GAS) drugs (antacids, H2 receptor antagonists, proton pump inhibitors), and narcotics were collected. Prior use was defined as any use in the 90 days prior to the encounter, and concomitant use was defined as any use during or within 60 days following the encounter. The 60-day follow-up period for concomitant medication use was chosen because this time period is likely to capture the majority of medication use between initial CDI and recurrence; recurrent CDI is most common within 1–3 weeks posttreatment discontinuation, but late recurrences are also common [6].
Data and Statistical Analyses
Data extraction and variable creation were conducted using SAS version 9.2 (SAS Institute, Cary, North Carolina). All other data and statistical analyses were conducted using JMP 13.0 (SAS Institute). First, baseline characteristics were presented descriptively and compared between CDI and control group patients using the χ2 or Wilcoxon rank-sum test as appropriate. Next, discharge dispositions were presented descriptively for each CDI episode and compared between episodes using the McNemar χ2 test. In CDI patients only, independent predictors for nonhome discharge following first occurrence were determined using logistic regression with the following covariates: age, sex, race/ethnicity, 21 comorbidities, 8 concomitant infections, severe CDI, and concomitant medications (antibiotics, GAS drugs, and narcotics) (Table 1).
Table 1.
Baseline Characteristics of Propensity Score–matched Veterans Health Administration Population
| Characteristic | CDI Group (n = 10970) |
Control Group (n = 10970) |
P Value |
|---|---|---|---|
| Age, y, median (IQR) | 66 (59–77) | 66 (59–77) | .2604 |
| Male sex, % | 95.6 | 95.8 | .4241 |
| Race/ethnicity, % | .0010 | ||
| Non-Hispanic white | 65.4 | 66.7 | |
| Non-Hispanic black | 21.0 | 19.6 | |
| Hispanic | 6.0 | 5.6 | |
| Other | 4.3 | 5.2 | |
| Missing | 3.2 | 3.0 | |
| Prior hospitalization, % | 32.9 | 32.7 | .7957 |
| Comorbidities, % | |||
| Hypertension | 73.9 | 74.6 | .2228 |
| Dyslipidemia | 53.5 | 53.1 | .5426 |
| Obesity | 16.1 | 15.5 | .1765 |
| Myocardial infarction | 8.2 | 8.2 | .9021 |
| Congestive heart failure | 19.0 | 19.1 | .7570 |
| Peripheral vascular disease | 15.1 | 15.0 | .7198 |
| Cerebrovascular disease | 15.2 | 14.9 | .4851 |
| Dementia | 2.7 | 2.3 | .0929 |
| COPD | 33.7 | 34.8 | .0878 |
| Rheumatologic disease | 2.4 | 2.3 | .4734 |
| Peptic ulcer disease | 3.8 | 3.9 | .6999 |
| Liver disease | 5.7 | 6.1 | .2173 |
| Diabetes | 36.0 | 36.1 | .8882 |
| Hemiplegia or paraplegia | 2.0 | 1.8 | .2369 |
| Renal disease | 19.8 | 20.0 | .7737 |
| Cancer | 25.6 | 26.6 | .1031 |
| HIV/AIDS | 1.7 | 1.8 | .4112 |
| GERD | 26.1 | 26.3 | .7588 |
| Transplant | 0.1 | 0.1 | .4129 |
| Inflammatory bowel disease | 2.0 | 2.0 | 1.0000 |
| Irritable bowel syndrome | 1.1 | 1.0 | .2285 |
| Charlson score, median (IQR) | 3 (1–5) | 3 (1–5) | .0170 |
| Concomitant infections, % | |||
| Bacteremia | 3.0 | 2.6 | .0693 |
| Pneumonia | 17.1 | 17.6 | .3449 |
| Skin infection | 8.5 | 8.6 | .8471 |
| Intra-abdominal infection | 3.6 | 3.7 | .4713 |
| Device-related infection | 1.2 | 1.1 | .4072 |
| Acute respiratory infection | 2.7 | 2.9 | .3065 |
| Endocarditis | 0.3 | 0.3 | 1.0000 |
| Urinary tract infection | 2.8 | 3.3 | .0296 |
| Medications, % | |||
| Prior antibiotics | 42.0 | 42.5 | .4440 |
| Prior GAS drugs | 45.8 | 45.7 | .8815 |
| Prior narcotics | 31.9 | 31.7 | .8505 |
Abbreviations: CDI, Clostridioides difficile; COPD, chronic obstructive pulmonary disease; GAS, gastric acid suppressant; GERD, gastroesophageal reflux disease; HIV, human immunodeficiency virus; IQR, interquartile range.
Results were validated using a propensity score–matched analysis. Specifically, logistic regression was used to generate propensity scores using the cohort (CDI vs control) as the dependent variable and the following covariates: age, sex, race/ethnicity, prior hospitalization, 21 comorbidities, 8 concomitant infections, and 3 prior medication classes (Supplementary Appendix 2). Propensity scores were then matched 1:1 CDI to control cohort using nearest neighbor matching without replacement and a caliper of 0.001. Following matching, discharge to a nonhome location was compared between groups using the χ2 test.
Houston External Validation Cohort
To validate and extend the findings from the VHA population, we utilized data from an ongoing, multicenter, cohort study of non-VHA patients with CDI in Houston, Texas [11]. In brief, patients were included in the study if they were at least 18 years old with a positive liquid stool test for C. difficile toxin(s), were receiving CDI therapy, and had at least 1 of the following signs or symptoms of CDI: diarrhea, fever, leukocytosis (white blood cell count >10000/mL3), nausea, anorexia, or abdominal pain, cramping, or discomfort. Data collected for this study included patient demographics, comorbidities, inpatient hospital admission, and discharge disposition. As part of this study, leftover stool samples from patients with CDI were collected. Stool samples were incubated under anaerobic conditions for 48 hours for C. difficile growth. Isolates were confirmed to be C. difficile on the basis of Gram stain results, typical odor, and the presence of C. difficile antigen on Microscreen latex agglutination (Microgen Bioproducts). Clostridioides difficile isolates were then strain typed using polymerase chain reaction–based ribotyping method as previously described [18]. This study was approved by the Institutional Review Board at the University of Houston.
Statistical Analysis
Proportion of patients with a nonhome discharge and each discharge disposition were calculated. Risk factors for nonhome discharge identified in the VHA cohort were tested in the Houston cohort using logistic regression. Finally, incidence of nonhome discharge for any C. difficile ribotype identified in at least 25 unique patients compared to all other ribotypes was assessed using logistic regression.
RESULTS
VHA Database Results
Baseline Characteristics
A total 15173 hospitalized patients admitted from the community or outpatient clinic were included in the CDI cohort and 48599 in the control group. Supplementary Appendix 2 describes the patients’ baseline characteristics. Patients with CDI were predominately older (median age, 67 years), male (96%), and non-Hispanic white (66%). Compared to the control group, CDI patients more commonly had chronic comorbidities and recent or concomitant medication use.
CDI Discharge Disposition and Clinical Risk Factor for a Nonhome Discharge
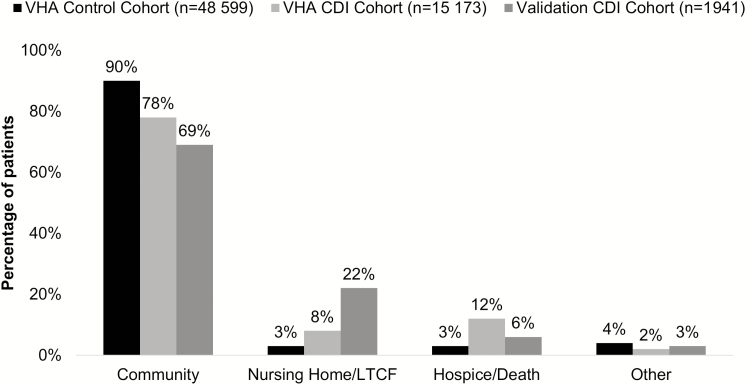
The most common discharge locations for CDI patients included community (78%), nursing home/LTCF (8%), hospice/death (12%), or other (2%) (Figure 1). Compared to non-CDI controls, CDI patients were more often discharged to a nursing home/LTCF (8% vs 3%; P < .0001) or hospice/death (12% vs 3%; P < .0001). A total of 3190 (18%) CDI patients were discharged to a nonhome location following their first CDI episode compared with 3686 (8%) in the control group (P < .0001).
Figure 1.
Discharge destination of Clostridioides difficile patients and controls admitted from the community in Veterans Health Administration and external validation cohorts. Abbreviations: CDI, Clostridioides difficile; LTCF, long-term care facility; VHA, Veterans Health Administration.
For propensity score analyses, 10970 CDI patients were matched to 10970 controls. CDI and control patients were well matched for demographics and comorbidities (Table 1). Compared to controls, CDI patients were more often discharged to a nursing home/LTCF (8% vs 4%; P < .0001) or hospice/death (10% vs 5%; P < .0001). Overall, discharge to a nonhome location was more common in the CDI cohort (19%) compared with the control cohort (11%) (P < .0001).
A total of 2712 CDI patients (15%) had a first recurrence, of whom 1213 (45%) were rehospitalized and 858 (32%) had discharge disposition data available for the subsequent hospitalization. These patients were most often admitted from the community (83%) for their recurrence. Compared to first episodes, the proportion of CDI patients discharged to a nonhome setting was significantly lower for first recurrences (8%; P < .0001). A total of 858 (32% of first recurrence patients) experienced a second recurrence, of whom 249 were rehospitalized (29%) and 15 (2%) had discharge disposition information available. Second recurrence patients were most often admitted from the community (20%) or outpatient clinics (67%). No (0%) second recurrence patients were discharged to a nonhome setting.
Overall, 17 variables independently predicted discharge to a higher level of care following the initial CDI episode (Table 2). Those with the strongest association included severe CDI (odds ratio [OR], 1.85 [95% confidence interval {CI}, 1.69–2.01]), liver disease (OR, 1.80 [95% CI, 1.55–2.09]), and age ≥65 years (OR, 1.74 [95% CI, 1.58–1.91]).
Table 2.
Independent Predictors of Discharge to Higher Level of Care Among Veterans Health Administration (VHA) Clostridioides difficile (CDI) Patients Admitted From the Community (n = 15173; VHA CDI Cohort) and Hospitalized Patients From a Quaternary Care Hospital (n = 574; Validation Cohort)
| Characteristic | VHA CDI Cohort | Validation Cohort | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Severe CDI | 1.85 (1.69–2.01) | <.0001 | 1.61 (1.03–2.50) | .025 |
| Liver disease | 1.80 (1.55–2.09) | <.0001 | ||
| Age ≥65 y | 1.74 (1.58–1.91) | <.0001 | 3.48 (2.20–5.53) | <.0001 |
| Concomitant antibiotics | 1.59 (1.41–1.78) | <.0001 | 2.06 (1.28–3.31) | .003 |
| Pneumonia | 1.59 (1.45–1.75) | <.0001 | ||
| Male sex | 1.52 (1.15–1.99) | .0028 | ||
| Hemiplegia/paraplegia | 1.48 (1.19–1.84) | .0004 | ||
| White race | 1.35 (1.21–1.51) | <.0001 | ||
| Congestive heart failure | 1.29 (1.17–1.43) | <.0001 | 3.33 (2.10–5.28) | <.0001 |
| Dementia | 1.25 (1.01–1.54) | .0413 | ||
| Concomitant narcotics | 1.24 (1.14–1.35) | <.0001 | ||
| Cancer | 1.20 (1.09–1.31) | <.0001 | ||
| Bacteremia | 1.18 (1.03–1.37) | .0238 | ||
| Concomitant acid suppressants | 1.15 (1.02–1.28) | .0160 | ||
| Cerebrovascular disease | 1.12 (1.00–1.25) | .0436 | ||
Abbreviations: CDI, Clostridioides difficile; CI, confidence interval; OR, odds ratio; VHA, Veterans Health Administration.
Houston External Validation Cohort
Baseline Characteristics and Discharge Disposition
Discharge status was available for 1953 hospitalized CDI patients aged 63 ± 18 years (mean ± standard deviation) (58% female) admitted from the community in the external validation cohort. Of those CDI patients admitted from the community, most (69%) were discharged back to the community followed by nursing home/LTCF (22%), hospice/death (6%), or other (3%).
Additional clinical metadata were available for 585 patients. Comorbidities present in this population with an incidence of at least 10% included chronic obstructive pulmonary disease (11%), cerebrovascular disease (16%), congestive heart failure (21%), solid, nonmetastatic tumor (12%), diabetes with no complications (16%), and hemodialysis (18%). Hospitalization variables present in at least 10% of the population included proton pump inhibitor use (46%), scheduled narcotics (29%), continued use of non–C. difficile antibiotics (62%), and scheduled steroid use (14%). Average (± standard deviation) Charlson score was 2.4 ± 2.2. These variables were included in a multivariate stepwise, logistic regression analysis to identify predictors of nonhome discharge disposition. Significant predictors included age >65 years (OR, 3.5 [95% CI, 2.2–5.6]; P < .0001), congestive heart failure (OR, 3.3 [95% CI, 2.1–5.2]; P < .0001), severe CDI (OR, 1.58 [95% CI, 1.02–2.45]; P = .04), and continued use of systemic antibiotics (OR, 2.10 [95% CI, 1.30–3.37]; P = .0022).
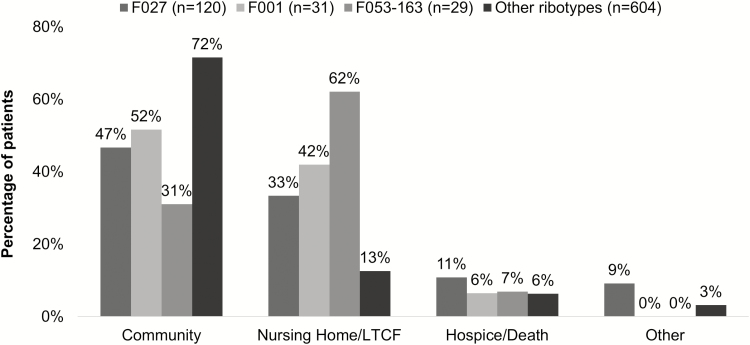
Seven hundred and eighty-four patients with discharge disposition information also had C. difficile ribotype data available. The most common ribotypes present identified in >25 unique patients included F014-020 (n = 126 [16%]), F027 (n = 120 [15%]), F106 (n = 97 [12%]), F002 (n = 83 [11%]); F001 (n = 31 [4%]), F053-163 (n = 29 [4%]), F054 (n = 27 [3%]), and F078 (n = 26 [3%]). In addition to age and gender, these ribotypes were included as unique variables compared to all other ribotypes combined in a multivariate logistic regression model to identify potential ribotype predictors of nonhome discharge. In addition to age >65 years, ribotypes associated with a nonhome discharge included ribotype F027 (OR, 2.60 [95% CI, 1.71–3.94]; P < .0001), F001 (OR, 2.49 [95% CI, 1.16–5.32]; P = .019), and F053-163 (OR, 4.40 [95% CI, 1.91–10.14]; P = .0005). Discharge disposition by ribotype is shown in Figure 2.
Figure 2.
Discharge destination of Clostridioides difficile patients stratified by polymerase chain reaction ribotype. Abbreviation: LTCF, long-term care facility.
DISCUSSION
CDI is common in the United States and associated with many poor outcomes during hospitalization [19]. Despite a high incidence and mortality rate due to CDI, very few studies have focused on transition of care for hospitalized patients with CDI, with a specific focus on discharge disposition in patients originally admitted from the community setting. In this study, we demonstrated that hospitalized patients with CDI have a higher likelihood of requiring a higher level of healthcare at discharge compared with non-CDI controls. These results were demonstrated in a large VHA database and validated using a secondary database comprised of hospitalized patients with CDI in Houston, Texas. Variables associated with a nonhome discharge reflected CDI disease severity, increased age, and patient comorbidities. After controlling for age and comorbidities, patients with CDI were still more likely to be discharged to a nonhome setting. Finally, the recent epidemic ribotype 027 strain was associated with a nonhome discharge, as were 2 other less frequently isolated ribotypes. Taken together, these data provide strong evidence that certain hospitalized patients with CDI are at a higher risk for discharge to a nonhome setting compared to hospitalized patients without CDI. Strengths of the study include a nationally representative, large VHA cohort to provide data that were validated using a separate database. Addition of strain typing to the analysis further helped to confirms these findings including identification of another poor patient outcome associated with ribotype 027.
Our findings are in line with prior single-center studies in the past. In 2003, Dubberke et al [20] demonstrated that CDI patients were more likely to be discharged to LTCFs or to another hospital compared with non-CDI control patients (32% vs 23%; OR, 1.62 [95% CI, 1.15–2.28]). Prior to this study, CDI patients with persistent colonization were also at increased risk for discharge to an LTCF [21]. During the recent ribotype 027 outbreak, 37% of CDI patients were discharged to LTCFs from hospitals in the Chicagoland area [22]. These results were remarkably similar to the 22%–31% of patients in our study discharged to a nonhome setting. The likelihood of a nonhome discharge decreased with subsequent recurrences, likely due to survivor bias; patients with a recurrence have a lower mortality rate compared with initial episodes, as seen in prior studies [11, 15, 23].
Although we were unable to determine the specific reasons for postdischarge site of care in this study, severe CDI disease presentation was a consistent risk factor in the VHA and validation cohorts. Ribotypes shown to increase the likelihood of a nonhome discharge have also been associated with severe CDI. The most recent 027/BI/NAP1 strain was often described as hypervirulent during the epidemic, although other ribotypes have been demonstrated to be equally virulent [2, 3, 14]. Ribotype 001 has been shown to cause severe disease during an epidemic in the Czech Republic [24], and has also been shown to be a significant causative ribotype for transmission events between patients [25]. Our ribotyping assay does not distinguish between ribotypes 053 and 163; however, both ribotypes have been associated with epidemic spread or severe disease in the past [26, 27]. This is one of the first studies documenting a higher level of discharge care associated with these ribotypes. Discharge to a nonhome setting increases global healthcare costs and also increases likelihood for mortality [28, 29].
Once validated, these findings also support routine strain typing of C. difficile isolates to identify patient populations at high risk for poor outcomes, including unfavorable discharge disposition, and additional studies identifying the most effective clinical treatment strategies for patients with high-risk strain types.
The study has potential limitations. Data for the primary VHA cohort were collected retrospectively from the VHA electronic medical record system. Relevant diagnoses and admission/discharge information might be subject to misclassification and could not be confirmed through individual chart review. Signs/symptoms associated with CDI and specialty care (ie, infectious diseases consultation) could not be confirmed to aid in CDI diagnosis, nor could recurrences be confirmed with additional laboratory testing or CDI therapy. Misclassification might also occur in patients who were admitted from an outpatient clinic, as it is possible these were LTCF or nursing home residents. The VHA population is predominately older and male and might not be representative of CDI patients in community hospitals; however, our findings were similar in the non-VHA validation cohort. Clinical metadata from our validation database was not available for many of the cases where ribotyping had been performed; therefore, more in-depth multivariate analyses with ribotype and clinical metadata were not possible. Last, study outcomes could not be determined as attributable to CDI; rather, these were all-cause outcomes.
In conclusion, hospitalized patients with CDI more frequently required a higher level of medical care residence at discharge compared with non-CDI controls. Risk factors for a nonhome discharge included CDI disease severity, comorbidities, and variables commonly associated with recurrent CDI (eg, older age, concomitant antibiotics).
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Xavier Jones, BS, for creating analytic variables for the Veterans Health Administration patient cohort.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH), the US Department of Veterans Affairs, or the US government.
Financial support. This study was supported in part by Merck & Co, Inc, and the NIH/National Institute of Allergy and Infectious Diseases (grant number 1UO1 AI-24290-01). This material is also the result of work supported with resources and the use of facilities at the Audie L. Murphy Memorial Veterans Affairs Hospital, San Antonio, Texas. K. R. R. is supported by the NIH/National Institute on Aging San Antonio Claude D. Pepper Older Americans Independence Center (grant number 1P30AG044271-01A1).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Magill SS, Edwards JR, Bamberg W, et al. Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med 2005; 353:2433–41. [DOI] [PubMed] [Google Scholar]
- 3. Pépin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ 2005; 173:1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang S, Palazuelos-Munoz S, Balsells EM, Nair H, Chit A, Kyaw MH. Cost of hospital management of Clostridium difficile infection in United States—a meta-analysis and modelling study. BMC Infect Dis 2016; 16:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shah DN, Aitken SL, Barragan LF, et al. Economic burden of primary compared with recurrent Clostridium difficile infection in hospitalized patients: a prospective cohort study. J Hosp Infect 2016; 93:286–9. [DOI] [PubMed] [Google Scholar]
- 6. Johnson S, Adelmann A, Clabots CR, Peterson LR, Gerding DN. Recurrences of Clostridium difficile diarrhea not caused by the original infecting organism. J Infect Dis 1989; 159:340–3. [DOI] [PubMed] [Google Scholar]
- 7. Crook DW, Walker AS, Kean Y, et al. Study 3/4 Teams Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis 2012; 55(Suppl 2):S93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eyre DW, Walker AS, Wyllie D, et al. Infections in Oxfordshire Research Database Predictors of first recurrence of Clostridium difficile infection: implications for initial management. Clin Infect Dis 2012; 55(Suppl 2):S77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lessa FC, Winston LG, McDonald LC; Emerging Infections Program C. difficile Surveillance Team. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:2369–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol 2002; 97:1769–75. [DOI] [PubMed] [Google Scholar]
- 11. Aitken SL, Joseph TB, Shah DN, et al. Healthcare resource utilization for recurrent Clostridium difficile infection in a large university hospital in Houston, Texas. PLoS One 2014; 9:e102848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garey KW, Aitken SL, Gschwind L, et al. Development and validation of a Clostridium difficile health-related quality-of-life questionnaire. J Clin Gastroenterol 2016; 50:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sethi S, Garey KW, Arora V, et al. Increased rate of irritable bowel syndrome and functional gastrointestinal disorders after Clostridium difficile infection. J Hosp Infect 2011; 77:172–3. [DOI] [PubMed] [Google Scholar]
- 14. Aitken SL, Alam MJ, Khaleduzzaman M, et al. In the endemic setting, Clostridium difficile ribotype 027 is virulent but not hypervirulent. Infect Control Hosp Epidemiol 2015; 36:1318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reveles KR, Lawson KA, Mortensen EM, et al. National epidemiology of initial and recurrent Clostridium difficile infection in the Veterans Health Administration from 2003 to 2014. PLoS One 2017; 12:e0189227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davis ML, Sparrow HG, Ikwuagwu JO, Musick WL, Garey KW, Perez KK. Multicentre derivation and validation of a simple predictive index for healthcare-associated Clostridium difficile infection [manuscript published online ahead of print 16 February 2018]. Clin Microbiol Infect 2018. doi: 10.1016/j.cmi.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 17. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45:613–9. [DOI] [PubMed] [Google Scholar]
- 18. Walk ST, Micic D, Jain R, et al. Clostridium difficile ribotype does not predict severe infection. Clin Infect Dis 2012; 55:1661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dubberke ER, Butler AM, Reske KA, et al. Attributable outcomes of endemic Clostridium difficile-associated disease in nonsurgical patients. Emerg Infect Dis 2008; 14:1031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med 1989; 320:204–10. [DOI] [PubMed] [Google Scholar]
- 22. Black SR, Weaver KN, Jones RC, et al. Clostridium difficile outbreak strain BI is highly endemic in Chicago area hospitals. Infect Control Hosp Epidemiol 2011; 32:897–902. [DOI] [PubMed] [Google Scholar]
- 23. McFarland LV, Surawicz CM, Rubin M, Fekety R, Elmer GW, Greenberg RN. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol 1999; 20:43–50. [DOI] [PubMed] [Google Scholar]
- 24. Krutova M, Matejkova J, Drevinek P, Kuijper EJ, Nyc O; Study Group. Increasing incidence of Clostridium difficile ribotype 001 associated with severe course of the infection and previous fluoroquinolone use in the Czech Republic, 2015. Eur J Clin Microbiol Infect Dis 2017; 36:2251–8. [DOI] [PubMed] [Google Scholar]
- 25. Martin JSH, Eyre DW, Fawley WN, et al. Patient and strain characteristics associated with Clostridium difficile transmission and adverse outcomes [manuscript published online ahead of print 12 April 2018]. Clin Infect Dis 2018. doi: 10.1093/cid/ciy302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagar A, Yew P, Fairley D, et al. Report of an outbreak of Clostridium difficile infection caused by ribotype 053 in a neurosurgery unit. J Infect Prev 2015; 16:126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng JW, Yang QW, Xiao M, et al. High in vitro activity of fidaxomicin against Clostridium difficile isolates from a university teaching hospital in China. J Microbiol Immunol Infect 2018; 51:411–6. [DOI] [PubMed] [Google Scholar]
- 28. Ziakas PD, Joyce N, Zacharioudakis IM, et al. Prevalence and impact of Clostridium difficile infection in elderly residents of long-term care facilities, 2011: a nationwide study. Medicine 2016; 95:e4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ricciardi R, Nelson J, Griffith JL, Concannon TW. Do admissions and discharges to long-term care facilities influence hospital burden of Clostridium difficile infection?J Hosp Infect 2012; 80:156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.