Abstract
Background: Circulating microRNAs (miRNA) are steady preserved in blood plasma. Multiple evidences have shown that miRNAs play a crucial role in cardiovascular disease including miRNA-378, which has been illustrated to participate in diverse physiological and pathological processes of cardiovascular disease. In the present study, we aim to explore the expression of plasma miRNA-378 and its clinical significance in patients with coronary artery disease (CAD).
Methods: MiRNA-378 expression in blood plasma was performed by quantitative real-time PCR (qRT-PCR) in 215 CAD patients and 52 matched controls of healthy populations. Medical information of all patients including the results of coronary angiography (CAG) was acquired through hospital information system (HIS). Spearman’s correlation, binary linear regression, and covariance analysis were used to examine the association between miRNA-378 and relative clinical risk factors. Receiver operating characteristic curve analysis was applied to evaluate the value of miRNA-378 in predicting the disease severity of coronary lesion.
Results: Plasma miR-378 expression was significantly down-regulated in CAD patients compared with healthy controls. Relative miR-378 level was shown conversely correlated with Gensini score, which present the severity of coronary artery lesions. Moreover, it is indicated that miR-378 expression can effectively distinguish patients with or without coronary artery stenosis.
Conclusions: Plasma miR-378 levels appear to be a promising non-invasive biomarker, but require to be further validated by a large cohort study in future.
Keywords: coronary artery disease, clinical significance, expression, miRNA-378
Introduction
Coronary artery disease (CAD), with a high incidence and mortality in the population, is an overwhelming disease that endangers human health throughout the world [1,2]. Coronary artery atherosclerosis is considered the main underlying cause of CAD [3]. However, CAD is diagnosed based on angiography results, which is invasive and technology dependent. Moreover, few of circulating indicators have been reported regarding to the diagnostic value of CAD [4]. Therefore, a circulating indicator for screening coronary lesions is required.
The Gensini scoring system is most widely used in quantifying CAD [5]. The Gensini score not only quantified the degree of stenosis, but also accurately and objectively represent the degree of coronary artery lesions according to different segments of the coronary artery. The calculation method is relatively simple and more suitable for the calculation of large clinical sample size. The higher the score is, the more severe the stenosis will be. Therefore, it proves to be a very practical and effective quantitative evaluation method [6].
MicroRNAs (miRNAs) have been proved to be a group of small non-coding single stranded RNAs of ∼22 nucleotides, which could negatively regulate gene expression by binding to the target mRNAs, degrading mRNA or cleaving target mRNA, repressing mRNA translation [7]. Multiple evidences have shown that miRNAs play a crucial role in cardiovascular disease [8–10]. Importantly, circulating miRNAs are steady preserved in blood plasma because they are either involved in microparticles or bound to proteins, which protected from RNase activity [11]. Therefore, miRNAs in circulation might be usefully served as promising diagnostic biomarkers [12].
Previous research has reported close relationship between miRNA-378 and atherosclerosis [13]. Circulating miRNA-378 was found to remarkably down-regulated in the plasma of CAD patients compared with normal population [14]. Although expression of circulating miRNAs in CAD patients has been reported, the relationship between miR-378 expression and severity of CAD has not been studied. Here, we detected plasma level of miRNA-378 in CAD patients, then investigated the association between miRNA-378 expression and the severity of atherosclerosis lesion.
Methods and materials
Ethics statement
The present study was approved by Medical Ethics Committee of Qilu Hospital of Shandong University. Written informed consent was obtained from all of participants before participation.
Clinical samples
Study participants were enrolled from Qilu Hospital of Shandong University prior to undergoing selective cardiac catheterization. Patients with valvular heart disease, cardiomyopathy, sever infectious disease, tumor, immune disease, and renal dysfunction were excluded. Finally, 267 patients were included in the present study. Patients whose maximal coronary stenosis <50% confirmed by coronary angiograms were included in the control group. Clinical data were collected through hospital information system (HIS). Angiographies were evaluated independently by three cardiologists based on AHA/ACC criteria. Severity of coronary stenosis was demonstrated by Gensini Score according to previous study [15], which was quantified for the degree of luminal narrowing along with a multiplier for specific coronary tree locations for prognostic significance. Each lesion score by weighting summed to give a final Gensini Score.
Blood sample and miRNA isolation
Venous blood samples (2 ml) were collected before coronary angiography (CAG) in EDTA containing tubes. The sample was centrifuged with 1500 × g for 15 min at 4°C. 200 μl obtained plasma was subjected to RNA extraction. MiRNA was isolated using miRNeasy Isolation Kit (Qiagen, Hilden, Germany) according to manufacturer’s protocol.
Measurement of miRNA-378
MiRNAs were reversed transcribed to cDNA using the Reverse Transcription TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, U.S.A.) according to manufacturer’s instructions. Then, quantitative real-time PCR (qRT-PCR) was performed by Hairpin-it™ microRNA and U6 snRNA Normalization RT-PCR Quantitation Kit (GenePharma, China) in triplicate using Light Cycler (Bio-Rad). U6 was performed as an internal control. The relative expression of miRNA-378 was calculated by methods [14]: F = 2−Δct, Δct = ct (miRNA-378) – ct (miRNA-U6). CT referred to cycle number inside reactor when the fluorescent signal of sample rose above baseline. MiRNA-378-5p, qPCR Primer Pairs, and U6 primers were synthesized by GenePharma (Shanghai, China). The U6 RT-PCR primers were following: 5′-CGCTTCACGAATTTGCGTGTCAT-3′ and 5′-GCTTCGGCAGCACATATACTAAAAT-3′. MiR-378-5p forward primer: CAAACCTCCTCCTGACTCCAG and reverse primer: TATGCTTGTTCTCGTCTCTGTGTC.
Statistics
All calculations were performed using SPSS statistical software package. Data from normal distribution were expressed by mean ± SD, and data from abnormal distribution were displayed through median (25th–75th percentile). One-way ANOVA, analysis of covariance (ANCOVA), and nonparametric test adding post LSD were used for comparison of continuous variables among multiple groups. In addition, Student’s t test was used for comparison of continuous variables between two groups. Spearman’s correlation was applied for exploring indicated associations. Binary liner regression and multiple linear regression were taken to determine variables that independently contributed to presence of CAD. The receiver operating characteristic (ROC) curve was applied to determine the ability of relative miR-378 levels in predicting coronary stenosis. A difference with P<0.05 was considered significant.
Results
Demographic characteristics of study patients
The detailed information of patients is demonstrated in Table 1. There were no differences between CAD patients and control group of basic clinical characteristics, including gender, age, blood pressure, heart rate, BMI, Smoking, Drinking, Diabetes, TG, and HDL. Obviously, Gensini Score was significantly higher in CAD group while LDL level was significantly elevated compared with control group. Because control group cannot exclude CAD before coronary arteriography, there was no difference between medication on admission, such as ACEI/ARB, CCB, statins, and nitrates.
Table 1. Baseline characteristics of patients with CAD and controls.
| Variables | All subjects | CAD | Non-CAD | P |
|---|---|---|---|---|
| N (male %) | 183 (68.5) | 153 (71.1) | 30 (57.7) | 0.06 |
| Age (years) | 61±10 | 61±10 | 61±12 | 0.66 |
| SBP (mmHg) | 135±20 | 135±19 | 136±21 | 0.68 |
| DBP (mmHg) | 76±12 | 75±12 | 77±12 | 0.49 |
| HR (beats/min) | 72±12 | 72±12 | 73±12 | 0.19 |
| BMI (kg/m2) | 22±10 | 23±10 | 22±10 | 0.81 |
| Smoking n (%) | 124 (46.4) | 105 (48.8) | 19 (36.5) | 0.11 |
| Drinking n (%) | 81 (30.3) | 63 (29.3) | 18 (34.6) | 0.47 |
| HBP n (%) | 125 (46.8) | 104 (48.4) | 21 (40.4) | 0.30 |
| Diabetes n (%) | 71 (26.6) | 61 (28.4) | 10 (19.2) | 0.18 |
| Gensini Score | 96±85 | 111±85 | 34±50 | <0.01 |
| TG (mmol/l) | 3.9±1.0 | 3.9±1.0 | 3.9±1.0 | 0.79 |
| HDL (mmol/l) | 1.1±0.4 | 1.1±0.5 | 1.2±0.3 | 0.20 |
| LDL (mmol/l) | 2.3±0.8 | 2.4±0.8 | 2.0±0.5 | <0.01 |
| FBG (mmol/l) | 5.9±1.9 | 6.0±2.0 | 5.6±1.6 | 0.12 |
| NTproBNP (ng/ml) | 402±761 | 435±835 | 264±296 | 0.15 |
| ACEI/ARB n (%) | 143 (53.6) | 119 (55.3) | 24 (46.2) | 0.32 |
| CCB n (%) | 93 (34.8) | 70 (32.6) | 23 (44.2) | 0.11 |
| Nitrates n (%) | 162 (60.1) | 135 (62.8) | 27 (51.9) | 0.15 |
| Statins n (%) | 26 (99.3) | 214 (100.0) | 51 (98.1) | 0.28 |
| Anti-diabetic agents n (%) | 71 (26.6) | 61 (28.4) | 10 (19.2) | 0.18 |
Abbreviations: ACEI/ARB-angiotensin converting enzyme inhibitor/angiotensin receptor blocker; BMI-body mass index; CAD-coronary heart disease; CCB-calcium channel blockers; DBP-diastolic blood pressure; FBG-fast blood glucose; HDL-high-density lipoprotein; HR-heart rate; LDL-low-density lipoprotein; SBP-systolic blood pressure; TC, total cholesterol; TG-total triglyceride.
Plasma miR-378 levels were down-regulated in CAD patients
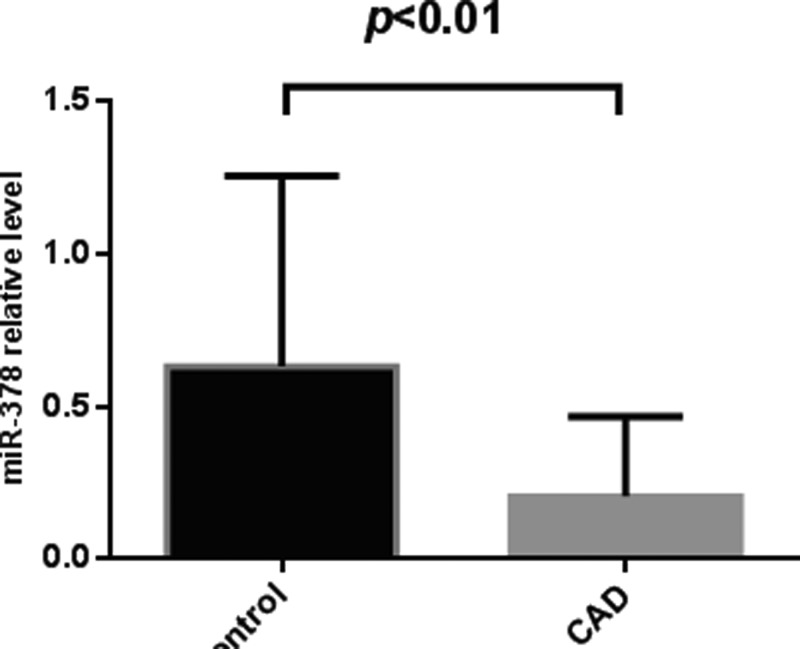
We studied the relative levels of miR-378 in plasma of CAD patients and control groups. Student’s t tests were used for statistical analysis. Results showed that plasma miR-378 levels were significantly decreased in CAD patients (0.6±0.6) compared with non-CAD controls (0.2±0.3) (Figure 1). In the CAD group the median of the Gensini score is 0.12, and in the control group the median of the Gensini score is 0.35.
Figure 1. Plasma miR-378 levels between CAD patients and control group.
Plasma miR-378 levels were detected different between CAD patients (0.6±0.6) and control group (0.2±0.3) by qRT-PCR. The expression levels of miR-378 were normalized to U6. P values were calculated using Student’s t test. Significant difference was observed about plasma miR-378 levels (P<0.01).
Correlation between relative miR-378 levels and coronary lesion severity
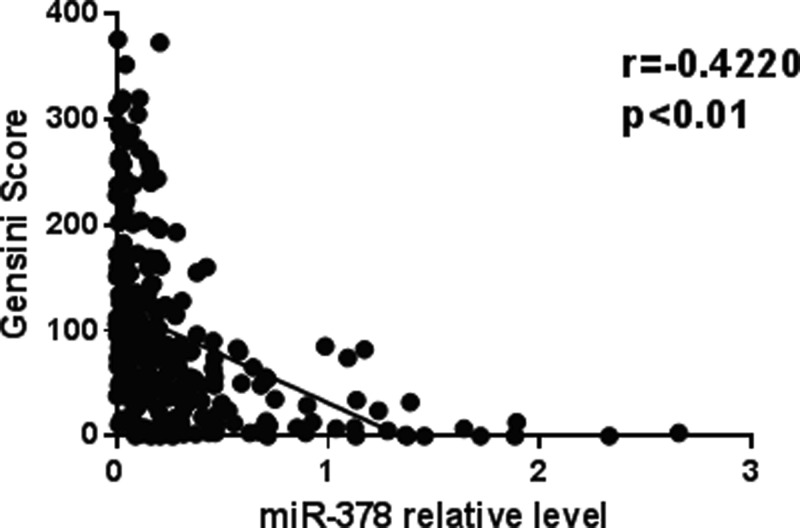
We further analyzed the association between plasma miR-378 levels and the severity of CAD. The severity of coronary lesion was evaluated with the Gensini Score in the present study (R = −0.4220; P < 0.01) (Figure 2).
Figure 2. Spearman’s correlation between relative miR-378 levels and Gensini Score.
The serum relative miR-378 levels were negatively correlated with Gensini Score (R = −0.4220; P < 0.01).
Plasma miR-378 level was an important risk factor and could predict coronary stenosis
To identify the factors contributed to coronary stenosis, we conducted binary linear regression, with/without CAD to be dependent variable. We found that independent variables (LDL, FBG, and relative miR-378 level) had significant impact on dependent variable (Table 2). After controlling relative covariance, the relative miR-378 level was different among groups (Table 3). To clarify the definite contribute of the risk factors to the miR-378 expression, we conducted multiple liner regression, relative miR-378 levels to be dependent variable. We found that LDL had significant but adverse effect on miR-378 expression, while others showed no significant efficacy in this model (Table 4).
Table 2. The risk factor by liner regression in patients with CAD and without CAD.
| Variables | P |
|---|---|
| LDL | <0.01 |
| FBG | 0.02 |
| Relative miR-378 level | <0.01 |
Table 3. The risk factor by covariance analysis with CAD and without CAD.
| Variables | Sum of squares (type III) | F | P |
|---|---|---|---|
| LDL | 29.07 | 1.39 | 0.36 |
| FBG | 31.48 | 1.00 | 0.51 |
| Relative miR-378 level | 38.52 | 2.03 | 0.04 |
Table 4. Relative miR-378 level and the risk factors are compared with multiple linear regression.
| β | t | P | |
|---|---|---|---|
| LDL | −0.138 | −2.266 | 0.02 |
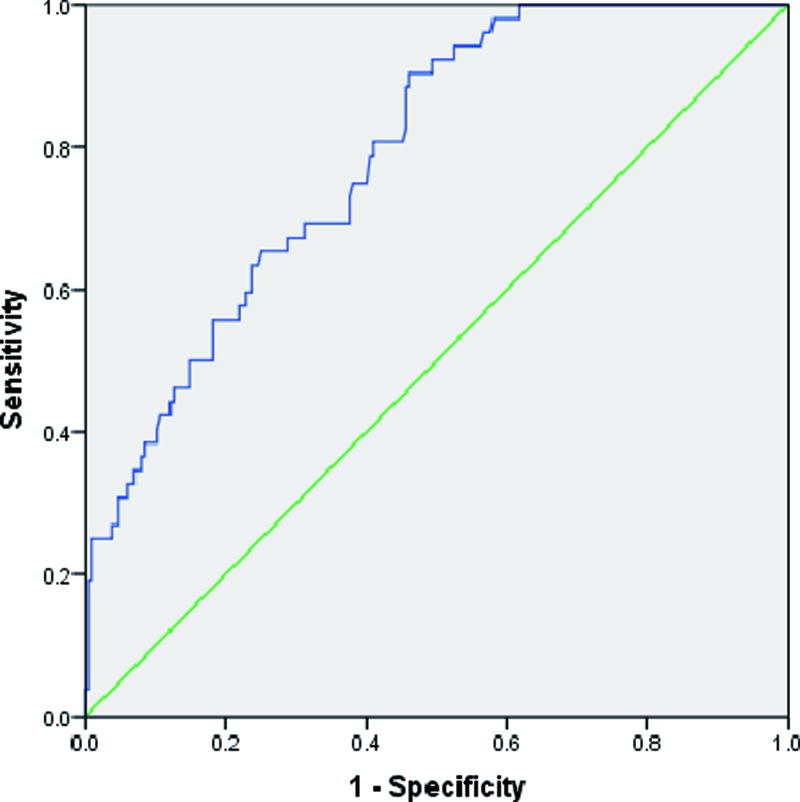
Then, ROC curve was applied to evaluate relative miR-378 levels in predicting coronary stenosis. The area under curve (AUC) is 0.789 (P<0.01) (Figure 3). MiR-378 expression could be used to predict coronary lesions (Table 5). These results indicated that miR-378 had an important prediction value for diagnosis of CAD and decreased plasma miR-378 levels were associated with increasing of severity of CAD.
Figure 3. AUC of ROC was used to study the predictive value of CAD patients.
The AUC is 0.789 (P<0.01).
Table 5. Evaluation of relative miR-378 level by ROC curve in diagnosis of CAD.
| AUC | 95%CI | P | |
|---|---|---|---|
| Relative miR-378 level | 0.789 | 0.728–0.851 | <0.01 |
Discussion
MiRNAs had been demonstrated highly conserved and played much vital roles in cardiovascular disease [8–10]. Recent studies had also shown that circulating miRNAs could act as novel biomarkers for diagnosis of CAD [12]. CAD currently was leading cause of mortality around the world, mainly caused by atherosclerosis. Therefore, early diagnosis was key to treatment of CAD. However, imaging technology was considered gold standard in routine clinical practice. Therefore, a potential biomarker for evaluating the presence and the severity of CAD was needed.
Circulating miRNA-378 was found to remarkably down-regulated in the plasma of CAD patients compared with normal population [16]. MiRNA-378 had been illustrated to participate in diverse physiological and pathological processes. It was reported that expression of miR-378 enhanced cell survival, reduced caspase-3 activity, and promoted angiogenesis [17–19]. Previous studies also demonstrated that miR-378 was as a regulator of cardiomyocyte hypertrophy, which exerted its activity by suppressing MAPK and Ras signaling pathway [20,21]. Kim et al. identified that miR-378 could be as a connective tissue growth factor (Ctgf) regulator in electrical stimulation cardiac stem cells. Ctgf was responsible for stem cell survival and adhesion [22]. Templin et al. reported that miR-378 regulated angiogenic capacity of CD34+ progenitor cells in myocardial infarction [23]. BMP4 was confirmed to be a direct target of miRNA-378 [24]. During the development of atherosclerosis, BMP4 produced in endothelial cells, which then leads to ICAM-1 induction and monocyte binding [25]. BMP4 accelerate the progression of atherosclerosis by induced macrophage foam cell formation through BMPR-2/Smad1/5/8 signaling [26]. Matkovich et al. predicted miRNA-378 should regulate miR-155 [27]; miR-155 have been suggested in high expression levels in pro-inflammatory macrophages in atherosclerotic plaques induced by oxidized LDL [28]. CD14+ monocytic cells have also shown increased miR-155 expression in patients with CAD compared with healthy subjects [28]. Those results indicated that miR-378 might exert a beneficial effect on CAD.
In the present study, we evaluated the relative level of plasma miR-378 in CAD patients whose angiography demonstrated at least one major epicardial artery with >50% stenosis and control group without CAD, which indicated that miR-378 might have a potential impact on predicting coronary lesions for CAD. Plasma miR-378 expression was significantly down-regulated in CAD patients than that of control group. Our result was consistent with Weber’s report [14]. Relative miR-378 level was shown conversely correlated with Gensini Score, which presented the severity of coronary artery lesions. Previous studies had shown that LDL and FBG are risk factors of CAD [29,30]. The same results were confirmed by our study. Moreover, our study revealed that miR-378 level, which was associated with LDL, was also a risk factor of CAD, and had significant difference with others. MiR-378 could effectively distinguish patients with or without coronary artery stenosis, which suggested that miRNA-378 could be used as a biological indicator for estimating coronary lesions. And further studies indicated that the association of miR-378 and CAD directly were required.
There were still several limitations in present study. Given significant cost involved in RT-PCR studies, clinical sample should be increased to enhance the power of statistical analysis. Secondly, we did not illustrate direct relationship in the present study between miR-378, LDL and CAD. Mechanistic studies were needed to confirm its potential as a biomarker.
In conclusion, we found that relative miR-378 levels were significantly lower in CAD patients compared with non-CAD subjects, which had significantly relationship with severity of coronary stenosis. Plasma miR-378 expression could provide further evidence for clinical practice in the diagnosis of CAD.
Abbreviations
- AUC
area under curve
- CAD
coronary artery disease
- Ctgf
connective tissue growth factor
- miRNA
microRNA
- qRT-PCR
quantitative real-time PCR
- ROC
receiver operating characteristic
Funding
The research was supported by National Natural Science Foundation of China [grant numbers 81470403, 81701404]; the Key Research and Development Plan of Shandong Province [grant number 2017GSF18165]; and the China Postdoctoral Science Foundation [grant number 2017M610431].
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Author Contribution
In the whole project, Rong Wang is responsible for designing and funding; Hongshi Li is responsible for collecting references and writing paper; Fei Gao and Lingling Ding are responsible for clinical data collection; Xiaowei Wang and Jiahong Wu are responsible for blood samples collection; Kunze Lu and Rongrong Li are responsible for extraction of RNA; and Minghao Liu is responsible for statistical analysis.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1211–1259 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M.. et al. (2016) Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 133, e38–360 [DOI] [PubMed] [Google Scholar]
- 3.Weber C. and Noels H. (2011) Atherosclerosis: current pathogenesis and therapeutic options. Nat. Med. 17, 1410–1422 10.1038/nm.2538 [DOI] [PubMed] [Google Scholar]
- 4.Sanz J., Moreno P.R. and Fuster V. (2013) The year in atherothrombosis. J. Am. Coll. Cardiol. 62, 1131–1143 10.1016/j.jacc.2013.06.045 [DOI] [PubMed] [Google Scholar]
- 5.Neeland I.J., Patel R.S., Eshtehardi P., Dhawan S., McDaniel M.C., Rab S.T.. et al. (2012) Coronary angiographic scoring systems: an evaluation of their equivalence and validity. Am. Heart J. 164, 547–552, e541 10.1016/j.ahj.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gensini G.G. (1983) A more meaningful scoring system for determining the severity of coronary heart disease. Am. J. Cardiol. 51, 606 10.1016/S0002-9149(83)80105-2 [DOI] [PubMed] [Google Scholar]
- 7.Bartel D.P. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 [DOI] [PubMed] [Google Scholar]
- 8.Welten S.M., Goossens E.A., Quax P.H. and Nossent A.Y. (2016) The multifactorial nature of microRNAs in vascular remodelling. Cardiovasc. Res. 110, 6–22 10.1093/cvr/cvw039 [DOI] [PubMed] [Google Scholar]
- 9.Small E.M. and Olson E.N. (2011) Pervasive roles of microRNAs in cardiovascular biology. Nature 469, 336–342 10.1038/nature09783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widmer R.J., Lerman L.O. and Lerman A. (2016) MicroRNAs: small molecule, big potential for coronary artery disease. Eur. Heart J. 37, 1750–1752 10.1093/eurheartj/ehw067 [DOI] [PubMed] [Google Scholar]
- 11.Gupta S.K., Bang C. and Thum T. (2010) Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ. Cardiovasc. Genet. 3, 484–488 10.1161/CIRCGENETICS.110.958363 [DOI] [PubMed] [Google Scholar]
- 12.Wang G.K., Zhu J.Q., Zhang J.T., Li Q., Li Y., He J.. et al. (2010) Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 31, 659–666 10.1093/eurheartj/ehq013 [DOI] [PubMed] [Google Scholar]
- 13.Cheng C.W., Yeh J.C., Fan T.P., Smith S.K. and Charnock-Jones D.S. (2008) Wnt5a-mediated non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochem. Biophys. Res. Commun. 365, 285–290 10.1016/j.bbrc.2007.10.166 [DOI] [PubMed] [Google Scholar]
- 14.Weber M., Baker M.B., Patel R.S., Quyyumi A.A., Bao G. and Searles C.D. (2011) MicroRNA expression profile in CAD patients and the impact of ACEI/ARB. Cardiol. Res. Pract. 2011, 532915 10.4061/2011/532915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niccoli G., Giubilato S., Di Vito L., Leo A., Cosentino N., Pitocco D.. et al. (2013) Severity of coronary atherosclerosis in patients with a first acute coronary event: a diabetes paradox. Eur. Heart J. 34, 729–741 10.1093/eurheartj/ehs393 [DOI] [PubMed] [Google Scholar]
- 16.Tsui N.B., Ng E.K. and Lo Y.M. (2006) Molecular analysis of circulating RNA in plasma. Methods In Mol. Biol. (Clifton, NJ) 336, 123–134 [DOI] [PubMed] [Google Scholar]
- 17.Lee D.Y., Deng Z., Wang C.H. and Yang B.B. (2007) MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. PNAS 104, 20350–20355 10.1073/pnas.0706901104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang J., Song X.W., Tian J., Chen H.Y., Li D.F., Wang J.F.. et al. (2012) Overexpression of microRNA-378 attenuates ischemia-induced apoptosis by inhibiting caspase-3 expression in cardiac myocytes. Apoptosis 17, 410–423 10.1007/s10495-011-0683-0 [DOI] [PubMed] [Google Scholar]
- 19.Wang S. and Olson E.N. (2009) AngiomiRs – key regulators of angiogenesis. Curr. Opin. Genet. Dev. 19, 205–211 10.1016/j.gde.2009.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganesan J., Ramanujam D., Sassi Y., Ahles A., Jentzsch C., Werfel S.. et al. (2013) MiR-378 controls cardiac hypertrophy by combined repression of mitogen-activated protein kinase pathway factors. Circulation 127, 2097–2106 10.1161/CIRCULATIONAHA.112.000882 [DOI] [PubMed] [Google Scholar]
- 21.Nagalingam R.S., Sundaresan N.R., Gupta M.P., Geenen D.L., Solaro R.J. and Gupta M. (2013) A cardiac-enriched microRNA, miR-378, blocks cardiac hypertrophy by targeting Ras signaling. J. Biol. Chem. 288, 11216–11232 10.1074/jbc.M112.442384 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Kim S.W., Kim H.W., Huang W., Okada M., Welge J.A., Wang Y.. et al. (2013) Cardiac stem cells with electrical stimulation improve ischaemic heart function through regulation of connective tissue growth factor and miR-378. Cardiovasc. Res. 100, 241–251 10.1093/cvr/cvt192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Templin C., Volkmann J., Emmert M.Y., Mocharla P., Muller M., Kraenkel N.. et al. (2017) Increased proangiogenic activity of mobilized CD34+ progenitor cells of patients with acute ST-segment-elevation myocardial infarction: role of differential microRNA-378 expression. Arterioscler. Thrombosis Vasc. Biol. 37, 341–349 10.1161/ATVBAHA.116.308695 [DOI] [PubMed] [Google Scholar]
- 24.Ju H., Yang Y., Sheng A. and Qi Y. (2016) Microrna-378 promotes myogenic differentiation by targeting bmp4. Mol. Med. Rep. 13, 2194–2200 10.3892/mmr.2016.4764 [DOI] [PubMed] [Google Scholar]
- 25.Sorescu G.P., Song H., Tressel S.L., Hwang J., Dikalov S., Smith D.A.. et al. (2004) Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based nadph oxidase. Circ. Res. 95, 773–779 10.1161/01.RES.0000145728.22878.45 [DOI] [PubMed] [Google Scholar]
- 26.Feng J., Gao J., Li Y., Yang Y., Dang L., Ye Y.. et al. (2014) Bmp4 enhances foam cell formation by bmpr-2/smad1/5/8 signaling. Int. J. Mol. Sci. 15, 5536–5552 10.3390/ijms15045536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matkovich S.J., Hu Y. and Dorn G.W. 2nd (2013) Regulation of cardiac micrornas by cardiac micrornas. Circ. Res. 113, 62–71 10.1161/CIRCRESAHA.113.300975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian F.J., An L.N., Wang G.K., Zhu J.Q., Li Q., Zhang Y.Y.. et al. (2014) Elevated microrna-155 promotes foam cell formation by targeting hbp1 in atherogenesis. Cardiovasc. Res. 103, 100–110 10.1093/cvr/cvu070 [DOI] [PubMed] [Google Scholar]
- 29.Nelson C.P., Hamby S.E., Saleheen D., Hopewell J.C., Zeng L., Assimes T.L.. et al. (2015) Genetically determined height and coronary artery disease. N. Engl. J. Med. 372, 1608–1618 10.1056/NEJMoa1404881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry C., Noble S., Grégoire J.C., Ibrahim R., Levesque S., Lavoie M.A.. et al. (2010) Glycaemic status influences the nature and severity of coronary artery disease. Diabetologia 53, 652–658 10.1007/s00125-009-1651-x [DOI] [PubMed] [Google Scholar]